Abstract
The effect of vitamin E intake on spatial learning and working memory performances of young rats has been investigated in the Morris water maze and locomotor activity has been assessed by an open-field test. A total of 45 young male Wistar albino rats aged two months were divided into three equal groups: control, olive oil, and vitamin E groups. These groups were treated for 30 days with a once daily intraperitoneal injection. The rats were then tested for their ability to find the location of the platform (spatial learning). The results revealed that there was no statistically significant difference between the time spent to find the platform and the time spent in half area of the tank, including the platform among the group, while the time spent to find the platform was found to have increased from the first day to the fourth day in all the groups. In the open-field test, the locomotor activity quite significantly increased in the peripheral area in the olive oil group. The supplementation with vitamin E for a short period had not improved the learning performance of the healthy young rats. It was concluded that the beneficial effect of vitamin E intake on learning is related to the beginning time and the duration of vitamin E intake.
Keywords: cognitive function, learning, memory, Morris water maze, open field, vitamin E
Introduction
Vitamin E is an essential nutrient for human beings. It functions as a natural antioxidant, scavenging free radicals in cell membranes and protecting unsaturated fatty acids from lipid peroxidation.1 Its dietary deficiency has been shown to be quite deleterious, especially in the central nervous system.2 Vitamin E and other antioxidants effectively improve cognitive performance in aged animals and prevent oxidative damage in animal models of Alzheimer’s disease (AD).3
The effects of antioxidants (β-carotene, vitamin E, and vitamin C) on learning have been widely reported in the literature.2 Some studies relate to the treatment of learning impairment, which causes neurological impairments constituted by practices such as ovariectomy and supplementation of quasi-proline substances or age-induced oxidative stress.4,5 But the effects of antioxidant vitamins on learning in human or animal health have not been widely reported in the literature. However, the exact effect of vitamin E is still not clear.5–7 This study is an attempt to investigate, especially, contradictions between age groups related to this condition. Therefore, investigations are done according to age groups. This study investigated the influence of antioxidant vitamin E intake on learning performance of healthy young male rats.
Material and Methods
The experiments were performed on male Wistar albino rats that were two-month old. The rats were obtained from the Erciyes University Experimental and Clinical Research Center. The animals were kept on a 12-hour light–dark cycle and allowed free access to food and water. All experiments were performed according to the guidelines of the Erciyes University Ethics Committee for the welfare of experimental animals.
In the first set of experiments, rats were randomly assigned to one of the following groups (15 animals in each group): control group (saline injection of only 1 mL/kg/day), olive oil group (olive oil injection of only 1 mL/kg/day), and experimental group (injection of vitamin E 40 mL/kg/day). The animals received a once daily intraperitoneal injection, between 11:00 am and 12:00 noon for 30 days.
After saline, olive oil, and vitamin E injections, animals were subjected to Morris water maze (MWM) testing to evaluate effects of vitamin E on spatial learning and memory. The water maze consisted of a circular water tank (125 cm diameter and 45 cm height) which was partially filled with water (25°C). Milk powder was used to render the water opaque. The tank was theoretically divided into four equal quadrants, labeled N, S, E, and W. Several distal visual cues were placed on the walls of the room.
An escape platform was hidden 2 cm under the water surface, in a fixed location in one of the four quadrants of the tank. Before the experiment, water in the tank was changed every day and made opaque by milk powder.
MWM task behavioral procedures
Reference memory task
The task consisted of four trials per session for five days. The rats had daily sessions of four trials to find the escape platform that was submerged 2 cm under the water surface and placed in the center of one of the quadrants of the tank, and the time spent in half area of the tank, including the platform, was measured by two chronometers. The interval between trials was 30 minutes. The task was created during two phases: acquisition phase and probe trial.
Acquisition phase: In this phase, the rats were submitted to daily sessions of four trials per day for four days to find the submerged platform.8 Several distal visual cues were placed on the walls of the room for the rats to know their environment. For each trial, the rat was placed in the north position (N) in the water maze that did not contain the platform and was allowed to swim for two minutes. If the rat did not find the platform within two minutes, it was manually placed and allowed to remain on it for 30 seconds. Two parameters were measured in each trial, namely, the time spent to find the platform and the time spent in half area of the tank, including the platform. The time spent in half area of the tank, including the platform, was calculated according to the percentage of the time spent to find the platform (total time). After each trial, the rats were removed, dried using a towel, and put back in their home cages.
