Abstract
The AMP-activated protein kinase (AMPK) is a sensor of cellular energy status expressed in essentially all eukaryotic cells. Once activated by energetic stress via a mechanism that detects increases in AMP:ATP and ADP:ATP ratios, AMPK acts to restore energy homeostasis by switching on catabolic pathways that generate ATP, while switching off ATP-consuming processes, including anabolic pathways required for cell growth and proliferation. AMPK activation promotes the glucose-sparing, oxidative metabolism utilized by most quiescent cells, rather than the rapid glucose uptake and glycolysis used by most proliferating cells. Numerous pharmacological activators of AMPK are known, including drugs in long use such as salicylate and metformin, and there is evidence that regular use of either of the latter provides protection against development of cancer. Tumor cells appear to be under selection pressure to down-regulate AMPK, thus limiting its restraining influence on cell growth and proliferation, and several interesting mechanisms by which this occurs are discussed. Paradoxically, however, a complete loss of AMPK function, which appears to be rare in human cancers, may be deleterious to survival of tumor cells. AMPK can therefore either be a friend and a foe in cancer, depending on the context.
Background
The AMP-activated protein kinase (AMPK) is a sensor of cellular energy status and a key regulator of energy homeostasis, which exists universally in eukaryotes as heterotrimeric complexes containing catalytic α and regulatory β and γ subunits (1, 2). In human, there are multiple isoforms of each subunit (AMPK-α1, -α2; -β1, -β2; -γ1, -γ2, -γ3) encoded by distinct genes (PRKAA1, PRKAA2; PRKAB1, PRKAB2; PRKAG1, PRKAG2, PRKAG3), generating up to twelve heterotrimeric combinations. In the yeast Saccharomyces cerevisiae, the AMPK ortholog is required for the response to glucose starvation, especially for the switch from rapid growth in high glucose using fermentative metabolism (i.e. glycolysis) to the slower growth using oxidative metabolism that occurs when glucose becomes limiting (3). This metabolic switch is equivalent to reversal of the “Warburg effect” that occurs in many rapidly proliferating mammalian cells, including tumor cells.
ATP and ADP can be likened to the chemicals in a rechargeable battery, with a high ratio of ATP:ADP representing a fully charged cellular “battery”, while any decrease indicates that the battery is becoming flat. Because the reaction catalyzed by adenylate kinase (2ADP ↔ ATP + AMP) operates close to equilibrium in most eukaryotic cells, any increase in ADP:ATP is always accompanied by a much larger rise in AMP:ATP (4), making the latter ratio a particularly sensitive indicator of energy stress. AMPK monitors cellular energy status by detecting increases in these ratios. In all species, it is activated >100-fold by phosphorylation of a conserved threonine residue (Thr172 in rat α2 (5)) located within the “activation loop” of the α subunit kinase domain. The primary upstream kinase phosphorylating this site in mammalian cells is a complex comprising the protein kinase LKB1 and two accessory subunits, STRAD and MO25 (6). Heterozygous mutations in STK11, the human gene encoding LKB1, had been identified as the cause of Peutz-Jeghers syndrome, an inherited susceptibility to cancer (7, 8). Thus, LKB1 is a tumor suppressor, and the findings that it acted upstream of AMPK introduced the first link between AMPK and cancer.
The γ subunits of AMPK contain three binding sites for AMP, with ADP and ATP binding in competition with AMP, at least at two of them (9, 10). AMP binding activates AMPK by three distinct mechanisms: (i) increased Thr172 phosphorylation by LKB1; (ii) decreased Thr172 dephosphorylation by protein phosphatases; (iii) allosteric activation (>10-fold) (11) (Fig. 1). This tripartite mechanism makes the system an exquisitely sensitive sensor of cellular energy status. Effects (i) and possibly (ii), but not (iii), are mimicked by binding of ADP, while all three are antagonized by ATP (11-13). All three are due to binding of AMP to AMPK itself, rather than to the upstream kinase or phosphatase. Thus, although LKB1 normally has to be present for cellular energy stress to activate AMPK, it is not itself activated by it (14). An alternate upstream kinase phosphorylating Thr172, the calmodulin-dependent kinase CaMKKβ (encoded by CAMKK2), is only active in cells when intracellular Ca2+ has been elevated (Fig. 1). This alternate, AMP-independent pathway mediates the effects of hormones that use Ca2+ as second messenger (15, 16).
