Abstract
Biomedical investigators require high quality human tissue to support their research; thus, an important aspect of the provision of tissues by biorepositories is the assurance of high quality and consistency of processing specimens. This is best accomplished by a quality management system (QMS). This article describes the basis of a QMS program designed to aid biorepositories that want to improve their operations. In 1983, the UAB Tissue Collection and Biobanking Facility (TCBF) introduced a QMS program focused on providing solid tissues to support a wide range of research; this QMS included a quality control examination of the specific specimens provided for research. Similarly, the Division of Laboratory Sciences at the Centers for Disease Control and Prevention (CDC) introduced a QMS program for their laboratory analyses, focused primarily on bodily fluids. The authors of this article bring together the experience of the QMS programs at these two sites to facilitate the development or improvement of quality management systems of a wide range of biorepositories.
Introduction
Many types of biomedical research depend upon the use of high quality human and/or animal tissues and associated annotation. A strong quality management system (QMS), sometimes referred to as a quality assurance (QA) program, is necessary to provide both high quality and consistently handled tissue specimens. Also, with the advent of certification (The Canadian Tumour Repository Network, CTRNet) and accreditation programs of biorepositories (College of American Pathologists, CAP), there is an increased need for understanding the use of a QMS to improve biorepository operations. All biorepositories should utilize a rigorous QMS.
The goal of this article is to update a prior publication on the applicability of QMS to biorepositories1 in order to clarify aspects of a QMS; this should aid personnel of biorepositories to integrate a QMS into biorepository operations and in certification and/or accreditation processes. Biorepositories that provide human and animal tissues for research utilize different approaches to meeting investigator needs for tissue specimens and associated annotation. QMS is a managerial approach that focuses on operational standards for all activities of an organization in order to ensure that a procedure, product, or service is of a defined quality. Quality Control (QC) is one component of QMS that includes the technical activities that measure the attributes and performance of a product, process, or service, compared to defined standards and to demonstrate that defined requirements are met fully.
In this article, the integration of QMS into two large biorepositories is described. One of these is a governmental biorepository that is focused on banking and analyzing human biofluids, and the other is an academic biorepository focused on providing solid human tissues, as well as biofluids, to investigators to support their research projects. This is described, followed by a general review of the principles of QMS and how QMS should be used to improve biorepository operations. Of note, although human tissues are emphasized in this article, almost all the information is directly applicable to biorepositories focusing on other tissues such as animal tissues and/or environmental monitoring.
Many biorepositories have evolved as satellites of clinical pathology or research analytical laboratory departments, in which QMS systems are a requirement for validity of assays. These laboratories routinely have utilized extensive QMS to ensure high quality experimental results based on the use of human tissue. For example, the Division of Laboratory Sciences (DLS), National Center for Environmental Health, is the part of the Centers for Disease Control and Prevention (CDC), which primarily performs assays on human specimens. As stated on its website: “By preventing disease from exposure to toxic chemicals in the environment; responding to terrorism and public health emergencies involving chemicals; and improving laboratory methods to diagnose and prevent disease, the laboratory has been in the vanguard of efforts to improve people's health across the nation and around the world.” Ensuring the quality of analyses which range in complexity from enzymatic assays of urine creatinine at concentrations of mg/dL to measurement of dioxins in serum by high-resolution tandem mass spectrometry at concentrations as low as parts per quintillion is demanding and requires an extensive QMS. Each validated assay in DLS is comprehensively described in a Clinical Laboratory Improvement Amendments (CLIA)-format standard operating procedure (SOP), and has multiple levels of “bench” (known to the analyst) and “blind” controls (QC pooled samples at different concentrations than those of the bench pools, and unknown to the analyst); the blind QC samples are prepared to be identical to actual specimens with similar vials and labels.
Additionally, DLS adheres to the requirements of CLIA, although few of the analytes of the DLS are considered regulated by CLIA. CLIA specifies many aspects of a QMS, including proficiency testing (PT). PT involves the analysis of unknown specimens for specified analytes. These unknown specimens are distributed by an external organization and results are compared with those from other sites participating in the PT program. Ideally, target values for the analyte concentrations in these PT programs are established by reference methods. Participation in PT is mandated in QMS all over the world. DLS even runs its own PT programs in trace elements in human blood and urine and neonatal screening of dried blood spots.
Similarly, CLIA principles such as calibration/verification exercises are required for each analyte every 6 months. If PT programs are not available for high complexity analytes, DLS laboratories participate in specimen exchanges with other reference laboratories world-wide. Approaches of the National Institute of Standards and Technology Standard Reference Materials (SRMs) also are routinely incorporated whenever they are available for external validation of assays. DLS routinely prepares its QC pools in quantities large enough to last for 2–3 years, so that results for all materials used can be plotted over time to gain maximum information about within-/among-run variation over time.2 Because DLS performs many analyses for the National Health and Nutrition Examination Surveys (NHANES), this degree of QMS complexity is critical for evaluating whether a perceived trend in U.S. population values for an analyte is real or an analytical artifact. This happened during NHANES II, 1976–1980, when lead levels in blood in the US population dropped in parallel with the removal of lead from gasoline during the same time period, and this observation was demonstrated not to be an analytical artifact.3,4
The DLS tracks all reagents, calibrators, major equipment, and components over time. All specimen collection, processing, storage, and analytical disposables are lot-tested for background levels of contamination from metals, phthalates, and other environmental toxins to minimize their contribution to inaccuracy.3,5 All of these efforts are then published to provide transparency to any other investigators or organizations wishing to emulate these assays.3,5 All aspects of laboratory analytical data generation, overall QMS, and data reporting are stringently monitored following the highest standards.
A goal for biorepositories should be to translate the level of quality management described for analytical laboratories appropriately to biorepositories, which may or may not be performing ancillary assays before distributing biospecimens. Specifically, a strong QMS is important in any aspect of biomedical research and must also be developed for facilities that collect, process, store, and distribute tissues to support biomedical research.
