Abstract
Demineralized lesions in tooth enamel around orthodontic brackets are caused by acids from cariogenic biofilm. This study aimed to develop a novel antibacterial orthodontic cement by incorporating a quaternary ammonium monomer dimethylaminododecyl methacrylate (DMADDM) into a commercial orthodontic cement, and to investigate the effects on microcosm biofilm response and enamel bond strength. DMADDM, a recently-synthetized antibacterial monomer, was incorporated into orthodontic cement at 0%, 1.5%, 3% and 5% mass fractions. Bond strength of brackets to enamel was measured. A microcosm biofilm model was used to measure metabolic activity, lactic acid production, and colony-forming units (CFU) on orthodontic cements. Shear bond strength was not reduced at 3% DAMDDM (p > 0.1), but was slightly reduced at 5% DMADDM, compared to 0% DMADDM. Biofilm viability was substantially inhibited when in contact with orthodontic cement containing 3% DMADDM. Biofilm metabolic activity, lactic acid production, and CFU were much lower on orthodontic cement containing DMADDM than control cement (p < 0.05). Therefore, the novel antibacterial orthodontic cement containing 3% DMADDM inhibited oral biofilms without compromising the enamel bond strength, and is promising to reduce or eliminate demineralization in enamel around orthodontic brackets.
Keywords: Antibacterial, microcosm biofilm, quaternary ammonium methacrylate, bond strength, lactic acid, metabolic activity
Introduction
The higher incidence of demineralized enamel areas around orthodontic brackets (white spot lesions) has been reported as a prevailing and challenging problem in fixed orthodontic therapy (Julien et al. 2013). This widespread situation is alarming and affects more than 60% of patients, counteracting the efforts for caries prevention under orthodontic treatment (Enaia et al. 2011). Furthermore, special attention is urgently needed in the use of orthodontic appliances for young patients (pre-adolescents) with a high risk for the development of incipient caries (Chapman et al. 2010).
The development of initial caries lesions in a relatively short period of time is attributed to prolonged accumulation and retention of bacterial plaques on the enamel surfaces adjacent to the orthodontic appliances which are difficult to be cleaned in tooth brushing (Ahn et al. 2010). Brackets, metal ligature ties, arch wires, and elastomeric rings lead to increases in biofilm accumulation and elevated levels of cariogenic bacteria (Papaioannou et al. 2007), which trigger the enamel demineralization process. White spot lesions can develop quite rapidly in about four weeks, and cavitated caries lesion can occur under continuous net mineral loss. The average orthodontic treatment time in adults and adolescents are usually 2.5 years, which is long enough to cause serious lesions. The development of caries lesions complicates the orthodontic treatment, and it illustrates the great need for oral biofilm control during orthodontic treatments. Moreover, biofilm degradation on resin-based orthodontic adhesives may contribute to premature de-bonding of the brackets due to the decomposition of the resin matrix by acid from bacteria.
Preventive approaches to avoid the increased caries risk for patients with fixed orthodontic treatment involve fluoride therapy via fluoridated over-the-counter products (such as dentifrices and oral rinses) or the professional topical fluoride applications (Derks et al. 2007). However, patient compliance is a limiting factor to achieving a successful outcome. Other strategies that do not depend on patient compliance could be more effective for preventing early demineralization. For example, fluoride-releasing materials, such as glass ionomer cements and fluoride-releasing composite materials can be used as orthodontic bonding agents (Rogers et al. 2010). However, it was noticed that a major drawback of glass ionomer materials was its low bond strength to dental substrate, resulting in high rates of de-bonding and bracket failures (Chin et al. 2009).
A promising alternative is the development of orthodontic bonding agents with antibacterial properties or microbial-repellent actions (Eliades 2006). Orthodontic bonding agents are in direct contact with the vulnerable enamel surface and their properties with respect to bacterial adhesion may play a key role on prevention of the starting process of net mineral loss. Preceding reports have suggested the incorporation of antibacterial agents such as nanosilver and nanohydroxyapatite into orthodontic cements to combat cariogenic biofilm (Ahn et al. 2009; Akhavan et al. 2013).
In previous studies, quaternary ammonium monomers successfully promoted antibacterial property in dental primers, including the maintenance of antibacterial effect after being photo-cured (Imazato et al. 1998). In recent investigations, novel polymers containing quaternary ammonium salts with different features have been synthesized with antibacterial activities (Xu et al. 2012; Zhou et al. 2013). Quaternary ammonium monomers (QAMs) present the advantage to be able to copolymerize with the dental resin matrix (Imazato 2003; Antonucci et al. 2012). This strategy forms antibacterial dental resin-based materials that can effectively reduce oral biofilm formation. In this approach, the antibacterial agent is immobilized in the resin and not released or lost over time, thus promoting a durable antibacterial capability to the dental material (Imazato et al. 2013). Recently, a new quaternary ammonium monomer, dimethylaminododecyl methacrylate (DMADDM) was synthesized (Cheng et al. 2013). Pilot studies have highlighted the promising results of substantial reductions in biofilm formation over dental materials containing DMADDM. However, there has been no report of developing antibacterial orthodontic bracket cement containing DMADDM. In addition, efforts in producing antibacterial orthodontic cement should not cause adverse effects on the mechanical properties such as the enamel bond strength.
Therefore, the objectives of this study were to develop a novel antibacterial orthodontic bracket cement containing DMADDM, and to investigate the effects of DMADDM mass fraction in orthodontic bracket cement on enamel bond strength and dental plaque microcosm biofilms for the first time. The following hypotheses were tested: (1) DMADDM could be incorporated into a resin-based orthodontic bracket cement without decreasing the enamel bond strength; (2) DMADDM-containing orthodontic adhesive would greatly reduce dental plaque microcosm biofilm growth, metabolic activity, and lactic acid production; and (3) the antibacterial potency is proportional to DMADDM mass fraction in the orthodontic cement.
