Abstract
Currently, the majority of diagnoses of malaria rely on a combination of the patient’s clinical presentation and the visualization of parasites on a stained blood film. Breath offers an attractive alternative to blood as the basis for simple, noninvasive diagnosis of infectious diseases. In this study, breath samples were collected from individuals during controlled malaria to determine whether specific malaria-associated volatiles could be detected in breath. We identified 9 compounds whose concentrations varied significantly over the course of malaria: carbon dioxide, isoprene, acetone, benzene, cyclohexanone, and 4 thioethers. The latter group, consisting of allyl methyl sulfide, 1-methylthio-propane, (Z)-1-methylthio-1-propene, and (E)-1-methylthio-1-propene, had not previously been associated with any disease or condition. Before the availability of antimalarial drug treatment, there was evidence of concurrent 48-hour cyclical changes in the levels of both thioethers and parasitemia. When thioether concentrations were subjected to a phase shift of 24 hours, a direct correlation between the parasitemia and volatile levels was revealed. Volatile levels declined monotonically approximately 6.5 hours after initial drug treatment, correlating with clearance of parasitemia. No thioethers were detected in in vitro cultures of Plasmodium falciparum. The metabolic origin of the thioethers is not known, but results suggest that interplay between host and parasite metabolic pathways is involved in the production of these thioethers.
Keywords: malaria, (E)-1-methylthio-1-propene, thioethers, volatile organic compound, odors, machine learning, diagnostic tool
Globally, an estimated 3.2 billion people in 97 countries are at risk of malaria [1], and in 2013, an estimated 198 million cases and 584 000 deaths were attributed to this infection [1]. Accurate diagnosis of malaria is important to provide adequate treatment, conserve valuable drugs, and help prevent the emergence of resistant strains of malaria parasites [2]. However, diagnosis continues to present challenges. Currently, the majority of diagnoses rely on a combination of clinical presentation and the century-old approach of visualizing parasites on a stained blood film. Approximately 197 million blood films were examined for malaria parasites in 2013 [1], and blood film examination is still considered the diagnostic gold standard. However, the method’s accuracy depends on the skill of the operator and the use of well-maintained equipment, and low levels of parasitemia can be challenging to detect. Rapid diagnostic tests represent a convenient point-of-care platform but suffer from suboptimal sensitivity in some settings [2]. There remains a need for a simple, inexpensive, and reliable diagnostic test for malaria that can be performed in the homes of patients or in other primary healthcare settings in remote areas [2].
Human breath offers an attractive alternative as the basis for simple, noninvasive diagnosis of infectious diseases. The breath of a healthy human contains up to 1849 volatile organic compounds (VOCs) [3]. In addition, there is evidence that the breath of infected people may contain VOCs indicative of specific diseases [4–6]. For example, some of the unique VOCs found in the headspace of Mycobacterium tuberculosis cultures have also been found in the breath of patients with tuberculosis [6]. Identification of such VOC biomarkers is an important step toward developing breath-based diagnostic methods. In the case of malaria, a range of intriguing reports suggests that humans [7, 8] and laboratory animals [9] infected with malaria parasites have elevated attractiveness to mosquitoes. Chemical cues have been hypothesized to drive at least some of the increased levels of attractiveness, but, so far, specific compounds have only been identified in a mouse model [9] and have not been found associated with malaria in humans [7, 8]. A study of blood cultures of Plasmodium falciparum did not reveal any VOCs associated with the presence of the parasite [10]. In-depth examination of the VOC profile of patients with malaria is ideal because it is possible that, unlike the situation with tuberculosis, specific VOC evolution may be affected by metabolic interactions between the parasite and the human host. However, this had not been done previously because breath is ephemeral and naturally acquired malaria largely occurs in locations remote from state-of-the-art analytical instrumentation.
In this research, breath samples were collected during controlled human malaria studies that are increasingly being used to aid vaccine and drug development [11, 12]. The experimentally induced blood-stage malaria (IBSM) paradigm uses direct intravenous inoculation of infected erythrocytes and has been used to investigate physiological and immunological status, drug efficacy, vaccine efficacy, and host-pathogen interactions in healthy, malaria-naive adults [11]. This was an ideal opportunity to test the hypothesis that malaria generates specific VOC signatures due to the host-pathogen interaction that might be detected by breath analysis but not in in vitro blood cultures. In this article, we present the results of a study in which breath samples were collected before and during early-stage malaria and after antimalarial drug administration, to determine whether malaria-associated VOCs can be detected in breath.
METHODS
Clinical Trial
The study was conducted as previously described [12, 13] and included 2 individual cohorts of 2 separate experimental studies aimed to test the in vivo activity of antimalaria drugs. The antimalaria drug investigated in cohort 1 was OZ439, and the drug used in cohort 2 was piperaquine. Data from these studies will be reported separately. Written informed consent was received from participants prior to inclusion in the study (clinical trials registration ACTRN12612000814875 and ACTRN12613000565741). Description of the clinical trials, ethics, and polymerase chain reaction (PCR) method for quantification of P. falciparum parasitemia [14] are presented in the Supplementary Materials.
