Abstract
Purpose
We studied incident atrial fibrillation (AF) in the prospective community-based Multi-Ethnic Study of Atherosclerosis (MESA). Reportedly, non-Hispanic blacks (NHBs) have a lower AF burden compared with their non-Hispanic white (NHW) counterparts. Information on the epidemiology of AF in Hispanic and Asian populations is much more limited.
Methods
We excluded participants with a history of AF at enrollment. A total of 6721 MESA participants were monitored for the first AF event ascertained according to hospital discharge International Classification of Diseases, Ninth Revision, codes. Age- and sex-adjusted incidence rates (IRs) of AF were calculated per 1000 person-years of observation. IR ratios were calculated using NHWs as the reference group. Age- and sex-adjusted population attributable fractions (PAFs) of established modifiable AF risk factors were ascertained.
Results
In the MESA cohort, 47.2% was male; at baseline, 25.7% had hypertension; 12.5% had diabetes. Three hundred five incident hospitalized AF events occurred over a mean follow-up of 7.3 years. Age- and sex-adjusted IRs and IR ratios showed that overall AF incidence was significantly lower among Hispanics, NHBs and Chinese compared with NHWs (all P < .001). Among participants 65 years of age or greater, Hispanics, Chinese, and blacks had significantly lower AF incidence than NHWs (all P ≥ 01), but IRs were similar among participants under age 65 years. The PAF for smoking was 27% among NHBs but lower among other race–ethnic groups. Among NHWs, the PAF for hypertension was 22.2%, but this was higher among NHBs (33.1%), Chinese (46.3%), and Hispanics (43.9%).
Conclusions
Overall, the incidence of hospitalized AF was significantly lower in Hispanics, NHBs, and Chinese than in NHWs. A larger proportion of AF events appear to be attributable to hypertension among nonwhite populations compared with NHWs.
Keywords: Atrial fibrillation, epidemiology, Hispanics, Chinese
Atrial fibrillation (AF) is the most common presenting sustained arrhythmia in clinical practice. More than 2.3 million adults in the United States have AF, and the number is expected to more than double over the next five decades [1]. Planning and budgeting for future health care expenditure imposed by AF requires clear understanding of the epidemiology of AF in the fast-growing nonwhite races/ethnicities in the United States and Europe. Current understanding of the pathophysiology and epidemiology of AF is based primarily on studies in non-Hispanic whites (NHWs) populations with limited data on the nonwhite populations. The few previous studies that addressed the risk of AF across different races/ethnicities focused on non-Hispanic black (NHB) versus NHW populations [2–8]. These few studies have consistently reported that NHB individuals have lower AF burden compared with their NHW counterparts.
In the Cardiovascular Health Study, black race/ethnicity was associated with lower risk of incident AF (hazard ratio: 0.55; 95% confidence interval [CI]: 0.35–0.88) compared with white race/ethnicity after adjustment for all considered AF risk factors) [7]. Data from the Atherosclerosis Risk in Communities study showed that compared with white participants, black participants had a lower age- and sex-adjusted risk of being diagnosed with AF (RR = 0.59; 95% CI, 0.38–0.92) despite AF risk factors being more prevalent among the latter [3]. In the Northern Manhattan Stroke Study, NHB patients had a 50% lower prevalence of AF than white patients [5]. Significantly less AF has been noted among NHBs compared with NHWs in a population of heart failure patients [4] as well as postoperatively in a large consecutive series of patients undergoing coronary artery bypass [6].
Information on AF incidence in Hispanic and Asian populations is much more limited. Currently 52 million Hispanics reside in the United States. At 16.7% of the total US population, the number of Hispanics now exceeds that of NHBs, Asian Americans, and Native Americans. By 2050, Hispanics are expected to make up at least 25% of the US population and the aging growth trend is greater for Hispanics. That is, although the NHW population over the age of 65 years is expected to grow by 83% between 2000 and 2030, Hispanics of this same age group are projected to grow by 328%, making Hispanics the fastest growing aging population in the United States [9,10]. Further knowledge regarding AF incidence in the Hispanic population will be important from a public health perspective. Furthermore, little is known about the differential contributions of AF risk factors in Hispanics and Asians versus NHW populations.
Our aims were to (1) examine the incidence of AF stratified by racial/ethnic group; and (2) explore the population attributable fractions (PAFs) of established key modifiable AF risk factors (hypertension, diabetes, smoking, and obesity) [2,11–18] with AF incidence across the race/ethnic groups. The Multi-Ethnic Study of Atherosclerosis (MESA) is uniquely suited to address our aims given its multi-ethnic composition inclusive of Hispanic and Chinese participants for adequate statistical comparisons and its longitudinal single cohort design with prospective collection of AF data.
Methods
MESA is a population-based multicenter longitudinal cohort investigating prevalence, correlates, and progression of subclinical cardiovascular disease. The cohort includes men and women (~50% each sex) of NHW (38%), NHB (28%), Hispanic (22%), and Chinese origin (12%), aged 45 to 84 years and without known clinical cardiovascular disease at baseline. Participants were recruited from six regions in the United States. Visit 1 occurred from 2000 to 2002. Details of MESA's design and objectives have been published [19]. The institutional review boards at all participating centers approved the study, and all participants gave informed consent.
