Abstract
Stroke represents a leading cause of long-term disability worldwide, with few therapeutic options available for improving behavioral recovery. Identification of endogenous neural stem and progenitor cells (NSPCs) that are capable of promoting reparative responses following brain injury and stroke make these cells attractive therapeutic targets for stimulating cell replacement and neuronal plasticity. Interest in the mechanisms that support NSPC survival and replenishment of damaged cells within the ischemic brain has led to elucidation of new roles for hypoxia-inducible factor-1α (HIF-1α) in NSPC function. HIF-1α is a well-studied mediator of adaptive cellular responses to hypoxia through direct transcriptional regulation of cellular metabolism and angiogenesis. Recent evidence also indicates novel roles for HIF-1α in stem cell differentiation through modulation of Notch and Wnt/β-catenin signaling pathways. In this review, we will explore the hypothesis that HIF-1α represents an important mediator of NSPC function under both non-pathological conditions and stroke; and plays a central role in the regulation of NSPC response to hypoxia, metabolism and maintenance of the vascular environment of the neural stem cell niche.
Keywords: Hypoxia, Focal cerebral ischemia, Middle cerebral artery occlusion, Neurogenesis, HIF
1. Introduction
Stroke is a leading cause of long-term disability worldwide, with approximately 70% of stroke survivors experiencing decreased work capacity and up to 30% requiring self-care assistance [1]. Focal cerebral ischemia caused by thrombotic or embolic occlusion of a cerebral artery accounts for approximately 80% of all strokes and results in immediate irreversible neuronal cell death and brain damage at the core of the infarct, followed by expansion of the area of brain damage through secondary injury that can continue for weeks and months following the initial ischemic event [2]. Currently, the only FDA-approved treatment for focal occlusive ischemia is administration of the thrombolytic agent, tissue plasminogen activator (tPA), which has a limited therapeutic window [2,3]. Thus, it is imperative to explore additional approaches to enhance long-term behavioral recovery.
Discovery of endogenous neural stem and progenitor cells (NSPCs) within the adult mammalian brain and their ability to mount regenerative responses following cerebral ischemia has generated much interest in therapeutic targeting of NSPCs to promote recovery of function after stroke [4]. NSPCs are multipotent cells that reside throughout the adult CNS, enriched within germinal centers of the subventricular zone (SVZ) lining the lateral ventricles and subgranular zone (SGZ) of the hippocampal dentate gyrus. NSPCs within the SVZ and SGZ generate new neurons of the olfactory bulb and dentate granule cell layer throughout life. In addition to providing neural progenitors for ongoing olfactory and hippocampal neurogenesis, NSPCs also mount regenerative responses following many types of metabolic and traumatic brain injuries. Focal cerebral ischemia stimulates proliferation and heterotypic migration of SVZ-NSPCs and their progeny into the ischemic brain parenchyma in both rodent [5–7] and human [8–11]. In rodents, SVZ-NSPCs give rise to new oligodendrocyte progenitors, astrocytes and neuroblasts that populate the peri-infarct region following middle cerebral artery occlusion (MCAO; e.g., see Fig. 1), even though only a small number of neuroblasts survive to maturity [7,12] reviewed in [4,13–15]. This regenerative response is temporally correlated with the onset of spontaneous improvements in behavioral deficits and cognitive function [5], but the mechanisms and extent to which NSPCs and their progeny contribute to spontaneous behavioral improvements through cell replacement or promotion of neuronal plasticity and reorganization have yet to be established. Apart from neuronal replacement, NSPCs may promote recovery of function through angiogenesis and stabilization of nascent vasculature [16–18], protection of penumbral neurons at risk of delayed cell death [19–22], or production of new glial cells [12] that may promote remyelination [23] and neurite outgrowth [24].
Fig. 1.
