Abstract
INTRODUCTION
Gemcitabine is a chemotherapeutic agent frequently used by for the treatment of several malignancies both in the adjuvant and metastatic setting. Although myelosuppression is the most adverse event of this therapy, gemcitabine might induce severe pulmonary toxicities. We describe a case of pulmonary veno-occlusive disease (PVOD) related to gemcitabine.
CASE PRESENTATION
The patient was an 83-year-old man with a metastatic pancreatic cancer who was treated by gemcitabine as first-line therapy. He was in good health and received no other chemotherapy. A dose of 1000 mg/m2 of gemcitabine was administered over a 30-minute intravenous infusion on days 1, 8, and 15 of a 28-day cycle. After a period of 6 months, a complete response was observed. Nevertheless, the patient developed a severe dyspnea, with arterial hypoxemia and very low lung diffusion for carbon monoxide. A CT scan showed diffuse ground glass opacities with septal lines, bilateral pleural effusion, and lymph node enlargement. On echocardiography, there was a suspicion of pulmonary hypertension with elevated systolic pulmonary artery pressure and normal left ventricular pressures. Right heart catheterization confirmed pulmonary hypertension and normal pulmonary artery occlusion pressure. Diagnosis of PVOD was made, and a gemcitabine-induced toxicity was suspected. A symptomatic treatment was started. At last follow-up, patient was in functional class I with near-normal of CT scan, arterial blood gases, and echocardiography. A gemcitabine-induced PVOD is the more likely diagnosis.
Keywords: gemcitabine, pancreatic cancer, pulmonary toxicities, veno occlusive disease
Introduction
Gemcitabine is a pyrimidine analog used in many solid tumors. It is relatively well tolerated, and pulmonary toxicities are usually mild and self-limiting. Although up to 25% of patients treated with gemcitabine may develop dyspnea, severe pulmonary adverse events remain rare and include mainly diffuse alveolar damage, acute respiratory distress syndrome, interstitial pneumonitis, or noncardiogenic pulmonary edema requiring steroid therapy.1–3 Recently, Roychowdhury et al analyzed the incidences of pulmonary toxicity events in 4,448 patients treated with gemcitabine in clinical trial databases.4 The authors reported 0.27% of other serious pulmonary toxicities. Based on an estimated 217,400 patients treated with commercial gemcitabine worldwide, the crude incidences of dyspnea and other serious pulmonary toxicity events were 0.02% and 0.06%, respectively.4 However, these publications lack a precise description of gemcitabine-induced lung toxicities. The clinical presentation of these pulmonary diseases is mostly a sub-acute clinical syndrome and is frequently nonspecific. There are several possible diagnoses, and the delay between gemcitabine introduction and the onset of pulmonary symptoms is variable as described in previous case reports (Table 1).1–15 We report a case of pulmonary veno-occlusive disease (PVOD) related to gemcitabine. To our knowledge, this is the second case reported in the literature. Written informed consent was obtained from the next of kin of the patient for publication of this case report and accompanying images.
Table 1.
Review of lung toxicity reported in the previous case report.
