Abstract
Purpose
Graves' orbitopathy (GO) is a sight-threatening autoimmune disorder causing extraocular muscle fibrosis, upper lid retraction and eye bulging due to orbital fat expansion. These clinical features are mediated by aspects of orbital fibroblasts differentiation, including adipogenesis and fibrosis. Our previous work suggested that this dual phenotype might be a manifestation of mixed cell populations, partially linked to the expression of mesenchymal stem cell (MSC) marker CD90. Thus, we set out to determine whether GO orbital fibroblasts displayed MSC properties.
Methods
Control and GO orbital fibroblasts previously characterized for CD90 and CD45 expression were analyzed by flow cytometry for classical MSC positive (CD73, CD105) and negative (CD14, CD19, HLA-DR, and CD34) markers. Graves' orbitopathy fibroblasts were tested further for their ability to undergo lineage specific differentiation following standard protocols.
Results
Control and GO fibroblasts strongly expressed CD73 and CD105, with a higher percentage of positive cells and stronger expression levels in GO. Neither cell type expresses CD14, CD19, and HLA-DR. Protein CD34 was expressed at low levels by 45% to 70% of the cells, with its expression significantly lower in GO cells. Graves' orbitopathy fibroblasts displayed features of osteogenesis (calcium deposits, and osteocalcin [BGLAP] and osteonectin [SPARC] expression), chondrogenesis (glycosaminoglycan production; SOX9 and aggrecan [ACAN] expression), myogenesis (α-smooth muscle actin expression), and neurogenesis (β-III tubulin expression) upon differentiation.
Conclusions
Our findings suggest that orbital fibroblasts contain a population of cells that fulfil the criteria defining MSC. This subpopulation may be increased in GO, possibly underlying the complex differentiation phenotype of the disease.
Keywords: mesenchymal stem cells, Grave's orbitopathy, orbital fibroblasts
This study reports the identification of cell populations with mesenchymal stem cell properties in orbital fibroblasts isolated from patients with Graves' orbitopathy.
Graves' orbitopathy (GO) is a disfiguring and potentially blinding disorder.1–3 Clinical features include expansion and fibrosis of the orbital tissues, leading to proptosis, eyelid retraction, dry eye syndrome, and diplopia.4 At the cellular level, pathological changes include adipogenesis, fibrosis, and hyaluronan production.5 We have developed an in vitro model for GO using primary fibroblast cultures derived from orbital fat, which uniquely allows the study of the fibrotic and the spontaneous adipogenic phenotype of orbital fibroblasts within 3 dimensional environments.6 We have shown that GO fibroblasts retain a complex phenotype in vitro, exhibiting hyaluronan production,7 adipogenesis, and increased contractile properties, and sensitivity to cytokine stimulation.6 Orbital fibroblasts are known to comprise a mixed population of cell subtypes, including fibrocytes,8 and Thy1+/Thy1− populations, which have been proposed to underlie the adipogenic and contractile phenotype.6,9 Thy1 (CD90) is a major marker of stromal and adipose derived stem cells.10–12 The high level of Thy1 expression in orbital cells in GO, as well as the diversity of the cell phenotypes observed, suggested that GO fibroblasts may possess mesenchymal stem cell-like (MSC) characteristics. We describe here how we use cell marker expression and in vitro differentiation to further investigate the presence of a potential MSC-like population in orbital fibroblasts.
Methods
Ethics Statement
This study was conducted according to the principles of the Declaration of Helsinki and reviewed by the National Research Ethics Service Committee London-Bentham (REC reference number 11/LO/1170). All participants gave their informed written consent before enrolment.
Cells
The 3 GO (HO1, HO2, HO3) and 3 control (CO2, CO3, CO4) orbital fibroblast lines used in this study and the clinical details of the donors have been described previously.6 Briefly, the GO lines were derived from patients with severely active disease, having undergone prior immunosuppressive steroid treatment, while the control samples were from patients undergoing removal of subconjunctival fat herniation.6 Unless otherwise stated, cells were grown in Dulbecco's modified Eagle's medium (DMEM, 4.5 g/L L-glutamine; Life Technologies, Thermo Fischer Scientific, Paisley, UK) with the addition of 10% fetal bovine serum (FBS; Sigma-Aldrich, Gillingham, UK), 100 IU/mL penicillin, 100 μg/mL streptomycin (Life Technologies, Thermo Fischer Scientific), and used between passages 3 and 8.
Flow Cytometry
Subconfluent orbital GO and control fibroblasts were trypsinized and 0.3 to 1 × 106 cells were placed in each vial and pelleted. Cells then were resuspended in 100 μL of PBS with addition of unlabeled primary (CD221; Biolegend, London, UK) or PE-conjugated (CD14, CD19, CD34, CD73, CD105, HLA-DR, and isotype controls IgG1 and IgG2; all from BioLegend) antibodies and incubated on ice for 1 hour. For CD221, this was followed by a PBS wash and 1-hour incubation with Cy5-conjugated goat anti-rabbit antibody (Jackson Laboratories, Bar Harbor, ME, USA). Cells were washed twice with PBS, resuspended in 300 μL of PBS, and transferred to FACS tubes (BD Falcon, BD Biosciences, Erembodegem, Belgium). Analysis was performed using FACSCalibur (Beckton Dickinson, Oxford, UK). At least 10,000 cells were analyzed per experiment, all experiments were repeated independently 3 times. The markers have been used previously to characterize multipotent stem cells from orbital fat tissue using FACS analysis,13,14 and are known to be insensitive to trypsin treatment,15–19 with the exception of CD14 for which immunofluorescence was used to confirm the absence of staining in orbital fibroblasts not treated with trypsin (Supplementary Fig. S1, Supplementary Methods and Legends). Protein CD73 was used as a positive control for immunofluorescence on orbital fibroblasts monolayers (Supplementary Fig. S2).
