Abstract
Introduction:
Adult patients with Obsessive Compulsive Disorder (OCD) have been shown to have gray matter (GM) volume differences from healthy controls in multiple regions – the orbitofrontal cortex (OFC), anterior cingulate cortex (ACC), medial frontal gyri (MFG), striatum, thalamus, and superior parietal lobule. However, there is paucity of data with regard to juvenile OCD. Hence, we examined GM volume differences between juvenile OCD patients and matched healthy controls using voxel based morphometry (VBM) with the above apriori regions of interest.
Method:
Fifteen right handed juvenile patients with OCD and age- sex- handedness- matched healthy controls were recruited after administering the Mini International Neuropsychiatric Interview-KID and the Children’s Yale-Brown Obsessive Compulsive Scale, and scanned using a 3 Tesla magnetic resonance imaging scanner. VBM methodology was followed.
Results:
In comparison with healthy controls, patients had significantly smaller GM volumes in left ACC. YBOCS total score (current) showed significant negative correlation with GM volumes in bilateral OFC, and left superior parietal lobule.
Conclusion:
These findings while reiterating the important role of the orbito-fronto-striatal circuitry, also implicate in the parietal lobe – especially the superior parietal lobule as an important structure involved in the pathogenesis of OCD.
Keywords: obsessive compulsive disorder, juvenile, childhood, gray matter, imaging, neurobiology
Résumé
Introduction:
Les patients adultes souffrant du trouble obsessionnel-compulsif (TOC) ont révélé des différences de volume de matière grise (MG) d’avec des sujets témoins en santé dans de multiples régions – le cortex orbitofrontal (COF), le cortex cingulaire antérieur (CCA), le gyrus frontal moyen (GFM), le striatum, le thalamus, et le lobule pariétal supérieur. Cependant, il y a pénurie de données à l’égard du TOC juvénile. Nous avons donc examiné les différences de volume de MG entre les patients du TOC juvéniles et des sujets témoins appariés en santé à l’aide de la morphométrie voxel à voxel (VBM) dans les régions d’intérêt mentionnées ci-dessus.
Méthode:
Quinze patients juvéniles droitiers souffrant du TOC et des sujets témoins en santé appariés selon l’âge, le sexe, et la manualité ont été recrutés après l’administration de la Mini International Neuropsychiatric Interview-KID et la Children’s Yale-Brown Obsessive Compulsive Scale (YBOCS). Les images ont été obtenues à l’aide d’un scanner d’imagerie de résonance magnétique 3 Tesla, selon la technologie VBM.
Résultats:
Comparativement aux témoins en santé, les patients avaient des volumes de MG significativement plus petits dans le CCA gauche. Le score total (actuel) d’YBOCS indiquait une corrélation négative significative avec les volumes de MG dans le COF bilatéral, et le lobule pariétal supérieur gauche.
Conclusion:
Ces résultats réitèrent le rôle important de la circuiterie orbito-fronto-striatale, mais ils impliquent aussi que le lobe pariétal, en particulier le lobule pariétal supérieur, est une importante structure participant à la pathogenèse du TOC.
Keywords: trouble obsessionnel-compulsif, juvénile, enfance, matière grise, imagerie, neurobiologie
Introduction
Obsessive Compulsive Disorder (OCD) is a debilitating mental disorder which affects 2–3% of the general population (Rasmussen & Eisen, 1992). There has been considerable progress in understanding the neuro-anatomical basis of OCD over the last two decades. OCD patients have been shown to have differences in multiple gray matter (GM) regions, in comparison with healthy controls. Most studies have implicated the orbitofrontal cortex (OFC), anterior cingulate cortex (ACC), caudate nucleus and thalamus, which are key areas in the orbitofrontostriatal circuit (Kwon, Jang, Choi, & Kang, 2009; Rotge et al., 2009). In addition, some studies have indicated involvement of the medial frontal gyri (Radua & Mataix-Cols, 2009) and parietal areas (Kim et al., 2001; Valente et al., 2005). Most of these findings are based on studies in adult patients with OCD and there is limited literature with regard to juvenile patients with OCD (Maia, Cooney, & Peterson, 2008). Examining younger subjects offers potential advantage to examine the neuroanatomical changes associated with this disorder at an earlier stage of illness and the potential confounding effects of chronicity and long-term exposure to antidepressants can be minimized. Also, it also provides an opportunity to examine if the GM changes in younger populations with OCD are any different from the changes reported in adults with OCD. In addition, it has also been suggested that juvenile OCD might be a developmental subtype of the disorder (Geller et al., 1998; Jaisoorya, Janardhan Reddy, & Srinath, 2003; Sobin, Blundell, & Karayiorgou, 2000); hence this group of patients might demonstrate brain abnormalities secondary to neurodevelopmental pathogenesis.