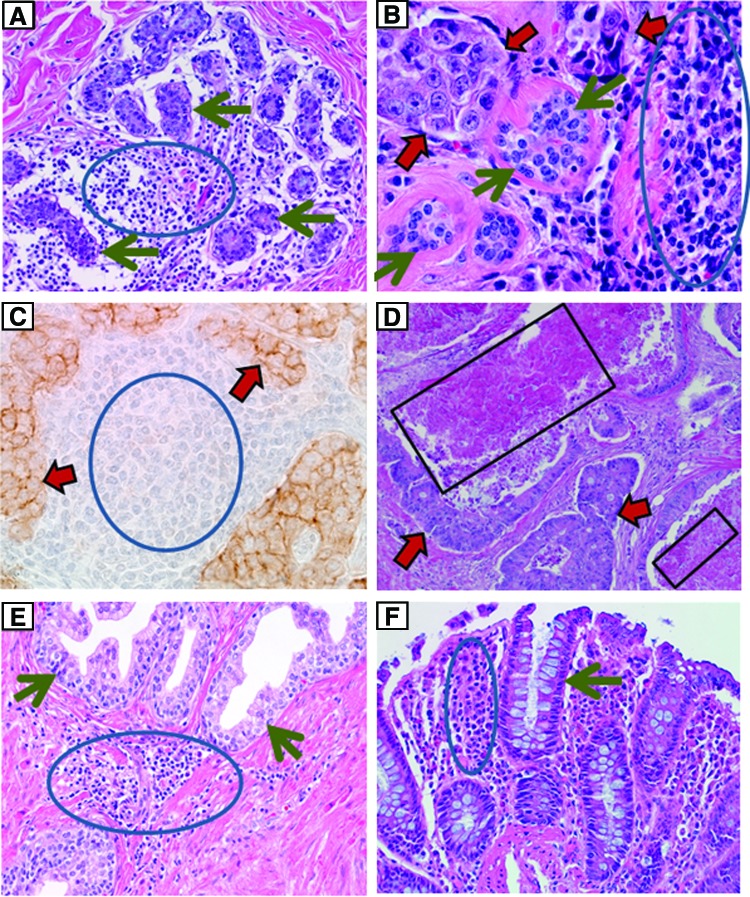
An organized biorepository program at the University of Alabama at Birmingham (UAB), the Tissue Collection and Banking Facility (TCBF), provides human tissues to support biomedical research; the TCBF has been developing since 1977 when the distribution of primarily solid human tissues to support biomedical research began. A QMS for biorepositories was introduced in 1983 by one of the authors (WEG) with a requirement for quality control (QC) of each solid tissue specimen distributed to a researcher. This initial approach to QC relied on the diagnosis of a mirror image tissue section that corresponded to the specific specimen provided to the investigator, instead of just relying on the diagnosis and diagnostic description in the surgical pathology report. This approach to QC was considered necessary because of tissue heterogeneity including variable areas of necrosis, fibrosis, and inflammation within tumors (Fig. 1).
FIG. 1.
demonstrates the heterogeneity of tissues that may affect the results of research. (A) (hematoxylin and eosin [H&E] stain; original magnification ×200) demonstrates that extensive lymphoid inflammation (blue circle) is present between uninvolved breast tissue (green arrows) from a case of cancer. (B) (H&E, ×400) shows lymphoid inflammation (blue circle) adjacent to breast cancer (red arrows) and uninvolved breast (green arrows). (C) (immuno-staining for E-cadherin with a hematoxylin counterstain, original magnification ×400) shows metastatic breast carcinoma to a lymph node. The carcinoma with brown immune-staining (red arrows) is mixed with lymphoid cells (blue circle). (D) (H&E, ×100) is a metastatic colorectal carcinoma (red arrows) to the liver with extensive necrosis (black boxes). (E) (H&E, ×100) demonstrates uninvolved glands of the prostate (green arrows) (cancer case) intermixed with lymphoid inflammation (blue circle). (F) (H&E, ×200) demonstrates lymphoid inflammation (blue circle) between uninvolved benign appearing colon glands (green arrow). A color version of this figure is available in the online article at www.liebertpub.com/bio.
Over the years, QC has identified that about 15% or more of specimens collected to support a specific research project based on gross appearance and the surgical pathology report, to be incorrectly interpreted. For banks with tumors that are difficult to identify by gross examination such as pancreas and prostate, over 50% of specimens may not contain adequate tumor and/or tissues collected as uninvolved (normal appearing) may be infiltrated by tumor or inflammatory cells. As QMS has expanded at UAB, multiple approaches have developed to improve tissue quality. This includes the development of written standard operating procedures (SOPs) for all activities of the TCBF and the use of audits to ensure that SOPs are being followed (e.g., specimens are stored at the locations described in the informatics system). Another component of the QMS is training of all personnel in multiple areas including SOPs, ethical and regulatory issues, and safety. Similarly, programs established to provide tissues from animals could easily follow the QMS models which UAB and other institutions have established.
In both the DLS and UAB programs, a strong QMS has been critical to ensure high quality products such as the tissues provided to support research projects. Because investigators may devote extensive research time and effort to tissues used in biomedical research, tissues of poor quality with incorrect diagnoses or inconsistent characteristics can significantly impede the progress of a specific research project or even an area of research.
Of importance, the quality of tissues provided for research should be judged based on whether the tissues meet the needs of an investigator's research; specifically, “quality” should not be confused with “availability”—these are very different concepts. Many biorepositories may not have specific types of tissues available. This unavailability of specimens does not equate with poor quality of tissues of the biorepository, but rather with decisions of the biorepository secondary to resources and the availability of specific tissues. For example, based on the standard operating procedure of a biorepository, solid tissue specimens of breast carcinomas may be collected and stored in 0.1–0.15 g aliquots, and only two aliquots may be stored per case (patient). The unavailability of 0.5–1.0 g specimens from each case of breast carcinoma needed for a project should not be confused with the quality of the smaller aliquots that are available.
Bias in Use of Tissue Collections
The variability of research using human tissues is in part caused by bias. Bias results when confounding variables are the cause of an observation rather than the variable being studied to which the observation is attributed. Biorepositories should be knowledgeable of the potential causes of bias resulting from the characteristics of the tissues they provide. Understanding bias should minimize the chances that analyses of tissues will result in biased results and conclusions. Specifically, there may be measured differences in tissues obtained from cases (patients) who have a disease when compared to tissues from individuals without the disease (i.e., controls); however, the differences may be secondary to confounding variables unrelated to the questions being evaluated concerning the disease. Thus, these confounding variables cause biases and may result in a wrong interpretation that the experimental differences in tissue from patients with disease are a result of the disease. Confounding variables may include unmatched differences in the population (e.g., comparing cases of one age range [50–80 years] with controls of a different age range [20–40 years]), in collection (e.g., different times of cold ischemia), processing (controls fixed in 70% ethanol, diseased cases fixed in 10% NBF), in storage (−20°C vs. −80°C), in distribution of specimens (shipped on blue ice versus dry ice), or in experimental procedures (e.g., samples of disease analyzed together on one day and samples from controls analyzed together a week later). Bias usually is identified when the experimental conclusion cannot be replicated and/or validated.6–12
Without detailed and accurate records, bias can be introduced easily into research projects and incorrect conclusions can be accepted as to the interpretation of the research results. For example, if pre-screening of collection/processing/storage/analytical materials was not performed routinely by the CDC's DLS, contaminated lots of reagents could be unknowingly used for trace metals and other ultra-low concentration analytes such as dioxin in human serum, and bias secondary to this contamination would result. Frequently, there may be multiple potential causes of bias in the same study so that the specific problematic confounding variable cannot be identified. For example, if the molecular features of a disease were to be evaluated using EDTA plasma collected prior to an operation (fasting) and the specimen had undergone multiple freeze/thaws during a storage time of over 10 years at −80°C, but the EDTA plasma controls had only one freeze/thaw cycle, a storage time of less than 1 year at −80°C and were collected from non-fasting volunteers using a mobile van in a community setting, multiplex methods might identify molecular differences caused by these sample differences and a biased conclusion might result.12
Also, these examples emphasize the importance of using detailed SOPs that are standardized across different collection sites, so that tissues are collected, processed, stored, and distributed uniformly. Continuity of the protocol and SOPs are an absolute requirement in any multi-site, long-term study. Of importance, careful records can emphasize the differences in specimens, and before reporting results, conclusions can be confirmed using a subset of samples collected, processed, stored, and distributed more uniformly. Even with great care, site to site bias is likely, so it is important to ensure that the number of controls equal the number of cases from each site. Some of the potential causes of bias of which biorepositories should be aware are listed in Table 1.