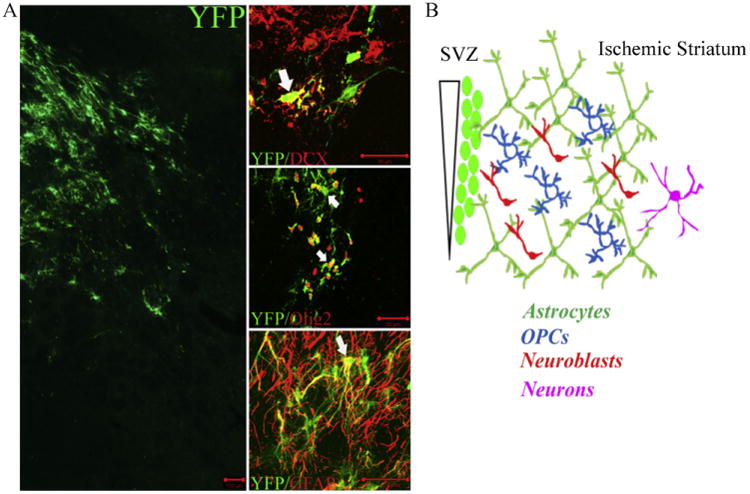
Stroke induces a multilineage response from adult SVZ. Nestin-CreERT2 :YFP mice were used to fate map nestin+ derivatives following stroke [12]. (A) Confocal images of YFP+ cells that populate the ischemic striatum by 2-weeks following 60 min transient MCAO. Phenotypic fate mapping was performed using markers for migrating neuroblasts (DCX), oligodendrocyte lineage (Olig2) and astrocytes (GFAP). Scale bars = 100 μm (YFP only and YFP/GFAP images), 20 μm (YFP/DCX and YFP/Olig2 images). (B) At 6-weeks post-MCAO, the relative distribution of YFP+ cells within the ischemic brain parenchyma includes approximately 45% astrocytes, 20% oligodendrocyte progenitors (OPCs), 20% neuroblasts and 5% postmitotic neurons.
Central to the regenerative response to stroke is the ability of NSPCs to withstand sudden onset hypoxia. Recent studies have demonstrated that NSPCs within the adult SVZ and SGZ constitutively express stabilized HIF-1α, a key transcriptional mediator of the cellular adaptive response to hypoxia [25,26]. HIF-1α regulates hundreds of genes involved in systemic, tissue and cellular adaption to low oxygen conditions; including genes that promote erythropoiesis, angiogenesis and glycolysis, respectively. Recently, HIF1α has been implicated in stem cell maintenance via non-canonical regulation of Notch and Wnt/β-catenin differentiation pathways [25,27–29]. This review will focus on the potential role of HIF-1α in the regulation of NSPC function under non-pathological conditions and stroke. This topic is also of potential clinical relevance with recent development of small molecule regulators of HIF-1α signaling for treatment of inflammation, chronic ischemic conditions and cancer [30–33], because these drugs might also be useful in regulating NSPC regenerative responses following brain injury.
2. HIF-1α stabilization in adult NSPCs under non-pathological and hypoxic conditions
2.1. Hypoxic regulation of HIF-1α stability
In most cell types, HIF-1α protein is constitutively expressed but is rapidly degraded under conditions of normoxia, with a half-life of <5 min in cultured cells (Fig. 2A; reviewed in [34–36]). Oxygen-dependent degradation occurs via prolyl hydroxylases that utilize O2 and α-ketoglutarate as substrates to hydroxylate HIF-1α at two proline residues (aa 402 and/or 564). Hydroxylated HIF-1α binds to the von Hippel-Lindau (VHL) protein, which recruits subunits of the E3 ubiquitin ligase, and thereby targets HIF-1α for ubiquitylation and degradation by the 26S proteasome. This allows cells to respond to conditions of hypoxia with rapid accumulation of HIF-1α, due to rate-limiting levels of intracellular O2 that inhibit prolyl hydroxylase activity. HIF-1α then dimerizes with the constitutively expressed HIF-1β subunit (ARNT; which is not susceptible to oxygen-dependent degradation), to form a heterodimeric HIF-1 transcriptional complex. This complex binds to cis-acting hypoxia-response elements (HREs) in target genes, and recruits the co-activator proteins p300/CBP, leading to increased transcription of genes encoding metabolic enzymes and pro-angiogenic factors. It should be noted that the HIF-1α paralogue, HIF-2α, is also O2-regulated, dimerizes with HIF-1β, and activates transcription of overlapping but distinct sets of target genes (reviewed in [37]).
Fig. 2.

(A) O2-dependent regulation of HIF-1α stability. Under normoxic conditions, HIF-1α is hydroxylated (-OH) by prolyl hydroxylase (PHD), leading to association with von Hippel-Lindau (VHL) protein and rapid proteasomal degradation. Under hypoxic conditions, HIF-1α is not hydroxylated and binds to HIF-1β (ARNT) to regulate target gene transcription through HRE (HIF response elements) in promoter regions. bHLH, basic helix-loop-helix; PAS, Per-Arnt-Sim; ODD, oxygen-dependent degradation domain; TAD, transactivator domain. (B) HIF-1α expression in SVZ under non-pathological conditions. Tangential section through adult mouse SVZ demonstrating localization of HIF-1α immunofluorescence (red) under non-pathological conditions (blue, DAPI nuclear stain). Scale bar= 20μm. For methodological details, please see Roitbak et al. [26]. (For interpretation of the references to color in this figure legend, the reader is referred to the web version of the article.)
