Abstract
Studies in animals and human volunteers demonstrate that antibodies against the repeat-region of the Plasmodium circumsporozoite protein (CSP) abrogate sporozoite infection. However, the realization that the N- and C- terminal regions flanking the repeats play essential roles in parasite infectivity raised the possibility that they could be targeted by protective antibodies. We characterized a monoclonal antibody (mAb5D5) specific for the N-terminus of the P. falciparum CSP, which inhibits the proteolytic cleavage of the CSP, a key requirement for parasite infection of hepatocytes. Adoptive transfer of mAb5D5 strongly inhibits the in vivo infection of sporozoites expressing the N-terminus of P. falciparum CSP, and this protection is greatly enhanced when combined with antirepeat antibodies. Our results show that antibodies interfering with molecular processes required for parasite infectivity can exert a strong in vivo protective activity and indicate that pre-erythrocytic vaccines against Plasmodium should include the CSP N-terminal region.
Keywords: antibodies, CSP, malaria, P. falciparum, sporozoites, vaccine
The circumsporozoite protein (CSP) is the major surface protein of Plasmodium sporozoites, forming a dense coat on the parasite's surface. This protein plays a critical role in the journey of Plasmodium parasites from the mosquito to the mammalian host and it is expressed in sporozoites of rodent, primate, and human Plasmodium species. The basic structure of the CSP consists of a central repeat domain flanked by an N-terminal portion containing a conserved proteolytic cleavage site, and a C-terminal flanking region containing a type I thrombospondin repeat (TSR) motif. As the parasites migrate from the mosquito salivary glands to the liver of the mammalian host, the CSP undergoes major conformational changes [1, 2]. Sporozoites injected into the skin display a folded CSP, such that the N-terminal region masks the C-terminal TSR. Upon reaching the liver, this protein undergoes proteolytic cleavage of the N-terminal domain, exposing the C-terminus region, in what appears to be a critical requirement for hepatocyte invasion by sporozoites [1, 2].
CSP has been intensively studied as a vaccine candidate, as it is well established that antibodies and T-cell responses against this antigen inhibit infection in mammalian hosts and provide protective immunity in experimental animal models and humans [3, 4]. The malaria vaccine candidate RTS,S is a virus-like particle consisting of the central repeat and C-terminal regions of the P. falciparum CSP fused to a hepatitis B virus surface antigen (HBVsAg). A number of phase 2 and 3 vaccine trials showed that 30%–50% of children were protected after vaccination [5–9]. These studies also indicated that anti-CSP antibody titers specific for the repeat domain strongly correlate with the protection observed among vaccinees. The protective efficacy of the RTS,S vaccine candidate is encouraging and suggests that further improvements of this subunit vaccine may provide increased protection. Importantly, the RTS,S vaccine does not include the CSP N-terminal region, which contains important functional domains, such as a ligand-binding domain [10] and a proteolytic processing site [2], and may be an important target for protective antibodies.
Here we describe the characterization of a monoclonal antibody, mAb5D5, which binds to a linear epitope located in the N-terminal region of the P. falciparum CSP and prevents its enzymatic processing. Using P. berghei transgenic parasite strains expressing a chimeric CSP that contains the N-terminal region of P. falciparum [11], we demonstrate that mAb5D5 strongly inhibits sporozoite infection in vivo. Moreover, we show that combining mAb5D5 with antibodies against the repeat domain of the P. falciparum CSP greatly enhances the in vivo neutralization of sporozoites.
Taken together, these data demonstrate that the CSP N-terminus represents a promising target for antibody-based protection against parasite infection of hepatocytes, and indicates that this CSP region should be included as part of a CSP-based, pre-erythrocytic vaccine construct.
MATERIALS AND METHODS
Antibody Development
Antibodies were prepared by Precision Antibody, a division of A&G Pharmaceutical Inc (Columbia, Maryland) using proprietary technology and mouse immunization protocols. Mice were immunized with full-length recombinant CSP (rCSP) [12] and serum titers determined by enzyme-linked immunosorbent assay (ELISA). Splenocytes were harvested and fused once titers exceeded 1:50 000. Hybridoma supernatants were screened for reactivity to rCSP by ELISA. A total of 14 clones were selected for monoclonal antibody (mAb) production and immunoglobin G (IgG) was purified using a Protein G column followed by a buffer change to phosphate-buffered saline (PBS).
Epitope Specificity Analysis
The fine epitope specificity of mAb5D5 was determined using 2 types of ELISAs.
Direct binding to short P. falciparum CSP peptides. An ELISA with CSP 15-mer peptides overlapping by 11 amino acids (Genscript, Piscataway Township, New Jersey), spanning the entire length of the protein (P. falciparum, 3D7 strain), was used to determine the epitope specificity. Briefly, MaxiSorp ELISA plates (Thermo Scientific Nunc, Rochester, New York) were coated with 100 microliters of a synthetic CSP peptides (1 µg/mL) and incubated overnight at room temperature. The wells were then washed and incubated with PBS and 1% bovine serum albumin (BSA) (Sigma-Aldrich) (PBS-1% BSA) for 1 hour at room temperature. Then, the wells were washed and incubated for 1 hour at room temperature with 250 ng/mL of mAb5D5 in PBS-1% BSA. After another washing step, the plate was incubated for 1 hour at room temperature with a peroxidase-labeled goat antimouse (IgG H + L) secondary antibody (KPL, Gaithersburg, Maryland) at 0.5 µg/mL in PBS-1% BSA. The assay was developed using a horseradish peroxidase substrate kit (KPL, Gaithersburg, Maryland), according to the manufacturer's specifications.
