Abstract
Changes in diet greatly affect the mucosal immune system, particularly in diseases such as Crohn’s disease and necrotizing enterocolitis. This article examines the hypothesis that alterations in the luminal environment of the intestine regulate the expression of genes in the enterocyte responsible for signaling to immune cells. Genes expressed by the epithelium orchestrate leukocytes in the lamina propria. For example, chemokine expression in the mouse intestinal epithelium, through transgenic means, induced the recruitment of neutrophils and lymphocytes into intestinal tissues. Diet alters the expression of the genes responsible for signaling by a variety of pathways. The introduction of a normal diet to a weanling mouse up-regulates MHC class II expression through a particular isoform of the class II transactivator, a protein that acts in the nucleus. SCFA concentrations in the intestinal lumen vary markedly with diet. SCFAs increase IL-8 and insulin-like growth factor binding protein-2 expression by inhibiting histone deacetylase activity in the enterocyte. Down-regulation of gene expression by butyrate can act through acetylation of the inhibitory transcription factor Sp3. The review therefore describes a number of molecular pathways, explaining how changes in diet may alter leukocyte recruitment by regulating enterocyte gene expression. Myofibroblasts enhance enterocyte chemotactic activity by cleaving inactive precursors; and myofibroblast genes also are regulated by SCFA. It is likely that other similar regulatory mechanisms remain to be discovered.
Introduction
It has always been inherently plausible that the food we eat should affect the mucosal immune system. The function of the gut-associated lymphoid tissue (GALT)4 is to respond to changes in the environment, and there are fewer changes on the external surface of the body than in the intestine caused by variations in diet. This article examines the possibility that the intestinal epithelium expresses signaling proteins that communicate with cells of the mucosal immune system. If such genes were regulated by diet, it is apparent that changes in nutrients could, through altering the expression of genes in the epithelium, modulate mucosal immune responses. This article, therefore, examines the hypothesis that diet-associated alterations regulate the expression of genes in the enterocyte responsible for signaling to immune cells. It describes the pathways within the cell by which changes in diet alter the expression of major histocompatibility complex class proteins in the intestinal epithelium. SCFA, particularly butyrate, concentrations in the intestinal lumen vary markedly with diet. This article describes pathways by which these products of bacterial fermentation both up-regulate and down-regulate specific immune genes in the epithelium. Finally, it presents evidence that myofibroblasts enhance the chemotactic signals expressed by the epithelium and that butyrate also regulates the expression of the protein responsible for such myofibroblast modulation. These concepts are illustrated with experimental evidence published from our laboratory.
We believe that signaling across the epithelium is important not only in health but also in the treatment of disease. The primary therapy for children with Crohn’s disease in the United Kingdom is treatment with enteral feeds. Randomized control studies showed in the 1980s (1,2) that an elemental diet was as effective in inducing remission as high-dose steroids with the added benefit that it did not suppress linear growth. More recent studies in children demonstrated a rapid fall in immune markers of disease activity, with IL-6 falling within 3 d of starting the diet (3). Changes in nutritional status, such as weight or skinfold thickness were not detectable until after 2 wk. It is established that it was not a change in nutritional body status that resulted in remission of inflammation but a rapid effect, most likely within the intestine itself. Although there are many mechanisms by which enteral feeds may have their activity, we think it possible that 1 of them is by radically altering the luminal environment to such an extent that it varies the signals from the intestinal epithelium to the mucosal immune system.
Genes expressed in the intestinal epithelial cell can be divided into 2 groups. There are the intrinsic proteins, which include those that are important for epithelial function, such as brush border proteins, solute transporters, and brush border enzymes. Proteins necessary for the function of any cell type such as histones, enzymes in the metabolic pathways, and cytoskeletal proteins are also part of this group. The proteins in the second group act as signals between the enterocyte and other cells of the intestine. These include surface molecules such as major histocompatibility complex (MHC) class II and secreted proteins including chemokines or insulin-like growth factor (IGF)-binding proteins.
This latter group of signaling proteins enables the epithelial cell to orchestrate events in the intestine. In our research group we have hypothesized that the expression of signaling molecules by the epithelial cell is regulated by changes in the intestinal lumen. By this means, the intestinal lumen acts through the epithelium to alter indirectly events in the intestine, particularly those of the mucosal immune system. It is also possible that the signaling molecules released have wider effects than on the local immune response.
This article first presents evidence that altering the expression of signaling genes in the enterocyte affects the mucosal immune system. Second, it describes how changes in the intestinal lumen (influenced by diet) alter the expression of these genes. The effects of the lumen on epithelial cell gene expression can, therefore, be considered as an afferent limb in this process; the effect of the epithelial cell on the mucosal immune system is the efferent limb.