Sex, age, body mass index, systolic blood pressure, use of antihypertensive medication, smoking, and history of diabetes were collected using standardized questionnaires and examinations at baseline. Height and weight were measured to the nearest 0.1 cm and 0.5 kg, respectively. Body mass index (kilogram per square meter) was used a measure of overall obesity. Three resting blood pressure readings were measured after 5 minutes in the seated position using an automated oscillometric method (Dinamap) and appropriate cuff size. The second and third readings were averaged to obtain the blood pressure levels used in analyses. Hypertension was defined as a self-report of physician-diagnosed hypertension along with use of antihypertensive medication or systolic blood pressure of 140 mm Hg or greater and/or diastolic blood pressure of 90 mm Hg or greater. Participants were categorized as having diabetes if their fasting blood glucose was 126 mg/dL or greater or they were taking medication for diabetes. Household annual income and educational attainment were each classified into four groups (<$20,000; $20,000 to $39 999; $40,000 to $75,000; and >$75,000) and less than high school; completed high school, technical school certificate or associate degree; and completed college or more).
All MESA participants are monitored for occurrence of hospitalizations and other health outcomes during follow-up. Regular follow-up contacts are made with MESA participants every 9 to 12 months, either by telephone or at field center visits. At these contacts, MESA staff inquires about all health events, including diagnostic tests and interim hospitalizations. A report of any hospitalization triggers a request to obtain medical records. A report of a death (by a participant's next of kin) triggers a request to obtain a death certificate and an interview with family members.
All discharge diagnoses for hospitalizations are cataloged and International Classification of Diseases codes are obtained, including codes from the second facility after transfer to another hospital or health care facility. In approximately 99% of cases, when a participant reports a hospitalization, we are able to obtain records of the encounter. In addition to this MESA follow-up, MESA obtains Medicare inpatient claims data from the Centers for Medicare and Medicaid Services for MESA participants aged 65 years or older and enrolled in fee-for-service Medicare. The purpose is to ascertain all hospitalizations including those not reported by participants as an additional source of information identifying incident AF. The inpatient files included institutional claims for inpatient services covered under fee-for-service Medicare on inpatient diagnoses of AF. For this analysis, AF is considered to be present when a discharge diagnosis code of 427.31 (AF) or 427.32 (atrial flutter) is present in any position among the hospital discharge diagnosis codes obtained either through MESA surveillance or from the Medicare claims data. A total of 6814 participants were enrolled at baseline. For this analysis, 36 participants did not have follow-up information, and 57 participants were identified with prevalent AF at baseline. Thus, the total number of MESA participants at risk for newly identified incident AF events was 6721. AF incidence was ascertained according to hospital discharge International Classification of Diseases, Ninth Revision, codes through December 31, 2010.
Statistical analysis
Continuous data are presented as mean ± SD and categorical data are presented as frequencies (percentages). Continuous variables were compared using unpaired student t tests and categorical data with the χ2 test. Incidence rates (IRs) of AF were ascertained for the total cohort and by age, sex, and racial–ethnic group per 1000 person-years of observation. We computed the person-years of follow-up from the date of the baseline examination to a diagnosis of AF, death, or end of follow-up, whichever came earlier. Person-years of follow-up were allocated to race–ethnicity and age groups. IRs of AF were calculated dividing the number of new AF cases in each race–ethnic group by the corresponding person-years. IR ratios (IRRs) were derived using NHWs as the reference group. Poisson regression analysis was used to obtain age- and sex-adjusted IRs and IRRs. Sensitivity analysis was performed with separate models including age-, sex-, and education-adjusted or age-, sex-, and income-adjusted IRRs.
Race–ethnic PAFs of established AF risk factors were ascertained. The PAF (total incidence in the population – incidence in the unexposed)/total incidence in the population (containing both exposed and nonexposed individuals) [20] reflects the fraction of event rate or risk in a given period that is attributable to exposure, assuming that the risk factor is causal. We chose key modifiable risk factors for AF including hypertension, diabetes, obesity, and smoking that have been established in multiple prospective cohort studies [2,11–18]. We hypothesized that the magnitude of the PAF for each key modifiable risk factor for incident AF may be different for Hispanics, Chinese and NHBs compared with NHWs. Age- and sex-adjusted PAFs were estimated using indirect standardization with the standardized morbidity ratio (Rsm) as ρ(Rsm – 1/Rsm), where ρ denotes the proportion of exposure among events, using the SAS procedure STDRATE (SAS/STAT version 9.3). To assess for certainty in the PAF estimates, complementary logarithmic transformation was used to derive approximate two-sided 95% CIs.[21]
Results
A total of 6721 MESA participants were included in this analysis. More than 40% of the cohort was older than 65 years of age and hypertensive. Approximately half were female, one-third were obese, and over a quarter had prevalent diabetes at baseline. Obesity, current smoking, hypertension, and diabetes were more prevalent among NHBs and Hispanics compared with the other groups (Table 1). The proportion of those older than 65 years, male, with hypertension, diabetes, former alcohol users, or former smokers was higher in those with incident AF compared with those without (Supplemental Table). These trends remained similar across all the race–ethnic groups except among the Chinese, where the majority were women.
