Abstract
Case series
Patient: Male, 59 • Male, 85 • Male, 27
Final Diagnosis: refractory metastatic rectal cancer
Symptoms: —
Medication: —
Clinical Procedure: Chemotherapy
Specialty: Oncology
Objective:
Challenging differential diagnosis
Background:
Rectal linitis plastica (RLP) is a rare disease with poor outcome. It is often accompanied by a delayed histopathological diagnosis, primarily due to submucosal disease. A concentric ring pattern or “target sign” on T2-weighted magnetic resonance imaging (MRI) has been proposed as being characteristic for early suspicion. Even though RLP is more aggressive and has poorer survival than other rectal adenocarcinomas, no specific treatment is recommended. In this case report of 3 patients, we challenge the sensitivity of the characteristic radiological pattern, and we review the existing data for a treatment strategy.
Case Report:
One patient presented classic clinical characteristics of RLP with young age and advanced stage at diagnosis, with chemo-refractory disease and rapid fatal evolution. Biopsies confirmed the RLP with the presence of signet-ring cells (SRC) in a strong desmoplastic stromal reaction. However, the characteristic concentric ring pattern was absent. Instead, he had a large vegetative lesion with important tumor infiltration in mesorectum and pelvic organs, with major lymph node involvement. The 2 other patients presented resectable locally advanced disease with characteristic concentric ring pattern.
No clinical and radiological responses were observed to neo-adjuvant chemoradiotherapy (CRT), including 1 patient with non-resectable disease at surgery and another with upstaged disease at pathological specimen after resection. However, data suggest 2 types of RLP: about half of patients are extremely sensitive to CRT with pathological complete response, and the other half are highly resistant with no response to CRT. Current data are insufficient to distinguish between these 2 populations.
Conclusions:
The absence of a concentric ring pattern should not eliminate the suspicion of RLP, especially in young patients with aggressive clinical presentation.
There are probably 2 types of RLP in terms of chemoradiosensitivity, and neoadjuvant CRT could delay the curative-intent surgery in refractory patients. Future molecular analysis of the tumor and its environment are required to characterize the 2 different forms of RLP to develop more personalized treatment strategies.
MeSH Keywords: Carcinoma, Signet Ring Cell; Linitis Plastica; Rectal Neoplasms
Background
Linitis plastica was initially a morphological definition referring to the macroscopic thickened and rubbery aspect of a hollow organ with loss of its ability to distend. Nowadays, linitis plastica is considered as an advanced stage of a subtype of adenocarcinoma called signet-ring cells carcinoma (SRCC), characterized by a massive involvement of the affected organ wall by tumor infiltration within a desmoplastic inflammatory-like reaction. Linitis plastica can occur in any organ but is more prevalent in hollow viscous organs, especially in the stomach [1,2].
Rectal linitis plastica (RLP) is a rare disease affecting less than 1% of colorectal cancer patients. It is frequently diagnosed in younger patients, at a more advanced stage, and with poorer survival when compared to other adenocarcinomas, including the mucinous subtype [3–6].
Since magnetic resonance imaging (MRI) has become a standard in rectal cancer workup, some radiological features have been suggested as characteristic of RLP, such as concentric ring pattern or “target sign” on T2-weighted images. However, these findings were suggested based in a limited number of cases and the sensitivity or the specificity of these radiological patterns is not known [7,8].
Besides, little information is available concerning chemo- or radio-sensitivity of RLP, and no particular strategy is recommended at the present time. We describe in this paper 3 cases of primary RLP with emphasis on MRI features and treatment strategy.