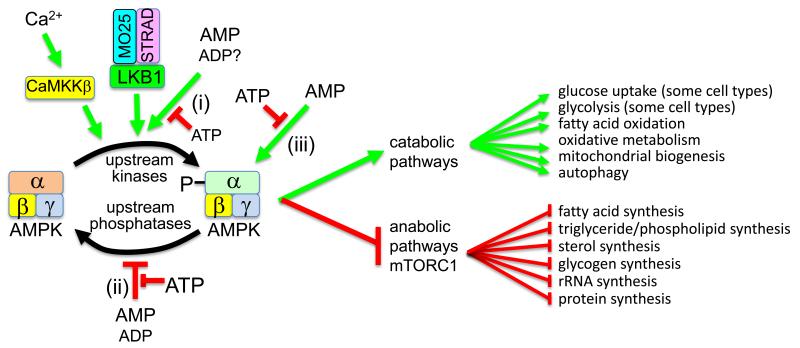
Figure 1.
Tripartite mechanism for AMPK activation by 5′-AMP. AMPK is phosphorylated at Thr172 and activated by upstream kinases, especially the constitutively active kinase LKB1 (which is only active in complex with MO25 and STRAD) and the Ca2+/calmodulin-dependent kinase kinase, CaMKKβ. Binding of AMP to AMPK activates the kinase by three mechanisms, all of which are antagonized by ATP: (1) binding of AMP (and possibly ADP) promotes Thr172 phosphorylation by LKB1; (2) binding of AMP (and ADP at higher concentrations) inhibits Thr172 dephosphorylation by phosphatases; (3) binding of AMP (but not ADP) causes 10-fold allosteric activation. Once activated by energy stress, AMPK acts to restore energy homeostasis by activating catabolic pathways (including oxidative metabolism) and by inhibiting anabolic pathways (including those downstream of mTORC1).
Once activated by energy stress, AMPK acts to restore energy homeostasis by promoting catabolic pathways generating ATP, while inhibiting ATP-consuming processes (1). The latter include most anabolic pathways, including those promoted by the mechanistic target-of-rapamycin complex-1 (mTORC1) signaling pathway, which is inhibited by AMPK (17, 18). Since AMPK switches off the synthesis of lipids, RNAs and proteins, it inhibits cell growth. It also causes a G1 cell cycle arrest by promoting phosphorylation of p53, thus blocking DNA synthesis (19, 20). Although AMPK can acutely enhance glucose uptake and glycolysis in some cell types, in the longer term it promotes (like its yeast ortholog) the more glucose-sparing, mitochondrial oxidative metabolism used by quiescent cells, rather than the rapid glucose uptake, glycolysis and pentose phosphate pathway used predominantly by proliferating cells (21).
Numerous pharmacological agents that activate AMPK have been identified, including many natural plant products, or their derivatives, used in traditional medicines (22). These include the anti-diabetic biguanides metformin (23) and phenformin (6), both derived from the natural product galegine, as well as salicylate, the active component of willow bark of which acetyl salicylic acid (ASA or aspirin) is a synthetic derivative as well as a pro-drug (24). Metformin, phenformin and galegine, and many natural products such as resveratrol and berberine, activate AMPK indirectly by inhibiting mitochondrial ATP synthesis, thus increasing cellular AMP (25). However, salicylate activates AMPK by direct binding in a cleft between the α and β subunits, with the same site being used by synthetic activators such as A-769662 and 991 (26, 27). A third activation mechanism is exemplified by 5-aminoimidazole-4-carboxamide ribonucleoside (AICAR), a nucleoside taken up by cells and phosphorylated to the nucleotide ZMP, which mimics the effects of AMP (28). Interestingly, ZMP is an intermediate in the pathway of purine nucleotide biosynthesis, and is metabolized by a transformylase that utilizes N10-formyl-tetrahydrofolate. Some antifolates used to treat cancer, including pemetrexed and methotrexate, inhibit this transformylase and thus cause ZMP accumulation and AMPK activation (29, 30).
As well as being required for activation of AMPK, LKB1 also activates a family of twelve AMPK-related kinases (ARKs) by phosphorylating the threonine residue equivalent to Thr172 (31). None of these appear to be activated by energy stress or to directly inhibit cell growth and division, and it therefore seems likely that most tumor suppressor effects of LKB1 are mediated by AMPK. However, reduced function caused by loss of LKB1 of two of the ARKs, MARK1 and MARK4, does contribute to increased migration and metastasis of epithelial tumor cells in mouse models (32).