Chondrogenic and Osteogenic Differentiation
Chondrogenic differentiation was performed as described previously.20 Briefly, 1.25 × 106 GO fibroblasts were centrifuged and resuspended in 1 mL of Chondrocyte Differentiation Medium (ZenBio, Inc., Research Triangle Park, NC, USA). A 200 μL amount of the cell suspension was dispensed per well into Nunc 96-well round bottom plates. Plates were centrifuged at 500g for 5 minutes to generate a pellet and differentiation was left to proceed for 21 days with the medium changed every other day. Alcian blue staining was used to identify chondrogenic differentiation.20 The cell pellets were fixed in formalin and embedded in paraffin. Sections were deparaffinized, and half of them were pretreated with 0.5 mg/mL hyaluronidase (Sigma-Aldrich) in a phosphate buffer pH 6.7. All sections then were stained with 1% alcian blue 8GX (TCS Biosciences, Botolph Claydon, UK) in 3% acetic acid glacial (Thermo Fischer Scientific). For osteogenic differentiation, GO fibroblasts were plated in 6 well plates (3 × 104 cells/cm2). After 24 hours, the medium was changed to Osteoblast Differentiation Medium (ZenBio, Inc.) and the differentiation was allowed to proceed for 21 days, with the medium changed every 3 to 4 days. Cells monolayers were fixed in graded ethanol concentrations (25, 50, 75, 100% in PBS) and incubated with alizarin red S (Sigma-Aldrich) at pH 4.2 for 10 minutes to identify calcium deposits. All images were taken using a Leica DMIL microscope (Leica Microsystems, Milton Keynes, UK) with Nikon DS-Fi1 camera (Nikon, Kingston Upon Thames, UK). These experiments were repeated independently 2 to 3 times.
Myogenic and Neuronal Differentiation
Graves' orbitopathy cells were seeded on glass coverslips (2 × 103 cells/cm2) in standard medium in 6-well plates. After 24 hours, the medium was supplemented with TGF-β1 (100 ng/mL; PeproTech, London, UK) for 48 hours (myogenic differentiation) or with neuronal differentiation inducer III (20 μM; Calbiochem, Merck KGaA, Darmstadt, Germany) for 5 days (neurogenic differentiation). The coverslips then were fixed in 3.7% formaldehyde, permeabilized in 0.5% Triton-X100 (Sigma-Aldrich), washed with 0.1 M glycine, and blocked with 1% FBS and 1% donkey serum in Tris Buffer Saline.21 Cells were incubated with primary antibodies against α-smooth muscle actin (α-SMA, mouse, 1:50; Sigma-Aldrich) and neuron-specific β III tubulin (rabbit, 1:200; Abcam, Cambridge, UK), followed by anti-mouse tetramethylrhodamine (TRITC)-conjugated and anti-rabbit fluorescein isothiocyanate (FITC)-conjugated secondary antibodies (both donkey, 1:100; Jackson Laboratories), respectively. Following washes, the coverslips were mounted with Fluoroshield mounting medium with 4′,6-diamidino-2-phenylendole (DAPI; Abcam). Cells were imaged using a Nikon Ti-E microscope with CoolSNAP HQ2 camera (Photometrics, Tucson, AZ, USA), using a ×20 air objective (20X Plan Fluor ELWD ADM with correction collar).
Real-Time PCR (RT-PCR)
Differentiated HO1, HO2, and HO3 cells (osteogenesis and chondrogenesis as above), matching undifferentiated control cells grown under the same conditions, but in the standard medium, and cells from standard monolayer cultures were homogenized in 700 μL of Trizol (Thermo Fischer Scientific). RNA was extracted using the miRNeasy kit (Qiagen, Hilden, Germany) according to the manufacturer's instructions. Concentration and purity of RNA was analyzed using NanoDrop 2000 (Thermo Fisher Scientific, Inc., Wilmington, DE, USA). Then, 200 ng of RNA was reverse-transcribed using QuantiTect Reverse Transcription kit (Qiagen) according to the manufacturer's instructions, except for the incubation time at 42°C, which was increased from 15 to 30 minutes. Then, 60 μL of water was added to the reaction, and 5 μL of this was mixed with 6.25 μL of water, 12.5 μL of TaqMan gene expression master mix (Applied Biosystems, DE, USA), and 1.25 μL of a primer targeting one of the following sequences: aggrecan (ACAN, Hs00153936_m1), SOX-9 (SOX9, Hs01001343_g1), osteonectin (SPARC, Hs00234160_m1), osteocalcin (BGLAP, Hs01587814_g1), hypoxanthine-guanine phosphoribosyltransferase (HPRT1, Hs02800695_m1), and splicing factor 3A subunit 1 (SF3A1, Hs01066327_m1; all from Applied Biosystems). The real-time PCR settings were as follows: 50°C for 2 minutes, 95°C for 10 minutes, 40 iterations of 95°C for 15 seconds, and 60°C for 1 minute. Data interpretation was done using the comparative ΔCT method.22 Transcription products were then run in 2% agarose gels with 1% TAE buffer in distilled water. Band intensities were measured using ImageJ (http://imagej.nih.gov/ij/), and the housekeeping genes HPRT1 (for ACAN and SPARC) or SF3A1 (for SOX9 and BGLAP) were used to normalize the values.
Statistical Analysis
Flow cytometry graphs show mean and SEM for three individual experiments. Statistical analysis was performed using ANOVA. Pearson product-moment correlation analysis was performed on averaged flow cytometry results from three separate experiments, and each of the 6 cell lines was shown as a separate data point. Levels of significance were determined using the Table of Critical Values for Pearson's correlation coefficient. Statistical analysis for RT-PCR measurements was performed using ANOVA and 2-tailed paired Student's t-test.