Table 1.
Examples of Potential Sources of Bias in Tissue Sets
| 1. Differences in population (e.g., race, age, sex) |
| 2. Homeostasis (e.g., fed versus fasting) |
| 3. Diurnal variations (i.e., time of collection) |
| 4. Co-morbidity (diabetes in cancer studies) |
| 5. Stress |
| 6. Collection container (e.g., red top Vacutainer® vs. serum separator tube) |
| 7. Time from collection to processing |
| 8. Time from processing to freezing |
| 9. Temperature and length of time of storage |
| 10. Freeze-thaw cycles |
| 11. Geographical sites of sample collection |
| 12. Environmental exposures |
| 13. Occupational exposures |
| 14. Medications, supplements, diet |
| 15. Changes in SOPs within a long-term study |
Biorepository Models
Some aspects of QMS may vary with the models under which biorepositories operate. Specifically, obtaining human or animal tissues and/or bodily fluids for biomedical research may be organized or disorganized. “Catch as catch can” best describes a disorganized model via which a pathologist, surgeon, veterinarian, or others randomly collect tissues for future research. Typically, these specimens have not been collected, processed, stored, or distributed using SOPs, and usually there is not a QMS including QC; therefore, these aliquots are frequently of poor quality and the diagnoses of the specimens may be wrong.
More concerning is that such specimens may be obtained without oversight of the Institutional Review Board (IRB) and may violate regulations such as the Health Information Privacy and Portability Act (HIPAA), which applies only in the United States of America, although similar privacy regulations may apply elsewhere. Similarly, projects for which animals are sacrificed specifically to obtain tissues should be under the review of animal use committees. Also, informatics programs with any identified personal health care information must meet very rigorous requirements of HIPAA.
Most organized biorepositories utilize one of the following models: In the 1) “banking” model, a specific tissue (e.g., breast cancer) or a wide range of tissues (e.g., any cancer and controls) are collected, processed, stored and distributed following SOPs. The 2) clinical trial model and the 3) epidemiology/population based and environmental model are subtypes of the banking model. The 4) prospective model collects, processes, and distributes tissue based on investigator requests. In the 5) combination model of prospective plus banking, specimens are both prospectively collected and/or banked; this model is currently used by the Tissue Collection and Banking Facility at UAB. A model that is developing is based not only on biospecimen collection and distribution, but also provision of data from some assays of the biospecimens.13 This model would have an advantage if data were available on specific molecules that degrade on storage of biospecimens.
In an organized banking model, SOPs are utilized for collecting, processing, storing, and distributing tissues. Typically, tissue banks store only frozen tissues, fixed tissues, stabilized tissue preparations, and/or paraffin blocks. Tissues that are processed by specific methods (e.g., fixed in 10% neutral buffered formalin [10% NBF]) and fresh unfrozen or unprocessed samples frequently are unavailable from a typical biobank. For example, size, extent of tumor, and special processing may be unavailable to meet specific requirements of an investigator.6–8,14,15 The banking model has an advantage in that multiple specimens may be available immediately.
Another advantage to a bank is that clinical information is often available at the time specimens are requested; tumors in most banks are allowed to “mature” until outcome data are available before distribution occurs. Alternatively, a portion of banked specimens may be analyzed when collected, and data can be made available prior to patient outcome. Unfortunately, many tissue banks do not focus on utilization of their specimens in research, which leads to an almost fatal flaw for the strict banking model; specifically, that most tissues that are put into a bank are never used in research.16,17 This is further complicated by molecular degradation of specific molecules during long-term storage and even after storage for 1 or 2 years.18 This has become an ethical issue with the implied promise to donors that their donated tissues will be used to support biomedical research.17 This also leads to a very inefficient model that is usually not cost effective.
The clinical trial model is a subtype of the banking model in which remnants of bodily fluids/solid tissues from one or more clinical trials are stored to support studies in the future that may be unrelated to the original clinical trial. The clinical trial model has all the disadvantages of the banking model; additionally, the patient consent for a clinical trial may not indicate that the specimens remaining after the clinical trial can be utilized in other types of research. The IRB may, therefore, prohibit use of such specimens for a different type of research for which they were consented.
The epidemiology/population based/environmental model usually focuses on answering a specific question(s) or future questions often via the use of patients or volunteers who are chosen to represent a statistical sample of a population or subpopulation. Typically, if a specific question is to be answered, tissues and extensive information are collected by SOPs from a specific group of volunteers. The specimens usually are samples of bodily fluids and the samples are associated with extensive annotations based upon information obtained directly from the individuals. This model has many of the same disadvantages as the banking model. Examples of an epidemiology/population based and environmental model are the NHANES study, the Prostate, Lung, Colorectal and Ovarian (PLCO) Cancer Screening Trial, and the UK Biobank. The PLCO trial involved collection of longitudinal samples of bodily fluids collected over several years with a goal of obtaining samples prior to the development of cancers in one or more of these organs.19 An interesting aspect of the UK Biobank is that a subset of specimens is analyzed “in house” similar to NHANES, so that both data and specimens are available through this facility.13
Prospective Collection is a biorepository model in which investigators specifically identify the tissues needed (e.g., diagnosis and size) and specify how the specimens are to be collected (e.g., only from fasting patients), processed (e.g., frozen), stored (e.g., in liquid nitrogen vapor phase), and distributed (e.g., shipped on dry ice). The prospective model has the disadvantage that when requested, specimens may not be immediately available, nor is clinical outcome—which may only be available after several years following collection of a specimen. Also, rare specimens are very problematic for prospective repositories. The advantage of a prospective collection model is that the tissues provided are specifically what the investigator requests (e.g., fresh, not frozen, ductal carcinoma of the breast minced in DMEM media plus antibiotics/antifungals).
A very important aspect of the prospective collection model is that almost all specimens collected are utilized in research,7 so it is very cost effective and efficient. For example, the Cooperative Human Tissue Network (CHTN) since 1987 has distributed over 1 million specimens to over 3300 investigators resulting in over 3700 publications.20 Also, prospectively collected specimens minimize molecular artifacts caused by storage. Although many tissue banks do not focus on utilization of their specimens in research, the combination model of tissue collection and banking combines prospective and banking models, and usually includes the advantages of both models and few of the disadvantages; however, there are the complicated administrative demands, including a need for a more complex bioinformatics system (Table 2). Each of the organized biorepository models needs a QMS if high quality and consistently processed tissues are to be distributed to investigators.
Table 2.