2.2. Stabilization of HIF-1α in adult NSPCs under non-pathological conditions
Unlike most cell types within the adult CNS, recent evidence suggests that HIF-1α is constitutively stabilized within NSPCs of adult brain. Both nestin- and Sox-2-expressing NSPCs of the adult mouse SVZ and SGZ express HIF-1α under non-pathological conditions [26] (Fig. 2B). Mazumdar et al. [25] recently reported that the adult mouse dentate gyrus and SGZ represent hypoxic zones, where SOX-2+ cells stain with pimonidazole hydrochloride, an oxygen-sensitive dye that detects intracellular O2 partial pressures of less than 10mmHg(∼1.3%) [25]. Pimonidazole staining was also found to be associated with the expression of stabilized HIF-1α and other hypoxia responsive genes such as carbonic anhydrase IX (CAIX) and vascular endothelial growth factor (VEGF) within the hippocampal dentate gyrus. These studies suggest that the NSPC niche environments of adult brain may represent areas of relatively low physiological oxygen tension where HIF-1α stabilization is maintained under non-pathological conditions.
Although the adult SVZ appears to be highly vascularized, cellular oxygen level within this niche environment has been estimated to be 2.5–3.0% under non-pathological conditions [38]. In the widely used mouse MCAO model of focal ischemia, the SVZ appears to be relatively spared from injury even though cellular pO2 within the SVZ falls to <1.3% [7]. Much evidence indicates that somatic stem cells throughout the body reside within hypoxic niches, where low oxygen tensions minimize oxidative stress and prevent premature differentiation and exhaustion of the stem cell pool [39]. Within bone marrow, hematopoietic stem cells (HSCs) maintain intracellular hypoxia and constitutive stabilization of HIF-1α [40]. Selective deletion of the HIF-1α gene within HSCs of adult mice results in depletion of the primitive stem cell population over time, and a near complete loss in their ability to provide long-term bone marrow reconstitution following transplantation [41]. Similarly, selective HIF-1α gene deletion within nestin-expressing NSPCs of adult mouse brain using an inducible Cre-loxP approach leads to an approximate 50% reduction in the number of SVZ-NSPCs under non-pathological conditions [42]. Conditional deletion of HIF-1α from postnatal mouse neurons using a calcium/calmodulindependent kinase (αCamKII) promoter also results in attenuated proliferation and neuroblast formation in adult SGZ [25]. These studies suggest that HIF-1α plays an important role in maintaining NSPC homeostasis and neurogenesis in adult brain under non-pathological conditions.
2.3. O2 -independent mechanisms regulating HIF-1α stability
Several O2-independent mechanisms for HIF-1α stabilization also exist, and the levels of HIF-1α protein under non-pathological conditions can vary among tissues and cell types [43]. For example, heat shock protein 90 (Hsp90) can stabilize HIF-1α in an oxygen-independent fashion, whereby Hsp90 binds to the PAS domain of HIF-1α and stabilizes it [44]. In the presence of pharmacological inhibitors of Hsp90, the Hsp90 binding site becomes occupied by RACK1 (receptor for activated C-kinase), which recruits ubiquitin ligase and targets HIF-1α for proteasomal degradation [45]. Interestingly, the ability of RACK1 to compete with Hsp90 under non-hypoxic conditions is linked to intracellular calcium levels and calcium-activated signal transduction cascades [46]. Hsp90 has been implicated in HIF-1α stabilization in embryonic neural stem cells [47]. HIF-1α activity is also regulated by cytokines, growth factors and other signaling events (reviewed in [48]). For example, PI-3K/AKT activity increases HIF-1α translation through mTOR (mammalian target of rapamycin) activation. The transactivation of HIF-1α is also regulated by the binding of FIH-1 (factor inhibiting HIF-1), which blocks binding of HIF-1α to the transcriptional co-activators necessary for target gene transcription.