Materials and methods
Synthesis of dimethylaminododecyl methacrylate (DMADDM)
DMADDM has an alkyl chain length of 12 (Fig. 1A) and was recently synthesized via a modified Menschutkin reaction, in which a tertiary amine group was reacted with an organohalide as described previously (Cheng et al. 2013). A benefit of this reaction is that the reaction products are generated at virtually quantitative amounts and require minimal purification. Briefly, a 20 mL scintillation vial was added with 10 mmol of 1-(dimethylamino)docecane (DMAD, Tokyo Chemical Industry, Tokyo, Japan), 10 mmol of 2-bromoethyl methacrylate (BEMA, Monomer-Polymer and Dajac Labs, Trevose, PA), and 3 g of ethanol. A magnetic stir bar was added, and the vial was capped and stirred at 70 °C for 24 h. After the reaction was complete, the solvent was removed via evaporation. This yielded DMADDM as a clear, viscous liquid (Cheng et al. 2013). The reaction and products were verified via Fourier transform infrared spectroscopy (FTIR) in a previous study (Zhou et al. 2013).
Figure 1.
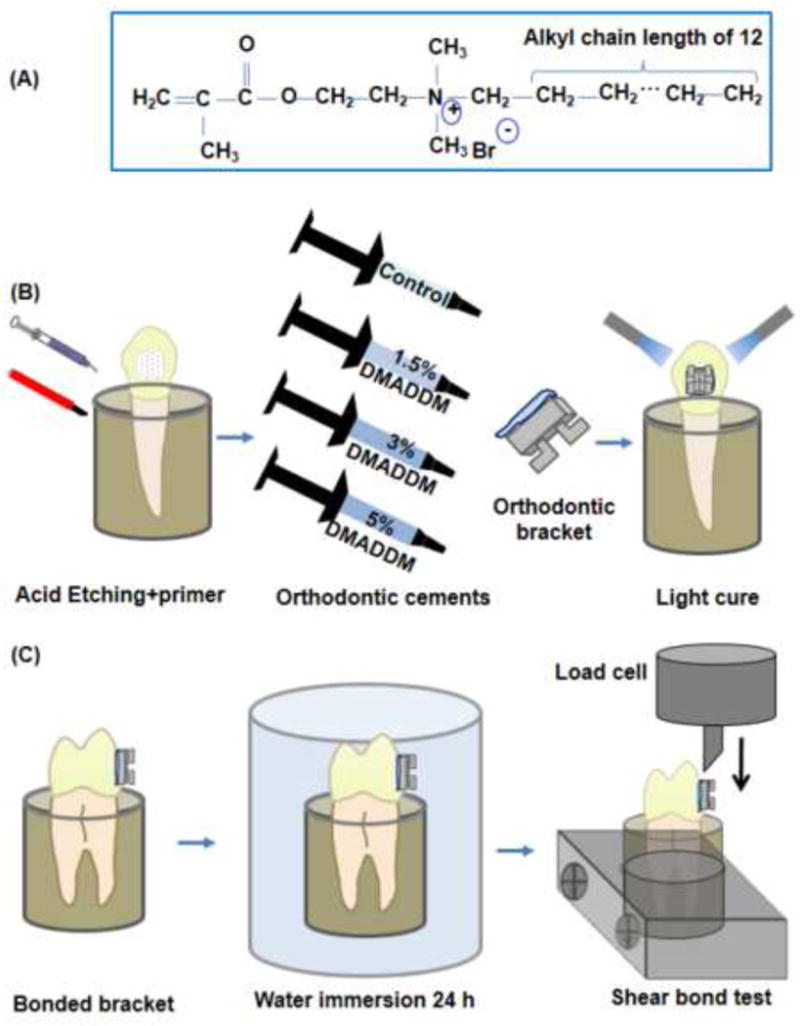
Schematic of QAM and orthodontic bonding. (A) Structure of dimethylaminododecyl methacrylate monomer (DMADDM); (B) Schematic of specimen preparation for shear bond strength test and bonding procedures for placement of brackets; (C) Schematic of bond strength testing.
Preparation of antibacterial orthodontic bracket cement
DMADDM was incorporated into a commercial orthodontic cement. Transbond XT (3M Unitek, Monrovia, CA) consisted of silane treated quartz (70–80 % by weight), bisphenol-A-diglycidyl ether dimethacrylate (10–20%), bisphenol-A-bis (2-hydroxyethyl) dimethacrylate (5–10%), silane-treated silica (< 2%) and diphenyliodonium hexafluorophosphate (< 0.2%), according the manufacturer. For enamel shear bond testing, DMADDM was mixed with the orthodontic cement at the following DMADDM/(DMADDM + orthodontic adhesive) mass fractions: 0%, 1.5%, 3% and 5%. They were selected following favorable results of preliminary tests. Therefore, four orthodontic cements were tested for shear bond strength:
-
(1)
Transbond XT control,
-
(2)
Transbond XT + 1.5% DMADDM (termed “1.5%DMADDM”),
-
(3)
Transbond XT + 3% DMADDM (termed “3%DMADDM”),
-
(4)
Transbond XT + 5% DMADDM (termed “5%DMADDM”).
The enamel shear bond strength results showed that groups 2 and 3 had similar shear bond strengths to the control, but the 5%DMADDM group had a slightly lower shear bond strength than the control. Therefore, for bacterial experiments, only groups 1–3 were tested.
For bacterial tests, disk specimens (n = 6) were fabricated using molds with a diameter of approximately 9 mm and a thickness of 2 mm, following a previous study (Melo et al. 2013). The specimens were photo-cured (Triad 2000, Dentsply, York, PA) for 1 min on each side. The disks were immersed in sterile water and agitated for 1 h to remove any uncured monomer, following a previous study (Imazato 1998). Then, the disks were sterilized via an ethylene oxide sterilizer (Anprolene AN 74i, Andersen, Haw River, NC) and de-gassed for 7 d following the manufacturer’s instructions.