Competitive ELISA by short synthetic CSP peptides against P. falciparum recombinant CSP. To further corroborate the mAb5D5 epitope specificity, we sought to inhibit antibody binding to rCSP [12] using short synthetic peptides. In brief, ELISA plates coated with 100 microliters of rCSP (200 ng/mL) were incubated overnight at room temperature. Then, the plates were incubated with PBS-1% BSA for an hour at room temperature. mAb5D5 (15 ng/mL) was incubated for 2 hours at 37°C with different concentrations of selected CSP15-mer peptides in a total volume of 100 microliters of PBS-1% BSA. Then, the antibody-peptide mixtures were transferred to the CSP-coated ELISA plate and incubated for 1 hour at room temperature. The plate was then washed and incubated with the peroxidase-labeled secondary antibody and developed as described above.
Transgenic Parasites
Plasmodium berghei NK65 strain expressing the repeat domain and a portion of the N-terminal region of P. falciparum CSP [11], P.b.-P.f. CSP–R, was kindly provided by Dr Elizabeth Nardin (New York University). A new transgenic strain, derived from P. berghei ANKA strain expressing the N-terminus of P. falciparum, was generated using the plasmid pR-CSPfNT, which carries the N-terminal region of the P. falciparum CSP. This plasmid was derived from plasmid pIC-CSPfNT, which resulted from the replacement of the P. berghei CSP N-terminus with the N-terminal region of the 3D7 strain of P. falciparum CSP. Briefly, a 570–base pair (bp) restriction fragment encompassing base pairs 65 to 634 of the P. berghei csp gene was excised from pIC-CSwt [13] using the restriction enzymes PflMI and EagI and then replaced with a fragment comprising the P. falciparum CSP N-terminal region. The P. falciparum CSP N-terminus was excised within a 579-bp PflMI-EagI restriction fragment from plasmid pPfNT (Genescript, Piscataway Township, New Jersey), synthesized to comprise the P. falciparum CSP N-terminal region flanked by a PflMI restriction site at its 5′ end and 372 bp from the P. berghei CSP (base pairs 267 to 639) at its 3′ end. Thus, the csp gene in the resulting plasmid, pIC-CSPPfNT, consists of the P. berghei CSP signal sequence (base pairs 1 to 69) followed by the N-terminal region of P. falciparum CSP (base pairs 70 to 285) and the remainder of the P. berghei CSP (base pairs 285 to 1032). We then excised the hybrid csp gene from pIC-CSPfNT as a KpnI-XhoI fragment and inserted it into the transfection plasmid, pR-CSPfNT. KpnI and SacI were used to release the inserted fragment from pR-CSPfNT prior to transfection of wild-type (WT) P. berghei (ANKA strain) parasites, as previously described [14].
Transgenic parasites were selected in Swiss Webster mice by treatment with pyrimethamine (MP Biomedicals, Solon, Ohio) in drinking water (0.07 mg/mL). Pyrimethamine-resistant parasites were then cloned by limiting-dilution. Successful recombination at the 5′ and 3′ ends of the locus was verified by polymerase chain reaction (PCR). The primers used to confirm 5′ integration were 5′-F (TCACCCTCAAGTTGGGTAAAA) and PbPfNT-R (TTATATAAATTAGTGCCTGCATTATCA); the primers to verify integration at the 3′ end were 3′-F (TGTAAAAATGTGTATGTTGTGTGC) and 3′-R (GTGCCCATTACGACTTTGCT). To verify that the cloned parasite population did not have contaminating WT P. berghei parasites, we developed a PCR assay using primers that flank the replaced N-terminal CSP sequence and then digested the resulting product using AflII. This restriction site is not present in the WT P. berghei CSP sequence but was inserted by replacement with our synthetic construct. The primers used for this PCR analysis were 5′-F and the reverse primer PbWT N-terminus (NT) (ACAATCCACAACCACAGC). Lastly, DNA isolated from the cloned chimeric parasites was sequenced to confirm the replacement of the P. berghei N-terminal region with the P. falciparum CSP N-terminus sequence.
Mosquito Infection, Parasite Development, and Sporozoite Infectivity of the P. berghei–P. falciparum CSP N-terminus
Anopheles stephensi female mosquitoes were fed on Swiss Webster mice infected with blood stages of cloned P. berghei–P. falciparum CSP N-terminus (P.b.-P.f. CSP-NT) parasites. The development of viable male gametocytes in blood was qualitatively determined by means of an exflagellation assay as described previously [15] and performed prior to mosquito feeding. The development of oocysts in mosquito midguts was assessed 14 days post blood meal. The midguts were dissected in sterile PBS and stained in a 0.1% mercury chromate solution.
The in vivo infectivity of chimeric P.b.-P.f. CSP-NT sporozoites was assessed in C57BL/6 mice. The liver parasite load was measured by reverse transcription followed by quantitative real-time PCR (RT-qPCR) upon intravenous challenge of mice with 104 chimeric sporozoites [16]. To evaluate the infectivity of P.b.-P.f. CSP-NT sporozoites by mosquito bite, 3 infected A. stephensi mosquitoes were allowed to feed on mice for 3 minutes. The development of blood-stage parasites was then determined by performing daily blood smears.
Immunofluorescence Assay
An indirect immunofluorescence assay (IFA) was used to characterize the reactivity of mAb5D5 against both live and air-dried P.b.-P.f. CSP-NT sporozoites. In brief, for live-sporozoite IFAs, 4 × 104 parasites were incubated on ice with different concentrations of mAb5D5. Sporozoites were then washed 3 times with cold PBS-1% BSA, resuspended in 0.2 mL, and placed into the well of the Lab-Tek chamber (Thermo Scientific Nunc). The chamber was then spun at 300 g for 2 minutes and, after discarding the supernatant, 0.2 mL of PBS-4% paraformaldehyde (Sigma) were added. Samples were incubated for 1 hour at room temperature, washed 3 times with PBS, and incubated with secondary antibody for 30 minutes. Samples were then washed and green-fluorescent sporozoites were visualized using a Nikon Eclipse 90i fluorescent microscope. IFAs using air-dried sporozoites were performed as previously described [15]. Plasmodium falciparum (3D7) sporozoites were obtained from A. stephensi mosquitoes infected with in vitro culture gametocytes of P. falciparum. IFA was performed as described in the preceding items.