Neuroanatomical studies in juvenile OCD patients have used the region of interest (ROI) (14 studies) and voxel based morphometry (VBM) (five studies) methods. VBM is a fully automated whole-brain measurement technique which provides a non-biased measure of well localized regions that may not be examined in hypothesis-based studies that utilize more labor-intensive ROI measurement techniques (Ashburner & Friston, 2000). As in adults, some studies in juvenile OCD have also reported GM volume changes in the orbitofrontostriatal circuit (Carmona et al., 2007; Szeszko et al., 2008), medial frontal gyri (Gilbert et al., 2008) and parietal cortex (Lazaro et al., 2009). In addition, other basal ganglia structures (putamen and globus pallidus), corpus callosum, hippocampus, amygdale and pituitary have also been implicated in juvenile OCD neuroimaging data (Huyser, Veltman, de Haan, & Boer, 2009).
This study was aimed to examine the GM volume differences between juvenile OCD patients and matched healthy controls using VBM with the most consistently reported brain areas as apriori regions of interest (ROI) (bilateral medial frontal gyrus, OFC, ACC, superior parietal lobule and striatum).We hypothesized that there will be decreased GM volume in the orbitofrontostriatal and parietal brain regions in juvenile OCD patients.
Methods
Sample
Fifteen children and adolescents with a DSM-IV diagnosis of OCD (American Psychological Association [APA], 1994) and 15 individually matched healthy controls were recruited for the study. Informed consent was obtained from their parents and assent from the child. The study was approved by the Ethics Committee of the National Institute of Mental Health and Neuro Sciences (NIMHANS), Bangalore, India.
Subjects
Children and adolescents (<18 years) with a primary diagnosis of OCD, diagnosed using DSM-IV criteria (APA, 1994), were invited to participate in the study. Study subjects (n = 15) were recruited from the Child and Adolescent Psychiatry (CAP) services and the speciality OCD clinic of the NIMHANS, Bangalore during the period of May 2008 to August 2009. A total of 52 children and adolescents with OCD were screened, and 32 were excluded based on exclusion criteria for the study. Of the 20 cases selected; five could not be scanned due to logistic reasons.
Subjects were right handed, established by the Edinburgh Handedness Inventory (Oldfield, 1971). The diagnosis of OCD in the index patient was established by a detailed clinical examination and administration of the Mini International Neuropsychiatric Interview- KID (MINI- KID) (Sheehan et al., 1998) and the Children’s Yale-Brown Obsessive Compulsive Scale (CY-BOCS) (Scahill et al., 1997). Diagnosis and associated features were confirmed by two experienced psychiatrists (YCJR or SS) by reviewing all the available information. The calculation of fluoxetine equivalents for those on drugs was done by following the method described by Weilburg et al. (Weilburg, O’Leary, Meigs, Hennen, & Stafford, 2003)t#. Exclusion criteria included presence of neurological disorders, mental retardation, learning disability, pervasive developmental disorders, attention deficit hyperactivity disorder, tic disorders, bipolar disorder or psychosis. The complete description of socio-demographic characteristics of the sample is given in Table 1.
Table 1.
Socio-demographic characteristics of sample
| Patients (n=15) Mean (SD) |
Controls subjects (n=15) Mean (SD) |
t | p | |
|---|---|---|---|---|
| Age at assessment | 14.13 (1.79) | 14.31 (2.15) | 0.25 | 0.80 |
| Age of onset of OCD | 12.73 (1.87) | -- | -- | -- |
| Years of education | 8.13 (1.8) | 8.87 (2.29) | 0.97 | 0.34 |
| Sex Ratio (M:F) | 8:7 | 8:7 | -- | 1.00 |
Healthy controls
The control sample consisted of 15 healthy subjects group matched for age, sex, handedness, and years of education. A clinical interview and MINI-KID was used to rule out any Axis I disorder. Family history of severe mental illnesses was excluded by using the Family Interview for Genetic Studies (FIGS) (Maxwell, 1992). Questions from the OCD section of the MINI-KID were used to exclude family history of OCD, since the FIGS does not have a section on OCD.
Magnetic Resonance Imaging (MRI) acquisition
MRI was done with a 3.0 Tesla scanner using a SENSE-Head 8 (Philips “Achieva”, Best, The Netherlands) channels coil. T1 weighted three-dimensional Magnetization Prepared Rapid Acquisition Gradient Echo sequence was performed (TR = 8.1 msec, TE = 3.7 msec, nutation angle = 8 degree, FOV = 256 mm, slice thickness = 1 mm without interslice gap, NEX = 1, matrix = 256*256) yielding 165 sagittal slices. The voxel size was 1mm*1mm*1mm.