As discussed below, NSPCs isolated from embryonic mouse telencephalon and postnatal SVZ also constitutively express HIF-1α under non-hypoxic conditions in culture. Biochemical analysis demonstrated that HIF-1α is not hydroxylated or ubiquitylated within cultured NSPCs, and does not associate with VHL, even though both 19 and 30 kD isoforms of VHL are expressed [26]. Immuno-electron microscopy of cultured NSPCs suggested that HIF-1α is sequestered in membranous cytoplasmic structures, which might prevent it from degradation processes [26]. It is important to note that in vitro regulation of HIF-1α may not reflect the same mechanisms that occur in vivo, since NSPCs are expanded under high growth factor conditions in culture, where mTOR signaling is robust [26]. On the other hand, NSPC niche environments in adult brain also represent areas of high growth factor signaling [49]. Further investigation is needed to determine the relative contribution of O2-dependent vs. O2-independent signaling pathways regulating NSPC-HIF-1α stability under in vitro and in vivo conditions.
3. Functional roles for HIF-1α in NSPCs
3.1. Does constitutive HIF-1 a stabilization maintain NSPC metabolic phenotype and ability to withstand sudden onset hypoxia?
It is becoming increasingly apparent that oxygen levels have a profound effect within the stem cell niche and strongly influence the proliferation, self-renewal, and phenotypic fate choice of neural stem cells during normal development and disease [50] (reviewed in [51–55]). Low oxygen tension promotes self-renewal of neural stem cells in culture and the preferential development of certain cell types upon differentiation. Disruption of oxygen availability by perinatal hypoxia/ischemia or following stroke in adulthood stimulates increased proliferation of cells within the SVZ and re-directed migration of SVZ-derivatives into the hypoxic brain region [56]. In culture, NSPCs are relatively resistant to brief periods of oxygen-glucose deprivation (OGD; a widely used in vitro model of cerebral ischemia) when compared to primary cortical neurons [19], which are highly dependent upon oxidative metabolism for survival [57].
Although studies have demonstrated that neural stem cells thrive under low oxygen conditions in cell culture, very little is known concerning the bioenergetics of NSPCs or how metabolic homeostasis is regulated in these cells. Such processes are likely to be fundamental for maintenance of the NSPC pool under normal conditions and following a metabolic insult such as cerebral ischemia and stroke. Much evidence indicates that adult stem cells from various tissues, embryonic stem cells derived from the inner cell mass of the blastocyst, and cancer stem cells all share common aspects of metabolic phenotype defined by high glycolytic flux, low oxygen consumption and minimal dependence on mitochondrial oxidative phosphorylation for ATP synthesis and survival [51,58–63]. It has been hypothesized that rapidly proliferating cells, such as cancer cells, utilize glycolysis under aerobic conditions to avoid free radical accumulation resulting from mitochondrial electron transport [64]. Reactive oxygen species are not only toxic to proliferating cells, but ROS production (e.g., superoxide and hydrogen peroxide), also signals stem cell differentiation [65,66]. This form of metabolism is thought to underlie self-renewal and maintenance of the undifferentiated state, but the molecular underpinnings driving this metabolic phenotype have not been fully established.
Neural stem cells are highly dependent upon the glycolytic and pentose phosphate arms of glucose metabolism, and display a relatively low requirement for oxidative metabolism. Both embryonic and adult neural stem cells survive prolonged periods of anoxic conditions in culture, but cannot withstand periods of glucose withdrawal even in the presence of the TCA substrate, pyruvate [67]. The high glucose requirement of NSPCs in culture appears to be due to dependence upon glycolysis for ATP production and for use of glycolytic intermediates in the pentose phosphate pathway (PPP), which provides ribose-5-phosphate (R5P) as substrate for nucleotide biosynthesis in proliferating cells and NADPH as an essential reducing equivalent for antioxidative processes and production of glutathione [68]. NSPC survival is impaired under conditions of glycolytic inhibition in the presence of pyruvate and by pharmacological inhibition of the PPP pathway. However, NSPCs display relative resistance to prolonged periods of hypoxia and pharmacological inhibition of mitochondrial respiration, and also display increased lactate production and lactate dehydrogenase activity compared to primary cortical neurons [67].
HIF-1α functions in many cell types to reprogram cellular metabolism to promote glycolysis, influence the PPP pathway, and repress oxidative metabolism (reviewed in [48]; Fig. 3). This is accomplished by HIF-1α-mediated transcriptional upregulation of genes encoding glucose transporters, glycolytic enzymes and lactate dehydrogenase, which replenishes NAD+ for further glycolysis. In addition, HIF-1α represses the flux of pyruvate into acetyl-CoA, diverting carbon away from mitochondria and suppressing O2 consumption, by stimulating the expression of pyruvate dehydrogenase kinase 1 (PDK-1). Further investigation is required to determine the extent to which HIF-1α drives metabolic phenotype in embryonic or adult NSPCs. Nevertheless, it appears that NSPCs are metabolically poised to withstand sudden onset hypoxia, yet their high dependence on glucose to provide glycolytic and PPP substrates may require close association with microvasculature.