Clinical-Translational Advances
Loss of a single AMPK-α1 allele accelerates development of lymphomas induced in mice by transgenic expression of Myc in B cells, while loss of both alleles has an even larger effect (33). Although this suggests that AMPK can act as a tumor suppressor, mutations in genes encoding AMPK subunits appear to be rather infrequent in human cancers. This might either be because of redundancy between AMPK isoforms, or perhaps more likely because a low level of AMPK is required to maintain viability during the metabolic stresses that tumor cells often experience. In support of the latter, mouse embryo fibroblasts (MEFs) totally deficient in LKB1 (34) or AMPK (35) are resistant to transformation by mutant H-Ras, although MEFs lacking only AMPK-α2 display increased susceptibility to transformation by mutant H-Ras in vitro, and increased growth as xenografts expressing mutant H-Ras in vivo (36). Thus, although a low level of AMPK function may be necessary for tumor cells to survive, reduction in normal expression levels may nevertheless promote tumorigenesis by reducing the restraining influence of AMPK on cell growth and division. Consistent with this, AMPK is often down-regulated in tumors by mechanisms other than somatic mutations. For example, immunohistochemical analysis of human breast cancer biopsies revealed reduced expression of AMPK-α subunits phosphorylated on Thr172, compared with surrounding normal tissue, in >90% of cases (37). The antibody used in this study does not distinguish between AMPK-α1 and -α2, and it was also not clear whether there was reduced expression of total AMPK-α subunits. However, reduced expression of AMPK-α2 has been found to be a frequent occurrence in hepatocellular carcinoma, which is associated with poor prognosis (38). The mechanisms by which down-regulation occurs in these cases remain unclear. One obvious mechanism is genetic loss of LKB1, which still allows some residual AMPK function due to the alternate CaMKKβ–mediated upstream pathway (15). However, while loss of LKB1 is relatively frequent in non-small cell lung cancer [≈30% (39, 40)] and cervical cancer [≈20% (41)], it appears to be less frequent in most other cancers, including breast cancer.
Another mechanism for down-regulation of AMPK involves the insulin/IGF1-regulated protein kinase Akt/PKB, which is hyper-activated in many tumors by gain-of-function mutations in phosphatidylinositol 3-kinases or loss-of-function mutations in PTEN. Akt phosphorylates rodent AMPK-α1 at Ser485 (Ser487 in humans) within a serine/threonine-rich loop (the “ST loop”) (42, 43). This inhibits subsequent Thr172 phosphorylation and activation by LKB1 or CaMKKβ, because the phosphorylated ST loop interacts with the kinase domain and blocks access to Thr172 (43). Ser487 hyper-phosphorylation occurs in several PTEN-deficient glioblastoma and breast cancer cell lines, and in these cells it is more difficult to activate AMPK (43). Consistent with this, in a mouse model in which PTEN was knocked out in thyrocytes, Ser485 phosphorylation was increased and Thr172 phosphorylation decreased. This was associated with thyroid gland hyperplasia at birth that was reduced by treatment with the AMPK activator, AICAR, and with occurrence of thyroid follicular adenomas by 6-8 months (44).
A third mechanism for AMPK down-regulation was observed in human melanoma cells carrying the B-Raf V600E mutation. This mutation activates B-Raf, causing activation of the downstream kinases Erk and RSK, which promote phosphorylation of sites in the C-terminal domain of LKB1 that appear to reduce its ability to activate AMPK (45). Interestingly, AMPK also phosphorylates B-Raf at a C-terminal site (Ser729), promoting its association with 14-3-3 proteins and disrupting its interaction with the scaffold protein KSR1, thus exerting a reciprocal negative effect that reduces proliferation and cell cycle progression in keratinocytes (46). These findings may have therapeutic implications, because the B-Raf inhibitor PLX4720 and the AMPK activator phenformin caused synergistic decreases in cell viability in melanoma cells in culture, and reduced growth of human melanoma cells as mouse xenografts, and growth of melanomas in a genetically engineered B-RafV600E mouse model (47).
Another intriguing mechanism by which AMPK is down-regulated in tumors has recently been reported (48). MAGE-A3 and -A6 are closely related members of the melanoma antigen family, encoded by neighboring genes on the X chromosome. Like most other MAGE proteins they are usually only expressed in testis, but become aberrantly re-expressed in many tumors, which is associated with enhanced viability of the tumor cells and poor prognosis for the patient. Expression of MAGE-A3/–A6 in NIH-3T3 cells promoted focus formation, while expression in immortalized human colon epithelial cells promoted anchorage-independent growth. MAGE-A3/–A6 are known to interact with the E3 ubiquitin ligase TRIM28, triggering polyubiquitylation and proteasomal degradation of p53 (49). However, many tumor cells in which MAGE-A3/–A6 expression enhances viability are p53-deficient, suggesting that they must have other targets. A screen for such targets identified AMPK-α1, and MAGE-A3 was found to interact with AMPK-α1, targeting it for polyubiquitylation by TRIM28 and proteasomal degradation. Consistent with this, knocking down MAGE-A3/A6 in tumor cells increased expression of total and Thr172 phosphorylated AMPK-α1, and produced many changes in downstream signaling and metabolism expected after AMPK activation. Analysis of the human cancer genome atlas showed that MAGE-A3/A6 were expressed in 20% of colorectal adenocarcinomas, 80% of lung squamous cell carcinomas, and 25% of breast invasive carcinomas, and expression correlated with marked reductions of total and Thr172-phosphorylated AMPK-α subunits, and with hyper-activation of mTORC1. Moreover, in immortalized human colon epithelial cells in which anchorage-independent growth was induced by expression of MAGE-A6, the AMPK activators AICAR and A-769662 reduced cell growth, while failing to do this in cells transformed with other oncogenes, such as MAGE-A10 (48).