Comparisons of Specific Types of Biorepositories
| Type of biorepository | Biobank, typical | Epidemiological, population-based, or environmental | Clinical trial | Prospective procurement | Combination model |
|---|---|---|---|---|---|
| 1) Usual types of specimens | Solid tissues and biofluids; Specimens may vary with goals of the biobank; Frequently only FFPE tissue and biofluids are available | Usually only biofluids – blood components and sometimes urine. | Solid tissues and biofluids depending on requirements of the clinical study. | Solid tissues and biofluids based on requests of investigators; Specimens can be provided fresh (non-frozen). | Specimens requested by investigators; Biobanked specimens collected, processed and stored based on biorepository goals. |
| 2) Specimen collection | Collected in a medical facility for future research; Usually disease-based. | Usually collected from individuals without disease, but can include specimens from individuals with an increased risk for a disease; Collected for future research; Longitudinal samples sometimes available. | Collected in a medical facility in association with therapeutic interventions for disease. | Collected at a medical facility based on the specific needs and requests of investigators. | Collected for future research or at request of investigators; Many specimens are rapidly distributed, but some are banked. |
| 3) Processing, storage and distribution | Processed via a SOP; Stored until distributed to an investigator. | Processed by a SOP; Stored for long periods until an endpoint is reached (e.g., development of a disease or study of an environmental agent); Distribution usually is restricted to research on specific issues; Tissues not associated with an endpoint (e.g., development of a disease) frequently are not initially made available for distribution. | Processed by a SOP; Stored until distributed to an investigator; Use may be restricted due to the informed consent. | Processed according to investigator requests; Distributed soon after collection; Specimens have to be collected so are not available when first requested. | Processed based on a SOP (bank) or according to investigator request; Distributed to a requesting investigator or stored in bank until requested; Strong emphasis on distribution. |
| 4) Data | Extensive data may be available upon request including outcome. | Extensive data available frequently based upon interview of participants; Usually participants are healthy; Outcome (e.g., development of a disease) will be variable. | Wide range of data available based on clinical trial; Data on response to intervention are available. | Demographic data immediately available; Clinical and other data available upon review of health record; Clinical data on outcome not available until outcome occurs. | Data models vary based on goals of biobanking component; Demographic data available and other data via health record; Outcome of prospective component not available when specimens first requested. |
| 5) Advantages | Specimens and data including outcome usually are immediately available. | Longitudinal specimens may be available; Extensive data on individual can be provided. | Specimens and extensive clinical data including outcome immediately available; Response to therapies immediately available. | Specimens are collected and processed to meet the specific needs of investigators; Fresh (non-frozen) specimens available; Most specimens utilized; No long-term storage artifacts. | Prospective specimens collected and processed based on investigator needs; Banked specimens and outcomes may be immediately available; Prospective specimens will not have long-term storage artifacts. |
| 6) Disadvantages | Many specimens are not utilized; Specimens may not meet needs of the investigator; Potential artifacts and molecular degradation based on long-term storage. | Many specimens are not utilized; Specimens subject to artifacts and molecular degradation on long-term storage. | Many specimens are not utilized; Original consent may limit use; potential specimen artifacts and degradation on long-term storage. | Specimens and outcome data not immediately available. | Specimen and outcome data not immediately available on prospective specimens; Potential artifacts and degradation of banked specimens on long-term storage; Banked specimens may not be utilized. |
Aids in Developing a QMS
There are several documents wthat can aid biorepositories in developing their QMS. The International Society of Biological and Environmental Repositories (ISBER) has developed several versions of its Best Practices to aid biorepositories in improving their operations and the quality of the products and services biorepositories provide. These guidelines are the most extensive available for biorepositories that specialize in providing human tissue specimens to support biomedical research and should be an important primary aid in developing a QMS for such biorepositories.21–23 In addition, the National Cancer Institute (NCI) has developed limited guidelines for human tissue biorepositories focusing on primary human tissues for research.24 A very useful and extensive collection of tested SOPs for biorepositories are available via the Canadian Tumor Research Network (CTRNet) web site.25 These SOPs are easily modified for use by other biorepositories.
The International Organization for Standardization (ISO), a worldwide federation of national standards organizations, has produced document ISO 900126 to provide organizations with useful internationally recognized information to aid in developing a QMS. Although the Federal Drug Administration's (FDA) Good Tissue Practices27 was developed to regulate human tissues that are used for transplantation, its regulations and requirements can be used to assure good tissue procurement practices for research, depending on the goals of specific biorepositories. Although developed originally for the pharmaceutical industry and regulated by the FDA, Good Manufacturing Practices (GMP), which are enforced in the United States by the US FDA under Section 501(B) of the 1938 Food, Drug, and Cosmetic Act (21 USCS § 351), also provide useful guidance for biorepositories.28
Components of these various regulations, guidelines, and best practices can be adapted, if appropriate, by biorepositories to meet their specific operational goals. Based upon the above documents, some important practices include the following:
• The biorepository is located in a secure area with limited access.
• The facility is locked when tissue resource personnel are not on site.
• Policies and procedures are documented in SOPs with appropriate references that are approved by designated personnel and changed or updated under strict document control rules.
• Personnel are trained in all SOPs associated with their job responsibilities.
• Safety and other operational issues, including training, are documented.
• The facility is subject to internal QMS audits as well as appropriate external audits; all audits are documented and evaluated carefully.
• An extensive audit trail is maintained for all specimens from receipt to distribution.
• Equipment maintenance and calibration procedures are performed and documented as required by manufacturers or by local SOPs.
• Access to the informatics system is limited and is controlled via a series of access codes which permit various levels of access by facility personnel. Per HIPAA requirements, each access to identified files in the informatics systems by biorepository personnel is documented.
• Deviation from SOPs is documented for all events that fall outside SOPs. Corrective action, if necessary, is recorded and follow-up monitoring is implemented to ensure that any incidents are not repeated.
QMS Personnel
Personnel assigned to work in QMS should have the responsibility for ensuring compliance with all SOPs and ethical and regulatory requirements, and should report directly to high levels of management concerning all issues in QMS. Personnel assigned to QMS should be responsible for designing, overseeing, and evaluating audits of overall operations regarding adherence to QMS requirements. In large repositories, a QMS or QA manager may report directly to the facility director or designate. In biorepositories, QMS personnel should aid in the development of SOPs for the collection, processing, storage, and distribution of specimens to ensure that each of these processes is performed in an appropriate and consistent fashion. In cases where different groups are collecting specimens for a single study, SOPs should be standardized across the different collection sites. When problems in these areas are identified and/or if any specimens of poor quality are identified, personnel should initiate and participate in efforts to correct these problems. Based on our experience, in monitoring long-term multisite studies, it is clear that some sites perform much better than other sites as to the quality of tissues they provide to a study. Therefore, it is important that some samples from each site be evaluated periodically during the performance period to ensure compliance with QMS requirements.