Case Report
Case 1
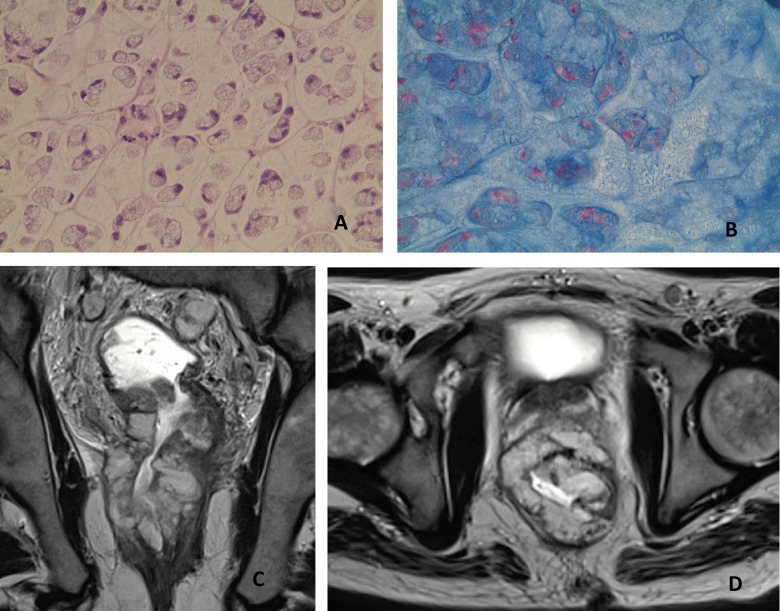
A 59-year-old man had a 3-month history of rectal bleeding, watery stools and weight loss. Digital rectal examination (DRE) was impossible due to severe pain. Colonoscopy under general anesthesia found a stenotic tumor at 10 cm from the anal verge. Biopsies showed a RAS (ras2 Kirsten rat sarcoma viral oncogene homolog) wild-type carcinoma characterized by scattered signet-ring cells (SRC) in a strong fibrous stromal reaction (Figure 1A). On pelvic MRI, T2-weighted sagittal plane showed a diffuse wall thickening of the rectum over 15 cm from the anal verge resulting in rigid tube morphology. T2-weighted axial plane showed a circumferential thickening over 11 mm with a concentric ring pattern and local extension to the bladder trigone (Figure 1B, 1C). No lymphadenopathy or distant metastases were detected on computed tomography (CT) scan. Upper gastrointestinal tract endoscopy was normal. Local extension to the trigone of the bladder was confirmed by cystoscopy. The disease was staged as cT4bN0M0. Neoadjuvant chemo-radiotherapy (CRT) followed by pelvectomy was decided in our multidisciplinary staff. Five weeks after 5-fluorouracil (5FU) based CRT, preoperative MRI showed no tumor response. At surgery, non-resectable locally advanced disease with peritoneal extension was observed. The multidisciplinary staff decided a FOLFOX regimen associated to panitumumab in this refractory cancer disease. A radiological partial response was then observed and the response was maintained over 10 months until peritoneal progression. Second line chemotherapy was ineffective and the patient died 3 months later.
Figure 1.
Pathology and MRI features in case 1. (A) Rectal biopsy shows scattered signet-ring cells (Hematoxylin and eosin staining, ×20). (B) T2-weighted sagittal plane MR image shows a diffuse wall thickening of the rectum over 15 cm. (C) T2-weighted axial plane shows a circumferential thickening over 11 mm with a concentric ring pattern.
Case 2
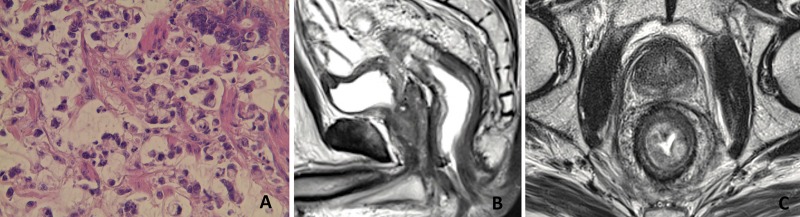
An 85-year-old man presented with a 3-week history of diarrhea, anal incontinence and a 10-kg weight loss. DRE revealed a lower rectal tumor. Rectosigmoidoscopy showed a circumferential tumor occluding the rectal lumen down to the pectinate line. Biopsies confirmed a SRCC with excessive fibrosis (Figure 2A). Pelvic MRI showed on T2-weighted coronal plane a diffuse wall thickening of the entire rectum over a length of 18 cm accounting for its markedly rigid tube aspect (Figure 2B). On T2-weighted axial plane, a circumferential wall thickening over 13 mm with infiltration of the mesorectum.
Figure 2.
Pathology and MRI features in case 2. (A) Scattered signet-ring cells (Hematoxylin and eosin staining, ×20). (B) Pelvic MRI shows on T2-weighted coronal plane a diffuse wall thickening of the entire rectum over a length of 18 cm, accounting for its markedly rigid tube aspect. (C) On T2-weighted axial plane, a circumferential wall thickening over 13 mm with infiltration of the mesorectum and a concentric ring pattern.
The characteristic concentric ring pattern was also observed (Figure 2C). The disease was staged as cT3N0M0 and upfront colostomy was performed due to a symptomatic tumor. Five weeks after 5FU-based CRT, pre-operative MRI and CT scan showed no radiological response. Abdomino-perineal resection was then performed. Pathological examination showed a SRCC infiltration in submucosa and muscularis propria with an important desmoplastic reaction. The tumor was upstaged to ypT3N1b suggesting a CRT resistance. Even though the complete total mesorectal dissection (TME) was hard due to circumferential mesorectal infiltration, thus impeding traction and countertraction maneuvers typically performed in TME, a complete resection was achieved. No adjuvant treatment was administered to this aged patient. The patient was disease-free 16 months after the initial diagnosis.