| REFERENCE | DISEASE | DELAY FROM GEMCITABINE INITIATION TO LUNG TOXICITY (DAYS) | DIAGNOSES | OUTCOMES |
|---|---|---|---|---|
| Pavlakis (1997) | Large cell lung carcinoma | 43 | Interstitial pneumonitis | Death |
| Ovarian cancer | 78 | Interstitial pneumonitis | Recovery | |
| Takada (1998) | Squamous cell lung carcinoma | 23 | Pulmonary fibrosis | Death |
| Marruchella (1998) | Squamous cell lung carcinoma | 42 | Diffuse alveolar damage | Death |
| Vander (1998) | Lung adenocarcinoma | 30 | Diffuse interstitial lung disease | Recovery |
| Rosado (2002) | Lung adenocarcinoma | 27 | Interstitial pneumonitis | Death |
| Sabria-Trias (2002) | Lung adenocarcinoma | 21 | Diffuse interstitial syndrome | Recovery |
| Maniwa (2003) | Lung adenocarcinoma | 3 | Acute respiratory distress syndrome | Death |
| Shaib (2008) | Pancreatic cancer | 7 | Pneumonitis | Recovery |
| Ho Kim (2008) | Pancreatic cancer | 28 | Diffuse interstitial infiltrates | Recover |
| Ko (2008) | Ovarian cancer | 56 | Interstitial pneumonitis | Recovery |
| Galvao (2010) | Gall bladder adenocarcinoma | 21 | Interstitial pneumonia | Death |
| Sherrod (2011) | Invasive ductal carcinoma | One year | Hypersensitivity pulmonary | Recovery |
| Yakabe (2013) | Pancreatic cancer | 60 | Eosinophilic pneumonia | Death |
Case Presentation
An 83-year-old man, without a history of cardiopulmonary disease, was admitted to our institution for epigastric pain. Only an antecedence of high blood pressure was reported and the concomitant medications were Plavix®, cholesterol-lowering therapy, finasteride for a benign prostatic hypertrophy, and an antihypertensive medication. Of note, the patient was a nonsmoker. After extensive medical tests, a pancreatic tumor was diagnosed, measuring 42 mm and associated with the involvement of lymph nodes. A biopsy revealed the presence of an undifferentiated adenocarcinoma of the pancreas. A CT scan identified the presence of four hepatic metastases. Baseline carcinoembryonic antigen and CA 19–9 were normal. Doses of 1000 mg/m2 of gemcitabine were administered as a monotherapy over a 30-minute intravenous infusion on days 1, 8, and 15 of a 28-day cycle. A total of 24 injections (eight cycles) of gemcitabine were administered without any extra-pulmonary side effects. Six months after the beginning of chemotherapy, an assessment of his disease was performed by a CT scan, which showed a complete response on liver metastases and partial response on primary pancreatic tumor. Gemcitabine was continued.
Seven months following gemcitabine introduction, the patient developed dyspnea on exertion (New York Heart Association [NYHA] Class III) and hemoptysis, slowly increasing for several weeks. The patient needed hospitalization, and an oxygen therapy was required. On physical examination, patient had crackles at both lung bases. No sign of fluid overload was noted. On arterial blood gases, the PO2 was 55 mmHg and the PCO2 was 42 mmHg in breathing room. Hematologic and biochemical tests were in the normal range. Brain natriuretic peptide was moderately high, at 800 ng/L. Electrocardiogram showed a right bundle branch block with a left anterior hemiblock.
A CT scan identified diffuse ground glass opacities with thickening of the interlobular septa and mild pleural effusion. There were also peripheral and posterior micronodules and mediastinal lymph nodes (Fig. 1).
Figure 1.


Chest computer tomography scanner showing diffuse ground glass opacities and interlobular septa thickening. There were also peripheral and posterior micronodules and mediastinal lymph nodes.
Infectious causes were ruled out because of the absence of fever and negative microbiological testing.
Antinative DNA antibodies, antinuclear antibodies, and anticardiolipin antibodies levels were normal.
Bronchoscopy was performed, and analysis of the bronchoalveolar lavage showed neutrophilia (49%) and small eosinophilia (10%). There was a moderate chronic alveolar hemorrhage with siderophages (Golde score = 63). A cytological examination did not find circulating tumor cells. The results of bacteriological tests were negative.
Pulmonary function tests after this procedure showed a slight restrictive pattern (vital capacity 65%, normal Tiffeneau ratio), whereas diffusing capacity of the lung for carbon monoxide (DLCO) was reduced to 32% of the predicted value.
Echocardiography revealed left ventricular hypertrophy with normal left ventricular systolic and diastolic functions. Right ventricular cavity was dilated, and systolic pulmonary artery pressure was estimated at 80–100 mmHg.
Right heart catheterization was carried out, which confirmed severe “precapillary” pulmonary hypertension with mean pulmonary artery pressure at 45 mmHg and pulmonary artery occlusion pressure at 7 mmHg. Cardiac index was maintained at 2.7 L/min/mL and venous oxygen saturation (SvO2) was measured as 57%. Blood was taken during right heart catheterization after occlusion of a pulmonary artery for cytological examination, but no circulating tumor cells were found.
The diagnosis of PVOD (group 1′ of the Nice classification) was then hypothesized and gemcitabine was stopped. High doses of diuretics and curative doses of heparin were started. In consequence, the clinical status of the patient was stabilized.