Pulse-Chase Metabolic Labeling Experiments
Pulse-chase metabolic labeling experiments were performed as previously described [2]. Briefly, freshly dissected P. falciparum sporozoites were metabolically labeled in Dulbecco's modified Eagle's medium (DMEM) without Cys/Met, 1% BSA and 400 µCi/mL L-[35S]-Cys/Met for 1 hour at 28°C and then kept on ice or chased at 28°C for 2 hours in DMEM with Cys/Met and 1% BSA in the presence of the indicated concentrations of mAb5D5, in the presence of the isotype control mAb3D11 (specific against the P. berghei CSP repeat region [17]), or in the absence of antisera. Chased sporozoites were lysed and labeled CSP was immunoprecipitated with mAb2A10 (specific against the P. falciparum CSP repeat region [18]) conjugated to sepharose, eluted from the beads, and analyzed by sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE) followed by autoradiography.
Inhibition of Sporozoite Infectivity by Passive Transfer of Monoclonal Antibodies
Monoclonal antibodies were passively transferred into C57BL/6 mice by intravenous injection. Immediately after antibody transfer, mice were challenged with 104 sporozoites, delivered by tail vein injection into each mouse. Forty-two hours after challenge, livers were harvested to assess the parasite burden by RT-qPCR. In addition to the newly developed chimeric P.b.-P.f. CSP-NT strain, these experiments were performed using another P. berghei–P. falciparum chimera, which also contains the amino acid sequence recognized by mAb5D5. This chimera “P.b.-P.f. CSP–R” expresses the repeat region and 23 residues upstream of the repeats of the P. falciparum 7G8 strain [11].
Data Analysis
Data were plotted using Graph Pad Prism 4 software. Unless otherwise stated, data were compared for significance using a Mann–Whitney test.
Study Approval
Animal studies were conducted at The Johns Hopkins University. Five- to 8-week old female C57BL/6 or Swiss Webster mice were purchased from NCI (Frederick, Maryland). All experimental procedures involving mice were approved by the Institutional Animal Care and Use Committee of The Johns Hopkins University.
RESULTS
mAb5D5 Targets an Epitope in the N-terminal Region of the P. falciparum CSP
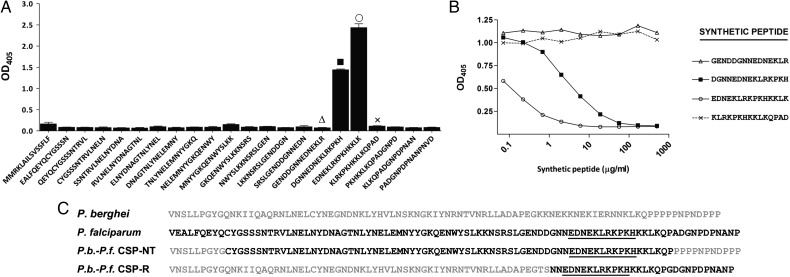
A series of monoclonal antibodies were isolated after immunization of mice with a full-length P. falciparum recombinant CPS (rCSP) [12]. Early experiments indicated that these antibodies bind to rCSP, as assessed by ELISA and Western blot analysis (data not shown). In initial studies, long synthetic peptides representing the N-terminal, repeat, or C-terminal regions of the P. falciparum CSP were used to determine the epitope specificity of selected anti-CSP monoclonal antibodies (Supplementary Figure 1). One of these antibodies, mAb5D5, recognized the peptide representing the N-terminal region of P. falciparum 3D7 CSP, residues 26 to 99. The fine epitope specificity of mAb5D5 was then defined by ELISA using immobilized 15-mer overlapping synthetic peptides representing residues 25 to 97 of the P. falciparum 3D7 CSP. Using this assay, we found that mAb5D5 bound to peptides 77DGNNEDNEKLRKPKH90 and 81EDNEKLRKPKHKKLK95, which represent a region spanning amino acids 81 to 90 in the P. falciparum 3D7 CSP (Figure 1A). Further confirmation of this epitope specificity was obtained from assays in which these synthetic peptides were used in soluble form, to inhibit the binding of mAb5D5 to immobilized rCSP. Consistent with the ELISA results using immobilized peptides, we found that peptides 77DGNNEDNEKLRKPKH90 and 81EDNEKLRKPKHKKLK95 inhibited the binding of mAb5D5 to rCSP. No such effect was observed with the peptides flanking these peptides, namely 73GENDDGNNEDNEKLR87 and 85KLRKPKHKKLKQPAD99 (Figure 1B). Taken together, these results indicate that the core sequence recognized by mAb5D5 in CSP is an epitope defined by the 11-amino-acid peptide 81EDNEKLRKPKH91 of the P. falciparum 3D7 CSP.
Figure 1.