Breath Analysis
Volunteers breathed through a cardboard mouthpiece connected to a chamber. After a few seconds of breathing, the chamber was connected to a 3-L impermeable aluminum gas bag (Shanghai Sunrise Instrument, Shanghai, China). Volunteers were asked to breath out a small amount, using a “ha” expiration; to pause; and, during the pause, to put the cardboard tube between their lips, and to continue the exhalation as far as they could, comfortably. In this way, only the last portion of the breath (ie, the alveolar air) was trapped in the bag. As soon as the volunteer finished breathing out, they closed the red valve (Supplementary Figure 1). Neither a nose clip nor a VOC filter were used. Days on which breath samples were collected are detailed in Supplementary Table 1. Breath from the bags was transferred to a sorbent tube, as shown in Supplementary Figure 2. Detailed description of gas chromatography–mass spectrometry analysis of the samples can be found in the Supplementary Materials.
Machine Learning Classifications and Cross-validations
Data used in this test were the peak area of the total ion chromatograms of the 4 thioethers, and the classes used for classifications and cross-validation were class 1, day 0 (collected before infection), and class 2, day 7 (collected in the afternoon; for cohort 1) or day 8 (collected in the afternoon; for cohort 2); these samples were obtained after drug administration. Two commonly used classifiers, a support vector machine and a k nearest neighbor algorithm [15–18], were used to train a model, using training data, and then to classify the test samples. Full description of these classifiers and cross-validation can be found in the Supplementary Materials.
Cell Culture
The 3D7 strain of P. falciparum was maintained in vitro as described previously [19], with modifications [20]. The serum substitute Albumax II was used instead of human serum [21]. Experiments were set up in Sarsted, canted-neck, polystyrene, 175-cm2 flasks with a PE Phenolic-style cap (catalog 83.1812.001) at a hematocrit of 1% and a starting parasitemia of 0.5%, in 100 mL of Roswell Park Memorial Institute 1640 medium. Experiments were initiated with parasites in the ring stage, except for those in which antimalarial drug or detergent were added, which were initiated with parasites mainly in the trophozoite stage. The flask headspace was flushed with a parasite-culture gas mix [20], the lid was closed and sealed with paraffin film, and the flask was incubated at 37°C for 96 hours with constant shaking [20]. The experiments designed to lyse the cells in the culture flask, inhibit growth with antimalaria drugs [22, 23], and spike red blood cell (RBC) cultures with the 4 thioethers are described in detail in the Supplementary Materials.
RESULTS
The study was conducted in 2 cohorts (n = 8 and n = 7), each of which received a different drug treatment regimen. In cohort 1, a single volunteer did not consent to provide breath samples, whereas in cohort 2, a single volunteer was unable to follow the procedure for providing breath samples. Therefore, 7 and 6 breath samples, respectively, were available for analysis from the 2 cohorts. All volunteers became PCR positive on approximately day 4 after inoculation and reached the protocol-designated treatment threshold of >1000 parasites/mL, as determined by quantitative PCR of morning samples collected on day 7 from cohort 1 and morning samples collected on day 8 from cohort 2. The allocated treatment then commenced, and the volunteers were admitted to the clinic (Supplementary Table 1). The mean peak pretreatment parasitemia levels were 4550 parasites/mL and 6218 parasites/mL for cohorts 1 and 2, respectively. The highest level of parasitemia between the 2 cohorts was 47 536 parasites/mL, a level that corresponds to approximately 0.001% parasitemia and is at about the threshold of microscopic detection.
VOCs in Breath of Volunteers
Using standard gas chromatography–mass spectrometry, we identified 9 compounds whose concentrations varied during the malaria episode. These compounds were carbon dioxide, isoprene, acetone, benzene, cyclohexanone, and 4 thioethers. The latter group was of particular interest because they have not previously been associated with any disease or condition and because their concentrations changed during infection for all individuals from both cohorts. The 4 thioether compounds were fully identified using commercially available and synthesized standards (Table 1) as allyl methyl sulfide, 1-methylthio-propane, (Z)-1-methylthio-1-propene, and (E)-1-methylthio-1-propene. These compounds have previously been reported at low levels in the breath of healthy individuals [3].
Table 1.
Putative Biomarkers of Malaria in Breath
| Putative Biomarker | Retention Time, mina | Characteristic Ionsb |
|---|---|---|
| Allyl methyl sulfide | 3.8–3.9 | 88, 73, 41 |
| 1-methylthio-propane | 4.09 | 61, 90, 48 |
| (E)-1-methylthio-1-propene | 4.30 | 45, 73, 88 |
| (Z)-1-methylthio-1-propene | 4.76 | 45, 73, 88 |
a Values denote the retention time of each putative biomarker in the chromatogram.
b Values are the 3 most-common characteristic ions for each putative biomarker.
In cohort 1 at day 0 (ie, in the sample collected just before infection), thioethers were either undetectable or present at very low levels (Figure 1). Four days after infection (ie, on day 4), the levels of the 4 thioethers increased, on average, by a factor of 21. Their levels reached a maximum on the day of treatment (ie, day 7 or 8), approximately 6.5 hours after initial drug administration. For cohort 1, (Z)-1-methylthio-1-propene was the compound with the largest change in intensity between day 0 and drug administration, increasing in concentration by >100-fold. The concentration of 1-methylthio-propane increased by 50-fold. For cohort 2, 1-methylthio-propane and (E)-1-methylthio-1-propene increased in concentration between day 0 and drug administration by 90-fold and 68-fold, respectively (Figures 2 and 3). Approximately 6.5 hours after the initial drug administration, the levels of the thioethers declined rapidly (Figure 2). Similar results were found in cohort 2 (Figure 3).