Results
GO Orbital Fibroblasts Display an MSC-Like Marker Profile
One of the criteria used to define MSCs is expression of CD73, CD105, and CD90 in at least 95% of cells, and lack of expression of CD14 or CD11b, CD19 or CD79α, CD34, CD45, and HLA-DR in at least 98% of cells.10 We have shown previously that GO and control orbital fibroblasts are negative for CD45, but positive for CD90 (57% to 96% of the cells), with varying levels of expression.6 We used here the same fibroblast lines to further analyze the expression of CD73, CD105, CD14, CD19, CD34, and HLA-DR. In addition, CD221 (IGF-1R) was used as a positive control/disease marker, as IGF-1R was shown previously to be overexpressed in GO fibroblasts, underlying some aspects of the disease.23–25
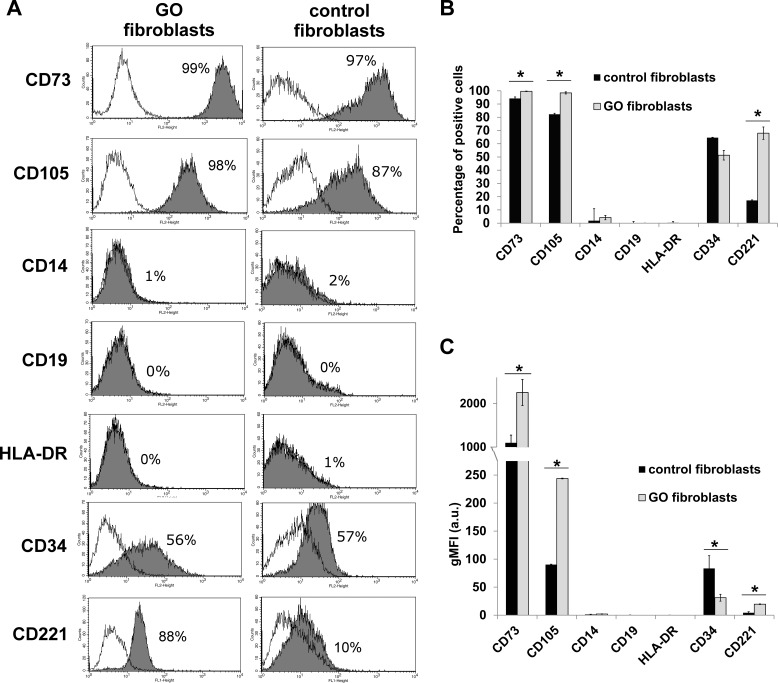
As expected, a significant proportion of GO fibroblasts expressed CD221 on average, as opposed to less than 20% for control fibroblasts (Figs. 1A, 1B), and the levels expressed by positive cells were significantly higher in GO cells (Fig. 1C). Control and GO fibroblasts displayed very high expression levels for positive markers CD73 and CD105 in the majority of the cells (Figs. 1A, 1B), but the percentage of positive cells and the geometric mean fluorescence intensity of the positive cells were significantly higher in GO fibroblasts (Figs. 1B, 1C).
Figure 1.
Graves' orbitopathy and control orbital fibroblasts express MSC markers. The expression of MSC markers CD105, CD73, CD14, CD19, HLA-DR, and CD34,10 as well as CD221 (IGF-1R) was analyzed in GO and control fibroblasts using flow cytometry. (A) Representative flow charts for individual markers in one GO (line HO1) and one control (line CO2) fibroblast line. Gray areas represent specific marker expression profile, with the percentage of positive cells as indicated. White areas show the distribution of the fluorescence using nonspecific matching IgG isoform control. (B, C) Percentage of cells expressing the indicated marker (B) and geometric mean fluorescence intensity (gMFI) for each marker (C). Shown is the mean ± SEM for 3 GO and 3 control fibroblast lines, with n = 3 for each marker in each cell line. *Statistically significant difference between control and GO cells (P < 0.05).
Only a minor fraction of cells expressed CD14 (0%–7.4%), CD19 (0%–1.6%) and HLA-DR (0%–1.2%), and at levels barely above background. Expression of CD34 was unexpectedly elevated, with 64.6% (SEM = 4.6) of CO cells and 51.3% (SEM = 3.6) of GO cells displaying the marker (Figs. 1A, 1B), although the levels of expression were significantly lower in GO cells (Fig. 1C).
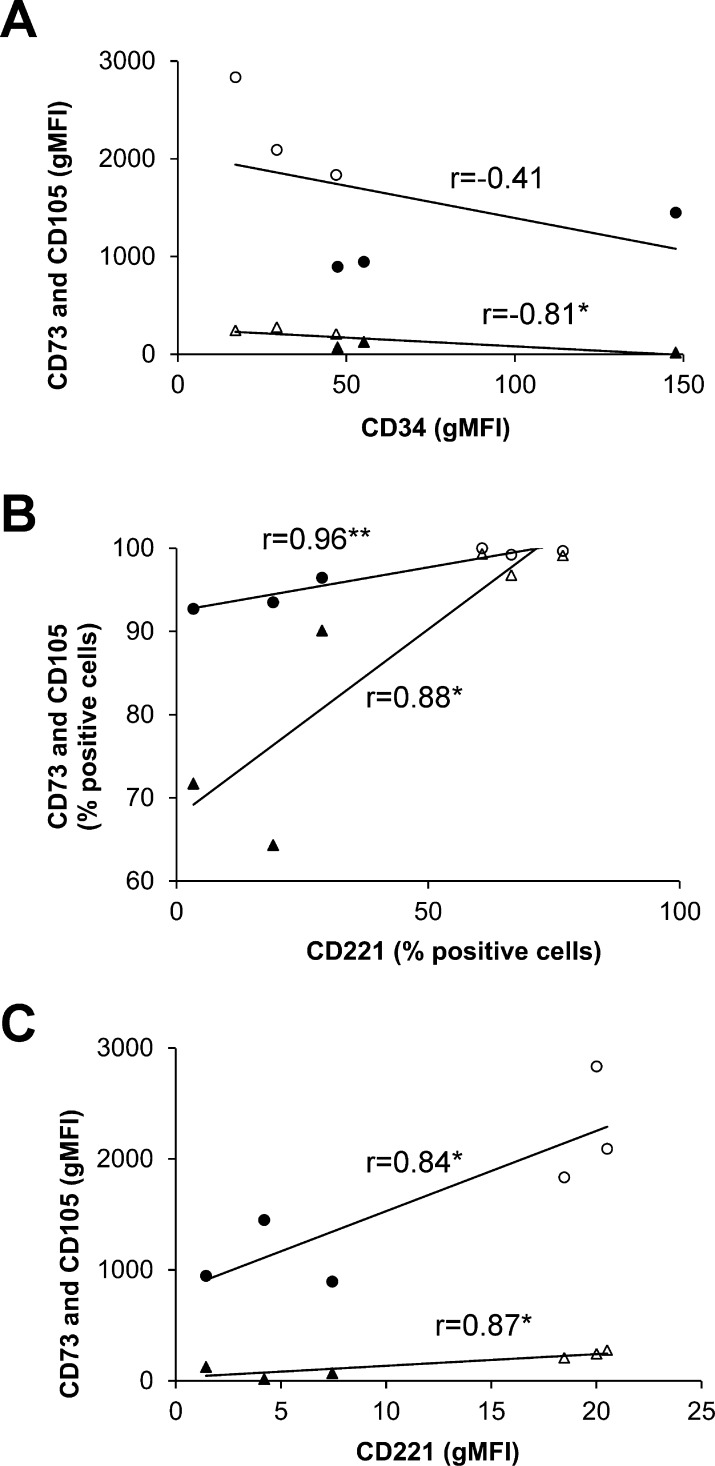
To determine the relationship between CD34 and CD221, and positive markers of MSCs, we analyzed separate marker expression using Pearson product-moment correlation (Fig. 2). There was a strong, negative correlation between levels of expression of CD34 and CD105 (r = −0.81, P < 0.05; Fig. 2A). Conversely, there was a strong positive correlation between the percentages of cells expressing CD221 and positive MSC markers CD73 (r = 0.96, P < 0.01) and CD105 (r = 0.88, P < 0.05; Fig. 2B). Similarly, levels of the expression of CD73 and CD105 markers were correlated strongly with CD221 expression levels (r = 0.84 and r = 0.87 respectively, P < 0.05; Fig. 2C). Overall, this showed that GO fibroblasts had a marker profile that more closely resembled a typical MSC profile than that of control orbital fibroblasts, suggesting that GO fibroblasts may comprise an MSC-like population capable of multilineage differentiation.