Epitope specificity of the mAb5D5 determined by ELISA. A, Direct binding of mAb5D5 to overlapping synthetic peptides representing the entire N-terminus of P. falciparum CSP. mAb5D5 specifically binds to the peptides 77DGNNEDNEKLRKPKH90 and 81EDNEKLRKPKHKKLK95. B, Competition ELISA between synthetic peptides and rCSP for mAb5D5 binding. Preincubation with different concentrations of synthetic peptides 77DGNNEDNEKLRKPKH90 and 81EDNEKLRKPKHKKLK95 was able to inhibit binding of mAb5D5 to P. falciparum CSP; no effect was observed with the flanking peptides 73GENDDGNNEDNEKLR87 and 85KLRKPKHKKLKQPAD99. Results are expressed as optical density at 405 nm. C, Comparison of CSP N-terminal region amino acid sequences beginning after the signal sequence of P. berghei (ANKA), P. falciparum (3D7), P.b.-P.f. CSP-NT, and P.b.-P.f. CSP-R. The P.b.-P.f. CSP-NT amino acid sequence was derived from DNA sequencing of clonal transgenic parasites. Underlining denotes the core sequence recognized by mAb5D5. Abbreviations: CSP, circumsporozoite protein; ELISA, enzyme-linked immunosorbent assay; mAb5D5, monoclonal antibody 5D5; OD, optical density; P.b.-P.f. CSP-NT, P. berghei–P. falciparum CSP N-terminus; P.b.-P.f. CSP-R, P. berghei–P. falciparum CSP repeat domain; rCSP, recombinant CSP.
mAb5D5 Recognizes the P. falciparum CSP Expressed in Live Sporozoites and Inhibits CSP Processing
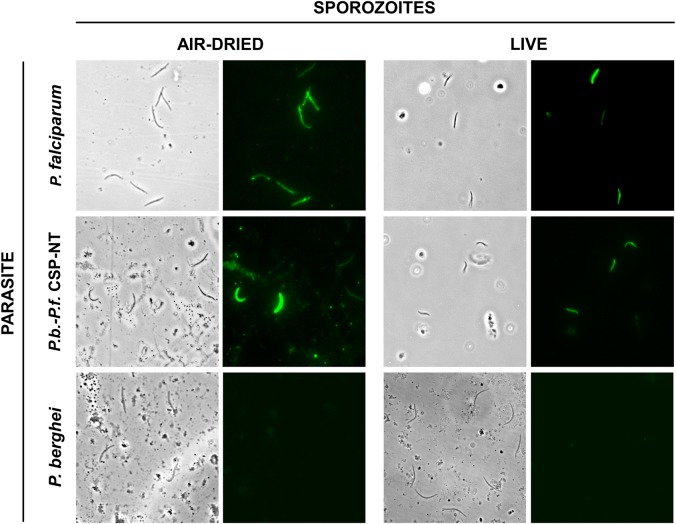
To determine whether this antibody recognized native CSP as expressed in sporozoites, we performed IFAs using P. falciparum sporozoites. The results of these assays using air-dried fixed and live sporozoites clearly indicated that mAb5D5 binds to the native P. falciparum CSP and, most important, that this epitope is exposed in live parasites (Figure 2).
Figure 2.
Recognition of air-dried and live sporozoites by mAb5D5. P. falciparum (3D7), chimeric P.b.-P.f. CSP-NT and P. berghei (ANKA) sporozoites were stained with mAb5D5 (1 µg/mL) live or after air-drying. Abbreviations: CSP, circumsporozoite protein; mAb5D5, monoclonal antibody 5D5; P.b.-P.f. CSP-NT, P. berghei–P. falciparum CSP N-terminus.
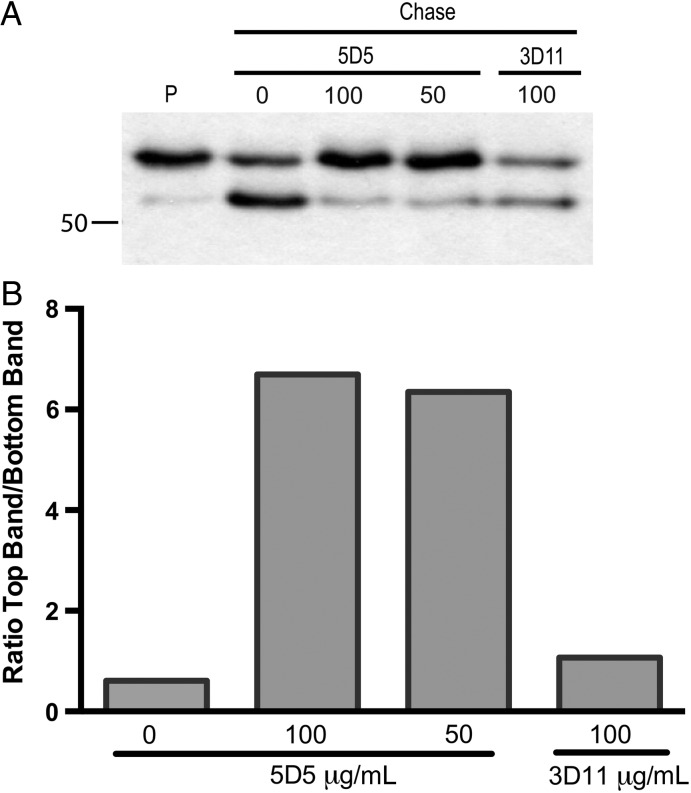
Given that the binding of mAb5D5 site lies in close proximity to Region I, which contains the CSP proteolytic processing site, we performed pulse-chase metabolic labeling experiments to determine if this antibody could inhibit processing of CSP by P. falciparum sporozoites. These sporozoites were metabolically labeled using 35S-cysteine/methionine and then chased in the presence of mAb5D5 or an isotype-control antibody. We found that 100 µg/mL and 50 µg/mL of mAb5D5 had a strong inhibitory effect on CSP processing (Figure 3). These results further highlight mAb5D5's fine epitope-specificity and indicate that antibody binding to, or close to, the proteolytic cleavage site sterically inhibit CSP processing.
Figure 3.