Probe trial: The day after the acquisition phase, a probe test was conducted by removing the platform. The rats were allowed to swim freely in the tank for 60 seconds. In this phase, only the time spent in half area of the tank, including the platform, a value representing the percentage in the total time, was calculated.9,10
Working memory task
One week after the probe test, the working memory version of MWM was performed. This task consisted of four consecutive trials per day, for four days. The interval between the trials was 30 seconds. The animals were placed in the tank and allowed to search for the platform that was submerged 2 cm under the water surface. The platform was positioned in the center of one of the quadrants, and its position was changed every subsequent day during the four testing days. Latencies to find the platform in every trial were calculated considering all testing days so as to assess working memory performance.11
Open-field task
Besides measurement of exploratory activity, the open-field test was performed as an indicator of drug toxicity, as well as to rule out any confounding effect on the cognitive tests. The apparatus consists of a square plastic board (100 × 100 cm) with plastic sides (30 cm high) and is divided into 16 squares. Each animal was placed in the center of the arena and was observed for lines crossed (with four paws), rearing, defecation, freezing, and grooming behaviors for five minutes.12,13
Statistical analysis
Reference memory, working memory, and open-field data were analyzed by one-way analysis of variance (ANOVA) followed by the Tukey’s multiple range test intergroups when indicated; P < 0.05 was considered significant. Reference memory and working memory results were analyzed by repeated measures ANOVA within groups. All analyses were performed using the Statistical Package for the Social Sciences (SPSS) software and GraphPad Instat 3.05 statistical programs.
Results
MWM reference memory task
Acquisition phase
There were significant differences within groups when compared latencies to find the platform. In all groups, latencies of first day were longer than all other days. These latencies showed significant decreases day to day (Table 1).
Table 1.
Effect of vitamin E on performance of spatial memory acquisition phase.a
| DAYS | SHAM GROUP (n = 15) (X ± SEM) (MINUTES) | OLIVE OIL GROUP (n = 15) (X ± SEM) (MINUTES) | VITAMIN E GROUP (n = 15) (X ± SEM) (MINUTES) | P |
|---|---|---|---|---|
| Day 1 | 57.05 ± 5.67* | 56.34 ± 5.26* | 58.63 ± 5.51* | >.05 |
| Day 2 | 22.70 ± 2.65 | 31.39 ± 3.99 | 25.03 ± 3.75 | >.05 |
| Day 3 | 17.16 ± 2.21 | 25.15 ± 5.96 | 15.43 ± 2.22 | >.05 |
| Day 4 | 10.61 ± 1.45 | 12.71 ± 1.53 | 12.44 ± 1.45 | >.05 |
| P | <.001 | <.001 | <.001 |
Notes: Data are latencies to find the platform on each day during the four testing days.
All values are the group mean ± SE for 15 animals in each group. There was no significant difference between groups, P > 0.05 (ANOVA). However, there were significant differences within groups (repeated measures ANOVA).
P < 0.001 when compared with other days (final three days).
Probe trial
In the probe trial, with the platform removed, vitamin E-treated rats showed poorer performance in remembering the precise location of the platform, spending less time in the half area of the former platform position than the sham and olive oil groups. But there were no significant differences between groups.
Working memory
There was no significant difference between groups; in the olive oil and vitamin E groups, latencies to find the platform showed an increase from the third day to the fourth day of testing, but these results did not significantly differ in the working memory task (Table 2).
Table 2.
Effect of vitamin E on performance in the working memory version of MWM spatial task.a
| DAYS | SHAM GROUP (X ± SEM) (MINUTES) | OLIVE OIL GROUP (X ± SEM) (MINUTES) | VITAMIN E GROUP (X ± SEM) (MINUTES) | P |
|---|---|---|---|---|
| Day 1 | 11.88 ± 1.73 | 17.66 ± 3.17 | 20.23 ± 3.64*,# | >.05 |
| Day 2 | 11.94 ± 1.32 | 15.05 ± 1.80 | 14.70 ± 2.66 | >.05 |
| Day 3 | 11.17 ± 1.41 | 12.20 ± 1.71 | 10.99 ± 2.11 | >.05 |
| Day 4 | 8.80 ± 1.01 | 16.16 ± 3.03 | 13.33 ± 2.51 | >.05 |
| P | >.05 | >.05 | >.05 |
Notes: Data are latencies to find the platform on each day during the four testing days.