Figure 2.
Mesenchymal stem cell marker expression is correlated with disease profile. Pearson product-moment correlation analysis was performed between (A) expression levels (mean gMFI) of CD34 versus CD73 (circles) and CD105 (triangles); (B) percentage of cells expressing CD221 versus CD73 and CD105; and (C) expression levels of CD221 versus CD73 and CD105. Each point represents averaged data (n = 3) for each control (filled symbols) and GO (empty symbols) cell line. Statistically significant correlations: *P < 0.05, **P < 0.01.
GO Orbital Fibroblasts Undergo Lineage Specific Differentiation
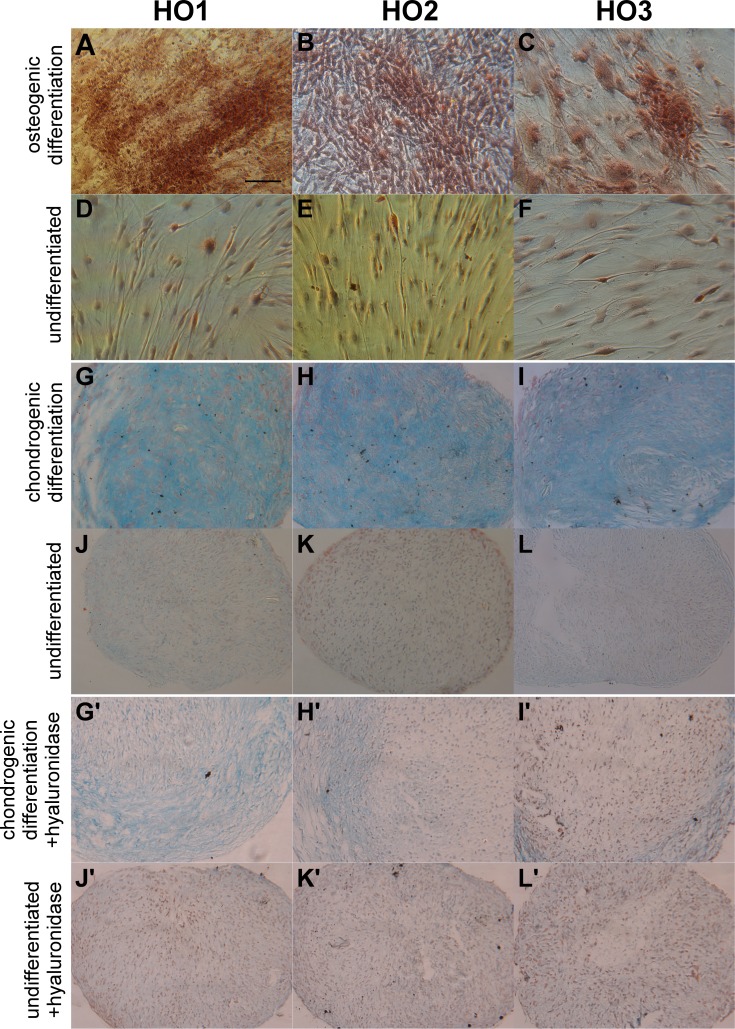
We have shown previously that GO fibroblasts (HO1, HO2, HO3 lines as used here) undergo adipogenesis, following standard stimulation with adipogenic differentiation medium in monolayer cultures, as well as spontaneously (without any chemical stimulation) when grown within 3 dimensional collagen matrices.6 To further explore the differentiation potential of GO fibroblasts, we tested the cells for chondrogenic, osteogenic, myogenic, and neurogenic potential. Following osteogenic differentiation for 21 days, alizarin red was used to stain calcium deposits that characterize bone mineral formations. Clusters of stained deposits were found in all 3 GO cell lines incubated in osteogenesis differentiation medium (Figs. 3A–C), but not in control medium (Figs. 3D, 3E). Additionally, the differentiated cells looked more irregular, forming clusters of cells with a 3-dimensional aspect compared to the characteristic flat, spindle-shaped morphology of the cells in control medium (Figs. 3A–F).
Figure 3.
Graves' orbitopathy fibroblasts demonstrate osteogenic and chondrogenic lineage differentiation. Graves' orbitopathy fibroblasts (lines HO1-3) were induced toward osteogenic (A–C) and chondrogenic (G/G'–I/ I') differentiation using specific media or kept in control medium under the same conditions (“undifferentiated”; [D–F] and [J/J'–L/L'], respectively). (A–F) Cells were stained with alizarin red to evaluate calcium deposits (brown areas). (G–L') Cell pellets were stained with Alcian blue without (G–L) or with (G'–L') prior treatment with hyaluronidase to evaluate glycosaminoglycan production (blue areas). Scale bar: 10 μm.
Following chondrogenic differentiation in cell pellets for 21 days, we used alcian blue staining to evaluate glycosaminoglycan production as a late chondrogenesis marker. Alcian blue produced a strong blue staining in the pellets incubated in differentiation medium (Figs. 3G–I), suggesting significant chondrogenic differentiation, while only faint blue spots were visible in the control pellets (Figs. 3J–L). The Alcian blue staining was largely absent when the sections were treated with hyaluronidase before staining (Figs. 3G'–L'), suggesting that most of the staining in the samples was due to the presence of glycosamiglycans (rather than nonspecific binding of the dye to lipids).