The patient was still in complete remission 24 months after the diagnosis of his pancreatic carcinoma. Eighteen months after stopping chemotherapy, an improvement in dyspnea (NYHA II) was noted, and moderate physical activity began once again. Clinical examination was normal except for some crackles on auscultation. No signs of heart failure were found, and no new pathology was detected. The CT scan did not show any abnormalities (Fig. 2). Oxygenotherapy was stopped.
Figure 2.
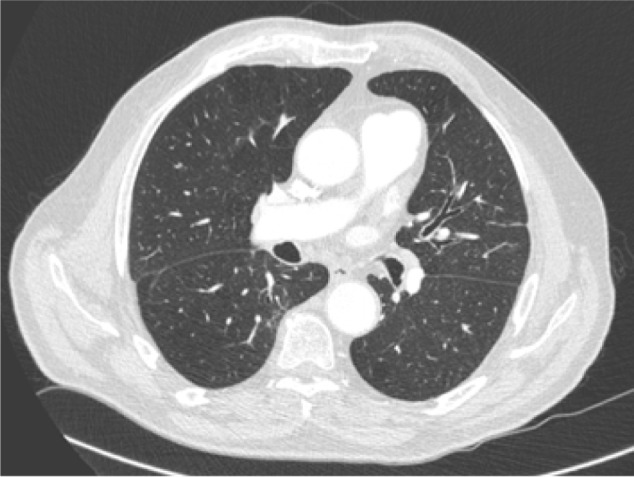
18 months after, CT scan showing the near disappearance of radiological signs.
Discussion
Gemcitabine (2′,2′-difluoro-2′-deoxycytidine [dFdC]) is a deoxycytidine analog with multiple modes of action inside the cell. As a prodrug, dFdC, is phosphorylated by deoxycytidine kinase to the active compounds gemcitabine di- and triphosphate. Gemcitabine diphosphate can be particularly toxic if the pool of cellular ATP decreases. However, many mechanisms are probably involved but they are still unknown. The principal pharmacological activity of the drug is its incorporation into DNA during replication in the S phase of the cell cycle. The results are the inactivation of DNA polymerases and the inhibition of DNA synthesis. Gemcitabine exerts its activity primarily by inducing cell cycle arrest and cell death. The induction of apoptosis through caspase signaling is also another important mechanism of action.16 The precise molecular mechanisms determining tumor cell responses to gemcitabine and the impact of mechanistic interactions with other chemotherapeutic agents remain unelucidated.17
Gemcitabine is generally well tolerated, with common side effects including nausea and vomiting, rash, fever, reversible elevation of liver transaminases, flu-like symptoms, and peripheral edema. Myelosuppression is the most common dose-limiting toxicity.18 Various pulmonary toxicities of gemcitabine have been reported. The frequencies vary between 0.7% and 13% in retrospective analyses. Pulmonary toxicities due to gemcitabine have been reported, including bronchospasm, capillary leak syndrome, non-cardiogenic pulmonary edema, hypersensitivity reaction, acute respiratory distress syndrome, diffuse alveolar damage, pleural effusion, and chronic interstitial pneumonitis. Belknap has reviewed the data from spontaneous reports to Research on Adverse Drug Events and Reports of pharmacovigilance (RADAR) program and appraised the clinical feature of gemcitabine-associated severe acute lung injury. Among 178 reports of gemcitabine-induced pulmonary injury (55 from clinical trials and 92 from spontaneous reports), dyspnea, fever, and pulmonary infiltrate were the most frequent symptoms. Eleven Phase II or Phase III clinical trials enrolling 317 patients identified pulmonary injury rates >10%.19
The present case report provides further evidence that chemotherapy may produce PVOD. To our knowledge, this is the second case of PVOD probably related to gemcitabine administration.20 In the first case, the patient was treated for lung cancer. Gemcitabine was the third line of chemotherapy. Seven cycles were administered with the standard regimen. Radiological exam showed disappearance of the tumor. Dyspnea appeared during the second cycle, and clinical and radiological signs were the same as in our patient. The right catheterization confirmed the diagnosis because the mean pulmonary artery pressure was elevated to 35 mmHg. We could identify many similarities in both case reports. The main difference was that their patient died and an autopsy confirmed diagnosis.