The efferent limb
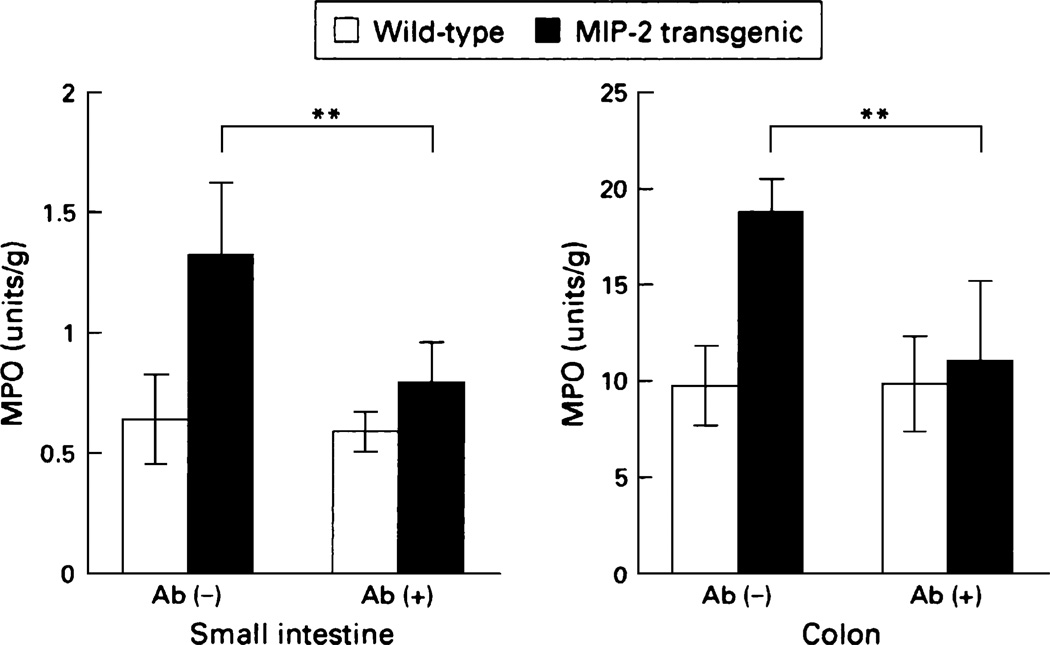
Evidence for the effect of epithelial cell gene expression on the mucosal immune system has come from the ability to selectively alter the expression of genes in the intestinal epithelial cell by transgenic techniques. We have used chemokine expression by the epithelium as a model to show that the epithelium can orchestrate the mucosal immune system. The chemokine IL-8, which in humans results in recruitment of neutrophils, was the first identified chemotatic cytokine. However, IL-8 is not expressed in the mouse. To examine the effects of chemokines on the mucosal immune system, a system was developed whereby the chemokine macrophage inflammatory protein-2 (MIP-2), whose effects are very similar to those of IL-8 in humans, was linked to a FABPI (fatty acid binding protein of the intestine) promoter (4). The promoter is active only in the epithelial cells of the small intestine and proximal colon. A construct was developed in which the FABPI promoter and MIP-2 cDNA were linked to an intron and a polyadenylation site. This construct was injected into mouse oocytes. The epithelium from the first generation of the founder was shown to express MIP-2 mRNA. Analysis showed effects both on neutrophil and on lymphocyte recruitment. The transgenic mice had increased myeloperoxidase activity in the lamina propria of the small intestine and proximal colon (Fig. 1), where the FABPI promoter is active. These findings are indicative of neutrophil infiltration, which was confirmed by histology. In contrast, there was no increase in myeloperoxidase activity and neutrophil recruitment in the distal colon, liver, and spleen, where the FABPI promoter is inactive.
FIGURE 1.
Neutrophil recruitment is increased in expressing MIP-2 in the intestinal epithelium. These effects were blocked with a MIP-2 antibody. Data are means ± SD of 8 animals for each group. **P < 0.01. Reproduced from (4) with permission from the BMJ publishing group.
These data show for the first time that the epithelial cell can, through the release of chemokines, alter themucosal immune function of the intestine in vivo. Interestingly, this effect of epithelial signaling is also seen in a background of inflammation. Wild-type mice fed dextran sodium sulfate exhibited less neutrophil recruitment than MIP-2 transgenic mice receiving the same agent (5).
Further analysis of the immune system in the MIP-2 transgenic mice demonstrated that the small intestine had increased lymphocyte infiltration in addition to neutrophils. Lymphocyte numbers in the lamina propria were significantly increased, and there was also a doubling of the numbers of intraepithelial lymphocytes. The increase of intraepithelial lymphocytes was caused by an increase in αβ-lymphocytes and in γδ-lymphocytes. Further examination of the receptors on the surface of the intraepithelial lymphocytes showed that they expressed the CXCR2, which is the receptor responsible for MIP-2 activity. Therefore, these experiments show that altering the expression of only 1 chemokine in the epithelium has marked effects on both lymphocyte and neutrophil function. However, changes in the intestinal lumen may affect many chemokines as well as other cytokines that alter immune function. It is likely, therefore, that the changes in gene expression in the epithelium have far-reaching effects on the rest of the mucosal immune system.
The afferent limb
In the preceding section we gave evidence that the epithelium can orchestrate the events of the mucosal immune system. It is the purpose of this section to show that alterations in the intestinal lumen can affect the expression of these genes. In our laboratory we examined 3 sets of genes in intestinal epithelium (6) and studied how luminal factors can alter their expression. These included the MHC Class II complex, the IGF-binding protein complex, and the chemokines IL-8 and monocyte chemotactic protein-1 (MCP-1).
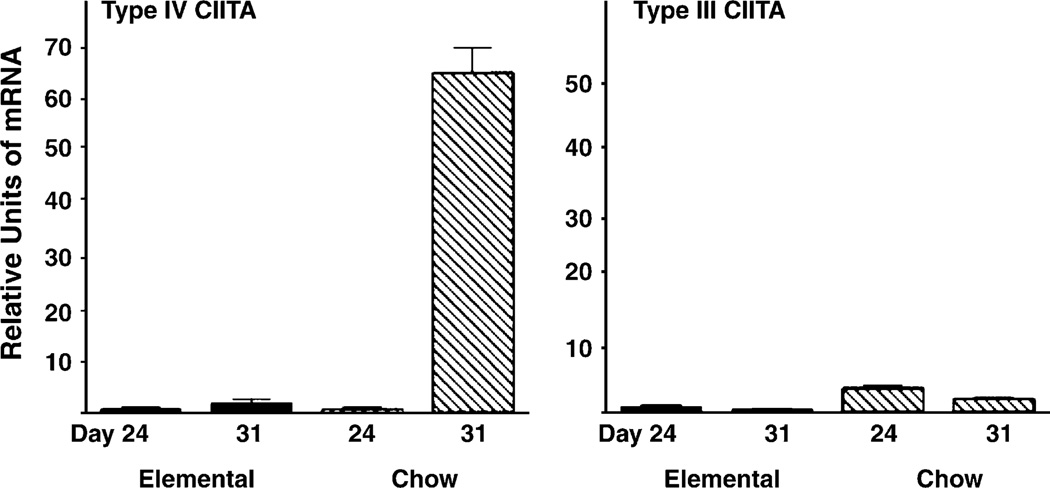
The MHC complex is the structure by which antigen is presented to the T-cell receptor on T lymphocytes. In the epithelial cell of the mouse, it is not expressed until after weaning. Our earlier experiments showed that the timing of its expression was altered by the time of weaning. Furthermore, weaning the mice onto an elemental diet prevented the expression of MHC class II and its associated genes (7), including the invariant chain (Ii chain) (except for some very limited expression at very late time points). The class II transactivator (CIITA) is a nuclear protein whose expression is both necessary and sufficient for MHC class II and Ii chain expression (8). Later experiments demonstrated that weaning up-regulated CIITA expression in mouse intestinal epithelium (9). This was the first demonstration that changes in diet could regulate the nuclear proteins that control gene expression in the intestinal epithelium. Interestingly, CIITA is present in 3 isotypes in mouse intestinal epithelium, and experiments showed that weaning induced only 1 of them, CIITA IV (Fig. 2). CIITA III increased slowly independent of diet, and its expression correlated with the late weak expression in elemental diet fed mice, mentioned earlier.