CSP processing is inhibited by mAb5D5. A, P. falciparum sporozoites were metabolically labeled with 35S-Cys/Met and then kept on ice (pulse, P) or chased for 2 hours in the absence (C) or presence of the indicated concentrations (µg/mL) of mAb5D5 or an isotype control (mAb3D11). Sporozoites were then lysed, and CSP was immunoprecipitated and analyzed by SDS-PAGE and autoradiography. Molecular mass is indicated in kilodaltons on the left-hand side of the autoradiograph. B, Densitometry analysis of scanned film using ImageJ software. Shown for each chased sample is the ratio of the density of the high MW CSP band to the low MW CSP band. A ratio of 1 indicates that the density of the top and bottom bands is the same. These results are representative of 2 independent experiments. Abbreviations: CSP, circumsporozoite protein; mAb5D5, monoclonal antibody 5D5; MW, molecular weight; SDS-PAGE, sodium dodecyl sulfate polyacrylamide gel electrophoresis.
The 5D5 Antibody Strongly Inhibits Infection of Hepatocytes
Because the regulated cleavage of CSP is an important step for efficient hepatocyte invasion by sporozoites, we next investigated whether mAb5D5 had an inhibitory effect on sporozoite infectivity in vivo. For this purpose, we performed infectivity assays using P. berghei parasites expressing a chimeric CSP in which the N-terminal region of the P. falciparum replaced the native P. berghei N-terminal sequence, P.b.-P.f. CSP-NT (Supplementary Figure 2A–C). We did not modify the P. berghei CSP's signal sequence (amino acids 1 to 23), thus in the chimeric CSP, amino acids 24 to 92 of the P. berghei CSP have been replaced with residues 25 to 97 from the P. falciparum 3D7 CSP (Figure 1C). The transgenic parasites develop normally in mosquitoes and efficiently infect naive C57BL/6 mice after intravenous injection of sporozoites (Supplementary Figure 2D). In addition, all mice developed blood-stage parasites by 5 days after feeding 3 infected mosquitoes on them, as also observed with mice infected with WT P. berghei (Supplementary Table 1). Furthermore, mAb5D5 (1 µg/mL) recognized live chimeric P.b.-P.f. CSP-NT sporozoites by IFA, thus confirming its epitope specificity. A weak cross-reactivity, possibly due to low sequence homology, was only observed with WT P. berghei parasites when using high concentrations of mAb5D5 (100 µg/mL) (data not shown). These results further corroborate the specificity of mAb5D5 and the recognition of an antigenic determinant that is displayed on the surface of live parasites.
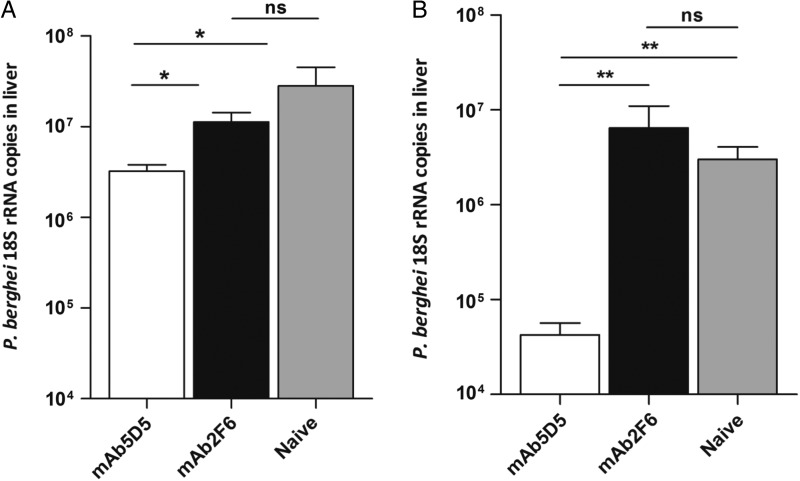
To evaluate the in vivo protective effect of mAb5D5, we passively transferred 300 µg of this antibody into naive C57BL/6 mice which were then challenged with the transgenic P. berghei strain, P.b.-P.f. CSP-NT. We found that mice receiving mAb5D5 and challenged with the P.b.-P.f. CSP-NT sporozoites had nearly 1 log reduction in parasite burden in the liver compared to naive controls (Figure 4A). Control mice receiving an irrelevant antibody, mAb2F6, had parasite loads comparable to those of naive controls. We also evaluated the protective effect of this antibody using a different strain of transgenic P. berghei sporozoites that express part of the N-terminal region (residues 97–111) and the repeat domain of P. falciparum 7G8 CSP, (P.b-P.f. CSP-R) [11] (Figure 1C). As determined by IFA, the sporozoites of this transgenic strain are also recognized by mAb5D5 (data not shown). As observed with P.b.-P.f. CSP-NT sporozoites, when this antibody was passively transferred into mice subsequently challenged with P.b.-P.f. CSP-R sporozoites, it reduces by approximately 2 logs the parasite burden in the liver (Figure 4B). The fact that mAb5D5 can inhibit sporozoite invasion in 2 different chimeric strains rules out the possibility that the observed inhibitory effect could be due to spurious reactivity of mAb5D5 with the repeat domain of P. falciparum or P. berghei CSP. All together, these results confirm the epitope-specificity and remarkable neutralizing capacity of mAb5D5 against sporozoites bearing the N-terminal region of the P. falciparum CSP.
Figure 4.
Protection against challenge with chimeric sporozoites by passive transfer of mAb5D5. 300 µg of mAb5D5 were inoculated intravenously into C57BL/6 mice immediately prior to injection of 104 sporozoites. Forty hours later, livers were harvested, RNA extracted, and liver parasite burden was determined by RT-qPCR. Passive transfer of mAb5D5 had a significant protective effect against P.b.-P.f. CSP-NT chimeric sporozoites (A), as well as P.b.-P.f. CSP-R (B), compared to mice that received irrelevant antibody (mAb2F6) or to naive controls. Mean ± SEM; n = 5, results are representative of 2 independent experiments. *P < .05; **P < .01. Abbreviations: CSP, circumsporozoite protein; mAb5D5, monoclonal antibody 5D5; ns, not significant; P.b.-P.f. CSP-NT, P. berghei–P. falciparum CSP N-terminus; P.b.-P.f. CSP-R, P. berghei–P. falciparum CSP repeat domain; rRNA, ribosomal RNA; RT-qPCR, quantitative real-time polymerase chain reaction; SEM, standard error of the mean.