Image processing and analysis
VBM analysis was performed using SPM8 (http://www.fil.ion.ucl.ac.uk/spm) and MATLAB 7.8 (Math-Works, Natick, MA, USA). MRI images were initially segmented into gray matter (GM), white matter (WM) and cerebrospinal fluid (CSF) using the standard unified segmentation model in SPM8 (Ashburner & Friston, 2005). GM population templates were then generated from the entire image dataset using the diffeomorphic anatomical registration using exponentiated Lie algebra (DARTEL) technique (Ashburner, 2007). Then, after an initial affine registration of the GM DARTEL templates to the tissue probability maps in Montreal Neurological Institute (MNI) space (http://www.mni.mcgill.ca/), non-linear warping of GM images was performed to the DARTEL GM template in MNI space. Next, images were modulated to ensure that relative volumes of GM were preserved following the spatial normalisation procedure. Lastly, images were smoothed with an 8mm full width at half maximum Gaussian kernel. After spatial pre-processing, the smoothed, modulated, normalised GM datasets were used for statistical analysis. The masks for the ROIs were constructed using Wake Forest University pick atlas [version 3.0.4] (Maldjian, Laurienti, & Burdette, 2004; Maldjian, Laurienti, Burdette, & Kraft, 2003). The following labels were selected from the IBASPM 71 atlas (http://www.thomaskoenig.ch/Lester/ibaspm.htm): the medial frontal gyrus, the medial & lateral orbitofrontal gyrus (for the OFC mask), precuneus, superior parietal lobule, caudate, thalamus and putamen & globus pallidus (for lenticular nuclei mask). The ROI for ACC was extracted from IBASPM 116 atlas (http://www.thomaskoenig.ch/Lester/ibaspm.htm) (Figures 1–3). The coordinates of significant areas of differences were transformed from MNI space into the stereotactic space of Talairach and Tournoux (Talairach & Tournoux, 1988) using nonlinear transform. (Brett et al 2002) within the Wake Forest University pick atlas interface (Lancaster, Woldorff, & Parsons, 2000). Volume differences were visualized at uncorrected p<0.001 and 10 voxel spatial threshold. Brain regions of significance surviving small volume correction for the respective mask volume (family-wise-error correction, p<0.05) were reported. Exploratory whole brain analysis was attempted. Areas surviving whole brain family-wise-error correction, p<0.05 were reported.
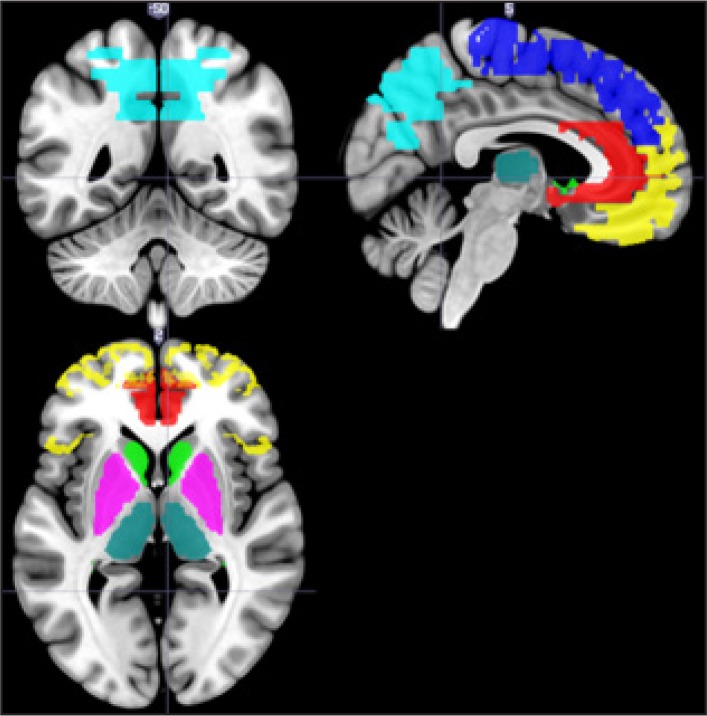
Figure 1.
Brain image showing the regions of interests used for analysis. Regions of interest have been labelled using coloured fills. Yellow: orbito-frontal cortex; dark blue: medial frontal gyrus; red: anterior cingulate gyrus; light green: caudate; cyan: lenticular nucleus; dark green: thalamus; light blue: superior parietal lobiule and precuneus.
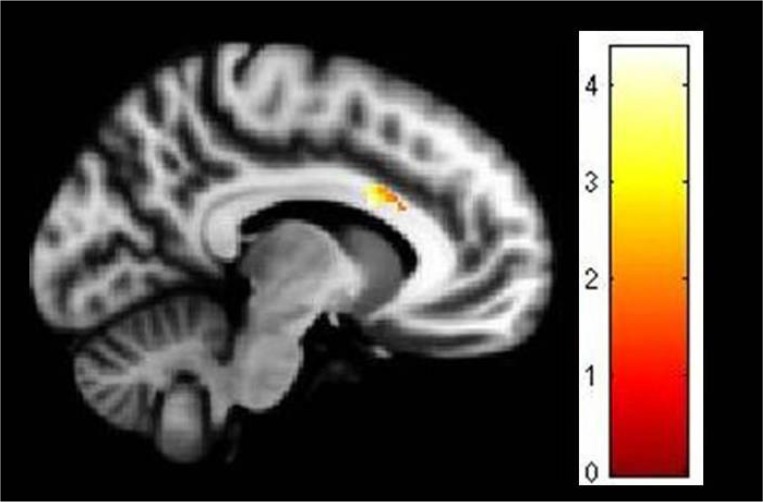
Figure 2.