FIGURE 2.
CIITA IV mRNA increases in the intestinal epithelium by d 31 in weanling mice being fed a complex diet, but CIITA III does not. The figure shows mRNA extracted at 2 experimental time points (d 24 and d 31). Bars represent units of mRNA as a proportion of the mRNA measured on d 24 in mice weaned to an elemental diet (mean and standard deviation). CIITA IV mRNA increased postweaning onto normal complex (semipurified diet) diet (P < 0.02, n = 3). Results are representative of 3 separate experiments. Reproduced from Sanderson et al. (9) with permission from the American Gastroenterological Association, Copyright 2004.
Bacterial fermentation in the intestine results in SCFA production. Butyrate levels therefore reflect changes in bacterial populations, in metabolic activity of the bacterial assemblages, and in the substrates available for bacterial metabolism. Butyrate levels vary greatly in response to external changes. For example, new-born babies have very low butyrate levels in either the small or large intestine. However, within 2 y butyrate levels rise to adult levels (10). Interestingly, butyrate levels are much higher in bottle-fed babies than they are in breast-fed babies during the first 6 mo of life (10).
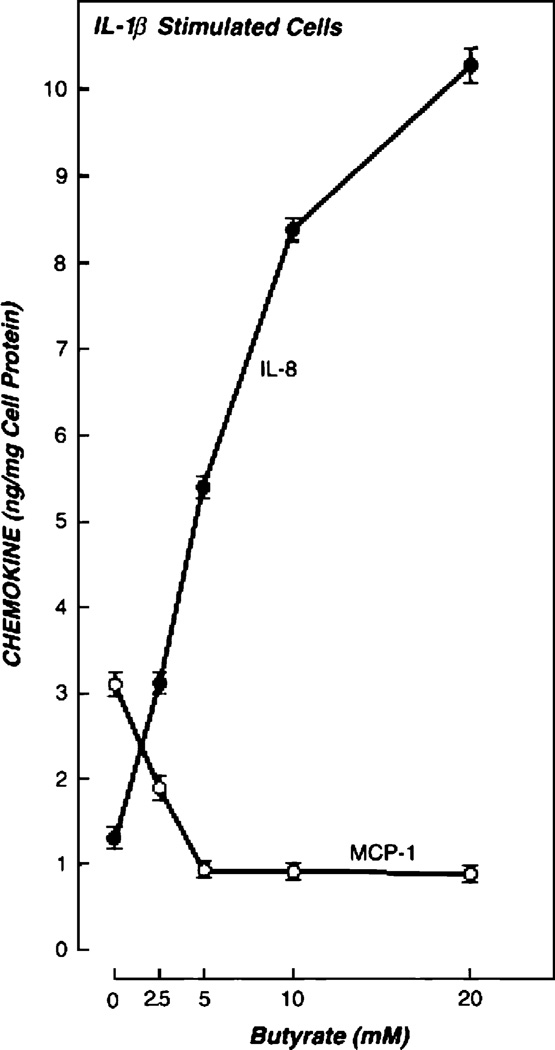
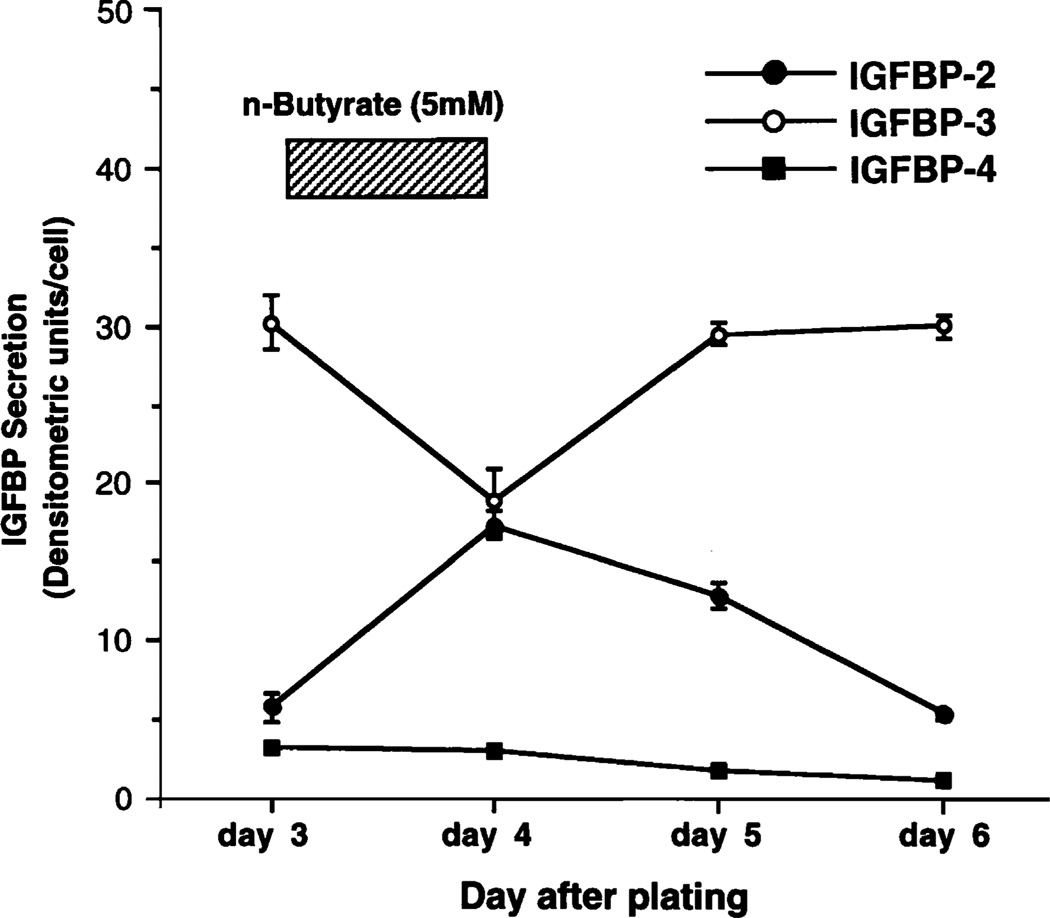
Butyrate levels therefore reflect events in the intestinal lumen, and we hypothesized that their concentrations may alter epithelial cell signaling. We therefore examined the effects of butyrate on the expression of IL-8 and MCP-1 (11,12). Increasing the concentration of sodium butyrate increased IL-8 secretion while it simultaneously decreased MCP-1 expression. These effects were seen in resting epithelial cell lines but were much more marked in cells that had been stimulated with a proinflammatory