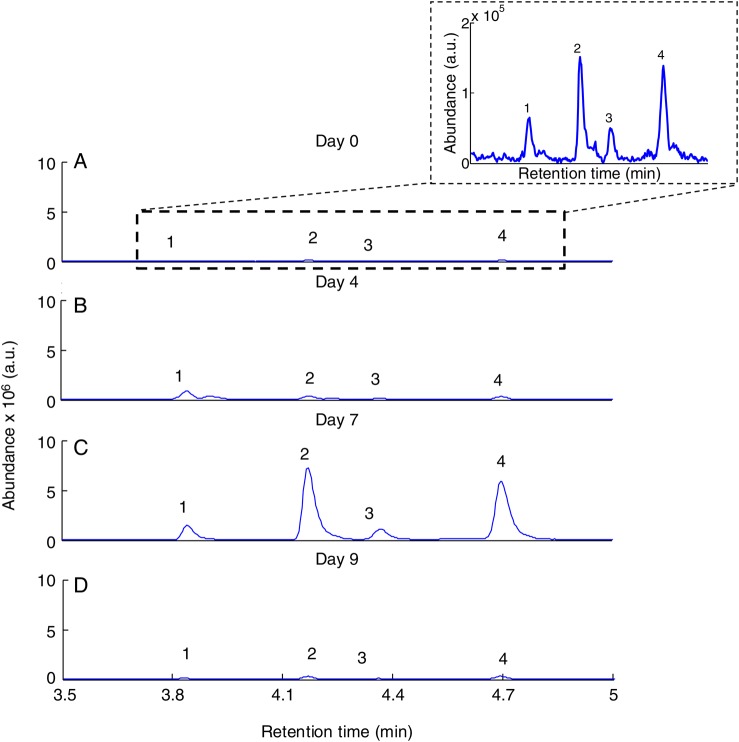
Figure 1.
Extracted ion gas chromatograms for a mass-to-charge ratio (m/z) of 88+90 for 1 typical subject on day 0, day 4, day 7 (after drug administration), and day 9. Peaks: 1, allyl methyl sulfide; 2, 1-methylthio-propane; 3, (E)-1-methylthio-1-propene; 4, (Z)-1-methylthio-1-propene.
Figure 2.
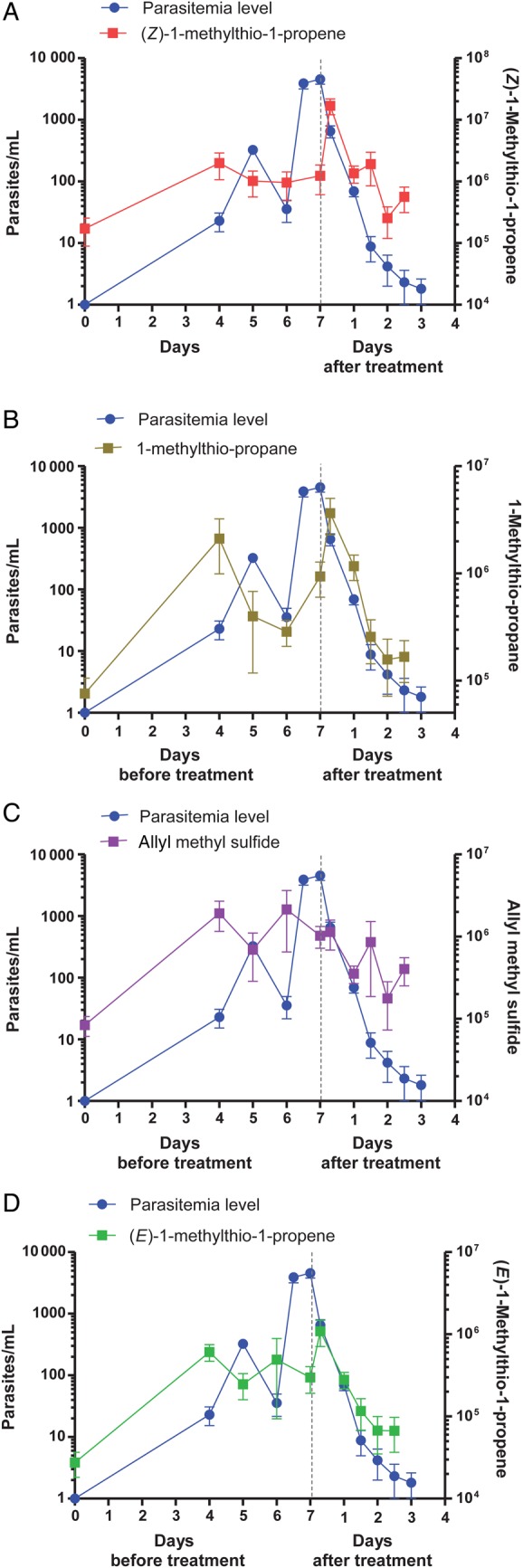
VOCs in breath (right y-axis) and parasite levels in blood (left y-axis) for cohort 1 during the course of experimental malaria before (during the first 7 days after infection onset) and after antimalarial treatment. Each participant in the cohort was inoculated on day 0 with approximately 1800 human erythrocytes infected with Plasmodium falciparum. Breath and blood samples were collected over the course of infection. Drug treatment (OZ439) started on day 7 for cohort 1 (n = 7), as indicated by dotted vertical lines in the plots. Multiplication and clearance of parasites are denoted by blue circles, and the abundance of compounds is denoted by squares: (Z)-1-methylthio-1-propene (m/z 88), 1-methylthio-propane (m/z 90), allyl methyl sulfide (m/z 88) and (E)-1-methylthio-1-propene (m/z 88). Error bars represent standard errors of the mean.