Enhanced In Vivo Sporozoite Neutralization by Combining mAb5D5 and mAb2A10
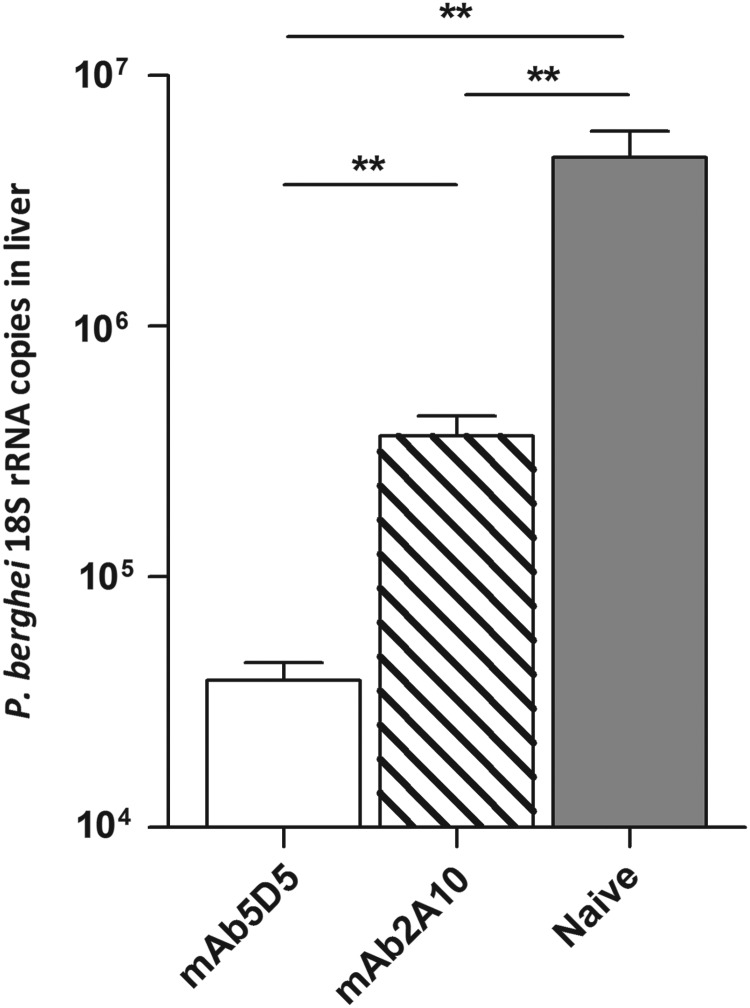
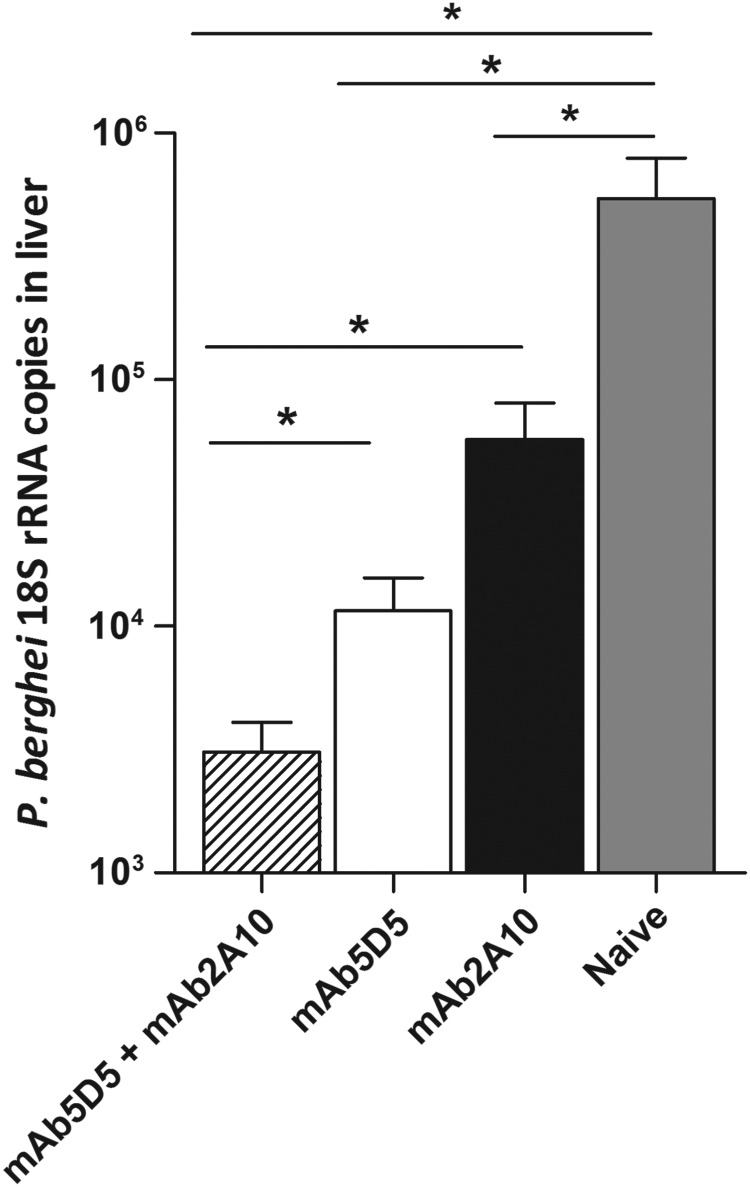
Next, we investigated whether the antibody-mediated sporozoite neutralization could be enhanced by combining antibodies that recognize different epitopes of the P. falciparum CSP. First, we performed passive transfer experiments to compare the neutralizing capacities of mAb5D5 and mAb2A10, specific for the repeat domain [18]. Recipient mice and control mice were challenged with the P.b.-P.f. CSP-R parasite strain, expressing both the N-terminal and repeat-domain epitopes. As expected, both antibodies inhibited parasite infection, although in repeated experiments we consistently observed that mAb5D5 displayed a higher inhibitory activity than mAb2A10 (Figure 5). To determine whether the neutralizing effect of these antibodies was additive, we compared liver parasite burden of mice that received 25 µg of mAb5D5 or 100 µg of mAb2A10 to that of mice receiving both antibodies (ie, mAb5D5 and mAb2A10). When compared to mice that received a single antibody, passive transfer of mAb5D5 together with mAb2A10 resulted in significantly enhanced inhibition of parasite infection (Figure 6).