A final mechanism for down-regulation of the LKB1-AMPK pathway in tumor cells involves microRNAs, short single-stranded RNAs that bind the 3′-untranslated regions (3′-UTRs) of specific mRNAs and reduce their translation into protein. One, miR-451, is over-expressed in many human glioblastomas. A key target for miR-451 was found to be the mRNA encoding MO25, one of the subunits of the LKB1 complex, and miR-451 over-expression reduced expression of MO25 and consequent Thr172 phosphorylation on AMPK (50). Another microRNA, miR-301a, appears to directly down-regulate AMPK-α1 in osteosarcoma cells (51).
Intriguingly, epidemiological studies in humans provide evidence that prolonged use of known AMPK activators provide protection against cancer development. Thus, type 2 diabetics taking metformin have a lower incidence of cancer (52), as do subjects taking aspirin in randomized control trials of its efficacy in protecting against cardiovascular events (53). It should be emphasized that there is currently no direct evidence that these apparent effects are mediated by AMPK activation, nor that they are direct effects on the tumor cells themselves. The metformin studies compared diabetics taking the drug with those on other medications, which would particularly include sulfonylureas and insulin. Subjects with untreated Type 2 diabetes usually exhibit hyperinsulinemia, and metformin (due to its insulin-sensitizing effects, mediated by AMPK activation in the liver (54)) reduces this. By contrast, sulfonylureas enhance insulin secretion and thus increase plasma insulin, as does therapy with insulin itself. As insulin is a promoter of cell growth, reduction of hyperinsulinemia has been proposed to explain the protective effects of metformin in cancer. Some evidence in favor of this came from studies of human colon carcinoma cells grown as mouse xenografts, whose growth was reduced by treatment with metformin in mice that had been made insulin-resistant by feeding a high-fat diet, but not in mice on a normal chow diet. The same effects were observed whether or not LKB1 had been previously knocked down in the cells using shRNAs, suggesting that the effect of metformin was not to activate AMPK in the tumor cells themselves (55).
Although the mechanism for the apparent protective effect of metformin on the incidence of cancer in humans remains uncertain, the association has triggered many trials of metformin treatment in cancer (over 200 listed in www.clinicaltrials.gov). Many of these are small-scale pilots, but the MA.32 trial is recruiting >3,000 women with early stage breast cancer, who will receive metformin or placebo for 5 years as an adjunct to existing therapy (56).
Most of the pre-clinical and clinical data discussed above support the idea that AMPK is a “friend” in cancer, since it is a tumor suppressor down-regulated in a high proportion of cancers. However, tumor cells often experience metabolic stresses that occur when their growth outstrips the ability of their blood supply to provide oxygen and nutrients, while many cytotoxic therapies also cause cellular stress. As discussed above, there is evidence that a low level of AMPK may be necessary to maintain viability of tumor cells under these circumstances. Here, AMPK is acting as a “friend” to the tumor cells but a “foe” to the patient. A possible example of this was provided by a mouse model of non-small cell lung cancer, in which treatment with phenformin prolonged survival when the tumors were caused by mutant K-Ras combined with loss of LKB1, but not by mutant KRas and loss of p53, where the LKB1-AMPK pathway would still be functional (57). In this scenario, phenformin is acting as a cytotoxic drug by inhibiting mitochondrial ATP synthesis, which kills LKB1-deficient tumor cells because they lack normal AMPK function to protect them, unlike surrounding normal cells.
In another study of the LKB1-deficient A549 lung cancer cell line, glucose deprivation was shown to cause cell death by generating oxidative stress, but this was relieved by re-expressing LKB1 to restore AMPK activation. The effect of AMPK on cell survival was ascribed to its ability to phosphorylate and inactivate acetyl-CoA carboxylases-1 and -2 (ACC1/ACC2), thus inhibiting fatty acid synthesis and preserving NADPH for the reduction of oxidized glutathione to counter oxidative stress (58). Finally, in an shRNA screen looking for human kinases whose loss caused synthetic lethality when combined with over-expression of Myc, two of the hits were AMPK-α1 and the AMPK-related kinase, Ark5/Nuak1. Although the authors chose to follow-up the latter rather than the former, these results suggest that AMPK-α1 is required for transformation by Myc over-expression (59).