Figure 3.
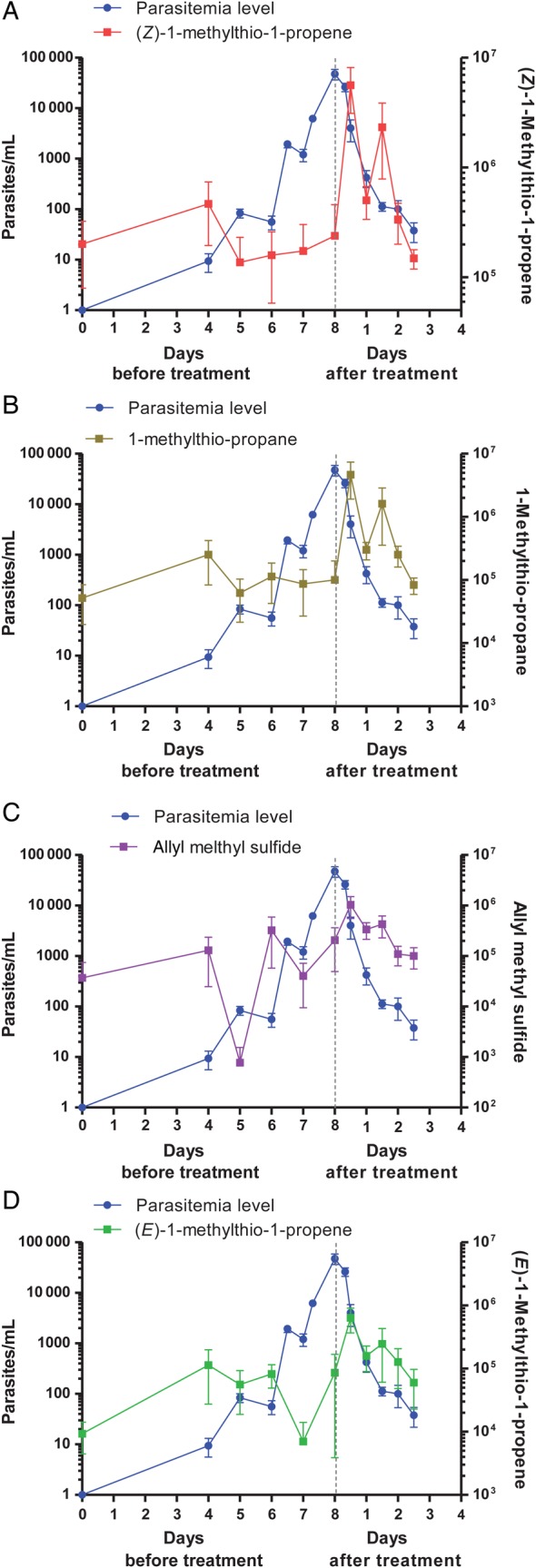
VOCs in breath (right y-axis) and parasite levels in blood (left y-axis) for cohort 2 during the course of experimental malaria before (during the first 8 days after infection onset) and after antimalarial treatment. Each participant in the cohort was inoculated on day 0 with approximately 1800 human erythrocytes infected with Plasmodium falciparum. Breath and blood samples were collected over the course of infection. Drug treatment (piperaquine) started on day 8 for cohort 2 (n = 6), as indicated by dotted vertical lines in the plots. Multiplication and clearance of parasites are denoted by blue circles, and the abundance of compounds is denoted by squares: (Z)-1-methylthio-1-propene (m/z 88), 1-methylthio-propane (m/z 90), allyl methyl sulfide (m/z 88) and (E)-1-methylthio-1-propene (m/z 88). Error bars represent standard errors of the mean.
Ambient air specimens collected during both clinical trials did not contain detectable levels of any of the thioethers, in agreement with observations by Suarez et al [24], who found that allyl methyl sulfide could not be detected in indoor air. These results exclude the possibility that these chemicals were a result of contamination of the ambient air.
In a separate study of breath samples from patients with fever due to bacterial infection who presented to hospital emergency departments, the thioethers were undetectable in fever and control groups, and the levels were below those observed after infection in the present study (unpublished data). It appears, therefore, that elevation of the levels of the detected thioethers is specific to malaria, rather than to bacterial infections.