Figure 5.
Comparison of the sporozoite-neutralizing effect of mAb5D5 and mAb2A10. A total of 300 µg of mAb5D5 or mAb2A10 were inoculated intravenously into C57BL/6 mice immediately prior to injection of 104 P.b.-P.f. CSP-R sporozoites. Passive transfer of both monoclonal antibodies had a significant protective effect compared to mice-naive controls. Mean ± SEM; n = 5, results are representative of 2 independent experiments. **P < .01. Abbreviations: CSP, circumsporozoite protein; mAb, monoclonal antibody; ns, not significant; P.b.-P.f. CSP-R, P. berghei–P. falciparum CSP repeat domain; rRNA, ribosomal RNA; SEM, standard error of the mean.
Figure 6.
Enhanced inhibitory effect upon combination of antibodies of different epitope specificity. The antibodies mAb5D5 (25 µg) and mAb2A10 (100 µg) were administered combined or independently to assess their additive effect against 104 P.b.-P.f. CSP-R sporozoites. Mean ± SEM; n = 5, results are representative of 2 independent experiments. *P < .05. Abbreviations: CSP, circumsporozoite protein; mAb, monoclonal antibody; P.b.-P.f. CSP-R, P. berghei–P. falciparum CSP repeat domain; rRNA, ribosomal RNA; SEM, standard error of the mean.
DISCUSSION
In this study, we report the characterization of a mAb specific for the P. falciparum CSP, which recognizes an epitope located in the N-terminal region of P. falciparum. This antibody binds to a linear epitope defined by the sequence 81EDNEKLRKPKH91, which is exposed on the surface of live sporozoites. Using transgenic P. berghei parasites, expressing the N-terminal region of the P. falciparum CSP we demonstrate, for the first time, that a single mAb against the N-terminal region of the CSP region can neutralize sporozoite infectivity in vivo. Importantly, we show that mAb5D5 blocks the enzymatic processing of CSP, as it recognizes an epitope adjacent to the proteolytic cleavage site of the CSP N-terminal region. These findings are in full agreement with previous studies demonstrating that proteolytic processing of the CSP is a required step for an efficient sporozoite invasion of hepatocytes [1, 2]. Finally, our results are also in agreement with in vitro observations reporting that polyclonal antibodies raised against the N-terminal region of P. falciparum CSP can inhibit sporozoite invasion of in vitro cultured hepatocytes. [10, 19–21].
The protective effect of RTS,S in humans appears to be largely mediated by antibody responses elicited against CSP's repeat region [5–9, 22]. Our data indicate that the neutralizing capacity of anti-CSP antibodies is enhanced when combining antibodies of different epitope specificity, suggesting that vaccines inducing anti-CSP antibodies responses of broad epitope specificities may result in increased protective efficacy. Our results strongly suggest that the N-terminal region of the P. falciparum CSP should be included in RTS,S or similar CSP-based vaccine candidates, as it is apparent that antibodies against this important region can have a major inhibitory effect on sporozoite infectivity. For years it has been suggested that the dominant antibody response to the CSP repeat region could represent a parasite strategy to divert the host immune response by preventing the recognition of regions of critical importance for parasite invasion. The availability of rodent parasites expressing distinct domains of human parasite orthologues should help answer some of these questions and advance research to study the immunogenic properties of the different CSP regions. Further studies should seek to determine whether induction of multidomain protective antibody responses can be better induced by immunizing with full-length recombinant CSP or with independent constructs representing individual CSP domains. In fact, while mAb5D5 recognizes a linear epitope and live sporozoites, it remains to be determined whether a similar antibody could be induced by native parasite protein.
Finally, our study shows that research aimed at defining the functional roles that molecules or specific domains may have on the parasite's biology and infection can provide critical information for identifying functionally important molecular domains that could be targeted by vaccine-induced protective antibody responses.
Supplementary Data
Supplementary materials are available at The Journal of Infectious Diseases online (http://jid.oxfordjournals.org). Supplementary materials consist of data provided by the author that are published to benefit the reader. The posted materials are not copyedited. The contents of all supplementary data are the sole responsibility of the authors. Questions or messages regarding errors should be addressed to the author.
Notes
Acknowledgments. The authors thank Dr Annie Mo, Program Officer for the Parasitology and International Programs Branch, Division of Microbiology and Infectious Diseases, National Institute of Allergy and Infectious Diseases (NIAID), for helpful insight and discussions. D. A. E., P. S. and F. Z. thank the Bloomberg Family Foundation for continued support.
Financial support. This work was supported in part by the NIAID/Division of Microbiology and Infectious Diseases (contract AI-N01-045210); and by the Nationals Institutes of Health/NIAID grant AI44375 (to F. Z.) and grant AI056840 (to P. S.). D. A. E. received a predoctoral fellowship from the Johns Hopkins Malaria Research Institute.
Potential conflicts of interest. All authors: No reported conflicts.