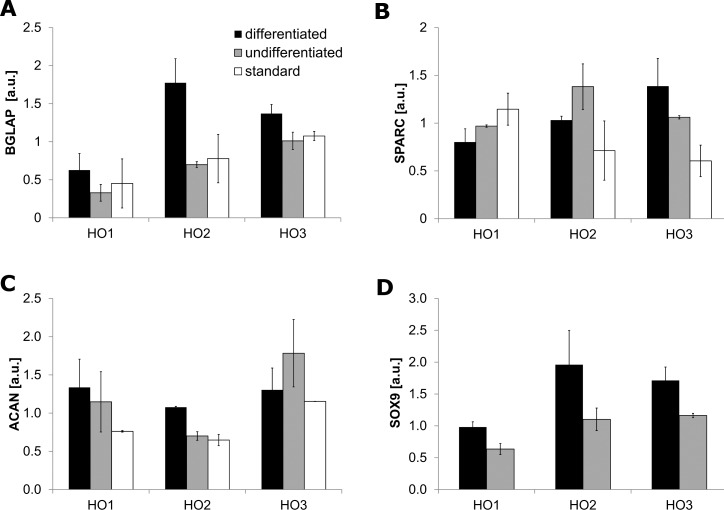
Osteogenesis and chondrogenesis were confirmed by analyzing the expression of specific differentiation marker genes, BGLAP and SPARC for the former, and ACAN and SOX9 for the latter respectively. Real-time PCR (Supplementary Table S1) and the subsequent gel electrophoresis (Fig. 4 and Supplementary Fig. S3) identified differentiation marker expression in all three fibroblasts cell lines. All cells cultured according to the differentiation protocols showed upregulation of BGLAP (P = 0.006) and SOX9 (P = 0.05), with upregulation of ACAN seen in HO1 and HO2, and upregulation of SPARC seen in HO3.
Figure 4.
Graves' orbitopathy fibroblasts express markers of osteogenesis and chondrogenesis. Graves' orbitopathy fibroblasts (lines HO1-3) were induced toward osteogenic (A, B), and chondrogenic (C, D) differentiation (“differentiated,” black bars) or kept in control medium under the same conditions (“undifferentiated,” gray bars); additionally, cells grown under standard cell culture conditions were tested (A–C, “standard,” white bars). Expression levels of markers of osteogenesis (BGLAP and SPARC) and chondrogenesis (ACAN, SOX9) were assessed by semi-quantitative analysis of agarose gel electrophoresis of RT-PCR products. Shown is the mean ± SEM, with n = 2 for each marker in each cell line.
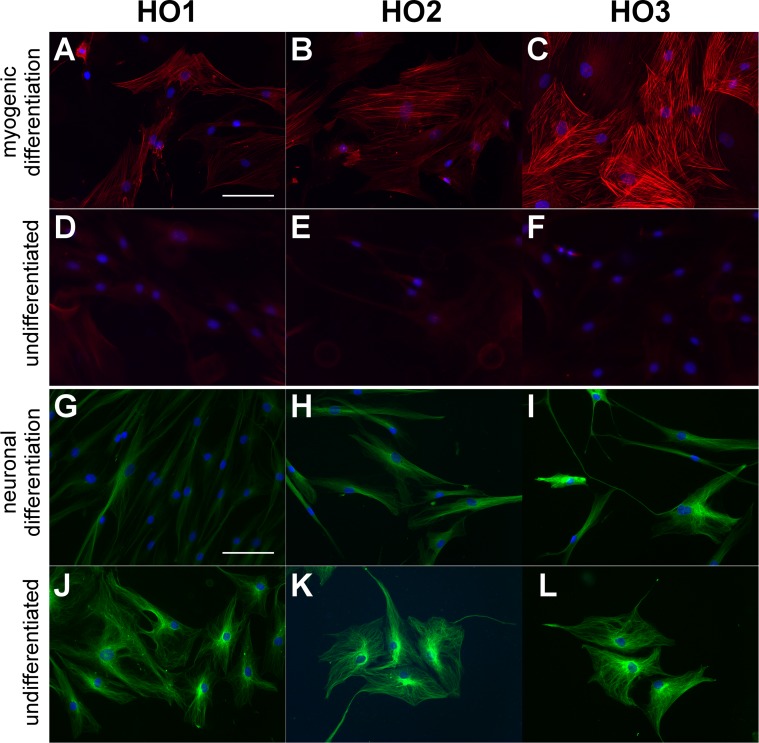
Transforming growth factor-β is a potent inducer of myogenic differentiation of MSCs,26,27 and we found that stimulation of GO cells with TGF-β led to a marked increase in α-SMA expression (marking the onset of myocyte differentiation), as well as its significant incorporation into actin stress fibers (Figs. 5A–F). After differentiation toward neuronal lineage using neuronal differentiation inducer III, the cells adopted a more elongated morphology (Figs. 5G–L), with some of them displaying long neuron-like protrusions (Fig. 5I). However, differentiated and undifferentiated cells expressed the neuron-specific β-III tubulin marker, suggesting a preexisting commitment toward a neuronal lineage (Figs. 5G–L).
Figure 5.
Graves' orbitopathy fibroblasts demonstrate myogenic and neuronal lineage differentiation. Graves' orbitopathy fibroblasts (lines HO1-3) were induced toward myogenic (A–C) and neuronal (G–I) differentiation using specific media or kept in control medium under the same conditions (“undifferentiated” [D–F] and [J–K], respectively). (A–F) Cells were immunostained for α-SMA (red) and DAPI (blue). (G–L) Cells were immunostained for β-III Tubulin (green) and DAPI (blue). Scale bar: 100 μm.
Discussion
The presence of stem cells within adipose tissue is well established and this includes orbital adipose tissue, where pluripotent cells have been identified that display a classical multilineage potential as well as the more unusual ability to differentiate into corneal epithelial cells.13,14,28 In addition, there is increasing evidence that fibroblasts display characteristics that define MSCs, including immunophenotype and multilineage differentiation,29–32 and that differences between classical MSC and fibroblasts are within variability seen among MSC lines of different topographical origin.30,33,34 We have shown previously that primary fibroblasts from the orbit of patients with active Graves' disease displayed a dual profibrotic/contractile and adipogenic phenotype when cultured within 3 dimensional (3D) collagen matrices,6 and produced hyaluronan when stimulated with IGF1 or patient serum (Ezra D, unpublished data). These cells also were largely positive for CD90 and negative for CD45,6 positive and negative markers of MSCs, respectively.10–12 Thus, we hypothesized that GO orbital fibroblasts contain a population of cells capable of pluripotent differentiation, potentially underlying the multifaceted phenotype of the disease.