Case 3
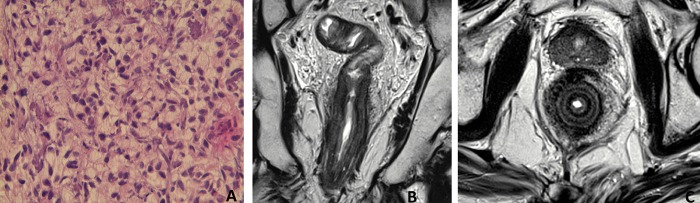
A 27-year-old man was referred for rectal bleeding and diarrhea. Colonoscopy showed an ulcerated tumor of the lower rectum extended to the anal canal. Biopsies showed a RAS mutated carcinoma characterized by scattered SRC in a strong desmoplastic stromal reaction (Figure 3A, 3B). MRI of the pelvis on T2-weighted showed a large vegetative circumferential tumor of the lower rectum and anal canal of 10 cm in length. The tumor was extended to the mesorectum, anal sphincters, prostate, and seminal vesicles with pelvic and inguinal lymph node enlargement (Figure 3C, 3D). However, the characteristic concentric ring pattern was not observed in this case. A second blinded independent pathological lecture confirmed a SRCC. Lung metastases were detected on CT scan and the disease was staged as cT4bN2M1. A tri-chemotherapy based on 5FU, oxaliplatin, and irinotecan was promptly started. No clinical and radiological efficacy was observed and the patient died 8 months after the initial diagnosis.
Figure 3.
Pathology and MRI features in case 3. (A) Scattered signet-ring cells (Hematoxylin and eosin staining, ×20). (B) Scattered signet-ring cells: mucus in blue, nuclei in red (Alcian blue, ×20). (C) Pelvic MRI shows on T2-weighted coronal plane a diffuse wall thickening with large lesion and lymph nodes enlargement. (D) On T2-weighted axial image: Hyper-intense vegetative circumferential tumor. No concentric ring pattern.
Discussion
The term linitis plastica was first introduced in 1854 by Brinton W to describe the “leather bottle” shape of the stomach, and was later extended to other hollow organs to describe the thickened, rigid and contracted aspect of their wall. Laufman and Saphir were the first to describe in 1951, the relation between this macroscopic aspect and the primary colorectal SRCC [9]. Presently, linitis plastica refers to SRCC with massive involvement of the hollow organ wall by infiltrating tumor cells [1].
Among our 3 cases, two patients presented the classic concentric ring pattern previously described [7]. However, this characteristic radiological sign was absent in the third case. MRI showed a large vegetative lesion with massive infiltration without recognizable layers of “target sign”. The clinical presentation was suggestive of RLP with young age and advanced stage at diagnosis, and rapid fatal progression, and a second blinded independent pathological lecture confirmed a SRCC of rectum.
Some published data are critical about the specificity of these radiological patterns of RLP since rectal infiltration by SRCC of another pelvic organ such as the bladder or the prostate and even rectal metastases of gastric SRCC and breast lobular carcinoma can mimic this pattern, as well as non-malignant diseases such as inflammatory or ischemic colitis [8,10–14]. However, the sensitivity of characteristic radiological patterns was not questioned until now. Thus, even though the concentric ring pattern is frequently observed in RLP, it is neither specific nor sensitive and should be interpreted carefully, especially in young patients with aggressive rectal carcinoma.
None of our two patients with locally advanced disease responded to neoadjuvant CRT at preoperative radiological assessment. Moreover, the disease was more advanced at surgery than at diagnosis in case 1, suggesting on-therapy progression. Other authors have reported the same isolated observations about probable chemo-radioresistance in RLP [15–17]. However, Bratland et al. reported 6 cases of SRCC among 120 rectal cancer patients who received neoadjuvant CRT. In this cohort, 3 patients (50%) had a pathological complete response (pCR), whereas other 3 patients had no response [18]. In a larger retrospective multicenter study, among 248 consecutive rectum cancer patients treated with neoadjuvant CRT or radiotherapy alone before surgery, 32 patients (12.9%) presented a pCR. On multivariate analysis, SRC histology was found to be an independent predictive factor of pathological response. In fact, 5 out of 11 (45%) SRCC patients presented pCR, in line with the previous report [19].