Table 1.
Baseline characteristics for the total cohort and by race–ethnic group
| All participants |
NHWs |
Chinese |
NHBs |
Hispanic |
P value* | |
|---|---|---|---|---|---|---|
| n = 6721 | n = 2580 | n = 795 | n = 1868 | n = 1478 | ||
| Mean age (SD.) | 62.0 (10.2) | 62.4 (10.2) | 62.3 (10.3) | 62.0 (10.0) | 61.2 (10.3) | .003 |
| Females (%) | 52.8 | 52.2 | 51.2 | 55.2 | 51.8 | .11 |
| BMI >30 (%) | 32.3 | 27.7 | 4.3 | 45.6 | 38.7 | <.001 |
| Mean BMI (SD) | 28.3 (5.5) | 27.7 (5.1) | 24.0 (3.3) | 30.2 (5.9) | 29.4 (5.1) | <.001 |
| Smoking | <.001 | |||||
| Never(%) | 50.3 | 44.4 | 75.2 | 45.2 | 53.8 | |
| Former | 36.6 | 44.0 | 19.1 | 36.9 | 32.6 | |
| Current | 13.1 | 11.6 | 5.7 | 17.9 | 13.6 | |
| Alcohol use | <.001 | |||||
| Never (%) | 20.4 | 9.3 | 53.7 | 17.2 | 25.9 | |
| Former (%) | 24.0 | 18.6 | 14.8 | 33.1 | 26.7 | |
| Current (%) | 55.6 | 72.1 | 31.5 | 49.7 | 47.4 | |
| Hypertension (%) | 44.8 | 38.4 | 37.4 | 59.5 | 41.5 | <.001 |
| Diabetes (%) | 26.4 | 17.2 | 29.9 | 32.4 | 33.1 | <.001 |
| Mean total cholesterol (SD) (%) | 194.2 (35.8) | 195.7 (35.2) | 192.7 (31.9) | 189.7 (36.3) | 198.0 (37.4) | <.001 |
| Hypertension medication (%) | 37.0 | 32.8 | 28.4 | 50.1 | 32.5 | <.001 |
| Mean SBP (SD) | 126.5 (21.5) | 123.3 (20.3) | 124.5 (21.6) | 131.7 (21.6) | 126.7 (21.9) | <.001 |
| Mean DBP (SD) | 71.9 (10.3) | 70.2 (10.0) | 72.0 (10.4). | 74.5 (10.2) | 71.6 (10.1) | <.001 |
| Mean glucose mg/dL (SD) | 97.3 (30.3) | 91.3 (21.5) | 98.8 (28.3) | 100.0 (32.1) | 103.6 (38.9) | <.001 |
| Mean physical activity met min/wk (SD) | 5762 (5898) | 5704 (5380) | 3768 (3875) | 6465 (6865) | 6051 (6107) | <.001 |
| Total caloric intake kcal (SD) | 1532 (805) | 1561 (726) | 1171 (571) | 1589 (905) | 1622 (875) | <.001 |
BMI = body mass index.
Comparing race–ethnic differences in demographic and clinical characteristics.
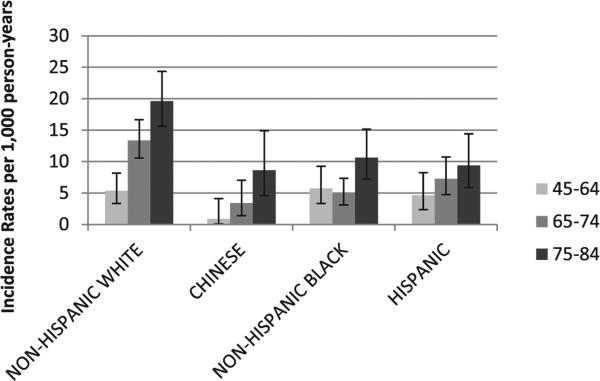
Overall, 305 incident hospitalized AF events occurred over a mean follow-up of 6.98 years. NHWs had the highest AF incidence, followed by decreasing stepwise incidence in Hispanics, NHBs, and Chinese, respectively. About a quarter of AF events were identified only by the Medicare claims data and not by MESA follow-up, and this proportion did not differ across the race–ethnic groups. The age- and sex-adjusted AF IR among NHWs was almost double that seen among Hispanics and almost four times greater than among Chinese participants (Table 2). Unadjusted AF IRs per 1000 person-years increased with increasing age (Fig. 1). Among those aged 65 to 74 and 75 to 84 years of age, higher age-specific IRs were seen among NHWs than the other race–ethnic groups, 13.4 (10.6–16.7) and 19.6 (15.6–24.3) for NHWs; 3.4 (1.4–7.0) and 8.6 (4.6–14.9) for Chinese; 4.9 (3.1–7.3) and 10.6 (7.2–15.1) for NHBs; 7.3 (4.7–10.7) and 9.4 (5.9–14.4) for Hispanics, respectively. However, among participants aged 45 to 64 years, unadjusted AF IRs were not statistically different among NHBs, Hispanics, and Chinese compared with NHWs but were somewhat lower for Chinese (Fig. 1).