We showed here that GO fibroblasts fulfil most of the proposed minimal criteria for MSC identification10: they are adherent to plastic under normal culture conditions, and express CD90,6 CD73, and CD105 while lacking the expression of CD45,6 CD14, CD19, and HLA-DR. However, a significant proportion of control and GO orbital cells expressed CD34, a commonly used negative marker of MSCs, albeit to low levels. Despite often being acknowledged as a negative marker for MSCs,10 adipose-derived MSCs (AMSCs) and committed preadipocytes have been shown to express CD34.12 Thus, the presence of CD34-positive cells within a putative MSC compartment in GO fibroblasts may be linked to the cells' proadipogenic phenotype and spontaneous adipogenic differentiation potential in 3D cultures.6 In addition, the presence/absence of CD34 on MSCs may depend on how, and from where, the cells have been isolated,35–37 and, indeed, CD34 previously was found expressed in orbital adipose tissue, particularly in the nasal fat area.14 Alternatively, CD34-positive cells may comprise a subpopulation of fibrocytes, having invaded the orbit from the circulation and contributing to the diversity of the orbital fibroblast phenotype,8 and to the local MSC pool.36 Although fibrocytes normally are CD45-positive and our GO populations have been found largely negative for CD45,6 we cannot entirely rule out some fibrocyte involvement as the expression of CD45 in fibrocytes is known to decrease after they enter the tissue in vivo as well as during culture in vitro.38 Overall, however, when marker profiles were analyzed for correlations, the frequency of the positive MSC markers CD105 and CD73 correlated positively with the presence of CD221 (IGF-1R), a marker that has been implicated in the pathology of the disease.24 Conversely, CD34 expression was inversely correlated with the positive markers expression. This suggests that the increased expression of MSC-positive markers and correlated decrease in MSC-negative markers in GO orbital fibroblasts reflect the emergence within these cells of a population with MSC characteristics that may be linked to disease progression.
When probed for their lineage-specific differentiation abilities, GO fibroblasts showed MSC-like pluripotency. In addition to their previously shown ability to differentiate into adipocytes,5,6,9 we show here that GO fibroblasts displayed significant levels of osteogenesis and chondrogenesis. Both RT-PCR quantitation and gene electrophoresis confirmed the presence of differentiation markers (BGLAP and SPARC for osteogenesis; ACAN and SOX9 for chondrogenesis) in all three GO lines, in cells exposed to the differentiation protocol and cells grown in control medium. Markers BGLAP and SOX9, which were expressed at lower levels than the other markers, were significantly upregulated in differentiated cells, confirming the differentiation toward osteogenesis and chondrogenesis, respectively. A possible explanation for the lack of significant upregulation of the other markers in response to the differentiation protocols is the high basal level of expression. As cell density has been reported to have a role in osteogenesis39,40 and chondrogenesis,41,42 we additionally tested expression of three of the markers in cells grown under standard conditions, that is, as subconfluent monolayers. Again, all markers tested were present in the cells, but most of them at levels lower than in the cells that had undergone differentiation (ACAN, P = 0.04; BGLAP, SPARC, and SOX9, P > 0.05). Therefore, we proposed that GO fibroblasts may constitutively express markers classically considered as markers for osteo- and chondrogenesis, but a further differentiation procedure may be required to induce functional differentiation, and production of calcium deposits and hyaluronan. Indeed, SPARC expression in mouse and human fibroblasts has been linked to skin and lung fibrosis,43,44 suggesting that constitutive expression of SPARC in GO fibroblasts may be linked to their profibrotic phenotype.
Myogenic differentiation was not originally one of the minimal criteria that define MSCs. Nevertheless, a number of studies have shown that MSCs could differentiate into the myogenic lineage, including smooth and striated muscle, under various conditions.45–48 Although α-SMA and MyoD have been described as early myogenic markers, there is some evidence that MyoD expression could be transient.46 In addition, α-SMA has been linked to fibrosis, which could again be of significant relevance in the pathology of Graves' orbitopathy. Graves' orbitopathy cells displayed minimal expression levels of α-SMA in normal cultures, but all three lines showed significantly elevated levels of α-SMA, as well as its incorporation into strong actin stress fibers, following stimulation with TGF-β, denoting a differentiation toward a myofibroblast/myogenic phenotype. This strong response to TGF-β stimulation may be linked to the profibrotic phenotype of the cells,6 possibly underlying some of the fibrotic pathology in GO. The ability of MSCs to differentiate into neurons is more controversial,49 but we found that GO fibroblasts appear to gain some morphological characteristics of neuronal cells upon stimulation with neuronal differentiation inducer III. However, they spontaneously expressed the neuronal marker β-III tubulin with little, if any, changes following stimulation with neuronal differentiation inducer III. This may be a reflection of the peculiar embryonic origin of orbital fat, as craniofacial adipose tissue is thought to originate from the neural crest cells rather than the mesoderm as for the most of the white adipose tissue.50,51
Overall, our findings suggested that GO orbital fibroblasts populations comprise cells that broadly fulfil the criteria defining MSCs, and have the potential for multilineage differentiation. Although this study still is preliminary and it is not clear whether the pluripotent cell population in GO represents true MSCs (with self-renewal potential), progenitors, or a mix of both, the identification of such cells suggests that they could underlie some of the complexity of the disease phenotype. Considering the emerging evidence of a role for MSCs in modulating inflammation, and particularly in the context of autoimmune diseases,52 it is tantalizing to speculate that MSCs/progenitors could be an important factor controlling disease progression in GO, as they have been proposed to for other fibrotic diseases.50
Supplementary Material
Acknowledgments
The authors thank Grazyna Galatowicz for help with the FACS analysis.
Supported by Fight For Sight (Grant 1341/1342; DGE, MB). Laboratories and imaging facilities were supported by the Wellcome Trust and Fight For Sight. Supported also by the National Institute for Health Research (NIHR) Biomedical Research Centre based at Moorfields Eye Hospital NHS Foundation Trust and the UCL Institute of Ophthalmology (DGE, GER). The views expressed are those of the authors and not necessarily those of the NHS, the NIHR, or the Department of Health.