PVOD is uncommon and belongs to group 1′ in new classification of pulmonary arterial hypertension (PAH).21 It is characterized by elevated pulmonary artery pressure leading to right heart failure. Several risk factors for PVOD have been described, including infection, genetic factors, autoimmune disorders, congenital heart disease, and exposure to toxins. PVOD has also been found in association with a variety of different tumors treated with chemotherapy protocols that mostly contained mitomycin, bleomycin, gemcitabine, or carmustine.19
Formal diagnosis of PVOD is based on histopathology22 and requires a lung biopsy or pathologic examination of pulmonary explants or postmortem lung samples. Vascular lesions dominate the post-capillary level of pulmonary vasculature. Histopathologic features are venous changes with intimal obstruction, congested capillaries, dilated lymphatics, and alveoli with hemosiderin-laden macrophages. However, lung biopsy is hazardous in patients with severe pulmonary hypertension, and this histological evidence is rarely available. Biopsy is absolutely contraindicated. There is a need for noninvasive diagnostic tools in this patient population.
The diagnosis of PVOD was considered highly probable if the patients fulfilled the characteristics previously described: hypoxemia, post-capillary pulmonary hypertension confirmed by right heart catheterization (with an increase of pulmonary pressure and a pulmonary artery occlusion pressure normal), presence of radiological abnormalities on high-resolution CT (HRCT) of the chest (including lymph node enlargement, centrilobular ground-glass opacities, and septal lines), low DLCO, and occult alveolar hemorrhage.
The diagnosis of drug-induced lung disease is made by the exclusion of other potential causes, including congestive heart failure, infections, auto-immune disease, or lymphangitic carcinomatosis. It has generally been accepted that a diagnosis of chemotherapy-induced pulmonary toxicity can be made when it develops shortly after the initiation of treatment, when there is lack of an alternative explanation of respiratory and/or cardiac failure, and, in some cases, when there is the resolution of symptoms after corticosteroid treatment and withdrawal of the presumed agent.23
In the present case report, the diagnosis was proposed on the basis of the presence of a severe pulmonary hypertension associated with typical clinical and radiological features. Differential diagnoses were ruled out by physical, biological, and radiological examinations. Indeed, an HRCT scan did not support any arguments in favor of pulmonary embolism, and immune infiltration of the lung could also be excluded. The absence of tumor cells in the bronchoscopy and the absence of disease progression during the follow-up ruled out a cancer-related lung infiltration. Finally, tumor emboli were unlikely because anatomo-pathological examination of arterial blood was negative and usually described with a precapillary pulmonary hypertension.
The evolution was an argument for drug toxicity because the clinical condition of patient improved after discontinuing gemcitabine. Moreover, no other therapy was introduced that could have been responsible for the development of respiratory problems.
When gemcitabine was stopped, the favorable clinical course was of drug toxicity. No specific treatment of PAH (such as epoprostenol) was introduced, which might have produced an adverse event.
A score of 7 was obtained using the Naranjo adverse drug reaction probability scale, suggesting gemcitabine as the most probable cause of PVOD in our patient.24
Clinical course of PVOD is often unfavorable, leading to death of the patient. Various treatment options have been recommended for PVOD, such as vasodilators, immunosuppressive medications, anticoagulant or antithrombotic agents, and, most of the time, oxygen therapy. While specific PAH therapies such as intravenous prostacyclin have established efficacy in the treatment of PAH, benefits of these treatments in patients with PVOD are unclear, as these patients may be refractory to PAH-specific therapy and may even deteriorate with it. Lung transplantation is the only curative treatment for PAH.25 Unfortunately, this treatment can only rarely be proposed and contraindicated in cases of cancer.
Conclusion
We presented a case of PVOD occurring following administration of gemcitabine for pancreatic cancer. Although this chemotherapy is very well tolerated, PVOD might be considered during the investigations performed to characterize any serious pulmonary toxicity of gemcitabine. This serious complication is rare, but can potentially be fatal or have a significant detrimental effect, resulting in severely reduced functional capacity and dependency on supplemental oxygen. This report illustrates the importance of early detection and treatment in maintaining the quality of life for patients.
Acknowledgments
This work was supported by the University Hospital of Besancon and the Clinical Investigation center of Besançon.
Footnotes
ACADEMIC EDITOR: William C S Cho, Editor in Chief
FUNDING: Authors disclose no funding sources.