All values are the group mean ± SE for 15 animals in each group. There was no significant difference between groups, P > 0.05 (ANOVA). However, there were significant differences within the vitamin group, P < 0.05 (repeated measures ANOVA). But there was no significant difference within sham and olive oil groups, P > 0.05 (repeated measures ANOVA).
P < 0.05 when compared with the other days (final three days).
Open-field task
The analysis of motor activity of rats was measured by the open-field task. The inter-group comparisons were analyzed by one-way ANOVA followed by Tukey’s post hoc test for the following measures: number of peripheral lines crossed, number of central lines crossed, peripheral rearings, central rearings, peripheral freezing number, central freezing number, peripheral defecation, central defecation, grooming number, freezing time, and grooming time (Fig. 1).
Figure 1.
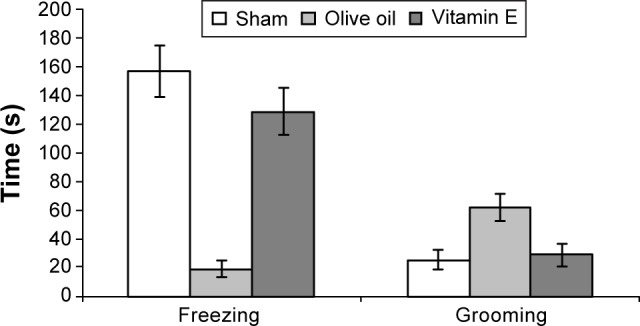
Comparison of freezing and grooming times of sham, olive oil, and vitamin E-treated rats (n = 15 rats/group). All values are the group mean ± SE. Freezing time significantly decreased (*P = 0.000), and grooming time significantly increased (**P = 0.002) in the olive oil group.
Number of lines crossed
There were no significant differences in the control and vitamin E groups, but there was a significant increase in the number of lines crossed (peripheral and central) in the olive oil group. The olive oil group had quite significantly higher number of lines crossed than the control and vitamin E groups in the peripheral area (P < 0.001). The olive oil group had a higher number of lines crossed (peripheral and central) than the other groups (Table 3).
Table 3.
Effect of vitamin E supplementation on performance [number of lines crossed, rearings, freezing number, defecation, grooming number, freezing time (seconds), and grooming time (seconds)] in the open-field task.a
| SHAM GROUP | OLIVE OIL GROUP | VITAMIN E GROUP | F | P | |
|---|---|---|---|---|---|
| Number of lines crossed (peripheral) | 13.00 ± 0.74* | 28.33 ± 1.18 | 6.26 ± 0.20* | 4,575 | .000 |
| Number of lines crossed (central) | 2.13 ± 2.85 | 4.26 ± 3.13 | 0.80 ± 1.82* | 18,041 | .016 |
| Peripheral rearings | 9.00 ± 2.04* | 27.60 ± 3.21 | 4.13 ± 1.04* | 4,575 | .000 |
| Central rearings | 0.33 ± 0.18 | 1.46 ± 0.70 | 0.13 ± 0.09 | 18,041 | .067 |
| Peripheral freezing number | 4.13 ± 0.44* | 1.13 ± 0.35 | 3.00 ± 0.36* | 2,888 | .000 |
| Central freezing number | 0.33 ± 0.18 | 0.00 ± 0.00 | 0.26 ± 0.59 | 15,144 | .214 |
| Peripheral defecation | 1.00 ± 0.30*,# | 3.33 ± 0.56 | 2.93 ± 0.56 | 0,089 | .004 |
| Central defecation | 0.20 ± 0.14 | 0.20 ± 0.10 | 0.13 ± 0.13 | 6,359 | .915 |
| Grooming number | 2.80 ± 0.57 | 3.66 ± 0.33 | 1.60 ± 0.30* | 6,095 | .005 |
| Freezing time | 156.55 ± 18.01* | 18.89 ± 6.93 | 128.52 ± 16.50* | 24,617 | .000 |
| Grooming time | 25.43 ± 5.38* | 61.75 ± 9.09 | 28.62 ± 7.65* | 7,131 | .002 |
Notes:
All values are the group mean ± SE.