Orthodontic bracket shear bond testing and the Adhesive Remnant Index (ARI)
Thirty extracted human maxillary first premolars were randomly allocated into 3 groups, n = 10. Each tooth was embedded vertically in a self-curing acrylic resin (Lang Dental Manufacturing, Wheeling, IL) taking into account the buccal axis of the clinical crown, so that that their labial surface would be parallel to the force during the shear bond test. The coronal portion was submitted to prophylaxis with oil-free pumice and rubber cups at a low speed for 10 s. Samples were washed and dried for 15 s. The teeth were etched with phosphoric acid at 35% (Scotchbond, 3M ESPE, St. Paul, MN) for 20 s, then washed and dried. Conventional brackets 0.022-inch stainless standard edgewise system (Ormco, Glendora, CA) were used and the average base surface areas of the brackets were calculated by measurements with a digital caliper (Mitutoyo, Miyazaki, Japan) (Ahn et al. 2009). Transbond XT primer was applied to the etched surfaces in a thin, uniform coat. Each orthodontic adhesive was applied to the bracket base and bonded in the most central area of the middle third of the anatomical crowns. After removing the excess of adhesive, both sides (mesial and distal) of the bracket were polymerized for 10 s using a light-curing unit (Optilux VCL 401, Demetron Kerr, Danbury, CT). After the bonding procedures, the tooth-bracket sets were incubated in distilled water at 37 °C for 24 h prior to mechanical testing (Akhavan et al. 2011).
Shear bond testing was conducted as shown schematically in Figs. 1B and 1C. The chisel was connected with a computer-controlled Universal Testing Machine (MTS, Eden Prairie, MN) and the chisel tip was positioned on the upper part of the bracket base. An occlusogingival load (1 kN; speed = 0.5 mm/min) was applied to the bracket, producing a shear load at the bracket-tooth interface until the bond failed. The shear bond strength = debonding force/bracket surface area.
After testing, each enamel surface was observed under a stereomicroscope (Leica Zoom 2000 – Leica Microsystems GmbH – Wetzlar, Germany) to examine the mode of bond failure. A magnification of 16× was used. Fracture surfaces were rated in accordance to the Adhesive Remnant Index (ARI) scores developed by Årtun and Berglund in 1984 and described in previous studies (Tuncer at al. 2009; Uysal et al. 2011). These scores quantified the remaining cement material on the enamel to assess the area where fracture had occurred during the shear bond strength test. The following scores were used: 0 = no amount of cement remaining in the enamel; 1 = less than half of the cement remaining in the enamel; 2 = more than half of cement remaining in the enamel; 3 = all the cement remaining in the enamel.
Oral microcosm biofilm assay
Whole, stimulated saliva was obtained from a healthy adult donor having natural dentition without active caries or periopathology, and without the use of antibiotics within the last 3 months, following a previous study (Cheng et al. 2012). The donor was instructed to not brushing for 24 h, and without eating/drinking for at least 2 h, prior to donating saliva. The saliva samples, collected in sterile 50-mL polypropylene tubes, were chilled in an ice bath. The saliva was mixed to sterile glycerol at a ratio of 7:3 in volume. The saliva-glycerol stock was added, with 1:50 final dilution, to a growth medium as inoculum. The growth medium contained mucin (type II, porcine, gastric) at a concentration of 2.5 g/L; bacteriological peptone, 2.0 g/L; tryptone, 2.0 g/L; yeast extract, 1.0 g/L; NaCl, 0.35 g/L, KCl, 0.2 g/L; CaCl2, 0.2 g/L; cysteine hydrochloride, 0.1 g/L; haemin, 0.001 g/L; vitamin K1, 0.0002 g/L, at pH 7 (Mc Bain. 2009). The inoculum was cultured at 37 °C in an incubator containing 5% CO2 for 24 h. Each disk specimen was placed into a well of 24-well plates. 1.5 mL of inoculum was added to each well, and incubated in 5% CO2 at 37 °C for 8 h. The disks were then transferred to new 24-well plates with fresh medium and incubated. After 16 h, the disks were transferred to new 24-well plates with fresh medium and incubated for 24 h. This totaled 2 days of culture which was shown to form mature biofilms on resins in previous studies (Cheng et al. 2012; Melo et al. 2013).
MTT assay of metabolic activity
The MTT (3-[4,5-Dimethylthiazol-2-yl]-2,5-diphenyltetrazolium bromide) assay is a colorimetric assay that measures the enzymatic reduction of MTT, a yellow tetrazole, to formazan. Each disk with the 2-d biofilm was transferred to a new 24-well plate, then 1 mL of MTT dye (0.5 mg/mL MTT in PBS) was added to each well and incubated at 37 °C in 5% CO2 for 1 h. During this process, metabolically active bacteria reduced the MTT to purple formazan. After 1 h, the disks were transferred to a new 24-well plate, 1 mL of dimethyl sulfoxide (DMSO) was added to solubilize the formazan crystals, and the plate was incubated for 20 min with gentle mixing at room temperature in the dark (Cheng et al. 2012) After mixing via pipetting, 200 μL of the DMSO solution from each well was transferred to a 96-well plate, and the absorbance at 540 nm (optical density OD540) was measured via a microplate reader (SpectraMax M5, Molecular Devices, Sunnyvale, CA). A higher absorbance is related to a higher formazan concentration, which indicates a higher metabolic activity in the biofilm on the disk (Zhang et al. 2013).
Lactic acid production and colony-forming unit (CFU) counts
Disks with 2-d biofilms were rinsed with cysteine peptone water (CPW) to remove loose bacteria, and then transferred to 24-well plates containing 1.5 mL buffered-peptone water (BPW) plus 0.2% sucrose. The samples were incubated in 5% CO2 at 37 °C for 3 h to allow the biofilms to produce acid. Lactate concentrations in the BPW solutions were determined using an enzymatic (lactate dehydrogenase) method, following a previous study (Cheng et al. 2012; Zhang et al. 2013). The microplate reader was used to measure the absorbance at 340 nm (optical density OD340) for the collected BPW solutions. Standard curves were prepared using a lactic acid standard (Supelco, Bellefonte, PA).