Volatile Levels in Breath Specimens Versus Parasite Levels in Blood Specimens
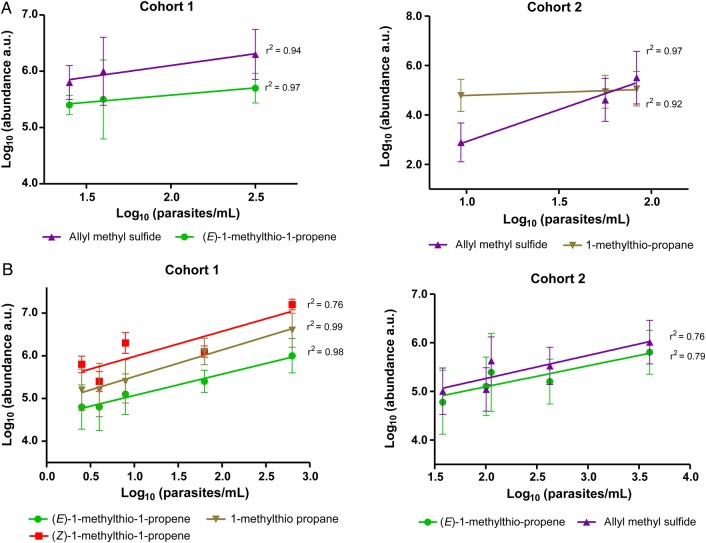
We investigated the relationship between thioether levels and parasitemia levels. Before treatment, there was evidence of cyclical changes in both the levels of thioethers, particularly allyl methyl sulfide and (E)-1-methylthio-1-propene, and, as expected, in the levels of parasitemia (Figures 2 and 3). In both cases, the period appeared to be approximately 48 hours, although the sampling regimen did not allow any greater resolution, and was imposed on a general trend of increased parasitemia levels, as determined by quantitative PCR, and increased levels of thioethers. The cyclical variation in thioether level appeared to be out of phase with the parasitemia. When the thioether data were subjected to a phase shift of 24 hours, a direct correlation between parasitemia and volatile levels was revealed (Figure 4A and 4B). For cohort 1, allyl methyl sulfide and (E)-1-methylthio-1-propene were the compounds whose levels had the highest correlations with parasitemia levels, with r2 values of 0.94 and 0.97, respectively. Also, for cohort 2, the allyl methyl sulfide concentration was highly correlated to the parasitemia level (r2 = 0.97), as was the 1-methylthio-propane concentration (r2 = 0.92).
Figure 4.
Correlation of parasite level and abundance of malaria VOCs before (A) and after (B) antimalarial treatment of the 2 cohorts. The thioether data were subjected to a phase shift of 24 hours, which revealed a direct correlation between parasitemia and volatile levels. In cohort 1, a fast-acting synthetic ozonide drug was used on day 7, and in cohort 2, a slower-acting piperaquine drug was administered on day 8. Points denote means and standard deviations.
In both cohorts, the highest levels of thioethers were seen in the first breath sample, collected approximately 6.5 hours after drug administration (Figures 2 and 3), possibly indicating a role for parasite death or lysis in driving thioether production. Volatile levels declined monotonically after initial drug treatment, correlating with clearance of parasitemia (Figures 2 and 3). For cohort 1, administration of a fast-acting synthetic ozonide resulted in a faster decline in parasitemia and thioether levels (Figure 2), compared with the decline observed with the slower-acting bisquinoline 4-aminoquinoline used in cohort 2 (Figure 3). For cohort 1, after treatment, there was a strong correlation between levels of parasitemia and the abundance of (Z)-1-methylthio-1-propene (r2 = 0.76), (E)-1-methylthio-1-propene (r2 = 0.98), and 1-methylthio-propane (r2 = 0.99; Figure 4C). For cohort 2, there was a lower but still significant correlation between the levels of thioethers and the levels of parasitemia, with r2 values of > 0.79 for (E)-methylthio-1-propene and > 0.76 for allyl methyl sulfide (Figure 4D).
Machine Learning Classification of Malaria, Based on Thioether Levels
Machine learning methods were used to verify our observation that changes in the levels of thioether (peak areas) are significant between day 0 before infection and days 7 or 8 after drug administration. As demonstrated in previous work [17, 25, 26], different classifiers’ performance on the same data set varies; therefore, 2 classifiers were used: a support vector machine and a k nearest neighbor classifier. It was found, that using leave-one-out cross-validation, all samples could be perfectly classified on the basis of volatile levels, demonstrating statistically significant differences between baseline and soon after drug administration. After performing data normalization to account for changes in instrument sensitivity over time, we trained the classifiers by using the cohort 1 data, and they were able to perfectly classify the data from cohort 2. In the same way, using cohort 2 data to train the classifiers gave perfect classification of cohort 1 data. This result is a strong indication that the classification was not due to overfitting of the data.
Thioethers Were Not Detected in the Headspace of In Vitro Blood Cultures of P. falciparum
Wong et al [10] previously reported that no change in headspace volatile levels could be detected when an in vitro erythrocyte culture was infected with P. falciparum. We specifically searched for the 4 thioethers in the headspace of 100 mL of a RBC suspension (1% hematocrit; starting parasitemia level, 0.5%) cultured for 4 days without changing medium (final parasitemia level, 38.8%). No thioethers were detected (Figure 5), and no significant changes in the entire volatile profile of the samples, compared with our control (noninfected blood), were observed. Thioethers were also not detected in the headspace of P. falciparum–infected cell cultures 5.5 hours following treatment with 1000 ng/mL of the antimalarial piperaquine tetraphosphate tetrahydrate or after lysing the cells with a combination of 0.8% saponin and 8% Triton X-100 (final parasitemia level, 21%). As a positive control, we spiked the 4 thioethers into the RBC cultures at the following levels: 2.7 nmol/L allyl methyl sulfide and 4.4 nmol/L 1-methylthio-propane (the same level of each was previously detected in healthy individuals [27]) and 2.5 nmol/L of each of the isomers (Z)-methylthio-1-propene and (E)-methylthio-1-propene. We then incubated the sample for 4 days and collected headspace specimens following procedures described in the Supplementary Materials. All of the thioethers were readily detected (Figure 5). Moreover, no thioether was detected in the ambient samples collected from the tissue culture laboratory (data not shown).
Figure 5.