All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1.Coppi A, Natarajan R, Pradel G, et al. The malaria circumsporozoite protein has two functional domains, each with distinct roles as sporozoites journey from mosquito to mammalian host. J Exp Med 2011; 208:341–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Coppi A, Pinzon-Ortiz C, Hutter C, Sinnis P. The Plasmodium circumsporozoite protein is proteolytically processed during cell invasion. J Exp Med 2005; 201:27–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Romero P, Maryanski JL, Corradin G, Nussenzweig RS, Nussenzweig V, Zavala F. Cloned cytotoxic T cells recognize an epitope in the circumsporozoite protein and protect against malaria. Nature 1989; 341:323–6. [DOI] [PubMed] [Google Scholar]
- 4.Zavala F, Tam JP, Barr PJ, et al. Synthetic peptide vaccine confers protection against murine malaria. J Exp Med 1987; 166:1591–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Agnandji ST, Lell B, Fernandes JF, et al. A phase 3 trial of RTS,S/AS01 malaria vaccine in African infants. N Engl J Med 2012; 367:2284–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Agnandji ST, Lell B, Soulanoudjingar SS, et al. First results of phase 3 trial of RTS,S/AS01 malaria vaccine in African children. N Engl J Med 2011; 365:1863–75. [DOI] [PubMed] [Google Scholar]
- 7.Asante KP, Abdulla S, Agnandji S, et al. Safety and efficacy of the RTS,S/AS01E candidate malaria vaccine given with expanded-programme-on-immunisation vaccines: 19 month follow-up of a randomised, open-label, phase 2 trial. Lancet Infect Dis 2011; 11:741–9. [DOI] [PubMed] [Google Scholar]
- 8.Guinovart C, Aponte JJ, Sacarlal J, et al. Insights into long-lasting protection induced by RTS,S/AS02A malaria vaccine: further results from a phase IIb trial in Mozambican children. PLOS One 2009; 4:e5165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Olotu A, Moris P, Mwacharo J, et al. Circumsporozoite-specific T cell responses in children vaccinated with RTS,S/AS01E and protection against P falciparum clinical malaria. PLOS One 2011; 6:e25786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rathore D, Sacci JB, de la Vega P, McCutchan TF. Binding and invasion of liver cells by Plasmodium falciparum sporozoites. Essential involvement of the amino terminus of circumsporozoite protein. J Biol Chem 2002; 277:7092–8. [DOI] [PubMed] [Google Scholar]
- 11.Persson C, Oliveira GA, Sultan AA, Bhanot P, Nussenzweig V, Nardin E. Cutting edge: a new tool to evaluate human pre-erythrocytic malaria vaccines: rodent parasites bearing a hybrid Plasmodium falciparum circumsporozoite protein. J Immunol 2002; 169:6681–5. [DOI] [PubMed] [Google Scholar]
- 12.Noe AR, Espinosa D, Li X, et al. A full-length Plasmodium falciparum recombinant circumsporozoite protein expressed by Pseudomonas fluorescens platform as a malaria vaccine candidate. PLOS One 2014; 9:e107764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cockburn IA, Tse SW, Radtke AJ, et al. Dendritic cells and hepatocytes use distinct pathways to process protective antigen from Plasmodium in vivo. PLOS Pathog 2011; 7:e1001318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ménard R, Janse C. Gene targeting in malaria parasites. Methods 1997; 13:148–57. [DOI] [PubMed] [Google Scholar]
- 15.Espinosa DA, Yadava A, Angov E, Maurizio PL, Ockenhouse CF, Zavala F. Development of a chimeric plasmodium berghei strain expressing the repeat region of the P. vivax circumsporozoite protein for in vivo evaluation of vaccine efficacy. Infect Immun 2013; 81:2882–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bruña-Romero O, Hafalla JCR, González-Aseguinolaza G, Sano GI, Tsuji M, Zavala F. Detection of malaria liver-stages in mice infected through the bite of a single Anopheles mosquito using a highly sensitive real-time PCR. Int J Parasitol 2001; 31:1499–502. [DOI] [PubMed] [Google Scholar]
- 17.Yoshida N, Nussenzweig RS, Potocnjak P, Nussenzweig V, Aikawa M. Hybridoma produces protective antibodies directed against the sporozoite stage of malaria parasite. Science 1980; 207:71–3. [DOI] [PubMed] [Google Scholar]
- 18.Zavala F, Tam JP, Hollingdale MR, et al. Rationale for development of a synthetic vaccine against Plasmodium falciparum malaria. Science 1985; 228:1436–40. [DOI] [PubMed] [Google Scholar]
- 19.Roggero MA, Filippi B, Church P, et al. Synthesis and immunological characterization of 104-mer and 102-mer peptides corresponding to the N- and C-terminal regions of the Plasmodium falciparum CS protein. Mol Immunol 1995; 32:1301–9. [DOI] [PubMed] [Google Scholar]
- 20.Bongfen SE, Ntsama PM, Offner S, et al. The N-terminal domain of Plasmodium falciparum circumsporozoite protein represents a target of protective immunity. Vaccine 2009; 27:328–35. [DOI] [PubMed] [Google Scholar]
- 21.Rathore D, Nagarkatti R, Jani D, et al. An immunologically cryptic epitope of Plasmodium falciparum circumsporozoite protein facilitates liver cell recognition and induces protective antibodies that block liver cell invasion. J Biol Chem 2005; 280:20524–9. [DOI] [PubMed] [Google Scholar]
- 22.Kester KE, Cummings JF, Ofori-Anyinam O, et al. Randomized, double-blind, phase 2a trial of falciparum malaria vaccines RTS,S/AS01B and RTS,S/AS02A in malaria-naive adults: safety, efficacy, and immunologic associates of protection. J Infect Dis 2009; 200:337–46. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.