Disks with biofilms were transferred into tubes with 2 mL CPW, and the biofilms were harvested by sonication (Fisher, Pittsburgh, PA). Serial dilutions (10−2 to 10−5) of sonified suspension were performed and aliquots were plated in three types of agar medium. First, tryptic soy blood agar culture plates were used to determine total microorganisms (Cheng et al. 2013). Second, mitis salivarius agar (MSA) culture plates, containing 15% sucrose, were used to determine total streptococci. Third, MSB agar culture plates containing bacitracin (0.2U/ml) was used to determine mutans streptococci. Bacitracin is an antibiotic that inhibits the growth of oral streptococci, except for mutans group streptococci, which makes it a coadjutant in the selective characteristic of bacitracin-containing media (Saraiva et al. 2013).
Live/dead staining of biofilms
The biofilms on the disks were gently washed three times with phosphate buffered saline (PBS), and then stained using a live/dead bacterial viability kit (Molecular Probes, Eugene, OR). Live bacteria were stained with Syto 9 to produce a green fluorescence, and bacteria with compromised membranes were stained with propidium iodide to produce a red fluorescence. The stained disks were examined using an epifluorescence microscope (TE2000-S, Nikon, Melville, NY) (Cheng et al. 2013).
Statistical Analysis
Statistical evaluations were performed with SigmaStat 3.5 (Systat, San Jose, CA). The normality and homogeneity were checked for each variable. Shear bond strengths and quantitative microbiological data were submitted to analysis of variance (ANOVA) and the Tukey test. ARI scores of 0 and 1 were combined (less than 50% adhesive left on the tooth surface) as were 2 and 3 (50% or more of the adhesive left on tooth surface) for the chi-square analysis. ANOVA and Tukey’s tests were performed at a significance level of p < 0.05.
Results
Fig. 2 shows the enamel shear bond strength results for the orthodontic cements (mean ± sd; n = 10). In (A), all groups had statistically similar shear bond strengths (p = 0.09), indicating that adding DMADDM to orthodontic adhesive up to 3% did not significantly compromise the bracket-enamel bond strength. In (B), a comparison of the ARI scores revealed no significant differences among all materials tested (p = 0.81). These results suggest that the new orthodontic adhesive containing 3% DMADDM did not cause significant changes to the bonding process. Using the antibacterial material, failure occurred frequently at the bracket-adhesive interface, similar to control group.
Figure 2.
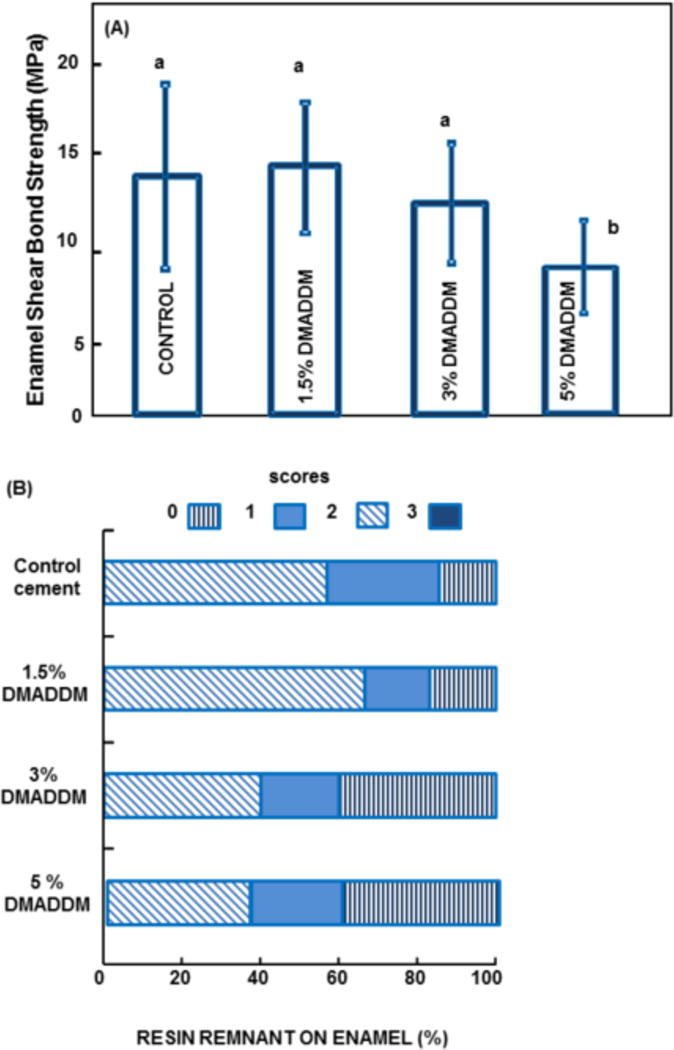
Orthodontic cement-enamel bond testing. (A) Mean and standard deviation for bond strength data (mean ± sd; n = 10). Similar letters indicate no statically significant difference (p=0.093). (B) Adhesive Remnant Index for the debonded specimens according to the following scales: 0 = no adhesive left on the tooth surface, the failure site was between the adhesive and enamel; 1 = less than half of the adhesive was left on the tooth surface; 2 = half or more of the adhesive was left on the tooth; 3 = all of the adhesive was left on the tooth surface, the failure site was between the adhesive and bracket base.
Fig. 3 shows results from microbiological experiments: (A) Schematic of biofilm growth on orthodontic adhesive, and (B) MTT metabolic activity and (C) lactic acid production of the dental plaque microcosm biofilms on orthodontic adhesive (mean ± sd; n = 6). In (B), Biofilms on the commercial orthodontic adhesive had a high metabolic activity. Incorporation of DMADDM at 1.5% reduced the metabolic activity (p < 0.05). Increasing the DMADDM mass fraction to 3% resulted in a much lower metabolic activity, which was 80% lower than control (p < 0.05). In (C), biofilms on control had the most acid production. Adding DMADDM decreased the acid production (p < 0.05). Incorporation of DMADDM at 3% yielded less acid than that at 1.5% (p < 0.05).
Figure 3.