P < 0.05 when compared with the olive oil group (one-way ANOVA).
P = 0.004 when compared with the vitamin E group (one-way ANOVA).
Rearings
Significant increases in the number of rearings were noted in the olive oil group in the peripheral area, which is higher than those in the control and vitamin E groups (P < 0.001). However, there was no significant difference in central rearings (Table 3).
Freezing number
There was no significant difference in peripheral and central freezing number between the control and vitamin E groups, while significantly lower peripheral freezing number (P < 0.005) no significant difference in central freezing number was onbserved in the olive oil group (Table 3).
Defecation
The olive oil and vitamin E groups had significantly higher defecation than the control group in the peripheral area (P < 0.05). There was no significant difference in the central area (Table 3).
Grooming number
The olive oil group showed significantly more grooming number than the vitamin group (P < 0.005). The olive oil group had a higher grooming number than the other groups.
Freezing time
There was a significant increase for the olive oil group, as increased freezing number (peripheral) (Table 3). The olive oil group showed quite significantly lower freezing time than the other groups (P < 0.001) (Fig. 1).
Grooming time
The olive oil group showed significantly more grooming time than the control and vitamin E groups (P < 0.01) (Fig. 1).
Discussion
Vitamin E supplementation has been found to have several health benefits, including improvement in mental and physical capacity of healthy aged individuals and those suffering from neurodegenerative conditions such as AD.13–15
In this study, we investigated the influence of vitamin E on cognitive performance of young male rats that are assessed with MWM, and exploratory activity has been measured by the open-field task.
Animals of all groups increased their learning performance, though the time spent to find the platform decreased from the first day to the fourth day of training within groups. Vitamin E supplementation did not improve spatial learning/memory in the reference memory task when compared to controls (olive oil and sham) (Table 1). Additionally, the results of probe trial did not vary between groups.
Clinical studies suggest that pretreatment with vitamins E and C has not altered memory when compared to controls (saline and vitamin E groups) but has prevented memory impairments caused by ovariectomy or proline.11 Adverse effects of therapeutic vitamin E supplementation have not been widely reported in the human or animal literature; however, it is noteworthy that in elderly patients with AD, studies with vitamin E indicate that high doses can slow the progression of AD.15
In addition, administration of a high-antioxidant diet at a later age would reverse age-related deficits in behavior and β-adrenergic function in the cerebellum.6
These results clearly indicate that mice fed control diets exhibit a deleterious effect of age on many different measures of cognitive functions.16 However, vitamin E intake did not lead to any improvement in the performance of any of the age-sensitive tests, even though the mice had been supplemented for up to 10 weeks prior to testing. These results contrast with earlier reports that vitamin E affords some protection against age-related losses of motor function and swim maze performance when supplementation began eight months prior to testing.17 Thus, the results of this study indicate that vitamin E supplementation is not beneficial unless it is implemented at ages prior to the appearance of losses in functional capacity.5
Confirming the working hypothesis, results show that vitamin E administration did not improve spatial navigation. It has been demonstrated that 60-day-old male rats did not show a significant difference between the control and experimental (administrated vitamin E and C) groups when compared latencies to find the platform in the working memory.11
Chronic administration of the hydroalcoholic extract of stems from Equisetum arvense (HAE) with demonstrated antioxidant properties in vitro seems to alleviate the performance deficits of the aged rats, because the old animals treated with HAE found the platform similar to young controls throughout the acquisition phase and spent time in the platform that was placed in one of the quadrants during retention phase. In the working memory version, the aged controls do not acquire the information throughout the four trials; however, in the old animals that received HAE, the acquisition performance was similar to that of the young controls. As verified in this work, antioxidants improved the performance of aged animals in the MWM.18 Socci et al showed that aged rats that received four to five months treatment with several antioxidants performed better than aged rats that received saline, in the MWM test.7
Clinical studies suggest that oxidative stress and reactive oxygen species might be involved in memory modulation mechanisms.6,19,20 Another line of evidence supporting the role of oxidative stress in behavior emerges from studies with vitamins. For example, α-tocopherol improves cognitive function of patients with temporal lobe radionecrosis21 and may be beneficial in lowering the cognitive impairment in patients with AD.22 It is well known that α-tocopherol is a lipid-soluble vitamin that interacts with cell membranes, traps free radicals, and interrupts the chain reaction that damages cells,23 preventing the uncontrolled propagation of lipid peroxidation by free radicals.24 The resultant tocopheroxil radical requires ascorbate, vitamin C, for its regeneration back to reduced tocopherol.25
The behavioral changes of rats were evaluated using the open-field test. Two of the open-field measures used, peripheral movement decreases and immobility increases, are generally regarded as related to attempts to escape,26 while central movement increases are more strongly related to exploratory behavior. This study demonstrated that there was a significant difference in peripheral defecation in the vitamin E group. However, it has been observed that open-field exploratory activity increases after olive oil injection in young male rats. Especially, there were significant differences in peripheral movements.