Disclosure: K. Kozdon, None; C. Fitchett, None; G.E. Rose, None; D.G. Ezra, None; M. Bailly, None
References
- 1. Trobe JD. Optic nerve involvement in dysthyroidism. Ophthalmology. 1981; 88: 488–492. [DOI] [PubMed] [Google Scholar]
- 2. Tallstedt L,, Lundell G,, Torring O,, et al. Occurrence of ophthalmopathy after treatment for Graves' hyperthyroidism. The Thyroid Study Group. N Engl J Med. 1992; 326: 1733–1738. [DOI] [PubMed] [Google Scholar]
- 3. El-Kaissi S,, Frauman AG,, Wall JR. Thyroid-associated ophthalmopathy: a practical guide to classification, natural history and management. Intern Med J. 2004; 34: 482–491. [DOI] [PubMed] [Google Scholar]
- 4. Regensburg NI,, Wiersinga WM,, Berendschot TT,, Potgieser P,, Mourits MP. Do subtypes of graves' orbitopathy exist? Ophthalmology. 2011; 118: 191–196. [DOI] [PubMed] [Google Scholar]
- 5. Eckstein AK,, Johnson KT,, Thanos M,, Esser J,, Ludgate M. Current insights into the pathogenesis of Graves' orbitopathy. Horm Metab Res. 2009; 41: 456–464. [DOI] [PubMed] [Google Scholar]
- 6. Li H,, Fitchett C,, Kozdon K,, et al. Independent adipogenic and contractile properties of fibroblasts in graves' orbitopathy: an in vitro model for the evaluation of treatments. PLoS One. 2014; 9: e95586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Ezra DG,, Rose GE,, Bailly M. Developing an in vitro model of tissue expansion in Graves ophthalmopathy: exploring the role of IGF1 receptor targeting as a novel treatment. Endocr Abstracts. 2011; 25(OC1.4).
- 8. Smith TJ. Potential role for bone marrow-derived fibrocytes in the orbital fibroblast heterogeneity associated with thyroid-associated ophthalmopathy. Clin Exp Immunol. 2010; 162: 24–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Koumas L,, Smith TJ,, Feldon S,, Blumberg N,, Phipps RP. Thy-1 expression in human fibroblast subsets defines myofibroblastic or lipofibroblastic phenotypes. Am J Pathol. 2003; 163: 1291–1300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Dominici M,, Le Blanc K,, Mueller I,, et al. Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy. 2006; 8: 315–317. [DOI] [PubMed] [Google Scholar]
- 11. Huang SJ,, Fu RH,, Shyu WC,, et al. Adipose-derived stem cells: isolation, characterization, and differentiation potential. Cell Transplant. 2013; 22: 701–709. [DOI] [PubMed] [Google Scholar]
- 12. Cawthorn WP,, Scheller EL,, MacDougald OA. Adipose tissue stem cells meet preadipocyte commitment: going back to the future. J Lipid Res. 2012; 53: 227–246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Ho JH,, Ma WH,, Tseng TC,, Chen YF,, Chen MH,, Lee OK. Isolation and characterization of multi-potent stem cells from human orbital fat tissues. Tissue Eng Part A. 2011; 17: 255–266. [DOI] [PubMed] [Google Scholar]
- 14. Korn BS,, Kikkawa DO,, Hicok KC. Identification and characterization of adult stem cells from human orbital adipose tissue. Ophthal Plast Reconstr Surg. 2009; 25: 27–32. [DOI] [PubMed] [Google Scholar]
- 15. Tabatabaei M,, Mosaffa N,, Nikoo S,, et al. Isolation and partial characterization of human amniotic epithelial cells: the effect of trypsin. Avicenna J Med Biotechnol. 2014; 6: 10–20. [PMC free article] [PubMed] [Google Scholar]
- 16. De Francesco F,, Tirino V,, Desiderio V,, et al. Human CD34/CD90 ASCs are capable of growing as sphere clusters, producing high levels of VEGF and forming capillaries. PLoS One. 2009; 4: e6537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Mulder WM,, Koenen H,, van de Muysenberg AJ,, Bloemena E,, Wagstaff J,, Scheper RJ. Reduced expression of distinct T-cell CD molecules by collagenase/DNase treatment. Cancer Immunol Immunother. 1994; 38: 253–258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Muczynski KA,, Ekle DM,, Coder DM,, Anderson SK. Normal human kidney HLA-DR-expressing renal microvascular endothelial cells: characterization, isolation, and regulation of MHC class II expression. J Am Soc Nephrol. 2003; 14: 1336–1348. [DOI] [PubMed] [Google Scholar]
- 19. Jena B,, Maiti S,, Huls H,, et al. Chimeric antigen receptor (CAR)-specific monoclonal antibody to detect CD19-specific T cells in clinical trials. PLoS One. 2013; 8: e57838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Solchaga LA,, Penick KJ,, Welter JF. Chondrogenic differentiation of bone marrow-derived mesenchymal stem cells: tips and tricks. Methods Mol Biol. 2011; 698: 253–278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Bailly M,, Macaluso F,, Cammer M,, Chan A,, Segall JE,, Condeelis JS. Relationship between Arp2/3 complex and the barbed ends of actin filaments at the leading edge of carcinoma cells after epidermal growth factor stimulation. J Cell Biol. 1999; 145: 331–345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Schmittgen TD,, Livak KJ. Analyzing real-time PCR data by the comparative C(T) method. Nat Protoc. 2008; 3: 1101–1108. [DOI] [PubMed] [Google Scholar]
- 23. Smith TJ,, Hegedus L,, Douglas RS. Role of insulin-like growth factor-1 (IGF-1) pathway in the pathogenesis of Graves' orbitopathy. Best Pract Res Clin Endocrinol Metab. 2012; 26: 291–302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Naik VM,, Naik MN,, Goldberg RA,, Smith TJ,, Douglas RS. Immunopathogenesis of thyroid eye disease: emerging paradigms. Surv Ophthalmol. 2010; 55: 215–226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Pritchard J,, Han R,, Horst N,, Cruikshank WW,, Smith TJ. Immunoglobulin activation of T cell chemoattractant expression in fibroblasts from patients with Graves' disease is mediated through the insulin-like growth factor I receptor pathway. J Immunol. 2003; 170: 6348–6354. [DOI] [PubMed] [Google Scholar]
- 26. Jeon ES,, Moon HJ,, Lee MJ,, et al. Sphingosylphosphorylcholine induces differentiation of human mesenchymal stem cells into smooth-muscle-like cells through a TGF-beta-dependent mechanism. J Cell Sci. 2006; 119: 4994–5005. [DOI] [PubMed] [Google Scholar]