Table 2.
Distribution of AF events during follow-up stratified by race and ethnicity
| Cases* | Person-years | Age- and sex-adjusted IRs (95% CIs) | |
|---|---|---|---|
| NHW | 171 | 18,562 | 11.23 (9.82-12.84) |
| Chinese | 18 | 5869 | 3.94 (2.54-6.11) |
| NHB | 64 | 13,528 | 5.77 (4.75-7.02) |
| Hispanic | 52 | 10,856 | 6.07 (4.71-7.84) |
**Per 1000 person-years
Fig. 1.
Age-specific unadjusted AF IRs per 1,000 person-years. IRs of AF for each race–ethnic group according to increasing age categories.
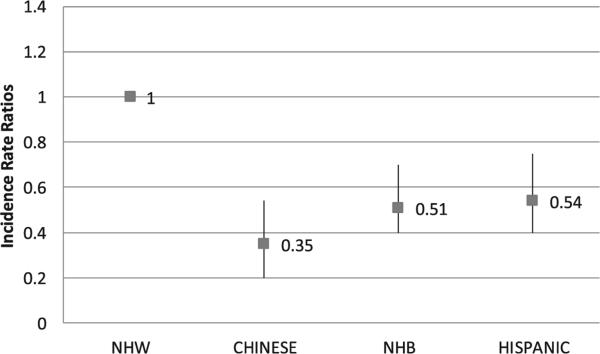
We evaluated the IRRs for AF among MESA participants using NHWs as the referent group. In age- and sex-adjusted analysis, compared with NHWs, overall Chinese had a 65% lower AF incidence and NHBs and Hispanics had a 49% and 46% lower incidence, respectively (Fig. 2). Adjusting the IRRs for income or educational attainment in separate models did not significantly alter the results (data not shown). In age-specific categories, un-adjusted IRRs for AF were significantly lower in nonwhite groups compared with NHWs for ages 65 years and older; 56% to 75% lower among Chinese, 46% to 63% lower among NHBs, and 46% to 52% lower among Hispanics (Table 3). Among participants younger than 65 years, unadjusted AF IRRs were not significantly different.
Fig. 2.
Age- and sex-adjusted AF incidence rate ratios. The incidence rate ratio provides a relative measure of AF IRs using NHWs as the reference groupdshowing that AF incidence is roughly halved among NHBs and Hispanics compared with NHWs; Chinese have about one-third the rate of incident AF events as NHWs.
Table 3.
Age-specific AF unadjusted incidence rate ratios with 95% CIs
| NHW |
Chinese |
NHB |
Hispanic |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Cases | Person-years | IRR (CI) | Cases | Person-years | IRR (CI) | Cases | Person-years | IRR (CI) | Cases | Person-years | IRR (CI) | |
| 45-64 | 19 | 3560 | Ref | * | * | 0.17 (0.02-1.24) | 15 | 2614 | 1.07 (0.55-2.11) | * | * | 0.87 (0.38-1.78) |
| 65-74 | 74 | 5545 | Ref | * | * | 0.25 (0.11 -0.59) | 21 | 4289 | 0.37 (0.23-0.61) | * | * | 0.54 (0.32-0.82) |
| 75-84 | 78 | 3977 | Ref | * | 1278 | 0.44 (0.24-0.84) | 28 | 2635 | 0.54 (0.33-0.79) | 19 | 2020 | 0.48 (0.29-0.80) |
Medicare data are not presented in these cells because of a small sample size.
We calculated age- and sex-adjusted PAFs to evaluate the relative contribution of established major AF risk factors among the different race–ethnic groups (Table 4). The PAFs for diabetes were quite small. The PAFs were highest among Hispanics for diabetes and obesity but the CIs crossed zero for all these age- and sex-adjusted estimates. The PAFs identified hypertension as the most important significant contributor to AF events; 22.2% among NHWs, and even higher in NHBs, Chinese, and Hispanics. The PAF for smoking was 27.4% among NHBs but was not significant among the other race–ethnic groups.
Table 4.