Conclusions
Although AMPK restrains the growth and proliferation of cells, and there appears to be selection pressure for tumor cells to down-regulate the pathway, a low residual level of AMPK function may be necessary for tumor cells to overcome the nutritional and energetic stresses that often occur during their development. Paradoxically, therefore, while treatment with AMPK activators may restrain the initial growth and proliferation of tumor cells, and there is selection pressure for the pathway to be down-regulated, a low level of residual AMPK function may be necessary for tumor tissue to survive the rigors of their existence. It is possible that, in such cases, AMPK inhibitors might be useful as adjuncts to conventional chemotherapy in treatment of cancer.
Acknowledgments
Grant Support
D.G. Hardie is supported by a Senior Investigator Award from the Wellcome Trust (097726) and a Programme Grant (C37030/A15101) from Cancer Research UK.
Footnotes
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
References
- 1.Hardie DG, Ross FA, Hawley SA. AMPK: a nutrient and energy sensor that maintains energy homeostasis. Nature Rev Mol Cell Biol. 2012;13:251–62. doi: 10.1038/nrm3311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hardie DG. AMPK - sensing energy while talking to other signaling pathways. Cell Metab. 2014;20:939–52. doi: 10.1016/j.cmet.2014.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Haurie V, Boucherie H, Sagliocco F. The Snf1 protein kinase controls the induction of genes of the iron uptake pathway at the diauxic shift in Saccharomyces cerevisiae. J Biol Chem. 2003;278:45391–6. doi: 10.1074/jbc.M307447200. [DOI] [PubMed] [Google Scholar]
- 4.Hardie DG, Hawley SA. AMP-activated protein kinase: the energy charge hypothesis revisited. BioEssays. 2001;23:1112–9. doi: 10.1002/bies.10009. [DOI] [PubMed] [Google Scholar]
- 5.Hawley SA, Davison M, Woods A, Davies SP, Beri RK, Carling D, et al. Characterization of the AMP-activated protein kinase kinase from rat liver, and identification of threonine-172 as the major site at which it phosphorylates and activates AMP-activated protein kinase. J Biol Chem. 1996;271:27879–87. doi: 10.1074/jbc.271.44.27879. [DOI] [PubMed] [Google Scholar]
- 6.Hawley SA, Boudeau J, Reid JL, Mustard KJ, Udd L, Makela TP, et al. Complexes between the LKB1 tumor suppressor, STRADa/b and MO25a/b are upstream kinases in the AMP-activated protein kinase cascade. J Biol. 2003;2:28. doi: 10.1186/1475-4924-2-28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hemminki A, Markie D, Tomlinson I, Avizienyte E, Roth S, Loukola A, et al. A serine/threonine kinase gene defective in Peutz-Jeghers syndrome. Nature. 1998;391:184–7. doi: 10.1038/34432. [DOI] [PubMed] [Google Scholar]
- 8.Jenne DE, Reimann H, Nezu J, Friedel W, Loff S, Jeschke R, et al. Peutz-Jeghers syndrome is caused by mutations in a novel serine threonine kinase. Nat Genet. 1998;18:38–43. doi: 10.1038/ng0198-38. [DOI] [PubMed] [Google Scholar]
- 9.Xiao B, Heath R, Saiu P, Leiper FC, Leone P, Jing C, et al. Structural basis for AMP binding to mammalian AMP-activated protein kinase. Nature. 2007;449:496–500. doi: 10.1038/nature06161. [DOI] [PubMed] [Google Scholar]
- 10.Scott JW, Hawley SA, Green KA, Anis M, Stewart G, Scullion GA, et al. CBS domains form energy-sensing modules whose binding of adenosine ligands is disrupted by disease mutations. J Clin Invest. 2004;113:274–84. doi: 10.1172/JCI19874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gowans GJ, Hawley SA, Ross FA, Hardie DG. AMP is a true physiological regulator of AMP-activated protein kinase by both allosteric activation and enhancing net phosphorylation. Cell Metab. 2013;18:556–66. doi: 10.1016/j.cmet.2013.08.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Oakhill JS, Steel R, Chen ZP, Scott JW, Ling N, Tam S, et al. AMPK is a direct adenylate charge-regulated protein kinase. Science. 2011;332:1433–5. doi: 10.1126/science.1200094. [DOI] [PubMed] [Google Scholar]