These data suggest that there are 2 types of RLP patients in terms of chemo-radiosensitivity: excellent responders and nonresponders. The tumor upstaging following neoadjuvant CRT suggests no cytotoxic or cytostatic effect of CRT in non-responder patients. Some available data explain the chemo-radioresistance of SRCC of colorectal origin. As has been suggested in gastric SRCC, the mucinous content could compete with drug-cell interactions within the tumor [20]. SRC contains abundant mucins characterized by the presence of a protein called MUC. Altered expression confers an important role to MUC in tumor progression, metastasis, and chemoresistance. Terada et al. examined the expression status of MUC1, MUC2, MUC5AC, and MUC6 in 30 cases of gastric SRCC and 12 cases of colorectal SRCC [21]. Interestingly, there was a significant tendency of primary gastric SRCC to express MUC5AC and MUC6 but not MUC1 and MUC2, while primary colorectal SRCC express MUC1, MUC2, and MUC5A, but not MUC6, suggesting the potential usefulness of MUC patterns for differential diagnosis of primary localization.
Only MUC2 is expressed in the normal colorectal epithelium, while MUC1, MUC5AC, and MUC6 are not. These findings suggest that up-regulation of MUC1 and MUC5AC is associated with the pathogenesis, carcinogenesis, and malignant behavior of colorectal SRCC. Moreover, MUC2 and MUC5AC expression observed in conventional adenocarcinoma is different from that seen in SRCC, suggesting different molecular mechanisms of carcinogenesis between these histological types.
Furthermore, SRCC aggressive behavior could be connected with genetic abnormalities different from that seen in classic adenocarcinoma. In particular, a higher prevalence of BRAF mutation, loss of heterozygosity, high-level microsatellite instability, and CpG island methylation phenotype-positive status was noted in SRCC [22].
Since there are clearly highly chemo-radio-resistant RLP patients, it would be important to distinguish at diagnosis from good-responders to perform an upfront surgical resection without CRT delay in resectable RLP. However, actual data do not justify a different strategy from classic adenocarcinoma since high chemo-radiosensitivity can be awaited with complete responses in half of the patients. There is an urgent need to explore SRCC biology to understand the resistance mechanisms involved in refractory patients and to identify specific signaling pathways that can be targeted.
Conclusions
On pelvic MRI, the concentric ring pattern is frequently observed in rectal SRCC. However, the absence of this “target sign” should not eliminate suspicion of RLP, especially with circumferential rectal infiltration and aggressive clinical presentation. There are probably 2 different chemo-radiobiology entities: extremely sensitive and highly resistant. Their distinction at diagnosis should be of importance for resectable disease to do prevent delay of curative-intent surgery. Future molecular biology studies of this tumor and its environment are needed.
Acknowledgments
The authors would like to thank Guadalupe Tizon for English writing assistance.
Footnotes
Conflict of interest
The authors declare that they have no conflict of interest.
References:
- 1.Lauwers GY, Carneiro F, Graham DY, et al. Gastric carcinoma. In: Bosman FT, Carneiro F, Hruban RH, Theise ND, editors. WHO Classification of Tumors of the Digestive System. 4th ed. Lyon: IARC; 2010. pp. 48–68. [Google Scholar]
- 2.El Absi M, Elouannani M, Elmdarhri J, et al. Primary linitis plastica of the rectum. Rev Med Liege. 2002;57:10–12. [PubMed] [Google Scholar]
- 3.Papp JP, Jr, Levine EJ, Thomas FB. Primary linitis plastica carcinoma of the colon and rectum. Am J Gastroenterol. 1995;90:141–45. [PubMed] [Google Scholar]
- 4.Kang H, O’Connell JB, Maggard MA, et al. A 10-year outcomes evaluation of mucinous and signet-ring cell carcinoma of the colon and rectum. Dis Colon Rectum. 2005;48:1161–68. doi: 10.1007/s10350-004-0932-1. [DOI] [PubMed] [Google Scholar]