Fig. 3.
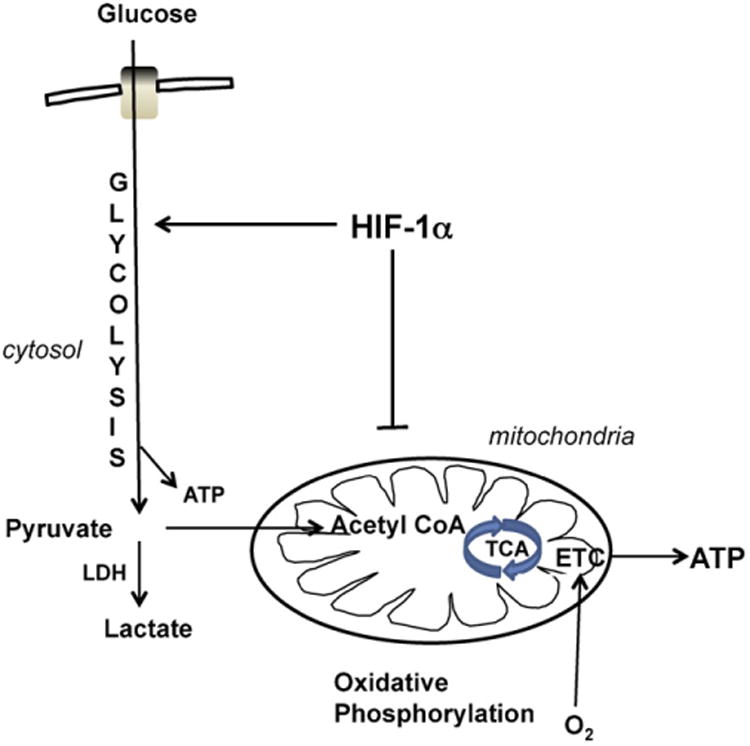
HIF-1α regulation of metabolic phenotype. HIF-1α regulates metabolism in many cell types by promoting glycolysis and inhibiting mitochondrial oxidative phosphorylation. This metabolic regulation facilitates cellular adaptations to low oxygen conditions. LDH, lactate dehydrogenase; TCA, tricarboxylic acid cycle; ETC, electron transport chain.
3.2. Vasculotrophic and neurotrophic support by NSPC-HIF-1α regulation of VEGF: role in maintaining the angiogenic niche and promoting brain repair following stroke
HIF-1α activates the transcription of vascular endothelial growth factor (VEGF) and other angiogenic growth factor genes [69] and is required for normal embryonic vascular development [70]. Systemic deletion of the HIF-1α gene is embryonic lethal, associated with malformation of the heart and cardiovascular system [71], whereas conditional deletion of HIF-1α within nestin+ stem cells of the developing nervous system results in regression of vasculature and massive neuronal apoptosis [72]. VEGF is not only a potent angiogenic factor, but also exerts direct neurotrophic signaling and stimulates adult neurogenesis [73,74]. The vascular and neurotrophic effects of VEGF are mediated by the receptor tyrosine kinase, VEGFR-2 (Flk-/KDR). Exogenous administration of VEGF following experimental stroke reduces infarct size and improves neurological performance, due to both direct neuroprotective effects of VEGF and stimulation of angiogenesis [75]. Transgenic mice that overexpress VEGF display increased neurogenesis, decreased infarct volume and improved motor function [76].
Embryonic and adult NSPCs constitutively express HIF-1α and release soluble VEGF under non-hypoxic conditions in culture [17,19]. HIF-1α and VEGF are coordinately upregulated approximately 2-fold within 24 h following transient exposure of NSPCs to oxygen and glucose deprivation, or following more prolonged periods of hypoxia alone. Reducing HIF-1α expression using siRNA or C re-mediated HIF-1α gene deletion attenuates the ability of NSPCs to survive ischemic conditions [26,42]. Although HIF-1α gene deletion attenuates VEGF release by at least 50%, pharmacological inhibition of VEGF signaling has no effect on the ability of NSPCs to withstand ischemia in culture. On the other hand, both brain endothelial cells and embryonic cortical neurons undergo cell death within 24 h following exposure to hypoxia/ischemia in culture, but are entirely protected by embryonic or adult NSPCs placed in transwell co-culture or by medium conditioned by NSPCs. Both the endothelial and neurotrophic effects are blocked by pharmacological inhibitors of VEGF-VEGFR2 signaling [17,19] (Fig. 4).