- 27. Kinner B,, Zaleskas JM,, Spector M. Regulation of smooth muscle actin expression and contraction in adult human mesenchymal stem cells. Exp Cell Res. 2002; 278: 72–83. [DOI] [PubMed] [Google Scholar]
- 28. Wester S. Orbital Stem Cells. Curr Ophthalmol Rep. 2014; 2: 107–115. [Google Scholar]
- 29. Blasi A,, Martino C,, Balducci L,, et al. Dermal fibroblasts display similar phenotypic and differentiation capacity to fat-derived mesenchymal stem cells, but differ in anti-inflammatory and angiogenic potential. Vasc Cell. 2011; 3: 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. De Luca A,, Verardi R,, Neva A,, et al. Comparative analysis of mesenchymal stromal cells biological properties. ISRN Stem Cells. 2013; 2013: 9. [Google Scholar]
- 31. Lorenz K,, Sicker M,, Schmelzer E,, et al. Multilineage differentiation potential of human dermal skin-derived fibroblasts. Exp Dermatol. 2008; 17: 925–932. [DOI] [PubMed] [Google Scholar]
- 32. Sabatini F,, Petecchia L,, Tavian M,, Jodon de Villeroche V,, Rossi GA,, Brouty-Boye D. Human bronchial fibroblasts exhibit a mesenchymal stem cell phenotype and multilineage differentiating potentialities. Lab Invest. 2005; 85: 962–971. [DOI] [PubMed] [Google Scholar]
- 33. Hematti P. Mesenchymal stromal cells and fibroblasts: a case of mistaken identity? Cytotherapy. 2012; 14: 516–521. [DOI] [PubMed] [Google Scholar]
- 34. Wagner W,, Wein F,, Seckinger A,, et al. Comparative characteristics of mesenchymal stem cells from human bone marrow, adipose tissue, and umbilical cord blood. Exp Hematol. 2005; 33: 1402–1416. [DOI] [PubMed] [Google Scholar]
- 35. Lin CS,, Ning H,, Lin G,, Lue TF. Is CD34 truly a negative marker for mesenchymal stromal cells? Cytotherapy. 2012; 14: 1159–1163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Diaz-Flores L,, Gutierrez R,, Garcia MP,, et al. CD34+ stromal cells/fibroblasts/fibrocytes/telocytes as a tissue reserve and a principal source of mesenchymal cells. Location, morphology, function and role in pathology. Histol Histopathol. 2014; 29: 831–870. [DOI] [PubMed] [Google Scholar]
- 37. Sidney LE,, Branch MJ,, Dunphy SE,, Dua HS,, Hopkinson A. Concise review: evidence for CD34 as a common marker for diverse progenitors. Stem Cells. 2014; 32: 1380–1389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Keeley EC,, Mehrad B,, Strieter RM. The role of fibrocytes in fibrotic diseases of the lungs and heart. Fibrogenesis Tissue Repair. 2011. ; 4: 2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Song W,, Lu HX,, Kawazoe N,, Chen GP. Gradient patterning and differentiation of mesenchymal stem cells on micropatterned polymer surface. J Bioact Compat Pol. 2011; 26: 242–256. [Google Scholar]
- 40. Kim K,, Dean D,, Mikos AG,, Fisher JP. Effect of initial cell seeding density on early osteogenic signal expression of rat bone marrow stromal cells cultured on cross-linked poly(propylene fumarate) disks. Biomacromolecules. 2009; 10: 1810–1817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Haas AR,, Tuan RS. Chondrogenic differentiation of murine C3H10T1/2 multipotential mesenchymal cells: II. Stimulation by bone morphogenetic protein-2 requires modulation of N-cadherin expression and function. Differentiation. 1999; 64: 77–89. [DOI] [PubMed] [Google Scholar]
- 42. Tsonis PA,, Goetinck PF. Cell-density dependent effect of a tumor promoter on proliferation and chondrogenesis of limb bud mesenchymal cells. Exp Cell Res. 1990; 190: 247–253. [DOI] [PubMed] [Google Scholar]
- 43. Zhou X,, Tan FK,, Guo X,, Arnett FC. Attenuation of collagen production with small interfering RNA of SPARC in cultured fibroblasts from the skin of patients with scleroderma. Arthritis Rheum. 2006; 54: 2626–2531. [DOI] [PubMed] [Google Scholar]
- 44. Wang JC,, Lai S,, Guo X,, et al. Attenuation of fibrosis in vitro and in vivo with SPARC siRNA. Arthritis Res Ther. 2010; 12: R60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Beier JP,, Bitto FF,, Lange C,, et al. Myogenic differentiation of mesenchymal stem cells co-cultured with primary myoblasts. Cell Biol Int. 2011; 35: 397–406. [DOI] [PubMed] [Google Scholar]
- 46. Gang EJ,, Jeong JA,, Hong SH,, et al. Skeletal myogenic differentiation of mesenchymal stem cells isolated from human umbilical cord blood. Stem Cells. 2004; 22: 617–624. [DOI] [PubMed] [Google Scholar]
- 47. Drost AC,, Weng S,, Feil G,, et al. In vitro myogenic differentiation of human bone marrow-derived mesenchymal stem cells as a potential treatment for urethral sphincter muscle repair. Ann N Y Acad Sci. 2009; 1176: 135–143. [DOI] [PubMed] [Google Scholar]
- 48. Jeon ES,, Park WS,, Lee MJ,, Kim YM,, Han J,, Kim JHA. Rho kinase/myocardin-related transcription factor-A-dependent mechanism underlies the sphingosylphosphorylcholine-induced differentiation of mesenchymal stem cells into contractile smooth muscle cells. Circulation research. 2008; 103: 635–642. [DOI] [PubMed] [Google Scholar]
- 49. Franco Lambert AP, Fraga Zandonai A, Bonatto D, Cantarelli Machado D, Pegas Henriques JA. Differentiation of human adipose-derived adult stem cells into neuronal tissue: does it work? Differentiation. 2009; 77: 221–228. [DOI] [PubMed] [Google Scholar]
- 50. Berry DC,, Stenesen D,, Zeve D,, Graff JM. The developmental origins of adipose tissue. Development. 2013; 140: 3939–3949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Billon N,, Iannarelli P,, Monteiro MC,, et al. The generation of adipocytes by the neural crest. Development. 2007; 134: 2283–2292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Singer NG,, Caplan AI. Mesenchymal stem cells: mechanisms of inflammation. Annu Rev Pathol. 2011. ; 6: 457–478. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.