Education of Biorepository Personnel and Investigators
The provision of high quality and consistent tissues for research or environmental monitoring requires a QMS including using SOPs so that tissues are collected, processed, stored, and distributed uniformly. All biorepository personnel collecting, processing, storing, and distributing tissues should be trained in biorepository operations, especially the QMS, including the importance of carefully following SOPs. Training of these staff should include safety as well as regulatory (HIPAA) and ethical (IRB) issues.6–8
Use of animals as a source of materials for research also requires training in the ethical use of animals and review of research projects by an animal use committee. All training must be documented. Education of users of the biorepository also is important; therefore, the biorepository should also serve as an educational resource for investigators who may need support concerning issues in the selection of the optimal tissues to support their research. For example, investigators should understand if their specimen requirements are too restrictive such that they could prevent the investigator from obtaining needed numbers of tissues and could significantly increase associated costs.6–8 This is especially important if investigator requirements are not supported scientifically.
Investigators also need to understand the limitations in obtaining tissues that may affect specific biorepositories. It is important for investigators to understand how tissues differ in their appropriateness to support a specific research project (e.g., smooth muscle from the wall of the uterus, which is estrogen sensitive, is not equivalent to smooth muscle of the wall of the stomach).
Human or veterinary pathologists or other equivalently knowledgeable professionals associated with the biorepository should review all requests for human and/or animal tissues. These professionals can provide guidance to investigators concerning the types of tissue they need for their specific research projects, and investigators should be able to request such guidance prior to designing their research projects. If a request is difficult to meet because of unnecessary requirements and/or restricted availability, an educational approach is an opportunity to explain to the investigators limitations as to obtaining the tissues requested.6–8
Standard Operating Procedures
The use of standard operating procedures that are detailed documented methods of each of the activities of a biorepository is necessary to ensure that all activities are performed uniformly. An SOP should be prepared in great detail so that trained personnel who have not performed the procedure previously can perform the method just as consistently and accurately as an employee experienced in the method. Changes in SOPs should be made only by authorized supervisors and documented. Most SOPs are currently computerized and changes should be made so that the date and the supervisor making the change are incorporated into the SOP. Also, the previous dated version of the SOP should be maintained as a historical record in order to identify any differences in tissues caused by changes in SOPs, especially in the collection, processing, storage, and/or distribution methods. The SOPs should be organized into a procedure manual that is easily available at the bench to all personnel so employees can follow them exactly. Each SOP should be reviewed at least annually, and revised as necessary. There should be a record in the SOP and procedure manual of the annual review by the supervisor, quality manager if different, and the director of the biorepository.
Audits as Part of QMS
Audits are written periodic evaluations of selected operating procedures and aspects of the infrastructure of the biorepository. QMS personnel of biorepositories should conduct regular audits such as those listed subsequently and should be responsible for designing, performing, evaluating, and documenting audits. Audits may be as simple as a biweekly re-check of the daily logs of freezer temperatures or liquid nitrogen levels (staff should monitor temperatures daily as a Best Practice), or may be more complex such as a review of specimens collected and their sites of storage over a 6 month period. QMS personnel should document problems quarterly and report them to upper management and the director of the facility.
The QMS program at a facility should describe how, when, and by whom audits are conducted. Examples of specific audits and documentation include reviews of the following:
• Updated SOPs and adherence to these procedures;
• Equipment maintenance, repair, and calibration;
• Equipment monitoring (e.g., determining the cutting thickness of microtomes);
• Training records and adherence of staff to required training (e.g. training in biohazards for appropriate personnel; refresher training of selected personnel in changes in shipping regulations);
• Record keeping, monitoring of records, and data management;
• Monitoring the accuracy of clinical and demographic data;
• Specimen tracking (e.g., monitoring/confirming specimen locations in freezer);
• Aspects of the safety plan (e.g., training in chemical hazards and biohazards including records of staff training); immunization records of staff;
• Shipping problems as reported by investigators;
• Procurement and receipt records for supplies;
• Reagent outdating;
• Surveys and reports from users.
Biorepositories should consider distributing an annual survey to determine the satisfaction of users/investigators who obtained tissue during the preceding year. It would be useful if the survey could be completed “on-line”; however, a paper survey also could be distributed to users. The results of the survey should be evaluated carefully. Investigators reporting unsatisfactory results should be contacted and their problems discussed, documented, and corrective action taken if practicable.
If the organization provides tissues to extramural investigators, each shipment should be monitored closely. Such monitoring can be accomplished by including a short questionnaire with each shipment that documents receipt of the shipment and provides an opportunity to report immediate feedback if problems were encountered (e.g., not enough dry ice in frozen shipments, incorrect IDs of specimens actually received vs. what is stated on the shipping manifest). These questionnaires could be returned via fax, e-mail, or the recipient could be referred to a website for completion of the questionnaire. Of note: transportation by air requires shipments to meet important standards established by the International Air Transport Association (IATA) and the Department of Transportation (DOT). IATA requires special training of personnel involved in preparation of air shipments and associated standards of these shipments (29,30).
Annotation of the Specimen
One of the most important aspects of a QMS for biorepositories is to ensure that all aliquots of tissues supplied for research are identified correctly and that the clinical and demographic information supplied with the specimen are correct and are from the specific patient from whom the tissue specimen was collected; thus, periodic audits of the accuracy of clinical and demographic data are required.
A labeling method should be used that (a) minimizes the chances that the label will separate from the specimen (e.g., specimen container), even at cryogenic temperatures, (b) prevents mislabeling due to personnel error and (c) avoids problems with reading the specimen identification (e.g., poor handwriting). For some biorepositories, this is best accomplished by the use of barcodes that link the specimen to a database containing pertinent information concerning the specimen. It should be understood that a barcode is only a “number or matrix,” and this identifier is primarily useful only by its linkage to a database through interface software that actually identifies information regarding the specimen. Thus, unless the same identical software is used at a second site, the second site can only read the specimen identifier without a link to information in the database. Information that does not identify the specimen source (i.e., no HIPAA identifiers) may be included on the printed label (e.g., race, age) via software instructions. Note, some governmental studies do not permit additional data to be printed on labels of specimens.
Quality Control of Solid Tissues
Monitoring the diagnoses and quality of the tissues provided for research (i.e., quality control) is a very important component of a QMS. Biorepositories have used various types of QC to help investigators in order to ensure that the tissues and associated information provided meet their needs.1,6–8,14 Many tissues, especially tumors, are heterogeneous; thus, specimens of tumors vary as to the extent of neoplastic cells, mucin production, fibrosis/desmoplasia, uninvolved cells, inflammatory cells, and/or necrosis. Fibrosis in and adjacent to tumors may be intermixed with or mistaken on gross examination for tumor; similarly, some tumors may infiltrate normal tissues diffusely, making areas of tumor difficult to identify grossly (Fig. 1).