Age- and sex-adjusted PAFs and 95% CIs of established AF risk factors in each stratum of race–ethnicity
| PAFs (%) and 95% CIs |
||||
|---|---|---|---|---|
| Diabetes | Hypertension | BMI >30 kg/m2 | Current smoking | |
| Chinese | 4.9 (–26.8 to 28.9) | 46.3 (24.9 to 76.5) | 2.2 (–9.0 to 12.3) | –0.7 (–17.7 to 46.9) |
| Hispanics | 9.6 (–9.9 to 25.7) | 43.9 (20.3 to 60.5) | 17.7 (–1.4 to 33.1) | –0.9 (–21.1 to 15.8) |
| NHB | 5.0 (–11.4 to 19.0) | 33.1 (20.6 to 53.8) | –0.6 (–16.3 to 13.0) | 27.0 (5.8 to 43.5) |
| NHW | 0.2 (–6.5 to 6.4) | 22.2 (10.8 to 32.3) | 2.9 (–6.1 to 11.9) | 6.9 (–1.3 to 14.4) |
BMI = body mass index.
95% CIs (lower limit, upper limit).
Discussion
There has been an increase in the incidence and prevalence of AF, and the public health burden of AF is expected to continue to rise [22,23]. There are many potential reasons as to why AF is estimated to increase [24]. With a growing percentage of the US populace being nonwhite, we believe it is important to consider race–ethnic differences in future studies of the societal burden of AF. Our results suggest that most of the population burden of AF occurs among NHWs. The IRs for AF in the nonwhite groups were 46% to 65% lower than in NHWs. This finding was most consistent across the 65+ years age strata where Hispanics, Asians, and NHBs had a significantly lower incidence of AF compared with NHWs.
To date, we know of few studies comparing incidence of AF across NHWs versus Hispanics and Asians. Shen et al. [25] studied the records of a California health maintenance organization and found a greater prevalence of AF among NHWs compared with blacks, Hispanics, and Asians. However, that study used Asian/Hispanic surnames to identify race–ethnicity and was cross-sectional in nature. Dewald et al. [26], using a large hospital-based retrospective cohort also had similar findings; however, this was a single-center study without the generalizability of a prospective, multicenter, community-based cohort. Many studies do suggest that AF is more prevalent among NHWs compared with NHBs [3,27,28]. In the Northern Manhattan Study, AF prevalence was more common in NHWs (29%) than in either NHBs (11%) or Hispanics (11%) [5]. There have been few studies examining AF risk in an Asian population in the United States. One study of male US veterans found the age-adjusted prevalence of AF to be 3.6% and 3.0% in Asian and Hispanics, respectively, compared with 5.7% in NHWs [29]. One European study reported low prevalence of AF on a study ECG among South Asians and NHBs [30]. Most studies on the Chinese and AF are from Asia [31,32] and report a low prevalence of AF among Chinese participants (~1%). However, these studies cannot be easily compared with our US-based study because of differences in case ascertainment and the fact that we examined AF incidence, not prevalence.
Although debatable [8], several explanations have been suggested to explain differences in AF incidence among NHBs and NHWs such as under ascertainment of events because of poorer access to care among NHBs, differential mortality, or under ascertainment of events because of a higher incidence of paroxysmal AF in NHBs [27]. In a population of NHBs and NHWs, ECG indices such as P wave duration, area, and amplitude did not appear to explain the race–ethnic differences in AF prevalence [33]. However, there may be pathophysiological mechanisms that can account for the consistent finding of differences in AF incidence by race or ethnicity. Racial differences in left atrial size and left atrial remodeling contributing to the initiation and perpetuation of AF [34] and differences in atrial automaticity contributing to the proarrhythmic substrate for AF [35] require further evaluation but may eventually help explain the observed differences in our study.
Our findings demonstrate potential differences in PAF estimates among the race–ethnic groups. The most significant difference was observed with hypertension, with 24.1% and 21.7% greater PAF estimates among Chinese and Hispanics, respectively, compared with NHWs. It is interesting that among NHWs, hypertension appeared somewhat less influential on AF incidence compared with nonwhite participants. Smoking carried a significant PAF among NHBs in this cohort but not among the other race–ethnic groups. Prior studies show a link between smoking and inflammation [36] which could extend to AF. Additionally, race–ethnic differences in inflammatory markers have been shown [37], supporting the notion that the axis between smoking, inflammation, and AF in minority populations needs further study. PAF estimates of AF risk factors have been previously noted to be higher for NHBs than for NHWs [38]. However, in our study and others [7,8], the race–ethnic differential distribution of AF risk factors does not explain the lower incidence of AF among nonwhite participants. Because there appears to be a genetic predisposition among NHWs for AF [28], our results support continued investigation for unexplained AF risk factors among NHWs and/or protective factors among nonwhite populations.
There are public health implications to our results. Planning and budgeting for future health care expenditure imposed by AF requires clear understanding of the epidemiology of AF in the fast-growing nonwhite groups in the United States. Hispanics are a large and growing aging population in the United States [9], and although we expect AF prevalence in this group to increase, it is important to note that among Hispanics as a whole, similar to NHBs, the disease burden of AF is not as high as that seen in NHWs. Ethnic variability in PAFs show that hypertension is a more important contributor among nonwhites thus suggesting that disease prevention strategies should emphasize blood pressure control in these populations.