agent such as LPS or IL-1β (Fig. 3). We also examined the effect of butyrate on IGF binding protein expression (13,14). Unlike the chemokines, IGF binding proteins (IGFBPs) do not require proinflammatory stimulation for expression, but they are constitutively secreted. Their function is to modulate the actions of IGFs, in addition to an inherent mild antiproliferative action on cells. Butyrate reversibly up-regulates IGFBP-2 and down-regulates IGFBP-3 (Fig. 4). Distinct IGF binding proteins have different affinities and differentially alter the bioavailability of IGF-I and IGF-II (14).
FIGURE 3.
Effect of IL-1β and butyrate on IL-8 and MCP-1 secretion by Caco-2 cells. IL-1β alone stimulated the secretion of both IL-8 and MCP-1. Butyrate differentially regulates the pattern of chemokine secretion in IL-1β-stimulated cells. IL-8 secretion was increased (P < 0.0001), whereas MCP-1 secretion was decreased (P < 0.0001). Bars represent standard deviations of 3 different wells for each point. The data are representative of 5 experiments. Reproduced from Fusunyan et al. (11) with permission.
FIGURE 4.
Effect of butyrate on IGFBP secretion in Caco-2 cells. The effect of butyrate on secretion of IGFBPs from Caco-2 cells is reversible. On d 3, conditioned media were collected, and 5 mM butyrate was added to each culture for 24 h. On d 4, conditioned media were collected, each culture well was washed to remove butyrate, and new medium without butyrate was added to culture. Results are means ± SE of triplicate wells and are representative of 3 experiments. Reproduced from Nishimura et al. (14) with permission from the American Physiological Society.
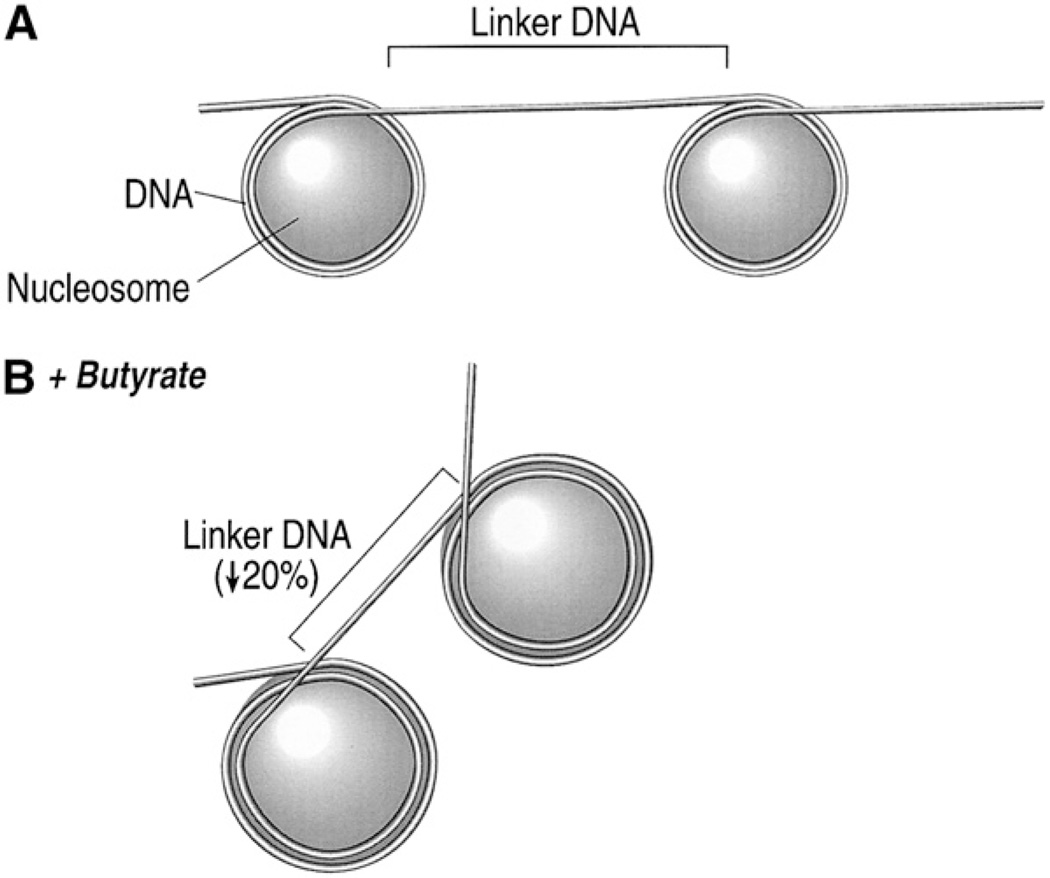
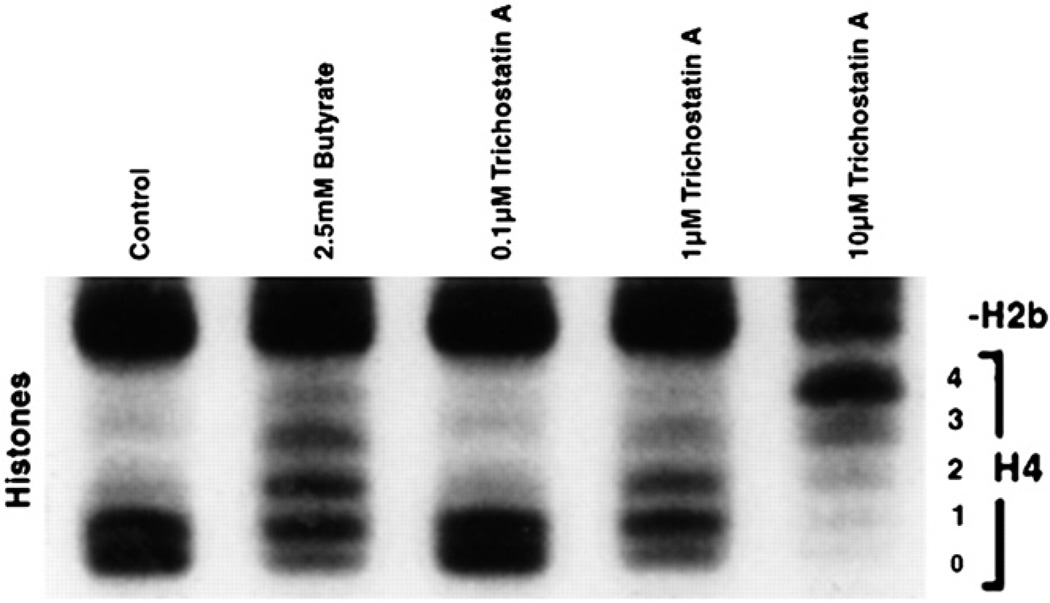
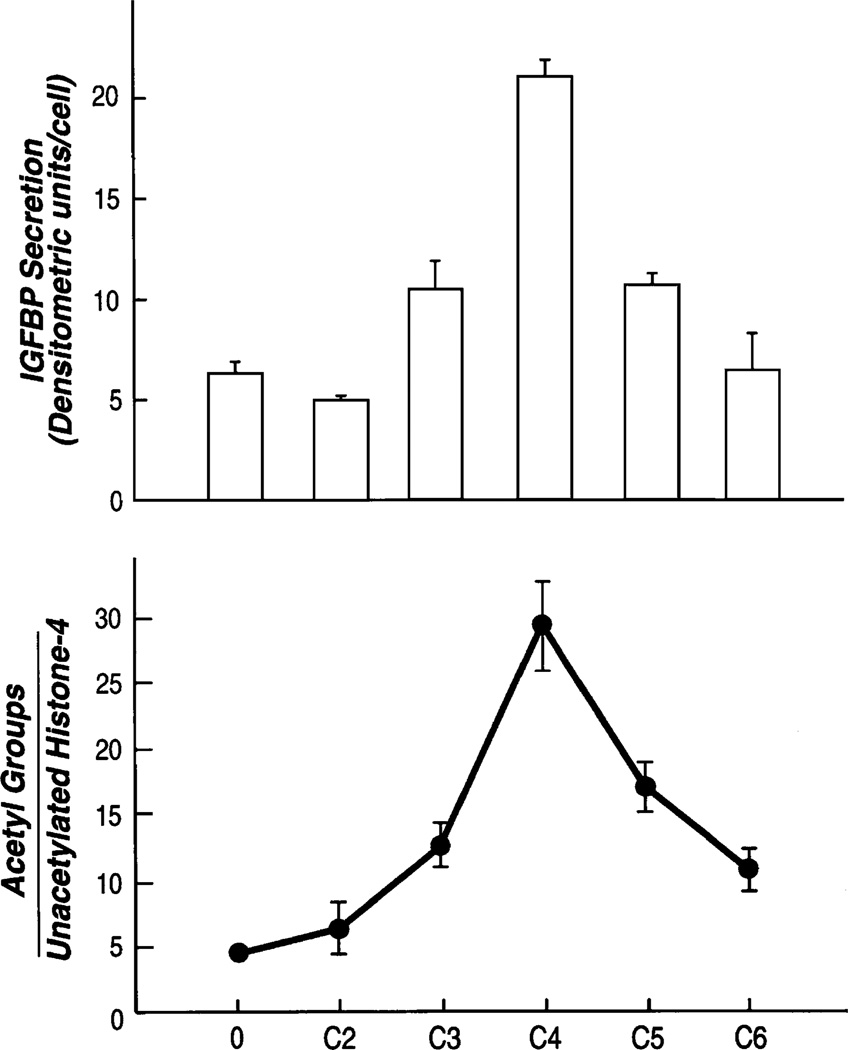