Deficient gray matter volume in left anterior cingulate cortex in patients in comparison to controls. Image has been obtained using mask for the anterior cingulate cortex (uncorrected P<0.05). The coloured bar is representative of t score mentioned in Table 2.
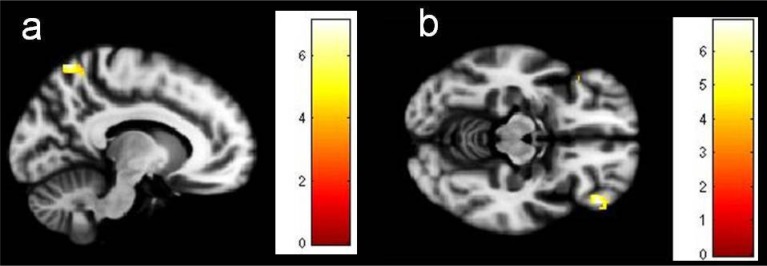
Figure 3.
Brain regions whose gray matter volume correlates negatively with YBOCS total score in patients; a) left superior parietal lobule; b) bilateral orbitofrontal cortex. Images have been obtained using mask for the respective brain regions (uncorrected P<0.001). The coloured bars are representative of t scores mentioned in Table 3.
Statistical analysis
Statistical package for social sciences (SPSS-13) was used to analyze the data. Kolmogorov Smirnov test revealed that the clinical data was normatively distributed, whereas the MRI data was non-normatively distributed. The socio-demographic data were compared using the Independent Sample t-test.
Results
Clinical parameters
The mean age at onset of OCD was 12.73 ± 1.87 years and the mean duration of illness was 1.40 ± 1.04 years (median-1.06). The most common obsessions were those of contamination (69%) and aggression (50%), whereas the most common compulsions were repeating rituals (81%), cleaning (63%), and checking (63%). The mean scores for obsessions and compulsions in the C-YBOCS scale were 10.87 ± 3.76 and 10.60 ± 3.72 respectively. The mean total score on the C-YBOCS was 21.47 ± 7.41. Co-morbid disorders were present in six (40%) patients, specific phobia in two and major depression, social phobia, oppositional defiant disorder and separation anxiety disorder in one each. No one had co-morbid attention deficit hyperactivity disorder (this was the exclusion criterion). There were two drug naive patients in the sample. The other patients were on treatment with fluoxetine (n=7), sertraline (n=4) and escitalopram (n=2). The median duration of treatment was 67 days.
MRI parameters
OCD patients had significantly larger gray matter volume (OCD patients: 705.1 ± 60.5 ml; Healthy controls: 636.0 ± 61.4 ml; p = 0.004) and white matter volume compared to healthy controls. (OCD patients: 484.6 ± 47.4 ml; Healthy controls: 441.9 ± 45.7 ml; p = 0.018) There was no significant difference in CSF volume (p = 0.120). In comparison with healthy controls, patients had significantly smaller GM volumes (Table 1) in left ACC (BA 24). YBOCS total score (current) showed significant negative correlation with GM volumes in bilateral OFC (left BA 47, right BA 11), and left superior parietal lobule (BA 7). These results are summarized in Tables 2–3. Whole brain analysis did not reveal any areas of significant difference.
Table 2.
Brain regions with significantly smaller volume in patients as compared to control subjects
| Region | Brodmann Area | Talairach & Tournoux co-ordinates | t | p | ||
|---|---|---|---|---|---|---|
|
| ||||||
| X | Y | Z | ||||
|
|
|
|
||||
| Left Anterior Cingulate Cortex | 24 | −6 | 7 | 27 | 4.23 | 0.033 |
Table 3.
Brain regions showing significant negative correlation between YBOCS total score (current) and gray matter volumes
| Region | Brodmann Area | Talairach & Tournoux co-ordinates | t | p | ||
|---|---|---|---|---|---|---|
|
| ||||||
| X | Y | Z | ||||
|
|
|
|
||||
| Left superior parietal lobule | 7 | −9 | −56 | 61 | 6.97 | 0.014 |
| Left orbitofrontal cortex | 47 | −41 | 25 | −19 | 6.71 | 0.020 |
| Right orbitofrontal cortex | 11 | 42 | 34 | −16 | 5.76 | 0.032 |
Discussion
Our study found GM volume reductions in the ACC in OCD patients compared to healthy controls. Greater illness severity was associated with greater volume reductions in bilateral OFC and superior parietal lobule. These results are broadly consistent with the proposed orbito-fronto-striatal model of OCD (Graybiel & Rauch, 2000; Saxena & Rauch, 2000), in addition parietal cortex – especially the superior parietal lobule has been implicated as another important structure involved in the pathogenesis.