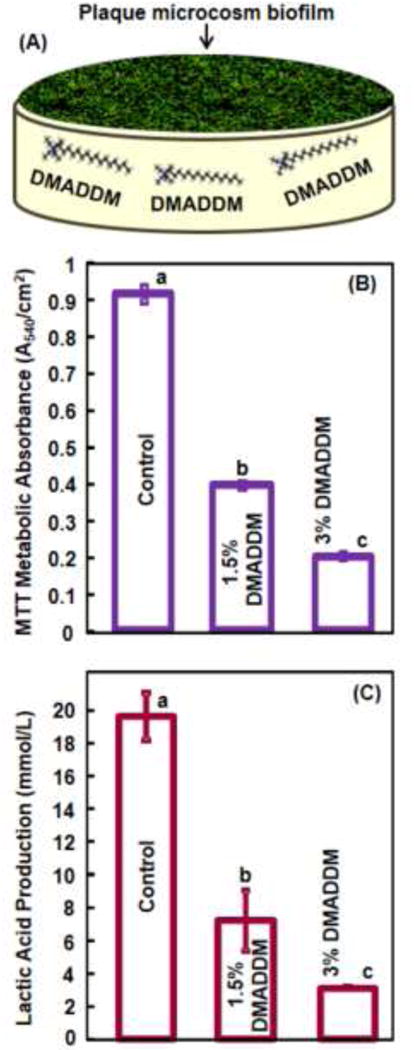
Results of biofilm experiments: (A) Schematic of biofilm on an orthodontic cement specimen; (B) MTT assay on metabolic activity of biofilms on specimens of tested groups, and (C) lactic acid production by biofilms (mean ± sd; n = 6). Different letters indicate statically significant differences (p < 0.05).
Fig. 4 plots dental plaque microcosm biofilm CFU counts for: (A) mutans streptococci, (B) total streptococci, and (C) total microorganisms (mean ± sd; n = 6). Adding DMADDM decreased the microorganism CFU of the biofilm, compared to control (p < 0.01). Increasing the DMADDM concentration to 3% decreased the mutans streptococci viability by two orders of magnitude, compared to the unmodified commercial orthodontic adhesive (p < 0.05).
Figure 4.
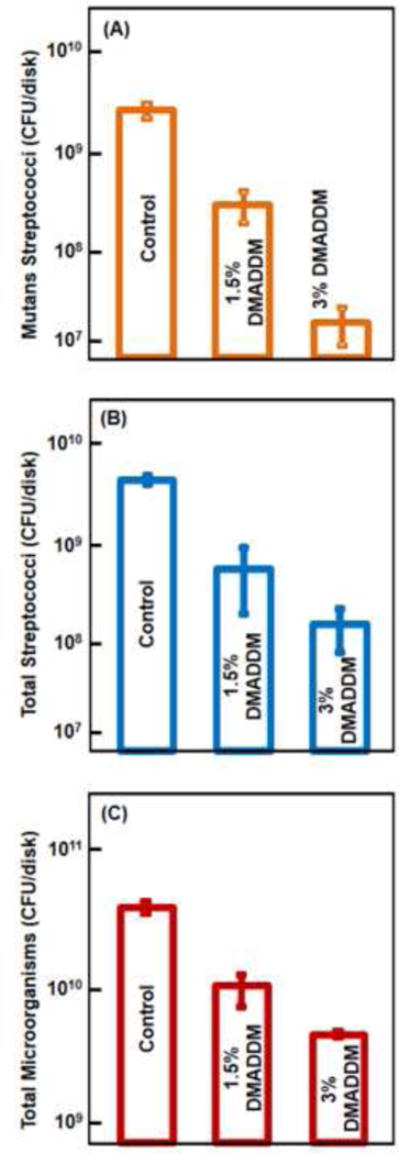
Microcosm biofilm CFU on orthodontic cement specimens: (A) mutans streptococci; (B) total streptococci, and (C) total microorganisms (mean ± sd; n = 6). In each plot, values with dissimilar letters are significantly different (p < 0.05).
Fig. 5 shows the live/dead staining images of the biofilms. Live bacteria were stained green, and dead bacteria were stained red. Live/dead bacteria that were close to, or on the top of, each other produced yellow and orange colors. In (A): Orthodontic adhesive control was covered with a thick layer of primarily live bacteria. In contrast, adhesives containing DMADDM at 1.5% had noticeably more compromised bacteria. Specimens with adhesive containing 3% DMADDM had mostly compromised bacteria. These qualitative observations visualized and supported the MTT, lactic acid and CFU results.
Figure 5.
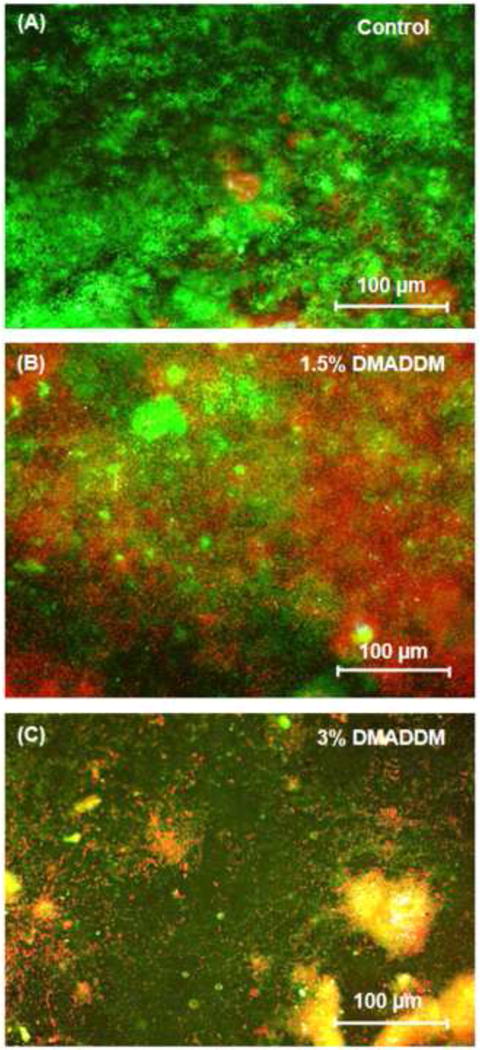
Live/dead images of dental plaque microcosm biofilms. Live bacteria were stained green, and dead bacteria were stained red. Live and dead bacteria in the proximity of each other produced yellow/orange colors.