- 13.Xiao B, Sanders MJ, Underwood E, Heath R, Mayer FV, Carmena D, et al. Structure of mammalian AMPK and its regulation by ADP. Nature. 2011;472:230–3. doi: 10.1038/nature09932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sakamoto K, Goransson O, Hardie DG, Alessi DR. Activity of LKB1 and AMPK-related kinases in skeletal muscle: effects of contraction, phenformin, and AICAR. Am J Physiol Endocrinol Metab. 2004;287:E310–E7. doi: 10.1152/ajpendo.00074.2004. [DOI] [PubMed] [Google Scholar]
- 15.Hawley SA, Pan DA, Mustard KJ, Ross L, Bain J, Edelman AM, et al. Calmodulin-dependent protein kinase kinase-beta is an alternative upstream kinase for AMP-activated protein kinase. Cell Metab. 2005;2:9–19. doi: 10.1016/j.cmet.2005.05.009. [DOI] [PubMed] [Google Scholar]
- 16.Stahmann N, Woods A, Carling D, Heller R. Thrombin activates AMP-activated protein kinase in endothelial cells via a pathway involving Ca2+/calmodulin-dependent protein kinase kinase beta. Mol Cell Biol. 2006;26:5933–45. doi: 10.1128/MCB.00383-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Inoki K, Zhu T, Guan KL. TSC2 mediates cellular energy response to control cell growth and survival. Cell. 2003;115:577–90. doi: 10.1016/s0092-8674(03)00929-2. [DOI] [PubMed] [Google Scholar]
- 18.Gwinn DM, Shackelford DB, Egan DF, Mihaylova MM, Mery A, Vasquez DS, et al. AMPK phosphorylation of raptor mediates a metabolic checkpoint. Mol Cell. 2008;30:214–26. doi: 10.1016/j.molcel.2008.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Imamura K, Ogura T, Kishimoto A, Kaminishi M, Esumi H. Cell cycle regulation via p53 phosphorylation by a 5′-AMP activated protein kinase activator, 5-aminoimidazole- 4-carboxamide-1-beta-d- ribofuranoside, in a human hepatocellular carcinoma cell line. Biochem Biophys Res Commun. 2001;287:562–7. doi: 10.1006/bbrc.2001.5627. [DOI] [PubMed] [Google Scholar]
- 20.Jones RG, Plas DR, Kubek S, Buzzai M, Mu J, Xu Y, et al. AMP-activated protein kinase induces a p53-dependent metabolic checkpoint. Mol Cell. 2005;18:283–93. doi: 10.1016/j.molcel.2005.03.027. [DOI] [PubMed] [Google Scholar]
- 21.Vander Heiden MG, Cantley LC, Thompson CB. Understanding the Warburg effect: the metabolic requirements of cell proliferation. Science. 2009;324:1029–33. doi: 10.1126/science.1160809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hardie DG. AMP-activated protein kinase: maintaining energy homeostasis at the cellular and whole-body levels. Annu Rev Nutr. 2014;34:31–55. doi: 10.1146/annurev-nutr-071812-161148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zhou G, Myers R, Li Y, Chen Y, Shen X, Fenyk-Melody J, et al. Role of AMP-activated protein kinase in mechanism of metformin action. J Clin Invest. 2001;108:1167–74. doi: 10.1172/JCI13505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hawley SA, Fullerton MD, Ross FA, Schertzer JD, Chevtzoff C, Walker KJ, et al. The ancient drug salicylate directly activates AMP-activated protein kinase. Science. 2012;336:918–22. doi: 10.1126/science.1215327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hawley SA, Ross FA, Chevtzoff C, Green KA, Evans A, Fogarty S, et al. Use of cells expressing gamma subunit variants to identify diverse mechanisms of AMPK activation. Cell Metab. 2010;11:554–65. doi: 10.1016/j.cmet.2010.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Xiao B, Sanders MJ, Carmena D, Bright NJ, Haire LF, Underwood E, et al. Structural basis of AMPK regulation by small molecule activators. Nature Commun. 2013;4:3017. doi: 10.1038/ncomms4017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Calabrese MF, Rajamohan F, Harris MS, Caspers NL, Magyar R, Withka JM, et al. Structural basis for AMPK activation: natural and synthetic ligands regulate kinase activity from opposite poles by different molecular mechanisms. Structure. 2014;22:1161–72. doi: 10.1016/j.str.2014.06.009. [DOI] [PubMed] [Google Scholar]
- 28.Corton JM, Gillespie JG, Hawley SA, Hardie DG. 5-Aminoimidazole-4-carboxamide ribonucleoside: a specific method for activating AMP-activated protein kinase in intact cells? Eur J Biochem. 1995;229:558–65. doi: 10.1111/j.1432-1033.1995.tb20498.x. [DOI] [PubMed] [Google Scholar]