Therefore, just knowing the surgical pathology diagnosis of a specimen from which aliquots are obtained is not adequate QC for the actual tissues provided for research. This is especially true for infiltrative tumors such as breast, prostate, pancreas, and sarcomas as well as tumors with typically large proportions of necrosis such as renal cell carcinomas and metastases to liver of colorectal cancer (Fig. 1). Similarly, a diagnosis based only on the surgical pathology report is not adequate for tumors or uninvolved tissues with extensive inflammation. Specifically, as demonstrated in Panels A, E, and F of Figure 1, many normal and uninvolved tissues may have focal primarily lymphoid inflammation that is intermixed in non-neoplastic tissues. Similarly, neoplastic cells can be intermixed with inflammatory as well as uninvolved cells. Both of these patterns (controls vs. cancers) can complicate some assays unless investigators are aware of these patterns.
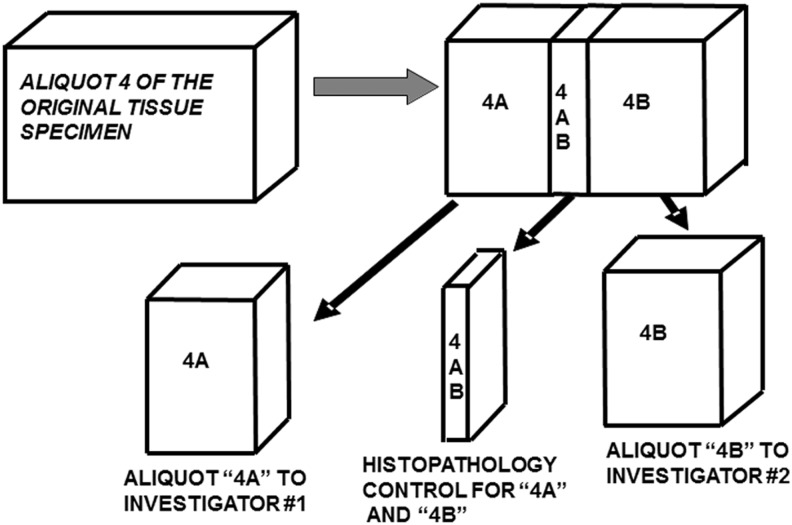
At UAB, the minimum QC of each tissue aliquot provided to an investigator is an accurate diagnosis and description of the aliquot based on a microscopic examination by a pathologist. For some sites, this approach may be too expensive. In such cases, an H&E slide of the aliquot or its digital image can be retained in the file to ensure an accurate description when the aliquot is provided for research. Figure 2 is an example of an approach to QC; it demonstrates the QC of a fourth aliquot of a tissue specimen in which 4AB is a mirror image of both aliquot 4A and aliquot 4B. Aliquot 4AB is processed to a paraffin block and the histopathologic diagnosis of 4AB is the quality control diagnosis for both specimen 4A and specimen 4B. As part of this diagnostic description, the proportion of malignant cells (tumor nuclei) and fibrosis, mucin and necrosis are specified. Unless the tissue is very small, a QC examination and diagnosis is made on a mirror image aliquot of the specific tissue that supplied for research.
FIG. 2.
A Minimum Quality Control examination for each tissue specimen provided for research. Aliquot 4 AB is processed to a paraffin block, and sections from it represent the QC diagnoses for both specimens 4A and 4B.
A quality control examination also may include a molecular analysis in which RNA, DNA, or protein and/or other molecules are extracted from small aliquots representative of the specimen provided for research followed by characterization of specific categories of these molecules. Molecular quality control may be performed if investigators request this level of QC, but investigators usually are required to pay for the increased associated costs; in addition, the quality of a sample of specimens provided by the facility could be evaluated periodically by molecular analysis as part of the overall quality assurance program of the facility. This can be aided by proficiency testing for specific measures of QC that are now available via ISBER. Molecular analysis also may be performed when investigators indicate that there is a problem with specific specimens if additional aliquots of those specimens are still available at the facility. Of note, as the required quality control examinations become more extensive, there is a “price” that includes increased time and effort by the tissue repository organization and increased “costs” of the specimen to the investigator.
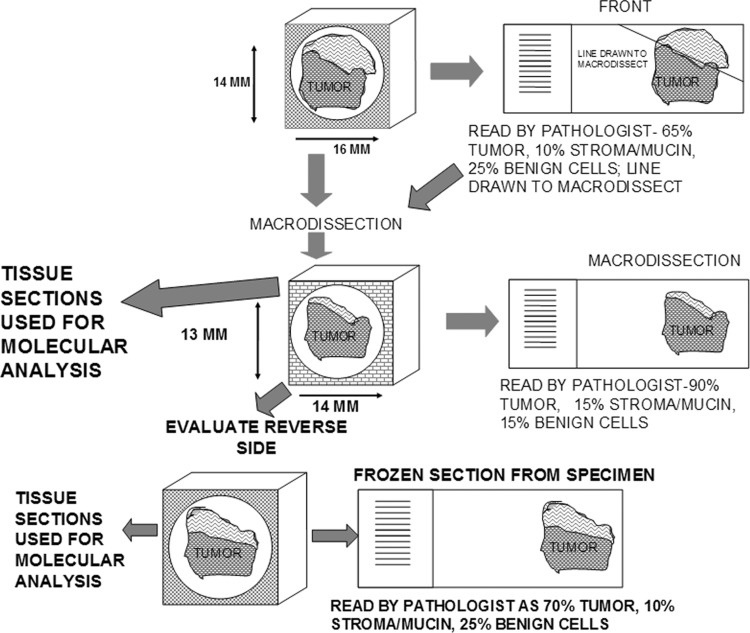
QC also can be tailored to individual investigators. A “platinum level” of quality control might include macrodissection of specimens to increase the proportion of tumor in specimens. In this approach, frozen sections of the whole specimen are made, followed by macrodissection of one or both sides of the specimen to enrich the specimen in diseased cells. Also, aliquots from the macrodissection could be analyzed molecularly (Fig. 3). Similarly, macrodissection can be performed on paraffin blocks based on paraffin sections. Both approaches increase the costs of processing specimens, as well as reduce the tissue available as the specimen is exhausted by the QC; however, this approach is much less costly than laser capture microdissection. If the research projects do not require or need such extensive quality control, this form of QC may not be cost effective and a more simple approach may be adequate. Alternatively, a local pathologist at the investigator's institution could aid by performing additional QC and/or macrodissection on the specimens provided.
FIG. 3.
demonstrates very extensive and expensive QC via use of frozen sections including macrodissection and molecular analysis.
The quality of mRNA is sometimes estimated by extracting RNA from frozen sections of tissue and evaluating surrogate measures such as RIN and/or the degradation factor. Obviously, if specimens are not to be used in RNA analyses, QC analysis at the RNA level adds unnecessary costs. In such molecular analysis, macrodissection and/or knowledge of the proportion of malignant cells is much more important to assays. Note that using short amplicons (<100 bps) in real time reverse transcriptase quantitative PCR techniques permits the use of degraded mRNA and even mRNA extracted from paraffin blocks can be analyzed and give equivalent results to those obtained from frozen tissues.31,32 Using QC, the TCBF has found that about 15%–20% of typical specimens of tumors cannot be used for the research for which the specimens were collected.1,6–8,14
In addition, in a specific example of banked tissues collected as “catch as catch can” without initial QC, when evaluated, over 50% of the specimens collected as tumor were found to contain less than 10% tumor. Specifically, a tissue aliquot that appears on gross examination to be free of cancer may be involved microscopically by cancer cells; in contrast, specimens that appear on gross examination to be tumor may not contain adequate malignant cells because of fibrosis/scarring and/or mucin. Other reasons to reject an aliquot of tissue for specific research because of the QC examination include extensive ischemia, inflammation, necrosis, or damage by surgery (e.g., cautery artifacts). For example, some focal areas of large or metastatic tumors (e.g., liver metastases of colorectal cancer) may be so necrotic so that inadequate recognizable tumor cells remain (Fig. 1).