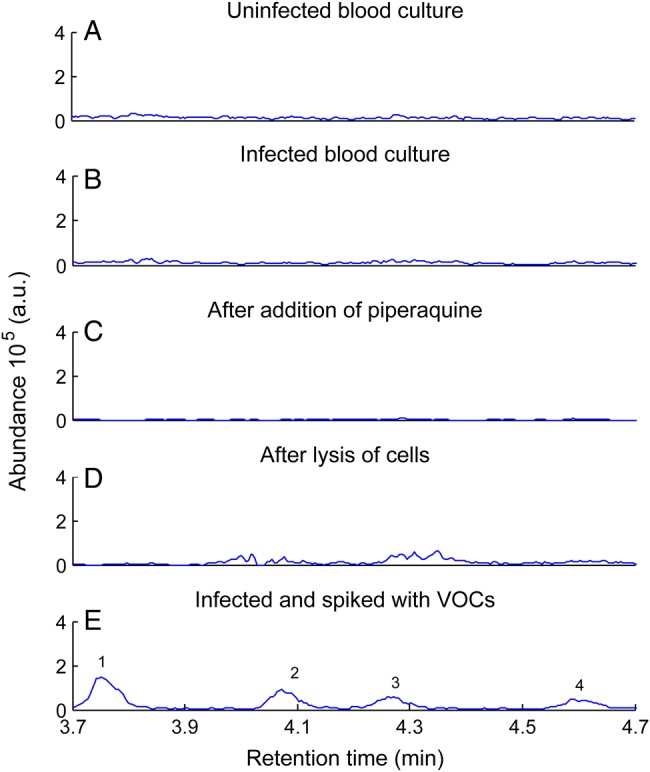
Screening of in vitro Plasmodium falciparum blood cultures under various conditions for thioethers (m/z 88+90). Thioethers were absent in the control (A) and in the headspace of 100 mL of a red blood cell suspension (1% hematocrit; B). Thioethers were neither present following treatment with 1000 ng/mL of the antimalarial piperaquine tetraphosphate tetrahydrate (C) nor after treatment-lysing of cells (D). As a positive control, red blood cell cultures were spiked at the levels previously detected in healthy individuals (E). Peaks: 1, allyl methyl sulfide; 2, 1-methylthio-propane; 3, (E)-1-methylthio-1-propene; and 4, (Z)-1-methylthio-1-propene. Data are representative of findings obtained in 2 independent experiments with average parasitemia levels of 29.9%. Abbreviation: VOC, volatile organic compounds.
The biomass of parasitized RBCs present in the blood cultures at the end of one of the in vitro experiments (0.1 L × a hematocrit of 1% × a parasitemia level of 39% = 0.39 g of infected erythrocytes) was approximately 18-fold higher than the biomass of infected RBCs estimated to be present in the volunteers (5 L × a hematocrit of 43% × a parasitemia level of 0.001% = 0.02 g of infected RBCs). Even if we consider sequestered parasites (not quantified by PCR) in the in vivo samples, the biomass would not affect the calculated value by >10%, especially considering that there is a known correlation between the sequestered parasites and the severity of the disease [28]. Approximately 200 mL of the available 400 mL of headspace was sampled in vitro, compared with the 1 L of breath sampled from volunteers.
DISCUSSION
Levels of 9 VOCs were found to change in the breath of volunteers after they were infected with blood-stage malaria parasites. Of these, acetone and isoprene are the most prominent volatile compounds in the breath of healthy humans. Acetone is produced by acetoacetate-decarboxylase during fatty acid breakdown [29]. Isoprene is a by-product of the mevalonate pathway, its concentration changes during exertion, and it can be an indicator of oxidative stress [3, 30–32]. Benzene is believed to be an exogenous contaminant of human breath. It is a common pollutant in the environment [3], can partition into human fat depots, and would have an elevated concentration during increased metabolic activity, particularly lipolysis. Cyclohexanone has also been reported previously in the breath of healthy individuals, as well as in urine samples [3]. CO2 flux is directly related to metabolic rate: the findings by Lavoisier form the basis for capnography, a breath test to monitor partial pressure of CO2 [29]. Of the VOCs whose levels changed during malaria, only the levels of thioethers—allyl methyl sulfide, (E)-1-methylthio-1-propene, (Z)-1-methylthio-1-propene, and 1-methylthio-propane—were correlated to parasitemia levels.
It is believed that allyl methyl sulfide and 1-methylthio-propane enter alveolar air from the blood, where they may have diet- or drug-related origins [27]. Allyl methyl sulfide has been associated with the consumption of garlic and is a metabolite of allicin [24, 33]. It is important to note that, in both cohorts, all volunteers had fasted before collection of morning samples and that, for the afternoon samples, which were collected when volunteers were confined in the clinic, samples were collected at least 3 hours after lunch. Lunch diets were different in both cohorts, yet the same pattern in volatile levels was observed, eliminating a possible dietary origin for the compounds. Moreover, in another (unpublished) study, thioethers were absent from the breath of patients with bacteremia/septicemia and controls, indicating that the thioethers are neither generic fever markers nor general markers of infection. In the present study, increased levels of thioethers were observed before drug administration. Thioethers could not be directly derived from the antimalarial drugs because neither of the drugs used in these trials contained a sulfur atom. Furthermore, none of the components of the parasite inoculum injected on day 0 contained thioethers.