Fig. 4.
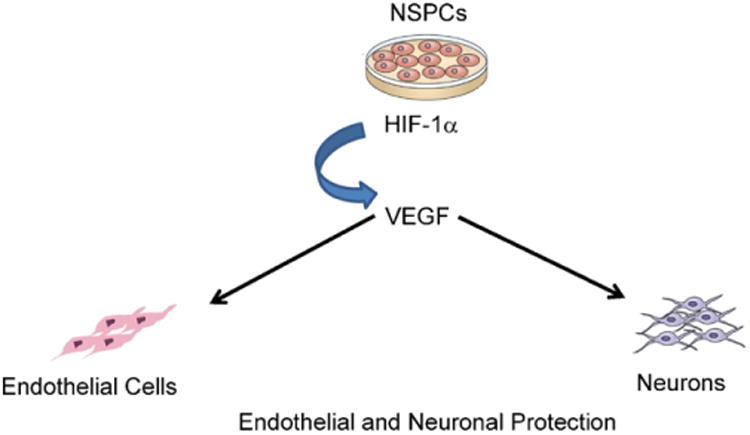
Vasculotrophic and neurotrophic influence of NSPCs. Embryonic and adult NSPCs protect endothelial cells and cortical neurons against ischemic conditions in culture via HIF-1α-regulated VEGF release [17,19].
Complex bi-directional signaling occurs between NSPCs and vasculature under non-pathological and stroke conditions (reviewed by [18,56,77,78]). NSPCs in adult brain reside in specialized microvascular niche environments that are important for supporting many aspects of neural stem cell function [77]. Within the adult SVZ, primitive stem cell astrocytes (type B cells), transit amplifying cells (type C) and neuroblasts (type A cells), display unique anatomical associations with endothelial cells [79]. Type B astrocyte stem cells maintain contact with SVZ endothelial cells through endfoot-like processes [80]. Transition of type B astrocyte stem cells to proliferative transit amplifying cells is thought to involve activation of type B astrocytes through stromal-derived factor 1 (SDF1)-CXC chemokine receptor 4 (CXCR4) signaling, where endothelial cells release SDF-1 to activate CXCR4 receptors expressed by NSPCs [81]. Endothelial cells within the SVZ also release factors that maintain the undifferentiated state and expand neural stem cells in culture [82]. Disruption of α6β1 integrin-laminin interaction blocks both NSPC adhesion to the vasculature and NSPC proliferation [82].
NSPCs are also found in close association with the vasculature during migration into ischemic brain regions following stroke [12,16] (Fig. 5). Re-routing of NSPCs toward areas of ischemic injury is dependent on SDF1-CXCR4 signaling [16,83,84]. SDF-1-induced migration of adult NSPCs is mediated by matrix metalloproteinases [85]. HIF-1α signaling increases CXCR4 receptor expression in many cell types [86,87] and may promote matrix metalloproteinase-mediated migration of neural stem cells in response to hypoxia [88]. Thus, potential roles for NSPC-HIF1α include (a) maintaining the vascular niche by driving constitutive VEGF release and (b) promoting activation and migration of NSPCs through CXCR4 and MMP regulation. If so, one would anticipate that interference with HIF-1α signaling in the adult NSPC population might destabilize the SVZ vasculature and also impair the activation and migration of NSPCs leading to an impairment of both the NSPC cytogenic response and the angiogenic component of stroke recovery.
Fig. 5.
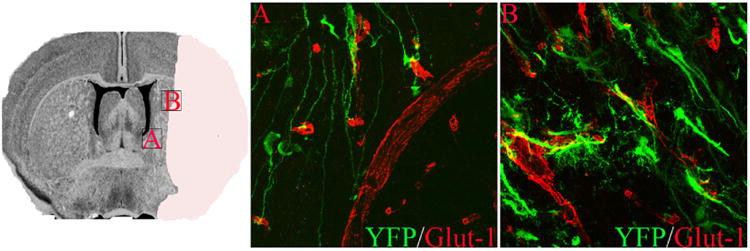
NSPC association with vasculature post-MCAO. Boxed regions on left depict areas shown in higher power in A and B. Dual immunofluorescence for YFP (green) and GLUT-1 (red) demonstrate YFP+ processes from radial glial-like cells in the SVZ that contact vasculature via endfeet (B). YFP reporter+ cells that migrate into the ischemic border zone are also associated with cerebral blood vessels (B) at 6 weeks following 60-min MCAO. For methodological details, please see [12].