Many studies confirmed that diet can influence behavior and specifically the manipulating dietary fat can cause behavioral changes.27 In human beings, reducing plasma cholesterol values is said to actually increase the incidence of death, and the association between low cholesterol levels and depression has been commented on several occasions.28,29 The above results strongly suggests that lipids have profound effects on the central nervous system and its activities.30 Also, most of the literature relates to the impact of dietary fat on cognitive behavior.28
In conclusion, previous studies on vitamin E intake concluded that prevented impairments of spatial learning and working memory were caused by oxidative stress.2,3 Although it was reported in the literature that vitamin E intake could improve deteriorated learning because of oxidative stress, healthy young rats pretreated with vitamin E for 30 days have not shown improved effects on spatial learning and working memory.
Footnotes
ACADEMIC EDITOR: Lora Talley Watts, Editor in Chief
PEER REVIEW: Three peer reviewers contributed to the peer review report. Reviewers’ reports totaled 1052 words, excluding any confidential comments to the academic editor.
FUNDING: This study was supported by Erciyes University Research Fund SBT-06-23. The authors confirm that the funder had no influence over the study design, content of the article, or selection of this journal.
COMPETING INTERESTS: Authors disclose no potential conflicts of interest.
Paper subject to independent expert blind peer review. All editorial decisions made by independent academic editor. Upon submission manuscript was subject to anti-plagiarism scanning. Prior to publication all authors have given signed confirmation of agreement to article publication and compliance with all applicable ethical and legal requirements, including the accuracy of author and contributor information, disclosure of competing interests and funding sources, compliance with ethical requirements relating to human and animal study participants, and compliance with any copyright requirements of third parties. This journal is a member of the Committee on Publication Ethics (COPE). Provenance: the authors were invited to submit this paper.
Author Contributions
Conceived and designed the experiments: ND, AK, SD. Analyzed the data: AK, SD. Wrote the first draft of the manuscript: ND, AK, SD. Contributed to the writing of the manuscript: AK, SD. Agree with manuscript results and conclusions: AK. Jointly developed the structure and arguments for the paper: ND, AK, SD. Made critical revisions and approved final version: AK. All authors reviewed and approved of the final manuscript.