COMPETING INTERESTS: Authors disclose no potential conflicts of interest.
Paper subject to independent expert blind peer review by minimum of two reviewers. All editorial decisions made by independent academic editor. Upon submission manuscript was subject to anti-plagiarism scanning. Prior to publication all authors have given signed confirmation of agreement to article publication and compliance with all applicable ethical and legal requirements, including the accuracy of author and contributor information, disclosure of competing interests and funding sources, compliance with ethical requirements relating to human and animal study participants, and compliance with any copyright requirements of third parties. This journal is a member of the Committee on Publication Ethics (COPE).
Author Contributions
Conceived and designed the experiments: CT. Analyzed the data: CT. Wrote the first draft of the manuscript: CT. Contributed to the writing of the manuscript: CB. Agree with manuscript results and conclusions: CB. Jointly developed the structure and arguments for the paper: CT, CB. Made critical revisions and approved final version: MJ, SK, MM, BD, TNG, PM, BG, M-BVR, BH. All authors reviewed and approved of the final manuscript.
REFERENCES
- 1.Pavlakis N, Bell DR, Millward MJ, Levi JA. Fatal pulmonary toxicity resulting from treatment with gemcitabine. Cancer. 1997;80:286–91. doi: 10.1002/(sici)1097-0142(19970715)80:2<286::aid-cncr17>3.0.co;2-q. [DOI] [PubMed] [Google Scholar]
- 2.Marruchella A, Fiorenzano G, Merizzi A, Rossi G, Chiodera PL. Diffuse alveolar damage in a patient treated with gemcitabine. Eur Respir J. 1998;11:504–6. doi: 10.1183/09031936.98.11020504. [DOI] [PubMed] [Google Scholar]
- 3.Barlesi F, Villani P, Doddoli C, et al. Gemcitabine-induced severe pulmonary toxicity. Fundam Clin Pharmacol. 2004;18:85–91. doi: 10.1046/j.0767-3981.2003.00206.x. [DOI] [PubMed] [Google Scholar]
- 4.Roychowdhury DF, Cassidy CA, Peterson P, Arning M. A report on serious pulmonary toxicity associated with gemcitabine-based therapy. Invest New Drugs. 2002;20:311–5. doi: 10.1023/a:1016214032272. [DOI] [PubMed] [Google Scholar]
- 5.Takada M, Negoro S, Kudo S, et al. Activity of gemcitabine in non-small-cell lung cancer: results of the Japan gemcitabine group (A) phase II study. Cancer Chemother Pharmacol. 1998;41(3):217–22. doi: 10.1007/s002800050731. [DOI] [PubMed] [Google Scholar]
- 6.Vander Els NJ Miller V. Successful treatment of gemcitabine toxicity with a brief course of oral corticosteroid therapy. Chest. 1998;114(6):1779–81. doi: 10.1378/chest.114.6.1779. [DOI] [PubMed] [Google Scholar]
- 7.Rosado MF, Kett DH, Schein RM, Baraona FJ, Sridhar KS. Severe pulmonary toxicity in a patient treated with gemcitabine. Am J Clin Oncol. 2002;25(1):31–3. doi: 10.1097/00000421-200202000-00005. [DOI] [PubMed] [Google Scholar]
- 8.Gupta N, Ahmed I, Steinberg H, Patel D, Nissel-Horowitz S, Mehrotra B. Gemcitabine-induced pulmonary toxicity: case report and review of the literature. Am J Clin Oncol. 2002;25(96):100. doi: 10.1097/00000421-200202000-00021. [DOI] [PubMed] [Google Scholar]
- 9.Maniwa K, Tanaka E, Inoue T, et al. An autopsy case of acute pulmonary toxicity associated with gemcitabine. Intern Med. 2003;42(10):1022–5. doi: 10.2169/internalmedicine.42.1022. [DOI] [PubMed] [Google Scholar]
- 10.Shaib W, Lansigan F, Cornfeld D, Syrigos K, Saif MW. Gemcitabine-induced pulmonary toxicity during adjuvant therapy in a patient with pancreatic cancer. JOP. 2008;9(6):708–14. [PubMed] [Google Scholar]