3.3. HIF-1α regulation of NSPC lineage fate by Notch1 and Wnt/β-catenin signaling
Hypoxia enhances the proliferation and multipotency of both human and rodent NSPCs, and can impact developmental outcome during NSPC differentiation. Recent studies have demonstrated that HIF-1α mediates the effects of low pO2 on proliferation and differentiation of several stem cell types in culture through direct physical association of the HIF-1α subunit with Notch and Wnt/β-catenin signaling components [25,27–29].
In neural stem cells, Notch signaling prevents terminal differentiation and preserves a pool of stem cells by preventing exit from the cell cycle and maturation [89,90]. In embryonic NSPCs and embryonic P19 carcinoma cells, hypoxia enhances Notch signaling via direct HIF-1α binding to activated Notch-1 (NICD), leading to enhanced stabilization of NICD and potentiated transcription of Notch-1 target genes [28]. Using mice genetically engineered to inducibly knock in or knock out Notch signaling in postnatal NSPCs, Breunig et al. [91] recently demonstrated that loss of Notch signaling depleted the progenitor pool and skewed differentiation toward the neuronal lineage, while over activation of Notch signaling decreased cell cycle exit and increased the size of the progenitor pool. Similar findings were recently reported by Ables et al., using inducible nestin-Cre:YFP:Notch1fl/fl transgenic mice [92]. Notch-1 signaling is initiated by ligand binding, which stimulates proteolytic cleavage of Notch-1 to liberate an intracellular domain (Notch intracellular domain; NICD). The NICD translocates to the nucleus and interacts with a transcriptional activation complex to inhibit transcriptional effectors such as neurogenin and Mash1, but can also stimulate neuronal differentiation of a small number of ependymal cells under conditions of focal ischemia [93]. Although previous studies have indicated that HIF-1α potentiates Notch signaling in embryonic NSCs, P19 carcinoma [28] and med-uloblastoma precursors [94], this has not been studied extensively in postnatal NSPCs, and the subset of Notch target genes regulated by HIF-1α potentiation have not been elucidated.
Components of the Wnt/β-catenin pathway are also expressed within the adult SVZ and SGZ [95–98], and are upregulated in SVZ following stroke [98]. Wnt/β-catenin stimulates neuronal lineage commitment in NSPCs via activation of the proneural transcription factor NeuroD1 [97,99,100]. Activation of the Wnt signaling pathway leads to dephosphorylation, stabilization and nuclear translocation of β-catenin. Stabilized β-catenin then complexes with the TCF-leukocyte enhancer factor (TCF/LEF) which binds to TCF/LEF response elements within the promoter region of proneural gene NeuroD1, and thereby triggers neuronal differentiation of adult NSPCs. In non-neuronal cell types, including colon carcinoma and hematopoietic stem cells, Wnt/β-catenin signaling is down-regulated in hypoxia leading to enhanced growth and impaired differentiation [27,29]. Hypoxic repression of Wnt/β-catenin signaling in non-neuronal cells is mediated by direct binding of HIf-1α to β-catenin and inhibition of β-catenin binding to TCF/LEF transcription factor. Mazumdar et al. [25], recently reported that in embryonic stem cells and isolated embryonic neural stem cells, HIF-1α modulates Wnt/β-catenin signaling by enhancing β-catenin activation and expression of TCF/LEF. HIF-1α gene deletion in postnatal NSPCs in culture stimulates reciprocal changes in the intracellular levels of NICD (decreased) and β-catenin (increase) [26], and shifts lineage fate in culture and following MCAO [42]. These finding suggest that HIF-1α regulation of Notch and Wnt signaling may be important in regulating the balance between self-renewal and differentiation of NSPCs under non-hypoxic conditions and stroke.