REFERENCES
- 1.Eidi A, Eidi M, Mahmoodi G. Effect of vitamin E on memory retention in rats: possible involvement of cholinergic system. Eur Neuropsychopharmacol. 2006;16(2):101–106. doi: 10.1016/j.euroneuro.2005.06.006. [DOI] [PubMed] [Google Scholar]
- 2.Meydani M. Antioxidants and cognitive function. Nutr Rev. 2001;59:75–80. doi: 10.1111/j.1753-4887.2001.tb05505.x. [DOI] [PubMed] [Google Scholar]
- 3.Grundman M. Vitamin E and Alzheimer’s disease: the basis for additional clinical trials. Am J Clin Nutr. 2000;71:630–636. doi: 10.1093/ajcn/71.2.630s. [DOI] [PubMed] [Google Scholar]
- 4.Delwing D, Bavaresco CS, Wannmacher CM, Wajner M, Dutra-Filho CS. Pro-line induces oxidative stress in cerebral cortex of rats. Int J Dev Neurosci. 2003;21:105–110. doi: 10.1016/s0736-5748(02)00109-0. [DOI] [PubMed] [Google Scholar]
- 5.Sumien N, Heinrich KR, Sohal RS, Forester MJ. Short-term vitamin E intake fails to improve cognitive or psychomotor performance of aged mice. Free Radic Biol Med. 2004;36:1424–1433. doi: 10.1016/j.freeradbiomed.2004.02.081. [DOI] [PubMed] [Google Scholar]
- 6.Bickford PC, Gould T, Briederick L, Chadman K, Pollock A. Antioxidant-rich diets improve cerebellar physiology and motor learning in aged rats. Brain Res. 2000;866(1–2):211–217. doi: 10.1016/s0006-8993(00)02280-0. [DOI] [PubMed] [Google Scholar]
- 7.Socci DJ, Crandall BM, Arendash GW. Chronic antioxidant treatment improves the cognitive performance of aged rats. Brain Res. 1995;69:88–94. doi: 10.1016/0006-8993(95)00707-w. [DOI] [PubMed] [Google Scholar]
- 8.Manschot SM, Biessels GJ, Cameron NE, Cotter MA, Kamal A. Angiotensin converting enzyme inhibition partially prevents deficits in water maze performance, hippocampal synaptic plasticity and cerebral blood flow in streptozotocin-diabetic rats. Brain Res. 2003;966(2):274–282. doi: 10.1016/s0006-8993(02)04211-7. [DOI] [PubMed] [Google Scholar]
- 9.Fergusun SA, Cada AM. Spatial learning/memory and social and nonsocial behaviors in the spontaneously hypertensive, Wistar-Kyoto and Spraque Dawley rat strains. Pharmacol Biochem Behav. 2004;28:463–483. doi: 10.1016/j.pbb.2003.12.014. [DOI] [PubMed] [Google Scholar]
- 10.Vasconcellos AP, Tabajara AS, Ferrari C, Rocha E, Dalmaz C. Effect of chronic stress on spatial memory in rats is attenuated by lithium treatment. Physiol Behav. 2003;79(2):143–149. doi: 10.1016/s0031-9384(03)00113-6. [DOI] [PubMed] [Google Scholar]
- 11.Delwing D, Bavaresco CS, Monteiro SC, Matté C, Netto CA, Wyse AT. Alpha-tocopherol and ascorbic acid prevent memory deficits provoked by chronic hyper-prolinemia in rats. Behav Brain Res. 2006;168(2):185–189. doi: 10.1016/j.bbr.2005.08.014. [DOI] [PubMed] [Google Scholar]
- 12.Volke V, Soosaar A, Koks S, Bourin M, Mannisto P, Vasar E. 7-Nitraindazole, a nitric oxide synthasevinhibitor, has anxiolytic-like properties in exploratory models of anxiety. Psychopharmacology. 1997;131:399–405. doi: 10.1007/s002130050309. [DOI] [PubMed] [Google Scholar]
- 13.Casadesus G, Shukitt-Hale B, Joseph JA. Qualiative versus quantitative caloric intake: are they equivalent paths to succesful aging? Neurobiol Aging. 2002;23:747–769. doi: 10.1016/s0197-4580(02)00079-9. [DOI] [PubMed] [Google Scholar]
- 14.Meydani M. Nutrition interventions in aging and age-associated disease. Ann N Y Acad Sci. 2001;928:226–235. doi: 10.1111/j.1749-6632.2001.tb05652.x. [DOI] [PubMed] [Google Scholar]
- 15.Sano M, Ernesto C, Thomas RG, Klauber MR, Schafer KA. A controlled trial of selegiline, alpha-tocopherol, or both as treatment for Alzheimer’s disease: the Alzheimer’s Disease Cooperative Study. N Engl J Med. 1997;336:1216–1222. doi: 10.1056/NEJM199704243361704. [DOI] [PubMed] [Google Scholar]