Limitations
A potential limitation of our analysis is incomplete AF ascertainment. Incident AF was based entirely on hospital discharge codes; so the true incidence of AF is underestimated because asymptomatic or minimally symptomatic arrhythmias may not require hospitalization. Such underestimation would generally result in attenuation of the association and therefore should not explain our significant findings. It is likely that some of the hospitalizations captured may be symptomatic paroxysmal AF. Upcoming studies using ambulatory electrocardiography may more accurately demonstrate the community prevalence of paroxysmal AF and asymptomatic AF and may possibly address race–ethnic differences. We adjusted for income or educational attainment in separate models, but this did not significantly alter the results. A prior study comparing race–ethnic differences in AF IRs by socioeconomic status found that within socioeconomic status strata (with presumably similar access to health care), NHBs consistently had a lower risk of AF than whites [39]. However, it is still possible that there is differential recording of AF as a discharge diagnosis by race. Genetic ancestry among Hispanics correlates with information by country of origin (e.g., Mexican, Puerto Rican, Cuban, and Dominican) [40]. However, we did not have enough power to study AF incidence by Hispanic nationality or country of origin given the limited number of AF events among Hispanics. Finally, PAF estimates are useful only if the risk factors under study are causal. The risk factors we studied are well-established, but it is difficult to ascertain causality definitively.
In conclusion, in a prospective, multi-ethnic, population-based cohort, we found a significantly lower incidence of AF among Hispanics and Chinese than in NHWs, similar to what has been previously observed for NHBs. Hypertension appears to be a somewhat more of a contributor to AF incidence among nonwhites and smoking appears to be more of a contributor among NHBs. This evidence supports race–ethnic differential AF risk in a fuller spectrum of the nonwhite US population and reinforces continued study of unexplained AF risk/protective factors. Future public health estimates of AF should be more inclusive of nonwhites. Our results also support heightened awareness and control of traditional AF risk factors, especially hypertension, among all populations.
Supplementary Material
Acknowledgments
This research was supported by contracts N01-HC-95159 through N01-HC-95169 from the National Heart, Lung, and Blood Institute and by grants UL1-RR-024156 and UL1-RR-025005 from NCRR. The authors thank the other investigators, the staff, and the participants of the MESA study for their valuable contributions. A full list of participating MESA investigators and institutions can be found at http://www.mesa-nhlbi.org.
Sources of Funding: This work was supported by a grant from the National Heart, Lung, and Blood Institute (R01 HL104199, Epidemiologic Determinants of Cardiac Structure and Function among Hispanics: C.J.R. principal investigator).
Footnotes
The authors have nothing to disclose.
References
- 1.Go AS, Hylek EM, Phillips KA, Chang Y, Henault LE, Selby JV, et al. Prevalence of diagnosed atrial fibrillation in adults: national implications for rhythm management and stroke prevention: the AnTicoagulation and Risk Factors in Atrial Fibrillation (ATRIA) Study. JAMA. 2001;285:2370–5. doi: 10.1001/jama.285.18.2370. [DOI] [PubMed] [Google Scholar]
- 2.Psaty BM, Manolio TA, Kuller LH, Kronmal RA, Cushman M, Fried LP, et al. Incidence of and risk factors for atrial fibrillation in older adults. Circulation. 1997;96:2455–61. doi: 10.1161/01.cir.96.7.2455. [DOI] [PubMed] [Google Scholar]
- 3.Alonso A, Agarwal SK, Soliman EZ, Ambrose M, Chamberlain AM, Prineas RJ, et al. Incidence of atrial fibrillation in whites and African-Americans: the Atherosclerosis Risk in Communities (ARIC) study. Am Heart J. 2009;158:111–7. doi: 10.1016/j.ahj.2009.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ruo B, Capra AM, Jensvold NG, Go AS. Racial variation in the prevalence of atrial fibrillation among patients with heart failure: the Epidemiology, Practice, Outcomes, and Costs of Heart Failure (EPOCH) study. J Am Coll Cardiol. 2004;43:429–35. doi: 10.1016/j.jacc.2003.09.035. [DOI] [PubMed] [Google Scholar]
- 5.Sacco RL, Kargman DE, Zamanillo MC. Race-ethnic differences in stroke risk factors among hospitalized patients with cerebral infarction: the Northern Manhattan Stroke Study. Neurology. 1995;45:659–63. doi: 10.1212/wnl.45.4.659. [DOI] [PubMed] [Google Scholar]