- 29.Racanelli AC, Rothbart SB, Heyer CL, Moran RG. Therapeutics by cytotoxic metabolite accumulation: pemetrexed causes ZMP accumulation, AMPK activation, and mammalian target of rapamycin inhibition. Cancer Res. 2009;69:5467–74. doi: 10.1158/0008-5472.CAN-08-4979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pirkmajer S, Kulkarni SS, Tom RZ, Ross FA, Hawley SA, Hardie DG, et al. Methotrexate promotes glucose uptake and lipid oxidation in skeletal muscle via AMPK activation. Diabetes. 2015;64:360–9. doi: 10.2337/db14-0508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lizcano JM, Göransson O, Toth R, Deak M, Morrice NA, Boudeau J, et al. LKB1 is a master kinase that activates 13 protein kinases of the AMPK subfamily, including the MARK/PAR-1 kinases. EMBO J. 2004;23:833–43. doi: 10.1038/sj.emboj.7600110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Goodwin JM, Svensson RU, Lou HJ, Winslow MM, Turk BE, Shaw RJ. An AMPK-independent signaling pathway downstream of the LKB1 tumor suppressor controls Snail1 and metastatic potential. Mol Cell. 2014;55:436–50. doi: 10.1016/j.molcel.2014.06.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Faubert B, Vincent EE, Griss T, Samborska B, Izreig S, Svensson RU, et al. Loss of the tumor suppressor LKB1 promotes metabolic reprogramming of cancer cells via HIF-1alpha. Proc Natl Acad Sci U S A. 2014;111:2554–9. doi: 10.1073/pnas.1312570111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bardeesy N, Sinha M, Hezel AF, Signoretti S, Hathaway NA, Sharpless NE, et al. Loss of the LKB1 tumour suppressor provokes intestinal polyposis but resistance to transformation. Nature. 2002;419:162–7. doi: 10.1038/nature01045. [DOI] [PubMed] [Google Scholar]
- 35.Laderoute KR, Amin K, Calaoagan JM, Knapp M, Le T, Orduna J, et al. 5′-AMP-activated protein kinase (AMPK) is induced by low-oxygen and glucose deprivation conditions found in solid-tumor microenvironments. Mol Cell Biol. 2006;26:5336–47. doi: 10.1128/MCB.00166-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Phoenix KN, Devarakonda CV, Fox MM, Stevens LE, Claffey KP. AMPKalpha2 suppresses murine embryonic fibroblast transformation and tumorigenesis. Genes Cancer. 2012;3:51–62. doi: 10.1177/1947601912452883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hadad SM, Baker L, Quinlan PR, Robertson KE, Bray SE, Thomson G, et al. Histological evaluation of AMPK signalling in primary breast cancer. BMC Cancer. 2009;9:307. doi: 10.1186/1471-2407-9-307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lee CW, Wong LL, Tse EY, Liu HF, Leong VY, Lee JM, et al. AMPK promotes p53 acetylation via phosphorylation and inactivation of SIRT1 in liver cancer cells. Cancer Res. 2012;72:4394–404. doi: 10.1158/0008-5472.CAN-12-0429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sanchez-Cespedes M, Parrella P, Esteller M, Nomoto S, Trink B, Engles JM, et al. Inactivation of LKB1/STK11 is a common event in adenocarcinomas of the lung. Cancer Res. 2002;62:3659–62. [PubMed] [Google Scholar]
- 40.Ji H, Ramsey MR, Hayes DN, Fan C, McNamara K, Kozlowski P, et al. LKB1 modulates lung cancer differentiation and metastasis. Nature. 2007;448:807–10. doi: 10.1038/nature06030. [DOI] [PubMed] [Google Scholar]
- 41.Wingo SN, Gallardo TD, Akbay EA, Liang MC, Contreras CM, Boren T, et al. Somatic LKB1 mutations promote cervical cancer progression. PLoS One. 2009;4:e5137. doi: 10.1371/journal.pone.0005137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Horman S, Vertommen D, Heath R, Neumann D, Mouton V, Woods A, et al. Insulin antagonizes ischemia-induced Thr172 phosphorylation of AMP-activated protein kinase alpha-subunits in heart via hierarchical phosphorylation of Ser485/491. J Biol Chem. 2006;281:5335–40. doi: 10.1074/jbc.M506850200. [DOI] [PubMed] [Google Scholar]