Our finding of reduced GM volume in the ACC is consistent with two previous VBM studies in pediatric OCD patients (Carmona, et al., 2007; Gilbert, et al., 2008), whereas one study in found increased ACC volumes (Szeszko, et al., 2008). Some of the potential factors that might have contributed to the differences in study findings include variations in sample characteristics and medication effects. ACC has also been implicated in structural MRI studies in adult OCD patients, including recent meta-analytic reports (Radua & Mataix-Cols, 2009; Rotge, et al., 2009). A recent study from our centre in drug-naïve adult OCD patients also implicated the ACC as a key region which differentiated OCD patients from controls (Venkatasubramanian et al., 2011). The ACC plays a crucial role in “error” detection, conflict-monitoring and response inhibition (Casey et al., 1997; Kiehl, Liddle, & Hopfinger, 2000). Neurocognitive models of OCD propose that obsessions arise due to exaggerated perception of negative consequences following an action which an OCD patient misinterprets as “erroneous” or faulty and compulsions arise due to an inability to inhibit responses in relation to this “error” perception (Menzies et al., 2007; Menzies et al., 2008). Aberrant ACC activity in OCD has also been documented using functional MRI during the performance of executive function tasks of response inhibition and decision making (Koch et al., 2012; Roth et al., 2007; Vandenbroucke & Gabriels, 2012).
Illness severity had a significant negative correlation with volume reductions in the bilateral OFC. Such associations have been reported in adult (Radua & Mataix-Cols, 2009; Rotge, et al., 2009) and pediatric (MacMaster, O’Neill, & Rosenberg, 2008) OCD studies. Such an association was also detected with left superior parietal lobule. One functional MRI study in pediatric OCD has also reported cognitive control in OCD to be associated with activation of the left parietal region (Viard et al., 2005). A dorsal frontal parietal network involving the ACC, dorsolateral Prefrontal Cortex and parietal brain regions is involved in self-generated thoughts, active inhibition of attentional processes and shift of attention across sensory modalities (Cavanna & Trimble, 2006; Mazoyer, Wicker, & Fonlupt, 2002). It has been suggested that derangement in this network may be linked to the OCD patients’ inability to shift their focus away from obsessions (Rotge et al., 2008).
Our study findings are very similar to adult VBM studies in OCD. Similar neuro-anatomical correlates in adult and juvenile OCD could mean that that juvenile and adult OCD may just reflect a continuum of psychopathology rather than distinct entities. However, one other reason why our results are in tune with adult OCD studies could be the inclusion of mainly young adolescents and very few of pediatric OCD subjects in this study. We also did not have any children with comorbid ADHD and tic disorders (very typical of pediatric OCD and one of the reasons why this group is called developmental subtype). In any case, it has been difficult to compare neural correlates of OCD among children and adults in view of sparse literature in children (Maia, et al., 2008). Hence, there is a need to longitudinally examine neuro-anatomical correlates in large samples of juvenile (especially pediatric onset) OCD patients to better understand the neurobiology of this complex disorder and its progression over time.
One limitation of the study is the small sample size of 15 subjects in each group. Several patients being on treatment is a potential confounding factor to the interpretation of these findings, especially in the light of insufficient data on the effect of medications on neuro-anatomical parameters. OCD subjects with diverse symptom profile were included which could prove a confounding factor given the heterogeneity nature of this complex disorder. This may also explain the variability of findings across studies. There is a need for studies that examine GM alteration across various OCD symptom dimensions as there is emerging evidence that various symptom dimensions of OCD could have distinct neural correlates (van den Heuvel et al., 2009). Even though major psychiatric illnesses (such as psychoses, bipolar disorder and tic disorders) were excluded, still 40% had a co morbid diagnosis. The children were not matched for intelligence. But developmental delay was ruled out during the clinical assessment. Also, there was no significant difference between the cases and controls in terms of educational levels. The cross-sectional nature of the study does not allow conclusions to be drawn regarding the causal relation between GM alterations and manifestation of symptoms.
Key points
There is limited literature on the neuroanatomical basis of juvenile OCD.
We examined grey matter volume in 15 juvenile OCD patients and matched healthy controls.
Voxel based morphometry was performed with apriori regions of interest.
Patients had smaller volumes in left anterior cingulate cortex
OCD severity correlated significantly with volume loss in orbitofrontal cortex/ superior parietal lobule.