Discussion
Cariogenic biofilms play a pivotal role in dental caries and they are inherently difficult to eradicate. Mechanical disruption by brushing is the main method for prevention of white spot lesions in contemporary orthodontic treatments. In spite of the persistent attempt in this field, the consensus view is that patients generally are unable to brush well enough to remove plaque around multi-bracket appliances. In addition, the adhesion of cariogenic streptococci was showed to be significantly higher for the orthodontic cements than for the bracket materials (Lim et al. 2008). Therefore, an antibacterial approach in the development of new orthodontic cements could benefit in the inhibition of biofilms and plaques.
The present study developed antibacterial orthodontic adhesive via the incorporation of a new quaternary ammonium monomer DMADDM and investigated its effects on bracket bond strength and microcosm biofilm response for the first time. Quaternary ammonium methacrylates can covalently copolymerize with the resin matrix (Imazato et al. 2013). Hence the antibacterial agent is immobilized in the resin and not lost over time, thus providing a durable antibacterial capability. This is a relevant property for orthodontic cements due to the relatively long duration (such as 2 years) of orthodontic treatment. A previous study investigated a DMADDM-containing dental adhesive and showed a potent anti-biofilm activity that did not decrease after 6 months of water-aging, compared to 1 d (Zhang et al. 2012). The long-term evaluation of the orthodontic cement containing DMADDM needs to be performed in a further study.
It is generally accepted that the mechanism of the bactericidal action of quaternary ammonium involves destructive electrostatic interactions with the bacterial cell wall and cytoplasmic membranes (Munoz-Bonilla and Fernández-García 2012). DMADDM presented a much stronger antibacterial potency than a previously-reported quaternary ammonium dimethacrylate (Zhang et al. 2012; Cheng et al. 2012). The increase in antibacterial activity can be attributed to the long antibacterial alkyl chain with 12 carbons for DMADDM, which increased the lipophilicity (Kenawy et al. 2007). Macromolecules may interact more effectively with the cell of Gram-positive bacteria as their polyglycan outer layer is sufficiently loosely packed to facilitate deep penetration of the polymer chain inside the cell to interact with the cytoplasmic membrane (Munoz-Bonilla and Fernández-García 2012). Recent report showed the role of quaternary ammonium chain length, which was correlated with the bactericidal effect (Li et al. 2013).
Bacteria live in the oral environment primarily in the form of complex and polymicrobial biofilms, commonly called dental plaque. Microcosm biofilm models using human saliva are representative of the microbial consortium and complex communities that colonize the oral cavity in vivo (Van de Sande et al. 2011). Biofilm bacteria are different from planktonic bacteria. The resistance of biofilm bacteria against antimicrobials is much higher than that of planktonic bacteria, highlighting the relevance of the dental plaque microcosm biofilm model (Roberts et al. 2010). In the present study, the dental plaque microcosm bacterial viability, metabolic activity, and lactic acid were greatly reduced via DMADDM, compared with the commercial orthodontic cement control, without compromising the bracket-enamel bond strength. Therefore, the new antibacterial orthodontic adhesive could be useful as an inhibitor of biofilms and caries.
Previous studies have investigated the antimicrobial activities of DMADDM incorporated into restorative bonding agents (primer and adhesive) as well as in dental composites (Cheng et al. 2012; Melo et al. 2013). These investigations showed substantial reductions in biofilm viability, consistent with the results of the present study on orthodontic cement. In addition, a previous study showed that the potent antibacterial properties of a DMADDM-incorporating bonding agent after 6 months of water-aging were the same as those at 1 d, hence the antibacterial activity was durable (Zhang et al. 2012). Previous studies reported antibacterial orthodontic adhesives containing other antibacterial agents, such as silver nanoparticles, benzalkonium chloride (BAC), chlorhexidine, and triclosan; however, agent release and its cytotoxicity effects are main concerns (Sehgal et al. 2007; Ahn et al. 2009). These issues could potentially be avoided when using DMADDM, showing no decrease in antibacterial potency in water-aging for 6 months due to copolymerization and immobilization in the resin matrix (Zhang et al. 2012).
Development of clinically acceptable orthodontic adhesives with antimicrobial activity could be achieved only if their mechanical and bonding properties have also been considered in comparison with conventional adhesives. Antibacterial monomers designed for orthodontic applications should have clinically acceptable mechanical properties. The present study examined the bracket bond strength of the new cements to human enamel. Since maxillary premolar teeth are the most frequently extracted teeth for orthodontic purposes (Proffit et al. 1994), these teeth were selected for the shear bond test. The concentrations of DMADDM tested in this study are consistent with other studies that have incorporated this monomer in resin-based restorative materials (Cheng et al. 2013). The present study sought to determine the best concentration of DMADDM in an orthodontic cement to keep the balance between antibacterial activity and enamel bonding properties. Under the experimental conditions of the present study, the DMADDM concentration of 3% in the orthodontic cement provided a similar bond strength to that of the control. It has been reported that the recommended bond strength of a conventional stainless steel bracket should be at least 8–9 MPa to permit adequate adhesion (Tuncer et al. 2009). In the present study, the shear bond strengths ranged from 13.1 to 14.6 MPa. Other previous studies using commercial orthodontic adhesive reported similar shear bond strengths values (Pithon et al. 2007; Akhavan et al. 2013). Hence, the new antibacterial DMADDM-containing orthodontic bracket cement of the present study with 3% DMADDM appeared to yield an adequate orthodontic bond strength to enamel.
A previous study indicated that the release of the antibacterial agent may cause weakening of the mechanical properties of the material (Daratsianos et al. 2013). Hence, the non-releasing property of quaternary ammonium monomers copolymerized and covalently bonded with the resin should help maintain the bond strength for a long period of time. Further study is needed to investigate the long-term properties of the DMADDM-containing orthodontic bracket cement during immersion in water for one to two years.