In a minimal QC examination, the proportion (percent) of the specimen that is diseased is specified. For example, for tumors, the percent fibrosis and mucin of the tumor as well as the percent necrosis should be specified together with the proportion of malignant cells sometimes called “tumor cells” or “tumor nuclei,” but more accurately designated as “malignant nuclei.” Specifically, a mucinous tumor may be composed of greater than 90% acellular mucin and the lack of malignant cellular representation may impede many specific forms of analysis. In general, the proportion of cells within the tumor that are malignant may vary extensively because a tumor may be infiltrated by large numbers of inflammatory cells and/or may contain other non-malignant cells so that the molecules from malignant cells may be diluted extensively. Similarly, the determination of the specific cells from which a molecule (e.g., microRNAs) arises may be difficult to ascertain.33
Typically, for frozen sections, tissue aliquots are embedded in frozen section support medium (e.g., Optimal Cutting Temperature [O.C.T.] Compound). O.C.T. or other similar heavy alcohol-based frozen section support mediums. Of importance, O.C.T. may superficially contaminate the specimen and subsequently interfere with some assays such as mass spectrometry or biological assays.
Quality Assurance/Control for the Collection of Bodily Fluids and Other Fluid Samples
The development of SOPs is a key for obtaining high quality bodily fluids to support biomedical research. Incorporated into these SOPs should be the parameters that have been identified as being important in collection, processing, storing, and distributing biological and other fluids. The first aspect of the SOPs is that samples of all bodily fluids should be frozen as rapidly as practicable, and the time between collection and freezing documented; specifically for blood, urine, and saliva, storage in general should be within 4 hours of collection. However, the importance of this time varies with the analyte and there are inadequate studies to support generalities.
Samples should be maintained at wet ice temperature (about 4°C) until processed and or/stored unless a specific use requires room temperature (e.g., immortalization of lymphocytes or clotting of serum).34 For samples of blood components, the samples, in general, should not be hemolyzed because hemolysis releases proteolytic enzymes as well as large quantities of proteins (e.g., hemoglobin). Again, the importance of minimizing hemolysis varies with the analyte.35 Thus, if it can be controlled, the collection needle should not be smaller than 23-gauge and the flow rate should not be too fast, or under too much pressure. Also, blood should not be frozen without additives prior to fractionation, if hemolysis is to be avoided. Note that not all assays are affected by hemolysis such as DNA.
All other aspects related to labeling, initial processing, and storage of bodily fluids should be incorporated into the SOPs. Similarly, the condition of storage should be documented. Finally, before distributing fluid specimens, as previously discussed, the involvement of bias in studies using fluid specimens should be considered.
Accreditation and Certification of Biorepositories
Quality management principles (QMP) provide guidelines that can be adopted and used by tissue repository organizations to aid in meeting their goals of providing high quality tissues and tissue related products. As discussed, ISO 9001:2008 provides biorepositories with useful, internationally accepted standards for developing and monitoring their QMS programs. Also, tissue repositories can obtain certifications in ISO 9001 or other ISO standards such as ISO 13485, 2003.36
A certification program also is being developed by the Canadian Tumour Repository Network (CTRNet). Open initially to Canadian tissue banks, it is now available internationally to biobanks.25 The CTRNet program is based upon the education of all biorepository personnel in multiple areas of biorepository operations (e.g., SOPs, bioethical and regulatory issues). Each educational component is written very generally, and has an associated test, so that the certification program can be adapted to the operations of any biorepository. The goal is to increase the quality of biorepository operations primarily by an educational approach of all personnel.25
A national accreditation program for biorepositories was developed in 2011 by the College of American Pathologists (CAP) in conjunction with ISBER. It involves adaptation of the CAP accreditation program currently used to accredit laboratories performing clinical testing of patient samples. Because of the long history of laboratory accreditation by the CAP in the US, CAP accreditation can be accepted by the Clinical Laboratory Improvement Amendments (CLIA) in lieu of CLIA inspections. The CAP accreditation program utilizes checklists to identify and solve problems as to quality of laboratory operations. All SOPs have to be developed in a standard format which must include control of reagents, equipment, disposables, calibration materials, and annotation of any subsequent modifications. Laboratory safety and quality control are especially emphasized in the CAP accreditation program.
Conclusion
In summary, it is our view that a strong QMS is necessary for a biorepository to provide high quality and consistent tissues and related products to investigators performing biomedical research. Otherwise, resources may be expended by investigators on studying tissues of poor quality, with incorrect diagnoses, or with biases, all of which are likely to delay the progress of a specific research project. In addition, poor quality tissues can cause artifactual and/or misleading conclusions, resulting in publications that cannot be reproduced. Without high quality tissues, biomedical research greatly suffers. Thus it is critical for biorepositories to have a plan to facilitate the distribution of their specimens to aid investigators in research. The leadership of a biorepository should realize that they play critical roles in research infrastructure and that this may involve education of investigators in the use, limitations, and potential bias of human tissues. We urge all biorepositories to accept the importance of a strong QMS with QC in their operations.
Acknowledgments
Supported in part by the Cooperative Human Tissue Network (CHTN Grant 5U01 CA44968-23, The Tissue Procurement Shared Facility of the UAB Comprehensive Cancer Center P30CA13148, The DOD Grant W81XWH-10-1-0543 and the NCI Specialized Program of Research Excellence (SPORE) Pancreatic P20 CA101955-05 and Breast 5P50 CA89019, the Skin Disease Research Center at UAB 5P30AR50948-08, the NHLBI 1R24HL123767-01 Grant and the U54 MSM/TU/UAB Comprehensive Cancer Center Partnership (2U54CA118948). Presented at the American College of Veterinary Pathologists and the American Society for Veterinary Clinical Pathology Concurrent Annual Meetings, Savannah Georgia, 2007.
Author Disclosure Statement
The authors report no financial conflict of interest.