Acknowledgements/Conflict of interest
The authors have no conflicts of interest to disclose.
Abbreviations
- OCD
Obsessive Compulsive Disorder
- GM
Gray Matter
- OFC
Orbitofrontal Cortex
- ACC
Anterior Cingulate Cortex
- ROI
Region of Interest
- VBM
Voxel Based Morphometry
- MINI
Mini International Neuropsychiatric Interview
- CYBOCS
Children’s Yale-Brown Obsessive Compulsive Scale
- FIGS
Family Interview for Genetic Studies
- MRI
Magnetic Resonance Imaging
References
- American Psychological Association . Diagnostic and Statistical Manual of Mental Disorders. 4th ed. Washington, DC: American Psychiatric Association; 1994. [Google Scholar]
- Ashburner J. A fast diffeomorphic image registration algorithm. Neuroimage. 2007;38(1):95–113. doi: 10.1016/j.neuroimage.2007.07.007. [DOI] [PubMed] [Google Scholar]
- Ashburner J, Friston KJ. Voxel-based morphometry - the methods. Neuroimage. 2000;11(6 Pt 1):805–821. doi: 10.1006/nimg.2000.0582. [DOI] [PubMed] [Google Scholar]
- Ashburner J, Friston KJ. Unified segmentation. Neuroimage. 2005;26(3):839–851. doi: 10.1016/j.neuroimage.2005.02.018. [DOI] [PubMed] [Google Scholar]
- Brett M, Johnsrude IS, Owen AM. The problem of functional localization in the human brain. Nature Reviews Neuroscience. 2002;3(3):243–249. doi: 10.1038/nrn756. [DOI] [PubMed] [Google Scholar]
- Carmona S, Bassas N, Rovira M, Gispert JD, Soliva JC, Prado M, Vilarroya O. Pediatric OCD structural brain deficits in conflict monitoring circuits: A voxel-based morphometry study. Neuroscience Letters. 2007;421(3):218–223. doi: 10.1016/j.neulet.2007.05.047. [DOI] [PubMed] [Google Scholar]
- Casey BJ, Trainor R, Giedd J, Vauss Y, Vaituzis CK, Hamburger S, Rapoport JL. The role of the anterior cingulate in automatic and controlled processes: A developmental neuroanatomical study. Developmental Psychobiology. 1997;30(1):61–69. [PubMed] [Google Scholar]
- Cavanna AE, Trimble MR. The precuneus: A review of its functional anatomy and behavioural correlates. Brain. 2006;129(Pt 3):564–583. doi: 10.1093/brain/awl004. [DOI] [PubMed] [Google Scholar]
- Geller D, Biederman J, Jones J, Park K, Schwartz S, Shapiro S, Coffey B. Is juvenile obsessive-compulsive disorder a developmental subtype of the disorder? A review of the pediatric literature. Journal of the American Academy of Child and Adolescent Psychiatry. 1998;37(4):420–427. doi: 10.1097/00004583-199804000-00020. [DOI] [PubMed] [Google Scholar]
- Gilbert AR, Keshavan MS, Diwadkar V, Nutche J, Macmaster F, Easter PC, Rosenberg DR. Gray matter differences between pediatric obsessive-compulsive disorder patients and high-risk siblings: A preliminary voxel-based morphometry study. Neuroscience Letters. 2008;435(1):45–50. doi: 10.1016/j.neulet.2008.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graybiel AM, Rauch SL. Toward a neurobiology of obsessive-compulsive disorder. Neuron. 2000;28(2):343–347. doi: 10.1016/s0896-6273(00)00113-6. [DOI] [PubMed] [Google Scholar]
- Huyser C, Veltman DJ, de Haan E, Boer F. Paediatric obsessive-compulsive disorder, a neurodevelopmental disorder? Evidence from neuroimaging. Neuroscience and Biobehavioral Reviews. 2009;33(6):818–830. doi: 10.1016/j.neubiorev.2009.01.003. [DOI] [PubMed] [Google Scholar]
- Jaisoorya TS, Janardhan Reddy YC, Srinath S. Is juvenile obsessive-compulsive disorder a developmental subtype of the disorder? Findings from an Indian study. European Child & Adolescent Psychiatry. 2003;12(6):290–297. doi: 10.1007/s00787-003-0342-2. [DOI] [PubMed] [Google Scholar]
- Kiehl KA, Liddle PF, Hopfinger JB. Error processing and the rostral anterior cingulate: An event-related fMRI study. Psychophysiology. 2000;37(2):216–223. [PubMed] [Google Scholar]
- Kim JJ, Lee MC, Kim J, Kim IY, Kim SI, Han MH, Kwan JS. Grey matter abnormalities in obsessive-compulsive disorder: Statistical parametric mapping of segmented magnetic resonance images. British Journal of Psychiatry. 2001;179:330–334. doi: 10.1192/bjp.179.4.330. [DOI] [PubMed] [Google Scholar]