The fact that thioethers were undetectable in the headspace above in vitro cultures of P. falciparum was unequivocally established. All attempts, including culturing for 2 intraerythrocytic cycles (96 hours), high parasitemia levels, and using antimalarial drug and lysing cells, failed to result in the detection of thioethers. Of course, in vivo, other factors are relevant. For example, lipophilic compounds such as the thioethers may partition into the plastic of the cell culture flasks. However, the spiking protocol we used mimicked the very low level of thioethers seen in the breath of healthy people [18]. Therefore, had thioethers been present, even at very low levels, we expect we would have detected them. In this respect, our findings support those of Wong et al [10], who, using culture conditions that differed slight from those in the present study, also found no differences in the VOC profile between control RBC cultures and parasitized cultures.
Thioethers are poorly soluble in water, and, although it is known that at least allyl methyl sulfide and 1-methylthio-propane reach the alveolar air from the blood in healthy individuals [27], we do not know whether (E)-1-methylthio-1-propene and (Z)-1-methylthio-1-propene would be detectable in the blood of infected volunteers. From a practical point of view, analysis of breath samples would be simpler and more convenient for patients.
The cycling of thioether levels, which appears to have the same periodicity as the 48-hour life cycle of the blood-stage parasite is intriguing. It suggests that thioether levels may be linked to a specific phase in the parasite’s intraerythrocytic cycle. Although our sampling regimen did not have the resolution to define the association precisely, the 180° phase shift from maximum parasitemia and the peak in thioether levels that occurs shortly after drug treatment suggests that the bursting of erythrocytes (schizont rupture) to release merozoites and infect new RBCs [2], accompanied by the death of parasite, may be the event that triggers maximal thioether release.
In the IBSM protocol, which involves malaria-naive volunteers, it is neither ethical nor safe to allow parasitemia levels to rise to those seen in field-acquired infections in human populations living in malaria-endemic regions, which may be 3 log units higher at symptom onset. It will be important to determine, in clinical trials with patients who have naturally acquired malarias, whether the same thioethers can be detected and whether their levels correlate with the much higher parasitemia levels expected. The fact that changes in VOC levels in infected volunteers’ breath occurred at low parasitemia levels indicates that analysis of breath specimens shows promise as a sensitive method for the diagnosis of malaria in patients with levels of parasitemia below the threshold of detection of rapid diagnostic tests used to detect the circulating biomarker of P. falciparum infection, PfHRP2 (<100 parasites/µL [34]).
The metabolic origin of the thioethers is unclear, and we cannot exclude the possibility that they may originate from 1 or more molecules in the breath that break down or rearrange during collection and analysis. However, our spiking protocols confirmed that the thioethers detected during our analytical procedure were stable. Our results suggest that some aspect of host response is involved in the production of specific VOCs, possibly during or because of schizont rupture. Just as parasite components released during lysis of infected RBCs (schizont rupture) and phagocytosis by immune cells generate the febrile response to malaria [35], other molecular events that occur during schizont rupture could generate the thioethers. In the absence of an in vitro model, it is highly desirable to identify a suitable animal model of malaria that leads to release of the same thioethers, to enable studies of the metabolic origins of these compounds.
Supplementary Material
Notes
Acknowledgments. We thank the volunteers and Q-Pharm staff, for their valuable contributions to this project; and the Canberra Branch of the Australian Red Cross Blood Service, for the provision of human erythrocytes.
Financial support. This work was supported by Medicines for Malaria Venture, the National Health and Medical Research Council, and CSIRO.
Potential conflicts of interest. All authors: No reported conflicts.
All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1. World Health Organization (WHO). World malaria report 2014 Geneva: WHO, 2014. [Google Scholar]
- 2. Penny L, Neuwald P, Urdea M. Biomarkers for infectious disease diagnostics in the developing world: rapid, home-based diagnosis of malaria in symptomatic inidividuals and screening of asymptomatic pregnant woman. http://www.halteresassociates.com/resources/white-papers/06-Infectious-Disease-Biomarkers-Malaria-20091112-v01.pdf. Accessed 02 December 2014.
- 3. Costello BD Amann A Al-Kateb H, et al. A review of the volatiles from the healthy human body. J Breath Res 2014; 8:1–29. [DOI] [PubMed] [Google Scholar]
- 4. Bousamra M Schumer E Li MX, et al. Quantitative analysis of exhaled carbonyl compounds distinguishes benign from malignant pulmonary disease. J Thorac Cardiovasc Surg 2014; 148:1074–80. [DOI] [PubMed] [Google Scholar]
- 5. Incalzi RA Pennazza G Scarlata S, et al. Reproducibility and respiratory function correlates of exhaled breath fingerprint in chronic obstructive pulmonary disease. PLoS One 2012; 7:e45396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Phillips M Basa-Dalay V Bothamley G, et al. Breath biomarkers of active pulmonary tuberculosis. Tuberculosis 2010; 90:145–51. [DOI] [PubMed] [Google Scholar]
- 7. Lacroix R, Mukabana WR, Gouagna LC, Koella JC. Malaria infection increases atractiveness of humans to mosquitoes. PLoS Biol 2005; 3:e298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Batista EPA, Costa EFM, Silva AA. Anopheles darlingi (Diptera: Culicidae) displays increased attractiveness to infected individuals with Plasmodium vivax gametocytes. Parasit Vectors 2014; 10.1186/1756-3305-7-251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. De Moraes CM Stanczyk NM Betz HS, et al. Malaria-induced changes in host odors enhance mosquito attraction. Proc Natl Acad Sci U S A 2014; 111:11079–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Wong R, Flematti G, Davis T. Investigation of volatile organic biomarkers derived from Plasmodium falciparum in vitro. Malar J 2012; 11:314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Engwerda CR, Minigo G, Amante FH, McCarthy JS. Experimentally induced blood stage malaria infection as a tool for clinical research. Trends Parasitol 2012; 28:515–21. [DOI] [PubMed] [Google Scholar]
- 12. McCarthy JS Sekuloski S Griffin PM, et al. A pilot randomised trial of induced blood-stage plasmodium falciparum infections in healthy volunteers for testing efficacy of new antimalarial drugs. PLoS One 2011; 6:e21914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Cheng Q Lawrence G Reed C, et al. Measurement of Plasmodium falciparum growth rates in vivo: A test of malaria vaccines. Am J Trop Med Hyg 1997; 57:495–500. [DOI] [PubMed] [Google Scholar]
- 14. McCarthy JS Griffin PM Sekuloski S, et al. Experimentally induced blood-stage plasmodium vivax infection in healthy volunteers. J Infect Dis 2013; 208:1688–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Cortes C, Vapnik V. Support vector networks. Mach Learn 1995; 20:273–97. [Google Scholar]
- 16. Chang C-C, Lin C-J. LIBSVM: a library for support vector machines 2011. http://www.csie.ntu.edu.tw/~cjlin/libsvm. Accessed 01 October 2014.