- 16.Forster MJ, Dubey A, Dawson KM, Stutts WA, Lal H, Sohal RS. Age-related losses of cognitive function and motor skills in mice are associated with oxidative protein damage in the brain. Proc Natl Acad Sci U S A. 1996;93:4765–4769. doi: 10.1073/pnas.93.10.4765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Joseph JA, Shukitt-Hale B, Denisova NA, et al. Long-term dietary strawberry, spinach, or vitamin E supplementation retards the onset of age-related neuronal signal-transduction and cognitive behavioral deficits. J Neurosci. 1998;18:8047–8055. doi: 10.1523/JNEUROSCI.18-19-08047.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Stackman RW, Eckenstein F, Frei B, Kulhanek D, Nowlin J, Quinn JF. Prevention of age-related spatial memory deficits in a transgenic Mouse model of Alzheimer’s disease by chronic Ginkgo Bioloba treatment. Exp Neurol. 2003;184:510–520. doi: 10.1016/s0014-4886(03)00399-6. [DOI] [PubMed] [Google Scholar]
- 19.Abidin I, Yargicoglu P, Agar A, Gumuslu S, Aydin S. The effect of chronic restraint stress on spatial learning and memory: relation to oxidant stres. Int J Neurosci. 2004;114:683–699. doi: 10.1080/00207450490430543. [DOI] [PubMed] [Google Scholar]
- 20.Cantuti-Castelvetri I, Shukitt-Hale B, Joseph JA. Neurobehavioral aspects of antioxidants in aging. Int J Dev Neurosci. 2000;18:367–381. doi: 10.1016/s0736-5748(00)00008-3. [DOI] [PubMed] [Google Scholar]
- 21.Chan AS, Cheung MC, Law SC, Chan JH. Phase II study of alpha-tocopherol in improving the cognitive function of patients with temporal lobe radionecrosis. Cancer. 2004;100:398–404. doi: 10.1002/cncr.11885. [DOI] [PubMed] [Google Scholar]
- 22.Mecocci P, Mariani E, Cornacchiola V, Polidori MC. Antioxidants for the treatment of mild cognitive impairment. Neurol Res. 2004;26:598–602. doi: 10.1179/016164104225017659. [DOI] [PubMed] [Google Scholar]
- 23.Ames BN, Shigenaga MK, Hagen TM. Oxidants antioxidants and the degenerative diseases of aging. Proc Natl Acad Sci U S A. 1993;90:7915–7922. doi: 10.1073/pnas.90.17.7915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.McCay PB, Vitamin E. Vitamin E: interactions with free radical and ascorbate. Annu Rev Nutr. 1995;5:323–340. doi: 10.1146/annurev.nu.05.070185.001543. [DOI] [PubMed] [Google Scholar]
- 25.Carr A, Frei B. Does vitamin C act as a pro-oxidant under physiological conditions? FASEB J. 1999;13:1007–1024. doi: 10.1096/fasebj.13.9.1007. [DOI] [PubMed] [Google Scholar]
- 26.Archer J. Tests for emotionality in rats and mice: a review. Anim Behav. 1973;21:205–235. doi: 10.1016/s0003-3472(73)80065-x. [DOI] [PubMed] [Google Scholar]
- 27.Coscina DV, Yehuda S, Dixon LM, Kish SJ, Leprohongreenwood CE. Learning is improved by a soybean oil diet in rats. Life Sci. 1986;38:1789–1794. doi: 10.1016/0024-3205(86)90130-x. [DOI] [PubMed] [Google Scholar]
- 28.Benton D. Dietary fat and cognitive functioning. In: Hillbrand M, Spitz RT, editors. Lipids, Health, and Behavior. Washington, DC: American Psychological Association; 1997. pp. 227–243. [Google Scholar]
- 29.LaRosa JC. Cholesterol lowering, low cholesterol and non cardiovascular disease. In: Hillbrand M, Spitz RT, editors. Lipids, Health and Behavior. Washington, DC: American Psychological Association; 1997. pp. 275–296. [Google Scholar]
- 30.Wurtman RJ. Nutrients that modify brain function. Sci Am. 1982;246:42–59. doi: 10.1038/scientificamerican0482-50. [DOI] [PubMed] [Google Scholar]