In conclusion, a new antibacterial monomer DMADDM was incorporated into an orthodontic bracket cement, and the enamel bonding properties as well as dental plaque microcosm biofilm response were determined for the first time. Within the limitations of this investigation, it can be concluded that the DMADDM-containing orthodontic bracket cement possessed a strong antimicrobial activity manifested by great reductions in metabolic activity, lactic acid production and CFU of biofilms, when incorporating 3% of DMADDM. The anti-biofilm potency increased with increasing the DMADDM mass fraction; however, the enamel bond strength had a slight decrease at 5% DMADDM. DMADDM could be useful in the development of antibacterial resin-based orthodontic materials for brackets bonding, lingual retainers and bands cementation. Strategies to inhibit cariogenic biofilms in orthodontic treatments are highly promising to meet the challenge of contemporary orthodontic practice and reduce or eliminate caries lesions in orthodontic patients.
Acknowledgments
This study was supported by NIH R01 DE17974 (HX), a bridge fund from the Department of Endodontics, Prosthodontics and Operative Dentistry (HX), and a seed grant from the University of Maryland Baltimore (HX).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict Of Interest Statement
No conflict of interest.
References
- Ahn SJ, Lee SJ, Kook JK, Lim BS. Experimental antimicrobial orthodontic adhesives using nanofillers and silver nanoparticles. Dental Materials. 2009;25:206–213. doi: 10.1016/j.dental.2008.06.002. [DOI] [PubMed] [Google Scholar]
- Ahn SJ, Lim BS, Leec SJ. Surface characteristics of orthodontic adhesives and effects on streptococcal adhesion. American Journal of Orthodontics and Dentofacial Orthopedics. 2010;137:489–95. doi: 10.1016/j.ajodo.2008.05.015. [DOI] [PubMed] [Google Scholar]
- Artun J, Bergland S. Clinical trials with crystal growth conditioning as an alternative to acid-etch enamel pretreatment. American Journal of Orthodontics. 1984;85:333–40. doi: 10.1016/0002-9416(84)90190-8. [DOI] [PubMed] [Google Scholar]
- Akhavan A, Sodagar A, Motjahedzadeh F, Sodagar K. Investigating the effect of incorporating nanosilver/nanohydroxyapatite particles on the shear bond strength of orthodontic adhesives. Acta Odontologica Scaninavia. 2013;11:1–5. doi: 10.3109/00016357.2012.741699. [DOI] [PubMed] [Google Scholar]
- Antonucci JM, Zeiger DN, Tang K, Lin-Gibson S, Fowler BO, Lin NJ. Synthesis and characterization of dimethacrylates containing quaternary ammonium functionalities for dental applications. Dental Materials. 2012;28:219–228. doi: 10.1016/j.dental.2011.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chapman JA, Roberts WE, Eckert GJ, Kula KS, González-Cabezas C. Risk factors for incidence and severity of white spot lesions during treatment with fixed orthodontic appliances. American Journal of Orthodontics and Dentofacial Orthopedics. 2010;138:188–94. doi: 10.1016/j.ajodo.2008.10.019. [DOI] [PubMed] [Google Scholar]
- Cheng L, Weir MD, Zhang K, Arola DD, Zhou X, Xu HH. Dental primer and adhesive containing a new antibacterial quaternary ammonium monomer dimethylaminododecyl methacrylate. Journal of Dentistry. 2013;41:345–55. doi: 10.1016/j.jdent.2013.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng L, Weir MD, Zhang K, Wu EJ, Xu SM, Zhou X, Xu HH. Dental plaque microcosm biofilm behavior on calcium phosphate nanocomposite with quaternary ammonium. Dental Materials. 2012;28:853–62. doi: 10.1016/j.dental.2012.04.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chin MYH, Sandham A, Rumachik EN, Ruben JL, Huysmans M-CDNJM. Fluoride release and cariostatic potential of orthodontic adhesives with and without daily fluoride rinsing. American Journal of Orthodontics and Dentofacial Orthopedics. 2009;136:547–53. doi: 10.1016/j.ajodo.2007.10.053. [DOI] [PubMed] [Google Scholar]
- Daratsianos N, Musabegovic E, Reimann S, Grüner M, Jäger A, Bourauel C. The influence of cyclic shear fatigue on the bracket-adhesive-enamel complex: an in vitro study. Dental Materials. 2013;29:506–13. doi: 10.1016/j.dental.2013.01.003. [DOI] [PubMed] [Google Scholar]
- Derks A, Kuijpers-Jagtman AM, Frencken JE, Van’t Hof MA, Katsaros C. Caries preventive measures used in orthodontic practices: an evidence-based decision. American Journal of Orthodontics and Dentofacial Orthopedics. 2007;132:165–70. doi: 10.1016/j.ajodo.2005.10.028. [DOI] [PubMed] [Google Scholar]
- Eliades T. Orthodontic materials research and applications: part 1. Current status and projected future developments in bonding and adhesives. American Journal of Orthodontics and Dentofacial Orthopedics. 2006;130:445–51. doi: 10.1016/j.ajodo.2005.12.028. [DOI] [PubMed] [Google Scholar]
- Enaia M, Bock N, Ruf S. White-spot lesions during multibracket appliance treatment: A challenge for clinical excellence. American Journal of Orthodontics and Dentofacial Orthopedics. 2011;140:17–24. doi: 10.1016/j.ajodo.2010.12.016. [DOI] [PubMed] [Google Scholar]
- Imazato S, Ehara A, Torii M, Ebisu S. Antibacterial activity of dentine primer containing MDPB after curing. Journal of Dentistry. 1998;26:267–271. doi: 10.1016/s0300-5712(97)00013-4. [DOI] [PubMed] [Google Scholar]
- Imazato S. Review: antibacterial properties of resin composites and dentin bonding systems. Dental Materials. 2003;19:449–457. doi: 10.1016/s0109-5641(02)00102-1. [DOI] [PubMed] [Google Scholar]