References
- 1.Grizzle WE, Sexton KC, Bell WC. Quality assurance in tissue resources supporting biomedical research. Cell Preserv Technol 2008;6:113–118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Centers for Disease Control and Prevention. Fourth Report on Human Exposure to Environmental Chemicals, 2009. Atlanta GA: US Department of Health and Human Services, Centers for Disease Control and Prevention; Available at http://www.cdc.gov/exposurereport/pdf/fourthreport.pdf Accessed 8April2015 [Google Scholar]
- 3.Gunter EW, McQuillan GM. Quality control in planning and operating the laboratory component of the Third National Health and Nutrition Examination Survey (NHANES III). J Nutr 1990;120:1451–1454 [DOI] [PubMed] [Google Scholar]
- 4.Pirkle JL, Brody DJ, Gunter EW, et al. The decline in blood lead levels in the United States: The National Health and Nutrition Examination Surveys (NHANES). JAMA 1994;272:284–291 [PubMed] [Google Scholar]
- 5.Gunter EW, Lewis BL, Konchikowski SM. Laboratory methods used for the Third National Health and Nutrition Examination Survey (NHANES III), 1988–1994. As contained in NHANES III Reference Manuals and Reports, NCHS CD-ROM, Centers for Disease Control and Prevention, Hyattsville, MD, October, 1996 [Google Scholar]
- 6.Bell WC, Sexton KC, Grizzle WE. Organizational issues in providing high-quality human tissues and clinical information for the support of biomedical research. Methods Mol Biol 2010;576:1–30 [DOI] [PubMed] [Google Scholar]
- 7.Grizzle WE, Bell WC, Sexton KC. Issues in collecting, processing and storing human tissues and associated information to support biomedical research. In: Srivastava S, Grizzle WE, eds. Translational Pathology of Early Cancer, Amsterdam: IOS Press BV; 2012, pp. 531–549 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Grizzle WE, Sexton KC, Bell WC. Human tissue biorepository. In: Cheng L, Zhang D, Eble J, eds. Molecular Genetic Pathology. New York: Springer Science+Business Media; 2013, pp. 483–497 [Google Scholar]
- 9.Ransohoff DF. Promises and limitations of biomarkers. Recent Results Cancer Res 2009;181:55–59 [DOI] [PubMed] [Google Scholar]
- 10.Ransohoff DF. How to improve reliability and efficiency of research about molecular markers: Roles of phases, guidelines, and study design. J Clin Epidemiol 2007;60:1205–1219 [DOI] [PubMed] [Google Scholar]
- 11.Ransohoff DF. Bias as a threat to the validity of cancer molecular-marker research. Nat Rev Cancer 2005;5:142–149 [DOI] [PubMed] [Google Scholar]
- 12.McLerran D, Grizzle WE, Feng Z, et al. Analytical validation of serum proteomic profiling for diagnosis of prostate cancer: Sources of sample bias. Clin Chem 2008;54:44–52 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.UK Biobank. Available at http://www.ukbiobank.ac.uk/ Accessed 8April2015
- 14.Bell WC, Sexton KC, Grizzle WE. How to efficiently obtain human tissues to support specific biomedical research projects. Cancer Epidemiol Biomarkers Prev 2009;18:1676–1679 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Grizzle WE, Srivastava S, Manne U. Translational pathology of neoplasia. In: Translational Pathology of Early Cancer. Eds. Srivastava S, Grizzle WE; IOS Press BV, Amsterdam, The Netherlands; 2012; pp. 7–20 [Google Scholar]
- 16.Henderson GE, Cadigan RJ, Edwards TP, et al. Characterizing biobank organizations in the US: Results from a national survey. Genome Med 2013;5:3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cadigan RJ, Lassiter D, Haldeman K, et al. Neglected ethical issues in biobank management: Results from a U.S. study. Life Sci Soc Pol 2013;9:1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Potter DM, Butterfield LH, Divito SJ, et al. Pitfalls in retrospective analyses of biomarkers: A case study with metastatic melanoma patients. J Immunol Methods 2012;376:108–112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Prostate, Lung, Colon and Ovary Study (PLCO). Available at http://prevention.cancer.gov/plco/ Accessed 8April2015
- 20.Hewitt RE, Grizzle WE, Watson PH, et al. Biobanking networks and global biobanking. In: Hainaut P, Vaught J, Pasterk M, Zatloukal K, eds. Biobanking of Human Specimens: Principle and Practice. New York: Springer; in press [Google Scholar]
- 21.Aamodt RL, Anouna A, Baird P, et al. Best practices for repositories I: Collection, storage and retrieval of human biological materials for research. Cell Preserv Technol 2005;3:5–48 [Google Scholar]
- 22.Pitt K, Campbell L, Skubitz A, et al. Best practices for repositories: Collection, storage, retrieval and distribution of biological materials for research. Cell Preserv Technol 2008;6:5–58 [DOI] [PubMed] [Google Scholar]
- 23.Campbell L, Betsou F, Garcia DL, et al. Best practices for repositories: Collection, storage, retrieval, and distribution of biological materials for research. Biopreserv Biobank 2012;10:1–85 [DOI] [PubMed] [Google Scholar]
- 24.Office of Biorepositories and Biospecimen Research, National Cancer Institute: Best Practices for Biospecimen Resources. Available at http://biospecimens.cancer.gov/bestpractices/ Accessed 8April2015
- 25.Canadian Tumour Repository Network (CTRNet). Available at https://www.ctrnet.ca Accessed 8April2015
- 26.International Organization for Standardization (ISO) – ISO 9001 and ISO 9001:2008. Available at http://www.iso.org/iso/home/standards/management-standards/iso_9000.htm Accessed 8April2015
- 27.Federal Drug Administration's (FDA) Good Tissue Practices (GTP, 21 CFR, Part 1271). Available at http://www.fda.gov/ Accessed 8April2015
- 28.Federal Drug Administration's Good Manufacturing Practices. Available at http://www.fda.gov Accessed 8April2015
- 29.International Air Transport Association (IATA). Available at http://www.ups.com/content/us/en/resources/ship/hazardous/biological_substances.html Accessed 8April2015
- 30.United States Department of Transportation (DOT). Available at http://www.ncsu.edu/ehs/dot/Bio_shipping.pdf Accessed 8April2015
- 31.Steg A, Wang W, Blanquicett C, et al. Multiple gene expression analyses in paraffin-embedded tissues by Taqman low density array: Application to Hedgehog and Wnt pathway analysis in ovarian endometrioid adenocarcinoma. J Mol Diagn 2006;8:76–83 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Steg A, Vickers SM, Eloubeidi M, et al. Hedgehog pathway expression in heterogeneous pancreatic adenocarcinoma: Implications for the molecular analysis of clinically available biopsies. Diagn Mol Pathol 2007;16:229–237 [DOI] [PubMed] [Google Scholar]
- 33.McNally LR, Manne U, Grizzle WE. Post-transcriptional processing of genetic information and its relation to cancer. Biotech Histochem 2013;88:365–372 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tuck MK, Chan DW, Chia D, et al. Standard operating procedures for serum and plasma collection: Early Detection Research Network consensus statement. Standard Operating Procedure Integration Working Group. J Proteome Res 2009;8:113–117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wenk RE. Mechanism of interference by hemolysis immunoassays and requirements for sample quality. Clin Chem 1998;44:2554. [PubMed] [Google Scholar]
- 36.International Organization for Standardization. Available at http://www.iso.org/iso/home.html Accessed 8April2015