- 43.Hawley SA, Ross FA, Gowans GJ, Tibarewal P, Leslie NR, Hardie DG. Phosphorylation by Akt within the ST loop of AMPK-α1 down-regulates its activation in tumour cells. Biochem J. 2014;459:275–87. doi: 10.1042/BJ20131344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Antico Arciuch VG, Russo MA, Kang KS, Di Cristofano A. Inhibition of AMPK and Krebs cycle gene expression drives metabolic remodeling of Pten-deficient preneoplastic thyroid cells. Cancer Res. 2013;73:5459–72. doi: 10.1158/0008-5472.CAN-13-1429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Zheng B, Jeong JH, Asara JM, Yuan YY, Granter SR, Chin L, et al. Oncogenic B-RAF negatively regulates the tumor suppressor LKB1 to promote melanoma cell proliferation. Mol Cell. 2009;33:237–47. doi: 10.1016/j.molcel.2008.12.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Shen CH, Yuan P, Perez-Lorenzo R, Zhang Y, Lee SX, Ou Y, et al. Phosphorylation of BRAF by AMPK impairs BRAF-KSR1 association and cell proliferation. Mol Cell. 2013;52:161–72. doi: 10.1016/j.molcel.2013.08.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Yuan P, Ito K, Perez-Lorenzo R, Del Guzzo C, Lee JH, Shen CH, et al. Phenformin enhances the therapeutic benefit of BRAF(V600E) inhibition in melanoma. Proc Natl Acad Sci U S A. 2013;110:18226–31. doi: 10.1073/pnas.1317577110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Pineda CT, Ramanathan S, Fon Tacer K, Weon JL, Potts MB, Ou YH, et al. Degradation of AMPK by a cancer-specific ubiquitin ligase. Cell. 2015;160:715–28. doi: 10.1016/j.cell.2015.01.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Doyle JM, Gao J, Wang J, Yang M, Potts PR. MAGE-RING protein complexes comprise a family of E3 ubiquitin ligases. Mol Cell. 2010;39:963–74. doi: 10.1016/j.molcel.2010.08.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Godlewski J, Nowicki MO, Bronisz A, Nuovo G, Palatini J, De Lay M, et al. MicroRNA-451 regulates LKB1/AMPK signaling and allows adaptation to metabolic stress in glioma cells. Mol Cell. 2010;37:620–32. doi: 10.1016/j.molcel.2010.02.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Zhang Y, Duan G, Feng S. MicroRNA-301a modulates doxorubicin resistance in osteosarcoma cells by targeting AMP-activated protein kinase alpha-1. Biochem Biophys Res Commun. 2015;459:367–73. doi: 10.1016/j.bbrc.2015.02.101. [DOI] [PubMed] [Google Scholar]
- 52.Evans JM, Donnelly LA, Emslie-Smith AM, Alessi DR, Morris AD. Metformin and reduced risk of cancer in diabetic patients. BMJ. 2005;330:1304–5. doi: 10.1136/bmj.38415.708634.F7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Rothwell PM, Fowkes FG, Belch JF, Ogawa H, Warlow CP, Meade TW. Effect of daily aspirin on long-term risk of death due to cancer: analysis of individual patient data from randomised trials. Lancet. 2011;377:31–41. doi: 10.1016/S0140-6736(10)62110-1. [DOI] [PubMed] [Google Scholar]
- 54.Fullerton MD, Galic S, Marcinko K, Sikkema S, Pulinilkunnil T, Chen ZP, et al. Single phosphorylation sites in ACC1 and ACC2 regulate lipid homeostasis and the insulin-sensitizing effects of metformin. Nat Med. 2013;19:1649–54. doi: 10.1038/nm.3372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Algire C, Amrein L, Bazile M, David S, Zakikhani M, Pollak M. Diet and tumor LKB1 expression interact to determine sensitivity to anti-neoplastic effects of metformin in vivo. Oncogene. 2011;30:1174–82. doi: 10.1038/onc.2010.483. [DOI] [PubMed] [Google Scholar]
- 56.Goodwin PJ, Stambolic V, Lemieux J, Chen BE, Parulekar WR, Gelmon KA, et al. Evaluation of metformin in early breast cancer: a modification of the traditional paradigm for clinical testing of anti-cancer agents. Breast Cancer Res Treat. 2011;126:215–20. doi: 10.1007/s10549-010-1224-1. [DOI] [PubMed] [Google Scholar]
- 57.Shackelford DB, Abt E, Gerken L, Vasquez DS, Seki A, Leblanc M, et al. LKB1 inactivation dictates therapeutic response of non-small cell lung cancer to the metabolism drug phenformin. Cancer Cell. 2013;23:143–58. doi: 10.1016/j.ccr.2012.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Jeon SM, Chandel NS, Hay N. AMPK regulates NADPH homeostasis to promote tumour cell survival during energy stress. Nature. 2012;485:661–5. doi: 10.1038/nature11066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Liu L, Ulbrich J, Muller J, Wustefeld T, Aeberhard L, Kress TR, et al. Deregulated MYC expression induces dependence upon AMPK-related kinase 5. Nature. 2012;483:608–12. doi: 10.1038/nature10927. [DOI] [PubMed] [Google Scholar]