- 5.Hyngstrom JR, Hu CY, Xing Y, et al. Clinicopathology and outcomes for mucinous and signet ring colorectal adenocarcinoma: analysis from the National Cancer Data Base. Ann Surg Oncol. 2012;19:2814–21. doi: 10.1245/s10434-012-2321-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nitsche U, Zimmermann A, Späth C, et al. Mucinous and signet-ring cell colorectal cancers differ from classical adenocarcinomas in tumor biology and prognosis. Ann Surg. 2013;258:775–83. doi: 10.1097/SLA.0b013e3182a69f7e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rudralingam V, Dobson MJ, Pitt M, et al. MR imaging of linitis plastica of the rectum. Am J Roentgenol. 2003;181:428–30. doi: 10.2214/ajr.181.2.1810428. [DOI] [PubMed] [Google Scholar]
- 8.Katsinelos P, Papaziogas B, Chatzimavroudis G, et al. Secondary rectal linitis plastica as first manifestation of urinary bladder carcinoma. Ann Gastroenterol. 2012;25:173–75. [PMC free article] [PubMed] [Google Scholar]
- 9.Laufman H, Saphir O. Primary linitis plastica type of carcinoma of the colon. AMA Arch Surg. 1951;62:79–91. doi: 10.1001/archsurg.1951.01250030082009. [DOI] [PubMed] [Google Scholar]
- 10.Dresen RC, Beets GH, Vliegen RF, et al. Linitis plastica of the rectum secondary to bladder carcinoma: a report of two cases and its MR features. Br J Radiol. 2008;81:e249–51. doi: 10.1259/bjr/59924178. [DOI] [PubMed] [Google Scholar]
- 11.Dadamessi I, Joly J-P, Dupas J-L. Rectal linitis as the first manifestation of a prostatic cancer. Gastroenterol Clin Biol. 2002;26:538–39. [PubMed] [Google Scholar]
- 12.Venturini F, Gambi V, Di Lernia S, et al. Linitis plastica of the rectum as a clinical presentation of metastatic lobular carcinoma of the breast. J Clin Oncol. 2014;pii doi: 10.1200/JCO.2013.50.6733. JCO.2013.50.6733 [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 13.Lim SW, Huh JW, Kim YJ, Kim HR. Laparoscopic low anterior resection for hematogenous rectal metastasis from gastric adenocarcinoma: A Case Report. World J Surg Oncol. 2011;9:148. doi: 10.1186/1477-7819-9-148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Balthazar EJ. CT of the gastrointestinal tract: principles and interpretation. Am J Roentgenol. 1991;156:23–32. doi: 10.2214/ajr.156.1.1898566. [DOI] [PubMed] [Google Scholar]
- 15.Samlani-Sebbane Z, Eddafali B, Guennoun N, Krati K. Primary linitis plastica of the rectum: a rare tumor. Gastroenterol Clin Biol. 2008;32:530–31. doi: 10.1016/j.gcb.2007.12.025. [DOI] [PubMed] [Google Scholar]
- 16.Keogh CF, Brown JA, Phang PT. Linitis plastica of the rectum: utility of transrectal ultrasonography. J Ultrasound Med. 2002;21:103–6. doi: 10.7863/jum.2002.21.1.103. [DOI] [PubMed] [Google Scholar]
- 17.Barabino G, Miggino M, Cuilleron M, et al. Rectal linitis. Surgery. 2012;154:641–42. doi: 10.1016/j.surg.2012.04.010. [DOI] [PubMed] [Google Scholar]
- 18.Bratland, Vetrhus T, Grøholt K, Ree AH. Preoperative radiotherapy in rectal signet-ring cell carcinoma – magnetic resonance imaging and treatment outcome: Report of six cases. Acta Oncol. 2010;49:42–49. doi: 10.3109/02841860903081897. [DOI] [PubMed] [Google Scholar]
- 19.Jayanand S, Seshadri RA, Tapkire R. Signet ring cell histology and non-circumferential tumors predict pathological complete response following neoadjuvant chemoradiation in rectal cancers. Int J Colorectal Dis. 2011;26:23–27. doi: 10.1007/s00384-010-1082-7. [DOI] [PubMed] [Google Scholar]
- 20.Messager M, Lefevre JH, Pichot-Delahaye V, et al. The Impact of Perioperative Chemotherapy on Survival in Patients with Gastric Signet Ring Cell Adenocarcinoma. Ann Surg. 2011;254:684–93. doi: 10.1097/SLA.0b013e3182352647. [DOI] [PubMed] [Google Scholar]
- 21.Terada T. An immunohistochemical study of primary signet-ring cell carcinoma of the stomach and colorectum: II. Expression of MUC1, MUC2, MUC5AC, and MUC6 in normal mucosa and in 42 cases. Int J Clin Exp Pathol. 2013;6:613–21. [PMC free article] [PubMed] [Google Scholar]
- 22.Kakar S, Deng G, Smyrk TC, et al. Loss of heterozygosity, aberrant methylation, BRAF mutation and KRAS mutation in colorectal signet ring cell carcinoma. Mod Pathol. 2012;25:1040–47. doi: 10.1038/modpathol.2012.44. [DOI] [PubMed] [Google Scholar]