- Koch K, Wagner G, Schachtzabel C, Peikert G, Schultz CC, Sauer H, Schlösser RG. Aberrant anterior cingulate activation in obsessive-compulsive disorder is related to task complexity. Neuropsychologia. 2012;50(5):958–964. doi: 10.1016/j.neuropsychologia.2012.02.002. [DOI] [PubMed] [Google Scholar]
- Kwon JS, Jang JH, Choi JS, Kang DH. Neuroimaging in obsessive-compulsive disorder. Expert Review of Neurotherapeutics. 2009;9(2):255–269. doi: 10.1586/14737175.9.2.255. [DOI] [PubMed] [Google Scholar]
- Lancaster JL, Woldorff MG, Parsons LM. Automated Talairach atlas labels for functional brain mapping. Human Brain Mapping. 2000;10:120–131. doi: 10.1002/1097-0193(200007)10:3<120::AID-HBM30>3.0.CO;2-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lazaro L, Bargallo N, Castro-Fornieles J, Falcon C, Andres S, Calvo R, Junqué C. Brain changes in children and adolescents with obsessive-compulsive disorder before and after treatment: A voxel-based morphometric MRI study. Psychiatry Research. 2009;172(2):140–146. doi: 10.1016/j.pscychresns.2008.12.007. [DOI] [PubMed] [Google Scholar]
- MacMaster FP, O’Neill J, Rosenberg DR. Brain imaging in pediatric obsessive-compulsive disorder. Journal of the American Academy of Child and Adolescent Psychiatry. 2008;47(11):1262–1272. doi: 10.1097/CHI.0b013e318185d2be. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maia TV, Cooney RE, Peterson BS. The neural bases of obsessive-compulsive disorder in children and adults. Development and Psychopathology. 2008;20(4):1251–1283. doi: 10.1017/S0954579408000606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maldjian JA, Laurienti PJ, Burdette JB. Precentral gyrus discrepancy in electronic versions of the talairach atlas. Neuroimage. 2004;21:450–455. doi: 10.1016/j.neuroimage.2003.09.032. [DOI] [PubMed] [Google Scholar]
- Maldjian JA, Laurienti PJ, Burdette JB, Kraft RA. An automated method for neuroanatomic and cytoarchitectonic atlas-based interrogation of fMRI data sets. Neuroimage. 2003;19:1233–1239. doi: 10.1016/s1053-8119(03)00169-1. [DOI] [PubMed] [Google Scholar]
- Maxwell M. Manual for the FIGS (Family Interview - for Genetics Studies) March 30, 1992. Clinical Neurogenetics Branch, Intramural Research Program, National Institute of Mental Health; 1992. [Google Scholar]
- Mazoyer P, Wicker B, Fonlupt P. A neural network elicited by parametric manipulation of the attention load. Neuroreport. 2002;13(17):2331–2334. doi: 10.1097/00001756-200212030-00032. [DOI] [PubMed] [Google Scholar]
- Menzies L, Achard S, Chamberlain SR, Fineberg N, Chen CH, del Campo N, Bullmore E. Neurocognitive endophenotypes of obsessive-compulsive disorder. Brain. 2007;130(Pt 12):3223–3236. doi: 10.1093/brain/awm205. [DOI] [PubMed] [Google Scholar]
- Menzies L, Chamberlain SR, Laird AR, Thelen SM, Sahakian BJ, Bullmore ET. Integrating evidence from neuroimaging and neuropsychological studies of obsessive-compulsive disorder: The orbitofronto-striatal model revisited. Neuroscience and Biobehavioral Reviews. 2008;32(3):525–549. doi: 10.1016/j.neubiorev.2007.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oldfield RC. The assessment and analysis of handedness: The Edinburgh inventory. Neuropsychologia. 1971;9(1):97–113. doi: 10.1016/0028-3932(71)90067-4. [DOI] [PubMed] [Google Scholar]
- Radua J, Mataix-Cols D. Voxel-wise meta-analysis of grey matter changes in obsessive-compulsive disorder. British Journal of Psychiatry. 2009;195(5):393–402. doi: 10.1192/bjp.bp.108.055046. [DOI] [PubMed] [Google Scholar]
- Rasmussen SA, Eisen JL. The epidemiology and clinical features of obsessive compulsive disorder. Psychiatric Clinic of North America. 1992;15(4):743–758. [PubMed] [Google Scholar]
- Rotge JY, Guehl D, Dilharreguy B, Cuny E, Tignol J, Bioulac B, Aouizerate B. Provocation of obsessive-compulsive symptoms: A quantitative voxel-based meta-analysis of functional neuroimaging studies. Journal of Psychiatry & Neuroscience. 2008;33(5):405–412. [PMC free article] [PubMed] [Google Scholar]