- Imazato S, Ma S, Chen JH, Xu HH. Therapeutic polymers for dental adhesives: Loading resins with bio-active components. Dental Materials. 2013;13:148–6. doi: 10.1016/j.dental.2013.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Julien KC, Buschang PH, Campbell PM. Prevalence of white spot lesion formation during orthodontic treatment. Angle Orthodontist. 2013;83:641–7. doi: 10.2319/071712-584.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li F, Weir MD, Xu HH. Effects of Quaternary Ammonium Chain Length on Antibacterial Bonding Agents. Journal of Dental Research. 2013;92:932–8. doi: 10.1177/0022034513502053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim BS, Lee SJ, Lee JW, Ahn SJ. Quantitative analysis of adhesion of cariogenic streptococci to orthodontic raw materials. American Journal of Orthodontics and Dentofacial Orthopedics. 2008;133:882–8. doi: 10.1016/j.ajodo.2006.07.027. [DOI] [PubMed] [Google Scholar]
- Kenawy el-R, Worley SD, Broughton R. The chemistry and applications of antimicrobial polymers: a state-of-the-art review. Biomacromolecules. 2007;8:1359–84. doi: 10.1021/bm061150q. [DOI] [PubMed] [Google Scholar]
- McBain AJ. In vitro biofilm models: an overview. Advances in Applied Microbiology. 2009;69:99–132. doi: 10.1016/S0065-2164(09)69004-3. [DOI] [PubMed] [Google Scholar]
- Melo MA, Cheng L, Weir MD, Hsia RC, Rodrigues LK, Xu HH. Novel dental adhesive containing antibacterial agents and calcium phosphate nanoparticles. Journal of Biomedical Materials Research B. 2013;101:620–9. doi: 10.1002/jbm.b.32864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munoz-Bonilla A, Fernández-García M. Polymeric materials with antimicrobial activity. Progress in Polymer Science. 2012;37:281–339. [Google Scholar]
- Papaioannou W, Gizani S, Nassika M, Kontou E, Nakou M. Adhesion of Streptococcus mutans to different types of brackets. Angle Orthodontics. 2007;77:1090–5. doi: 10.2319/091706-375.1. [DOI] [PubMed] [Google Scholar]
- Pithon MM, Oliveira MV, Ruellas AC, Bolognese AM, Romano FL. Shear bond strength of orthodontic brackets to enamel under different surface treatment conditions. Journal of Applied Oral Science. 2007;15:127–30. doi: 10.1590/S1678-77572007000200010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Proffit WR. Forty-year review of extraction frequencies at a university orthodontic clinic. Angle Orthodontics. 1994;64:407–14. doi: 10.1043/0003-3219(1994)064<0407:FROEFA>2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- Roberts AP. Mullany POral biofilms: a reservoir of transferable, bacterial, antimicrobial resistance. Expert Review of Anti-infective Therapy. 2010;8:1441–50. doi: 10.1586/eri.10.106. [DOI] [PubMed] [Google Scholar]
- Rogers S, Chadwick B, Treasure E. Fluoride-containing orthodontic adhesives and decalcification in patients with fixed appliances: a systematic review. American Journal of Orthodontics and Dentofacial Orthopedics. 2010;138:390. doi: 10.1016/j.ajodo.2010.02.025. [DOI] [PubMed] [Google Scholar]
- Saravia ME, Nelson-Filho P, Silva RA, De Rossi A, Faria G, Silva LA, Emilson CG. Recovery of mutans streptococci on MSB, SB-20 and SB-20M agar media. Archives of Oral Biology. 2013;58:311–6. doi: 10.1016/j.archoralbio.2012.10.010. [DOI] [PubMed] [Google Scholar]
- Sehgal V, Shetty VS, Mogra S, Bhat G, Eipe M, Jacob S, Prabu L. Evaluation of antimicrobial and physical properties of orthodontic composite resin modified by addition of antimicrobial agents–an in-vitro study. American Journal of Orthodontics and Dentofacial Orthopedics. 2007;131:525–9. doi: 10.1016/j.ajodo.2005.06.033. [DOI] [PubMed] [Google Scholar]
- Tuncer C, Tuncer BB, Ulusoy C. Effect of fluoride-releasing light-cured resin on shear bond strength of orthodontic brackets. American Journal of Orthodontics and Dentofacial Orthopedics. 2009;135:14. doi: 10.1016/j.ajodo.2008.09.016. [DOI] [PubMed] [Google Scholar]
- Uysal T, Amasyali M, Ozcan S, Koyuturk AE, Sagdic D. Effect of antibacterial monomer-containing adhesive on enamel demineralization around orthodontic brackets: an in-vivo study. American Journal of Orthodontics and Dentofacial Orthopedics. 2011;139:650–6. doi: 10.1016/j.ajodo.2009.06.038. [DOI] [PubMed] [Google Scholar]
- Van de Sande FH, Azevedo MS, Lund RG, Huysmans MC, Cenci MS. An in vitro biofilm model for enamel demineralization and antimicrobial dose-response studies. Biofouling. 2011;27:1057–63. doi: 10.1080/08927014.2011.625473. [DOI] [PubMed] [Google Scholar]
- Xu X, Wang Y, Liao S, Wen ZT, Fan Y. Synthesis and characterization of antibacterial dental monomers and composites. Journal of Biomedical Materials Resarch B. 2012;100:1151–62. doi: 10.1002/jbm.b.32683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang K, Cheng L, Wu EJ, Weir MD, Bai Y, Xu HH. Effect of water-ageing on dentine bond strength and anti-biofilm activity of bonding agent containing new monomer dimethylaminododecyl methacrylate. Journal of Dentistry. 2013;41:504–13. doi: 10.1016/j.jdent.2013.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou C, Weir MD, Zhang K, Deng D, Cheng L, Xu HH. Synthesis of new antibacterial quaternary ammonium monomer for incorporation into CaP nanocomposite. Dental Materials. 2013;29:859–70. doi: 10.1016/j.dental.2013.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]