- 17. Wang XR, Lizier JT, Nowotny T, Berna AZ, Prokopenko M, Trowell SC. Feature selection for chemical sensor arrays using mutual information. PLoS One 2014; 9:e89840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Russell S, Norvig P. Artificial intelligence: a modern approach. Englewood Cliffs, NJ: Pentice Hall, 1995. [Google Scholar]
- 19. Trager W, Jensen JB. Human malaria parasites in continuous culture. Science 1976; 193:673–5. [DOI] [PubMed] [Google Scholar]
- 20. Allen RJW, Kirk K. Plasmodium falciparum culture: The benefits of shaking. Mol Biochem Parasit 2010; 169:63–5. [DOI] [PubMed] [Google Scholar]
- 21. Cranmer SL, Magowan C, Liang J, Coppel RL, Cooke BM. An alternative to serum for cultivation of Plasmodium falciparum in vitro. T Roy Soc Trop Med H 1997; 91:363–5. [DOI] [PubMed] [Google Scholar]
- 22. Lee TMN Huang LS Johnson MK, et al. In vitro metabolism of piperaquine is primarily mediated by CYP3A4. Xenobiotica 2012; 42:1088–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Hofer S, Brun R, Maerki S, Matile H, Scheurer C, Wittlin S. In vitro assessment of the pharmacodynamic properties of DB75, piperaquine, OZ277 and OZ401 in cultures of Plasmodium falciparum. J Antimicrob Chemoth 2008; 62:1061–4. [DOI] [PubMed] [Google Scholar]
- 24. Suarez F, Springfield J, Furne J, Levitt M. Differentiation of mouth versus gut as site of origin of odoriferous breath gases after garlic ingestion. Am J Physiol-Gastr L 1999; 276:G425–30. [DOI] [PubMed] [Google Scholar]
- 25. Wang XR, Lizier JT, Berna AZ, Bravo FG, Trowell SC. Human breath-print identification by E-nose, using information-theoretic feature selection prior to classification. Sens Actuators B: Chem2014; 10.1016/j.snb.2014.09.115. [DOI]
- 26. Smolinska A, Hauschild A-C, Fijten RRR, Dallinga JW, Baumbach J, Schooten FJv. Current breathomics—a review on data pre-processing techniques and machine learning in metabolomics breath analysis. J Breath Res 2014; 8:027105. [DOI] [PubMed] [Google Scholar]
- 27. Mochalski P King J Klieber M, et al. Blood and breath levels of selected volatile organic compounds in healthy volunteers. Analyst 2013; 138:2134–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Dondorp AM Desakorn V Pongtavornpinyo W, et al. Estimation of the total parasite biomass in acute falciparum malaria from plasma PfHRP2. PLoS Med 2005; 2:e204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Amann A Costello B Miekisch W, et al. The human volatilome: volatile organic compounds (VOCs) in exhaled breath, skin emanations, urine, feces and saliva. J Breath Res 2014; 8:034001. [DOI] [PubMed] [Google Scholar]
- 30. King J Kupferthaler A Unterkofler K, et al. Isoprene and acetone concentration profiles during exercise on an ergometer. J Breath Res 2009; 3:027006. [DOI] [PubMed] [Google Scholar]
- 31. King J Mochalski P Unterkofler K, et al. Breath isoprene: muscle dystrophy patients support the concept of a pool of isoprene in the periphery of the human body. Biochem Bioph Res Co 2012; 423:526–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Lourenco C, Turner C. Breath analysis in disease diagnosis: methodological considerations and applications. Metabolites 2014; 4:465–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Filipiak W Ruzsanyi V Mochalski P, et al. Dependence of exhaled breath composition on exogenous factors, smoking habits and exposure to air pollutants. J Breath Res 2012; 6:036008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Maltha J, Gillet P, Jacobs J. Malaria rapid diagnostic tests in travel medicine. Clin Microbiol Infect 2013; 19:408–15. [DOI] [PubMed] [Google Scholar]
- 35. Oakley MS, Gerald N, McCutchan TF, Aravind L, Kumar S. Clinical and molecular aspects of malaria fever. Trends Parasitol 2011; 27:442–9. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.