- 6.Sun X, Hill PC, Lowery R, Lindsay J, Boyce SW, Bafi AS, et al. Comparison of frequency of atrial fibrillation after coronary artery bypass grafting in African Americans versus European Americans. Am J Cardiol. 2011;108:669–72. doi: 10.1016/j.amjcard.2011.04.013. [DOI] [PubMed] [Google Scholar]
- 7.Jensen PN, Thacker EL, Dublin S, Psaty BM, Heckbert SR. Racial differences in the incidence of and risk factors for atrial fibrillation in older adults: the cardiovascular health study. J Am Geriatr Soc. 2013;61:276–80. doi: 10.1111/jgs.12085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lipworth L, Okafor H, Mumma MT, Edwards TL, Roden DM, Blot WJ, et al. Race-specific impact of atrial fibrillation risk factors in blacks and whites in the southern community cohort study. Am J Cardiol. 2012;110:1637–42. doi: 10.1016/j.amjcard.2012.07.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.US Census Bureau 65+ in the United States: 2005. Current population reports. 2005 [Google Scholar]
- 10.National Alliance for Hispanic Health . Delivering health care to Hispanics: a manual for providers. National Alliance for Hispanic Health; Washington, DC: 2003. [Google Scholar]
- 11.Benjamin EJ, Levy D, Vaziri SM, D'Agostino RB, Belanger AJ, Wolf PA. Independent risk factors for atrial fibrillation in a population-based cohort. The Framingham Heart Study. JAMA. 1994;271:840–4. [PubMed] [Google Scholar]
- 12.Heeringa J, Kors JA, Hofman A, van Rooij FJ, Witteman JC. Cigarette smoking and risk of atrial fibrillation: the Rotterdam Study. Am Heart J. 2008;156:1163–9. doi: 10.1016/j.ahj.2008.08.003. [DOI] [PubMed] [Google Scholar]
- 13.Wang TJ, Parise H, Levy D, D'Agostino Sr RB, Wolf PA, Vasan RS, et al. Obesity and the risk of new-onset atrial fibrillation. JAMA. 2004;292:2471–7. doi: 10.1001/jama.292.20.2471. [DOI] [PubMed] [Google Scholar]
- 14.Frost L, Hune LJ, Vestergaard P. Overweight and obesity as risk factors for atrial fibrillation or flutter: the Danish Diet, Cancer, and Health Study. Am J Med. 2005;118:489–95. doi: 10.1016/j.amjmed.2005.01.031. [DOI] [PubMed] [Google Scholar]
- 15.Chamberlain AM, Agarwal SK, Folsom AR, Duval S, Soliman EZ, Ambrose M, et al. Smoking and incidence of atrial fibrillation: results from the Atherosclerosis Risk in Communities (ARIC) study. Heart Rhythm. 2011;8:1160–6. doi: 10.1016/j.hrthm.2011.03.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Asghar O, Alam U, Hayat SA, Aghamohammadzadeh R, Heagerty AM, Malik RA. Obesity, diabetes and atrial fibrillation; epidemiology, mechanisms and interventions. Curr Cardiol Rev. 2012;8:253–64. doi: 10.2174/157340312803760749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Manolis AJ, Rosei EA, Coca A, Cifkova R, Erdine SE, Kjeldsen S, et al. Hypertension and atrial fibrillation: diagnostic approach, prevention and treatment. Position paper of the Working Group 'Hypertension Arrhythmias and Thrombosis' of the European Society of Hypertension. J Hypertens. 2012;30:239–52. doi: 10.1097/HJH.0b013e32834f03bf. [DOI] [PubMed] [Google Scholar]
- 18.Sun Y, Hu D. The link between diabetes and atrial fibrillation: cause or correlation? J Cardiovasc Dis Res. 2010;1:10–1. doi: 10.4103/0975-3583.59978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bild DE, Bluemke DA, Burke GL, Detrano R, Roux AVD, Folsom AR, et al. Multi-Ethnic Study of Atherosclerosis: objectives and design. Am J Epidemiol. 2002;156:871–81. doi: 10.1093/aje/kwf113. [DOI] [PubMed] [Google Scholar]
- 20.Rockhill B, Newman B, Weinberg C. Use and misuse of population attributable fractions. Am J Public Health. 1998;88:15–9. doi: 10.2105/ajph.88.1.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Laaksonen MA, Harkanen T, Knekt P, Virtala E, Oja H. Estimation of population attributable fraction (PAF) for disease occurrence in a cohort study design. Stat Med. 2010;29:860–74. doi: 10.1002/sim.3792. [DOI] [PubMed] [Google Scholar]
- 22.Go AS, Mozaffarian D, Roger VL, Benjamin EJ, Berry JD, Borden WB, et al. Heart disease and stroke statisticse2013 update: a report from the American Heart Association. Circulation. 2013;127:e6–245. doi: 10.1161/CIR.0b013e31828124ad. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Miyasaka Y, Barnes ME, Gersh BJ, Cha SS, Bailey KR, Abhayaratna WP, et al. Secular trends in incidence of atrial fibrillation in Olmsted County, Minnesota, 1980 to 2000, and implications on the projections for future prevalence. Circulation. 2006;114:119–25. doi: 10.1161/CIRCULATIONAHA.105.595140. [DOI] [PubMed] [Google Scholar]