It is known that sodium butyrate alters histone acetylation. The nucleosome consists of a solenoid of histones wrapped around by an integral multiple of 2 turns of DNA (Fig. 5). Butyrate increases histone acetylation, and this reduces the compactness of the histone. The DNA cannot wrap around the large nucleosome in an integral number of turns. The nucleosome can no longer be packaged into tight bundles. This exposes the DNA and makes it more amenable to transcription factors. We hypothesized that butyrate altered the expression of chemokines by this process. To test this hypothesis, we used a fungicide, trichostatin A (TSA), which is 700 times more potent in inducing histone acetylation than butyrate. If the effects of butyrate on chemokine secretion were mediated by increased histone acetylation, we would expect them to be reproduced by the TSA. Experiments with TSA showed that TSA increased IL-8 secretion (12) after IL-1. In addition, it increased IGFBP-2 (14). Figure 6 shows that both TSA and butyrate increased the acetylation of histone 4. Nonacetylated histones move rapidly through the gel and form a single band, whereas acetylated histones form a ladder depending on the degree of acetylation. The histone 4 has 4 lysine residues that are acetylated, and thus, acetylation of histones will result in a ladder of 5 bands. This can be seen in the cells given butyrate or TSA. The effects of butyrate on histone acetylation were reversible (11). After removal of butyrate, histone acetylation returns to normal. Different-length SCFAs have differing effects on histone acetylation. Butyrate is the most effective SCFA in inducing histone acetylation, whereas longer and shorter carbon lengths have lesser effects. This effect on histone acetylation is reflected by effects on the expression of IL-8 (11) and on IGFBP-2 (Fig. 7).