- 11.Ho Kim D, Shiozawa S, Tsuchiya A, et al. A case of drug-induced interstitial pneumonitis after adjuvant chemotherapy with gemcitabine for pancreatic cancer. Gan To Kagaku Ryoho. 2008;35:133–6. [PubMed] [Google Scholar]
- 12.Ko E, Lee S, Goodman A. Gemcitabine pulmonary toxicity in ovarian cancer. Oncologist. 2008;13(7):807–11. doi: 10.1634/theoncologist.2008-0049. [DOI] [PubMed] [Google Scholar]
- 13.Galvão FH, Pestana JO, Capelozzi VL. Fatal gemcitabine-induced pulmonary toxicity in metastatic gallbladder adenocarcinoma. Cancer Chemother Pharmacol. 2010;65:607–10. doi: 10.1007/s00280-009-1167-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sherrod AM, Brufsky A, Puhalla S. A case of late-onset gemcitabine lung toxicity. Clin Med Insights Oncol. 2011;5:171–6. doi: 10.4137/CMO.S6643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yakabe T, Kitahara K, Komiya K, et al. Severe eosinophilic pneumonia presenting during gemcitabine adjuvant chemotherapy. World J Surg Oncol. 2013;11:167. doi: 10.1186/1477-7819-11-167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Noble S, Goa KL. Gemcitabine. A review of its pharmacology and clinical potential in non-small cell lung cancer and pancreatic cancer. Drugs. 1997 Sep;54(3):447–72. doi: 10.2165/00003495-199754030-00009. [DOI] [PubMed] [Google Scholar]
- 17.Hamed SS, Straubinger RM, Jusko WJ. Pharmacodynamic modeling of cell cycle and apoptotic effects of gemcitabine on pancreatic adenocarcinoma cells. Cancer Chemother Pharmacol. 2013 Sep;72(3):553–63. doi: 10.1007/s00280-013-2226-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Saif MW. Pulmonary toxicity associated with gemcitabine. J Pancreas. 2010;11(2):189–190. [PubMed] [Google Scholar]
- 19.Belknap SM, Kuzel TM, Yarnold PR, Slimack N, Lyons EA, Raisch DW, et al. Clinical features and correlates of gemcitabine-associated lung injury: findings from the RADAR project. Cancer. 2006 May 1;106(9):2051–7. doi: 10.1002/cncr.21808. [DOI] [PubMed] [Google Scholar]
- 20.Vansteenkiste JF, Bomans P, Verbeken EK, Nackaerts KL, Demedts MG. Fatal pulmonary veno-occlusive disease possibly related to gemcitabine. Lung Cancer. 2001 Jan;31(1):83–5. doi: 10.1016/s0169-5002(00)00159-8. [DOI] [PubMed] [Google Scholar]
- 21.Simonneau G, Gatzoulis MA, Adatia I, Celermajer D, Denton C, Ghofrani A, et al. Updated clinical classification of pulmonary hypertension. J Am Coll Cardiol. 2013 Dec 24;62(25 Suppl):D34–41. doi: 10.1016/j.jacc.2013.10.029. [DOI] [PubMed] [Google Scholar]
- 22.Sanchez O, Humbert M, Sitbon O, Jais X, Simonneau G. Guidelines for the diagnosis and treatment of pulmonary hypertension. Rev Mal Respir. 2010 Feb;27(2):141–50. doi: 10.1016/j.rmr.2009.11.013. [DOI] [PubMed] [Google Scholar]
- 23.Hiraya D, Kagohashi K, Sakamoto N, Kondo T, Satoh H. Gemcitabine-induced pulmonary toxicity in a patient with pancreatic cancer. JOP. 2010 Mar 5;11(2):186–8. [PubMed] [Google Scholar]
- 24.Naranjo CA, Busto U, Sellers EM, Sandor P, Ruiz I, Roberts EA, et al. A method for estimating the probability of adverse drug reactions. Clin Pharmacol Ther. 1981 Aug;30(2):239–45. doi: 10.1038/clpt.1981.154. [DOI] [PubMed] [Google Scholar]
- 25.Montani D, Price LC, Dorfmuller P, Achouh L, Jaïs X, Yaïci A, et al. Pulmonary veno-occlusive disease. Eur Respir J. 2009 Jan;33(1):189–200. doi: 10.1183/09031936.00090608. [DOI] [PubMed] [Google Scholar]