- Rotge JY, Guehl D, Dilharreguy B, Tignol J, Bioulac B, Allard M, Aouizerate B. Meta-analysis of brain volume changes in obsessive-compulsive disorder. Biological Psychiatry. 2009;65(1):75–83. doi: 10.1016/j.biopsych.2008.06.019. [DOI] [PubMed] [Google Scholar]
- Roth RM, Saykin AJ, Flashman LA, Pixley HS, West JD, Mamourian AC. Event-related functional magnetic resonance imaging of response inhibition in obsessive-compulsive disorder. Biological Psychiatry. 2007;62(8):901–909. doi: 10.1016/j.biopsych.2006.12.007. [DOI] [PubMed] [Google Scholar]
- Saxena S, Rauch SL. Functional neuroimaging and the neuroanatomy of obsessive-compulsive disorder. Psychiatric Clinics of North America. 2000;23(3):563–586. doi: 10.1016/s0193-953x(05)70181-7. [DOI] [PubMed] [Google Scholar]
- Scahill L, Riddle MA, McSwiggin-Hardin M, Ort SI, King RA, Goodman WK, Leckman JF. Children’s Yale-Brown Obsessive Compulsive Scale: Reliability and validity. Journal of the American Academy of Child and Adolescent Psychiatry. 1997;36(6):844–852. doi: 10.1097/00004583-199706000-00023. [DOI] [PubMed] [Google Scholar]
- Sheehan DV, Lecrubier Y, Sheehan KH, Amorim P, Janavs J, Weiller E, Dunbar GC. The Mini-International Neuropsychiatric Interview (M.I.N.I.): The development and validation of a structured diagnostic psychiatric interview for DSM-IV and ICD-10. Journal of Clinical Psychiatry. 1998;59(Suppl 20):22–33. quiz 34-57. [PubMed] [Google Scholar]
- Sobin C, Blundell ML, Karayiorgou M. Phenotypic differences in early- and late-onset obsessive-compulsive disorder. Comprehensive Psychiatry. 2000;41(5):373–379. doi: 10.1053/comp.2000.9009. [DOI] [PubMed] [Google Scholar]
- Szeszko PR, Christian C, Macmaster F, Lencz T, Mirza Y, Taormina SP, Rosenberg DR. Gray matter structural alterations in psychotropic drug-naive pediatric obsessive-compulsive disorder: An optimized voxel-based morphometry study. American Journal of Psychiatry. 2008;165(10):1299–1307. doi: 10.1176/appi.ajp.2008.08010033. [DOI] [PubMed] [Google Scholar]
- Talairach J, Tournoux P. A Co-Planar Stereotaxic Atlas of a Human Brain. Stuttgart: Thieme; 1988. [Google Scholar]
- Valente AA, Jr, Miguel EC, Castro CC, Amaro E, Jr, Duran FL, Buchpiguel CA, Busatto GF. Regional gray matter abnormalities in obsessive-compulsive disorder: A voxel-based morphometry study. Biological Psychiatry. 2005;58(6):479–487. doi: 10.1016/j.biopsych.2005.04.021. [DOI] [PubMed] [Google Scholar]
- van den Heuvel OA, Remijnse PL, Mataix-Cols D, Vrenken H, Groenewegen HJ, Uylings HB, Veltman DJ. The major symptom dimensions of obsessive-compulsive disorder are mediated by partially distinct neural systems. Brain. 2009;132(Pt 4):853–868. doi: 10.1093/brain/awn267. [DOI] [PubMed] [Google Scholar]
- Vandenbroucke CL, Gabriels L. The decision-making process in obsessive compulsive disorder. Tijdschrift voor Psychiatrie. 2012;54(1):39–49. [PubMed] [Google Scholar]
- Venkatasubramanian G, Zutshi A, Jindal S, Srikanth SG, Kovoor JME, Keshav kumar J, Janardhan Reddy YC. Comprehensive evaluation of cortical structure abnormalities in drug-naïve, adult patients with obsessive-compulsive disorder: A surface-based morphometry study. Journal of Psychiatric Research. 2011;46(9):1161–1168. doi: 10.1016/j.jpsychires.2012.06.003. [DOI] [PubMed] [Google Scholar]
- Viard A, Flament MF, Artiges E, Dehaene S, Naccache L, Cohen D, Martinot JL. Cognitive control in childhood-onset obsessive-compulsive disorder: A functional MRI study. Psychological Medicine. 2005;35(7):1007–1017. doi: 10.1017/s0033291704004295. [DOI] [PubMed] [Google Scholar]
- Weilburg JB, O’Leary KM, Meigs JB, Hennen J, Stafford RS. Evaluation of the adequacy of outpatient antidepressant treatment. Psychiatric Services. 2003;54(9):1233–1239. doi: 10.1176/appi.ps.54.9.1233. [DOI] [PubMed] [Google Scholar]