FIGURE 5.
Relation between nucleosomes and DNA. (A) DNA is wrapped 2 full turns around nucleosomes made up of unacetylated histones. (B) With butyrate-induced acetylation, the nucleosome expands, reducing the number of turns of DNA around the nucleosome to 1.8, with less linker DNA connecting each nucleosome. The result of this is that DNA cannot pass linearly from nucleosome to nucleosome but turns at an angle after every nucleosome, leading to disruption of nucleosome packaging. Reprinted from Sanderson and Naik (6), with permission, from the Annual Review of Nutrition, Volume 20, ©2000 by Annual Reviews, www.annualreviews.org.
FIGURE 6.
Effect of butyrate and trichostatin-A in the induction of histone acetylation in Caco-2 cells. Both butyrate and trichostatin A increased the acetylation of histones. Trichostatin-A, a specific histone deacetylase inhibitor, acted in a manner similar to that of butyrate when given at concentrations that produced a comparable change in histone acetylation. Reproduced from Nishimura et al. (14) with permission from the American Physiological Society.
FIGURE 7.
SCFA of different chain lengths (C2–C6) alter IGFBP-2 production according to their effects on histone acetylation. Here 2.5 mmol/L each of acetate (C2), propionate (C3), butyrate (C4), valerate (C5), and hexanoate (C6) were added to Caco-2 cells for 24 h. Acetate and hexanoate had little effect on acetylation and on chemokine expression (P not significant). Valerate and propionate altered both chemokine secretion (P < 0.01) and histone acetylation, but the effects were less than those of butyrate. Bars represent standard deviations of 3 different wells for each point. The data are representative of 3 experiments. Reproduced from Nishimura et al. (14) with permission from the American Physiological Society.
The effects on epithelial cell signaling genes correlate with those seen in other genes regulated by butyrate (15,16), where it enhances gene expression through histone acetylation. These experiments, however, do not exclude the possibility that additional effects of sodium butyrate may occur through promoter systems. It is a challenge of future work to examine the interaction between chromosomal regulation, as is seen in these experiments, and promoter-based regulation with both butyrate and other luminal molecules. In particular, down-regulation of gene expression is difficult to explain by changes in histone acetylation. Loosening protein-DNA interactions would enable DNA to bind to transcription factors and to the machinery of RNA polymerase activity. A possible explanation of how butyrate may down-regulate gene transcription is if it were to induce a repressor, to inhibit gene expression indirectly. However, experiments using cycloheximide to inhibit translation did not alter butyrate’s ability to down-regulate signaling genes, including human IGF binding protein-3 (17). If butyrate were to alter the expression of a repressor though new transcription, interruption of translation of the resulting mRNA would be expected to remove the inhibitor effect of the butyrate. Furthermore butyrate did not change mRNA stability, excluding another possible mechanism. Thus, it must directly act on the target gene itself. One possibility is that it alters the acetylation state of a transcription factor that is already fully synthesized. Certain transcription factors are now known to be capable of acetylation. They include p300 and SP3 (18). It is possible, therefore, that butyrate may down-regulate by also inhibiting deacetylases that act specifically on acetylated transcription factors. Data in our laboratory have shown that butyrate alters the binding of SP3 to DNA and that this is related to SP3 acetylation. Thus, we proposed a model (17) of butyrate action on down-regulation of genes that contain an SP3 site in their promoters. Although we have concentrated on acetylation in this article, there are likely to be other important epigenetic pathways. For example, Waterland (19) has examined the effect of diet on gene methylation in GI development, and such pathways may be relevant in immune signaling.