- 24.Wong CX, Brooks AG, Leong DP, Roberts-Thomson KC, Sanders P. The increasing burden of atrial fibrillation compared with heart failure and myocardial infarction: a 15-year study of all hospitalizations in Australia. Arch Intern Med. 2012;172:739–41. doi: 10.1001/archinternmed.2012.878. [DOI] [PubMed] [Google Scholar]
- 25.Shen AY, Contreras R, Sobnosky S, Shah AI, Ichiuji AM, Jorgensen MB, et al. Racial/ethnic differences in the prevalence of atrial fibrillation among older adultsea cross-sectional study. J Natl Med Assoc. 2010;102:906–13. doi: 10.1016/s0027-9684(15)30709-4. [DOI] [PubMed] [Google Scholar]
- 26.Dewland TA, Olgin JE, Vittinghoff E, Marcus GM. Incident atrial fibrillation among Asians, Hispanics, blacks, and whites. Circulation. 2013 doi: 10.1161/CIRCULATIONAHA.113.002449. [DOI] [PubMed] [Google Scholar]
- 27.Soliman EZ, Alonso A, Goff DC., Jr Atrial fibrillation and ethnicity: the known, the unknown, and the paradox. Future Cardiol. 2009;5:547–56. doi: 10.2217/fca.09.49. [DOI] [PubMed] [Google Scholar]
- 28.Marcus GM, Alonso A, Peralta CA, Lettre G, Vittinghoff E, Lubitz SA, et al. European ancestry as a risk factor for atrial fibrillation in African Americans. Circulation. 2010;122:2009–15. doi: 10.1161/CIRCULATIONAHA.110.958306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Borzecki AM, Bridgers DK, Liebschutz JM, Kader B, Kazis LE, Berlowitz DR. Racial differences in the prevalence of atrial fibrillation among males. J Natl Med Assoc. 2008;100:237–45. doi: 10.1016/s0027-9684(15)31212-8. [DOI] [PubMed] [Google Scholar]
- 30.Gill PS, Calvert M, Davis R, Davies MK, Freemantle N, Lip GY. Prevalence of heart failure and atrial fibrillation in minority ethnic subjects: the Ethnic-Echocardiographic Heart of England Screening Study (E-ECHOES). PLoS One. 2011;6:e26710. doi: 10.1371/journal.pone.0026710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hu D, Sun Y. Epidemiology, risk factors for stroke, and management of atrial fibrillation in China. J Am Coll Cardiol. 2008;52:865–8. doi: 10.1016/j.jacc.2008.05.042. [DOI] [PubMed] [Google Scholar]
- 32.Chien KL, Su TC, Hsu HC, Chang WT, Chen PC, Chen MF, et al. Atrial fibrillation prevalence, incidence and risk of stroke and all-cause death among Chinese. Int J Cardiol. 2010;139:173–80. doi: 10.1016/j.ijcard.2008.10.045. [DOI] [PubMed] [Google Scholar]
- 33.Soliman EZ, Prineas RJ, Case LD, Zhang ZM, Goff DC., Jr Ethnic distribution of ECG predictors of atrial fibrillation and its impact on understanding the ethnic distribution of ischemic stroke in the Atherosclerosis Risk in Communities (ARIC) study. Stroke. 2009;40:1204–11. doi: 10.1161/STROKEAHA.108.534735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Marcus GM, Olgin JE, Whooley M, Vittinghoff E, Stone KL, Mehra R, et al. Racial differences in atrial fibrillation prevalence and left atrial size. Am J Med. 2010;123:375, e1–7. doi: 10.1016/j.amjmed.2009.05.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lau CP, Tse HF, Siu CW, Gbadebo D. Atrial electrical and structural remodeling: implications for racial differences in atrial fibrillation. J Cardiovasc Electro-physiol. 2012;23(Suppl 1):S36–40. doi: 10.1111/jce.12022. [DOI] [PubMed] [Google Scholar]
- 36.Levitzky YS, Guo CY, Rong J, Larson MG, Walter RE, Keaney JF, Jr, et al. Relation of smoking status to a panel of inflammatory markers: the Framingham offspring. Atherosclerosis. 2008;201:217–24. doi: 10.1016/j.atherosclerosis.2007.12.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Albert MA. Inflammatory biomarkers, race/ethnicity and cardiovascular disease. Nutr Rev. 2007;65:234–8. doi: 10.1111/j.1753-4887.2007.tb00369.x. [DOI] [PubMed] [Google Scholar]
- 38.Huxley RR, Lopez FL, Folsom AR, Agarwal SK, Loehr LR, Soliman EZ, et al. Absolute and attributable risks of atrial fibrillation in relation to optimal and borderline risk factors: the Atherosclerosis Risk in Communities (ARIC) study. Circulation. 2011;123:1501–8. doi: 10.1161/CIRCULATIONAHA.110.009035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Misialek JR, Rose KM, Everson-Rose SA, Soliman EZ, Clark CJ, Lopez FL, et al. Socioeconomic status and the incidence of atrial fibrillation in whites and blacks: the Atherosclerosis Risk in Communities (ARIC) study. J Am Heart Assoc. 2014;3:e001159. doi: 10.1161/JAHA.114.001159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Manichaikul A, Palmas W, Rodriguez CJ, Peralta CA, Divers J, Guo X, et al. Population structure of Hispanics in the United States: the multi-ethnic study of atherosclerosis. PLoS Genet. 2012;8:e1002640. doi: 10.1371/journal.pgen.1002640. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.