Myofibroblast interaction
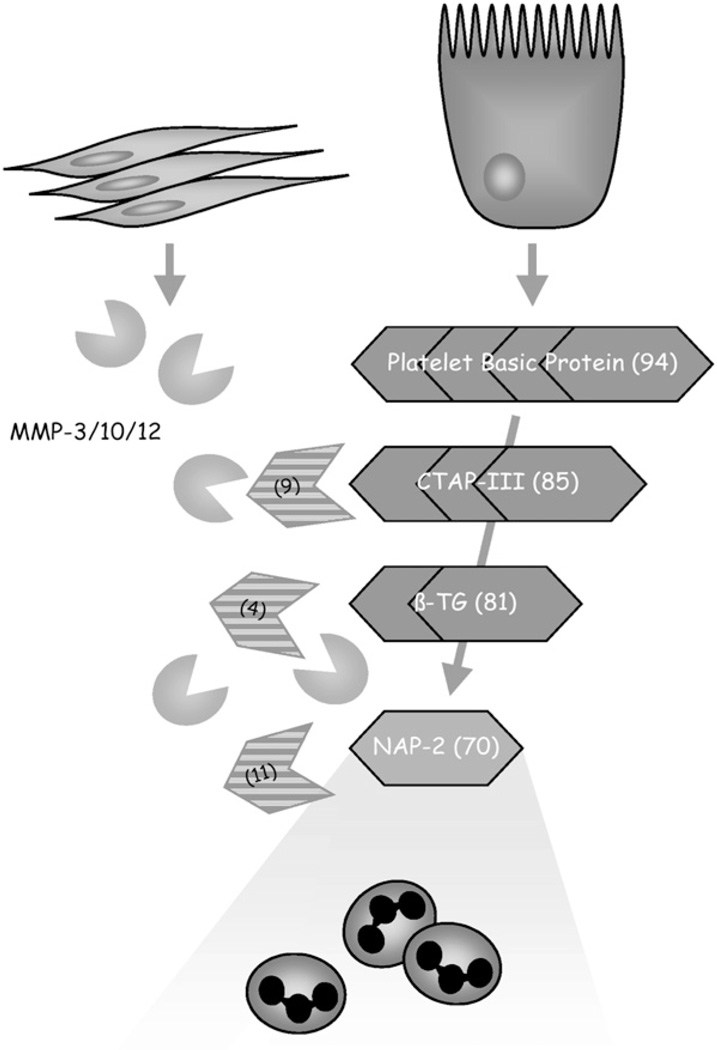
The signaling mechanisms described in the article thus far have assumed that proteins expressed by the enterocyte interact unchanged with the cells of the immune system of the intestine. However, this is not the case. In vitro experiments coincubating myofibroblasts with Caco-2 cells greatly enhanced the chemotactic properties of the cultured supernatant. Analysis of this effect was based on the expression of matrix metalloproteinases (MMPs) by the myofibroblasts that acted on an inactive chemokine precurser. Only after this molecule had been cleaved into the active chemokine NAP-2 (20) was full activity observed (Fig. 8).
FIGURE 8.
Model of MMP-mediated epithelial immune activation through platelet basic protein (PBP) and NAP-2 (CXCL7). Intestinal epithelial cells under proinflammatory conditions synthesize and secrete PBP, which in itself has no chemotactic activity. However, when MMPs are released by activated subepithelial myofibroblasts, epithelial PBP is processed into chemotactically active CXCL7, resulting in the recruitment of neutrophils to the site of inflammation. Reproduced from Kruidenier et al. (20) with permission from the American Gastroenterological Association, Copyright 2006.
These observations are relevant to the action of butyrate in the intestine. Not only does it alter chemokine expression in the intestine, as described earlier; it also regulates MMP expression by intestinal myofibroblasts (21). Here again, the action of butyrate is to increase certain MMPs and to down-regulate others. We also showed that the butyrate alters histone acetylation in a manner similar to that seen in epithelial cells. The question remains, however, to what extent does butyrate enter the stromal cells beneath the epithelium. The tissue concentrations of butyrate are likely to be very low in healthy intestine; but where the epithelium is breached in inflammatory conditions such as ulcerative colitis, Crohn’s disease, or necrotising enterocolitis, its effects could be substantial.
It is likely that inulin and oligofructose significantly alter the luminal environment. Our experiments examine the hypothesis that changes in the intestinal lumen can alter the expression of molecules in the enterocyte that direct the mucosal immune system. The intestinal epithelium acts as a relay for transducing the information of the intestinal environment to the mucosal immune system. Such a relay is also seen in surface receptors that signal the presence of microbial-associated molecular patterns through the epithelium (22). This mechanism has advantages over other forms of immune surveillance in the gut that require the breach of the mucosal barrier. Such breaches can be manipulated by invading organisms to enter the body. The classic example of this is the polio virus, which enters the intestine through the M cell to the immune system of Peyer’s patch, which is designed as a sampling system of the mucosal environment.
Footnotes
Published in a supplement to The Journal of Nutrition. Presented at the conference “5th ORAFTI Research Conference: Inulin and Oligofructose: Proven Health Benefits and Claims” held at Harvard Medical School, Boston, MA, September 28–29, 2006. This conference was organized and sponsored by ORAFTI, Belgium. Guest Editors for the supplement publication were Marcel Roberfroid, Catholique University of Louvain, Brussels, Belgium and Randal Buddington, Mississippi State University, USA. Guest Editor disclosure: M. Roberfroid and R. Buddington, support for travel to conference provided by ORAFTI.
Supported by the following research grants: NIH (AI43472, DK47753, DK43351, DK40561, DK 33506) and the Medical Research Council (48475).
Author disclosure: I. R. Sanderson, support for travel to conference provided by ORAFTI, and an honorarium of €250 was paid to his university research account.
Abbreviations used: CIITA, class II transactivator; FABPI, fatty acid binding protein of the intestine; GALT, gut-associated lymphoid tissue; IGF, insulin-like growth factor; IGFBP, Insulin-like growth factor binding protein; MHC, major histocompatibility complex; MIP-2, macrophage inflammatory protein-2; MMP, matrix metalloproteinase; PBP, platelet basic protein; TSA, trichostatin A.
Literature Cited
- 1.Sanderson IR, Udeen S, Davies PS, Savage MO, Walker-Smith JA. Remission induced by an elemental diet in small bowel Crohn’s disease. Arch Dis Child. 1987;62:123–127. doi: 10.1136/adc.62.2.123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sanderson IR, Boulton P, Menzies I, Walker-Smith JA. Improvement of abnormal lactulose/rhamnose permeability in active Crohn’s disease of the small bowel by an elemental diet. Gut. 1987;28:1073–1076. doi: 10.1136/gut.28.9.1073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bannerjee K, Camacho-Hübner C, Babinska K, Dryhurst KM, Edwards R, Savage MO, Sanderson IR, Croft NM. Anti-inflammatory and growth-stimulating effects precede nutritional restitution during enteral feeding in Crohn disease. J Pediatr Gastroenterol Nutr. 2004;38:270–275. doi: 10.1097/00005176-200403000-00007. [DOI] [PubMed] [Google Scholar]
- 4.Ohtsuka Y, Lee J, Stamm DS, Sanderson IR. MIP-2 secreted by epithelial cells increases neutrophil and lymphocyte recruitment in the mouse intestine. Gut. 2001;49:526–533. doi: 10.1136/gut.49.4.526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ohtsuka Y, Sanderson IR. Dextran sulfate sodium-induced inflammation is enhanced by intestinal epithelial cell chemokine expression in mice. Pediatr Res. 2003;53:143–147. doi: 10.1203/00006450-200301000-00024. [DOI] [PubMed] [Google Scholar]
- 6.Sanderson IR, Naik S. Dietary regulation of intestinal gene expression. Annu Rev Nutr. 2000;20:311–338. doi: 10.1146/annurev.nutr.20.1.311. [DOI] [PubMed] [Google Scholar]
- 7.Sanderson IR, Ouellette AJ, Carter EA, Harmatz PR. Ontogeny of Ia messenger RNA in the mouse small intestinal epithelium is modulated by age of weaning and diet. Gastroenterology. 1993;105:974–980. doi: 10.1016/0016-5085(93)90939-a. [DOI] [PubMed] [Google Scholar]
- 8.Steimle V, Otten LA, Zufferey M, Mach B. Complementation cloning of an MHC class II transactivator mutated in hereditary MHC class II deficiency (or bare lymphocyte syndrome) Cell. 1993;75:135–146. [PubMed] [Google Scholar]
- 9.Sanderson IR, Bustin SA, Dziennis S, Paraszczuk J, Stamm DS. Age and diet act through distinct isoforms of the class II transactivator gene in mouse intestinal epithelium. Gastroenterology. 2004;127:203–212. doi: 10.1053/j.gastro.2004.04.014. [DOI] [PubMed] [Google Scholar]
- 10.Midtvedt AC, Midvedt T. Production of short chain fatty acids by the intestinal microflora during the first 2 years of human life. J Pediatr Gastroenterol Nutr. 1992;15:395–403. doi: 10.1097/00005176-199211000-00005. [DOI] [PubMed] [Google Scholar]
- 11.Fusunyan RD, Quinn JJ, Fujimoto M, MacDermott RP, Sanderson IR. Butyrate switches the pattern of chemokine secretion by intestinal epithelial cells through histone acetylation. Mol Med. 1999;5:631–640. [PMC free article] [PubMed] [Google Scholar]
- 12.Ohno Y, Lee J, Fusunyan RD, MacDermott RP, Sanderson IR. Macrophage inflammatory protein-2: chromosomal regulation in rat small intestinal epithelial cells. Proc Natl Acad Sci USA. 1997;94:10279–10284. doi: 10.1073/pnas.94.19.10279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Oguchi S, Walker WA, Sanderson IR. Profile of IGF-binding proteins secreted by intestinal epithelial cells changes with differentiation. Am J Physiol. 1994;267:G843–G850. doi: 10.1152/ajpgi.1994.267.5.G843. [DOI] [PubMed] [Google Scholar]
- 14.Nishimura A, Fujimoto M, Oguchi S, Fusunyan RD, MacDermott RP, Sanderson IR. Short-chain fatty acids regulate IGF-binding protein secretion by intestinal epithelial cells. Am J Physiol. 1998;275:E55–E63. doi: 10.1152/ajpendo.1998.275.1.E55. [DOI] [PubMed] [Google Scholar]
- 15.de Ruijter AJ, van Gennip AH, Caron HN, Kemp S, van Kuilenburg AB. Histone deacetylases (HDACs): characterization of the classical HDAC family. Biochem J. 2003;370:737–749. doi: 10.1042/BJ20021321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Davie JR. Inhibition of histone deacetylase activity by butyrate. J Nutr. 2003;133:2485S–2493S. doi: 10.1093/jn/133.7.2485S. [DOI] [PubMed] [Google Scholar]
- 17.White NR, Mulligan P, King PJ, Sanderson IR. Sodium butyrate-mediated Sp3 acetylation represses human insulin-like growth factor binding protein-3 expression in intestinal epithelial cells. J Pediatr Gastroenterol Nutr. 2006;42:134–141. doi: 10.1097/01.mpg.0000189345.31010.89. [DOI] [PubMed] [Google Scholar]
- 18.Braun H, Koop R, Ertmer A, Nacht S, Suske G. Transcription factor Sp3 is regulated by acetylation. Nucleic Acids Res. 2001;29:4994–5000. doi: 10.1093/nar/29.24.4994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Waterland RA. Epigenetic mechanisms and gastrointestinal development. J Pediatr. 2006;149(5) Suppl:S137–S142. doi: 10.1016/j.jpeds.2006.06.064. [DOI] [PubMed] [Google Scholar]
- 20.Kruidenier L, Macdonald TT, Collins JE, Pender SL, Sanderson IR. Myofibroblast matrix metalloproteinases activate the neutrophil chemoattractant CXCL7 from intestinal epithelial cells. Gastroenterology. 2006;130:127–136. doi: 10.1053/j.gastro.2005.09.032. [DOI] [PubMed] [Google Scholar]
- 21.Pender SL, Quinn JJ, Sanderson IR, MacDonald TT. Butyrate upregulates stromelysin-1 production by intestinal mesenchymal cells. Am J Physiol Gastrointest Liver Physiol. 2000;279:G918–G924. doi: 10.1152/ajpgi.2000.279.5.G918. [DOI] [PubMed] [Google Scholar]
- 22.Sanderson IR, Walker WA. TLRs in the gut, I. The role of TLRs/Nods in intestinal development and homeostasis. Am J Physiol Gastrointest Liver Physiol. 2007;292:G6–G10. doi: 10.1152/ajpgi.00275.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]