4. Conclusions
Focal cerebral ischemia stimulates proliferation and heterotypic migration of SVZ-derived progenitors into the ischemic brain parenchyma. This is a multilineage cytogenic response in which NSPCs of the SVZ generate new oligodendrocyte progenitors, astrocytes and neuroblasts that persist within the peri-infarct region. Successful therapeutic targeting of NSPCs for functional brain repair will require the ability to maintain an adequate and viable stem cell pool and the ability to direct the differentiation and survival of desired lineages. Ultimately, an understanding of the complex molecular regulation of these processes will be needed. Here, we have focused on potential mechanisms by which HIF-1α facilitates stem cell survival, self-renewal and differentiation (Fig. 6). Constitutive stabilization of HIF-1α in adult neural stem cells may render these cells poised to survive sudden onset hypoxia and promote the activation and migration of these cells into the injured brain parenchyma. HIF-1α may also be important in maintaining the vascular niche environment and promoting angiogenesis through transcriptional modulation of VEGF. Finally, HIF-1α represents an intrinsic regulator of NSPC multipotency and developmental outcome upon differentiation. Future development of small molecule regulators of HIF-1α stability and signaling may ultimately prove useful to therapeutically target endogenous NSPCs for enhancing recovery and repair in the adult brain.
Fig. 6.
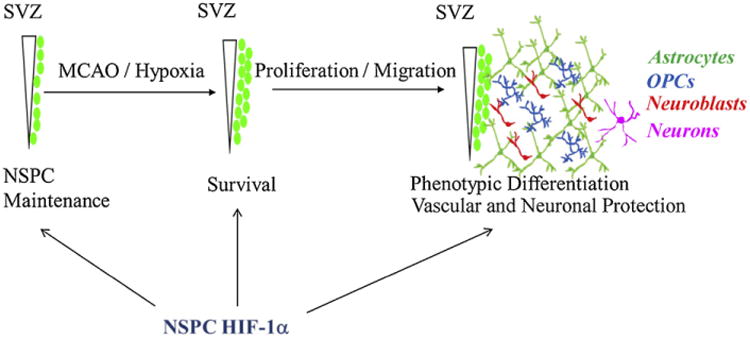
Multiple roles for HIF-1α in NSPC function and SVZ response to focal cerebral ischemia. Schematic depicting multiple potential roles for HIF-1α in NSPC function and SVZ response to focal cerebral ischemia. NSPC-HIF-1α is important for the maintenance of NSPCs in both SVZ and SGZ and modulates lineage fate of NSPCs in culture. NSPC-HIF-1α is also likely to be important in viability as well as proliferative and migratory responses of NSPCs following ischemic injury.
Acknowledgments
Supported by The American Heart Association, 09GRNT229017.
References
- 1.Lloyd-Jones D, Adams RJ, Brown TM, Carnethon M, Dai S, De Simone G, et al. Heart disease and stroke statistics - 2010 update: a report from the American Heart Association. Circulation. 2010;121:e46–215. doi: 10.1161/CIRCULATIONAHA.109.192667. [DOI] [PubMed] [Google Scholar]
- 2.Moskowitz MA, Lo EH, Iadecola C. The science of stroke: mechanisms in search of treatments. Neuron. 2010;67:181–98. doi: 10.1016/j.neuron.2010.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lo EH. A new penumbra: transitioning from injury into repair after stroke. Nat Med. 2008;14:497–500. doi: 10.1038/nm1735. [DOI] [PubMed] [Google Scholar]
- 4.Lindvall O, Kokaia Z. Stem cells in human neurodegenerative disorders - time for clinical translation? J Clin Invest. 2010;120:29–40. doi: 10.1172/JCI40543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Arvidsson A, Collin T, Kirik D, Kokaia Z, Lindvall O. Neuronal replacement from endogenous precursors in the adult brain after stroke. Nat Med. 2002;8:963–70. doi: 10.1038/nm747. [DOI] [PubMed] [Google Scholar]
- 6.Kokaia Z, Thored P, Arvidsson A, Lindvall O. Regulation of stroke-induced neurogenesis in adult brain - recent scientific progress. Cereb Cortex. 2006;16(Suppl. 1):i162–7. doi: 10.1093/cercor/bhj174. [DOI] [PubMed] [Google Scholar]
- 7.Thored P, Wood J, Arvidsson A, Cammenga J, Kokaia Z, Lindvall O. Long-term neuroblast migration along blood vessels in an area with transient angiogenesis and increased vascularization after stroke. Stroke. 2007;38:3032–9. doi: 10.1161/STROKEAHA.107.488445. [DOI] [PubMed] [Google Scholar]
- 8.Jin K, Wang X, Xie L, Mao XO, Zhu W, Wang Y, et al. Evidence for stroke-induced neurogenesis in the human brain. Proc Natl Acad Sci U S A. 2006;103:13198–202. doi: 10.1073/pnas.0603512103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Macas J, Nern C, Plate KH, Momma S. Increased generation of neuronal progenitors after ischemic injury in the aged adult human forebrain. J Neurosci. 2006;26:13114–9. doi: 10.1523/JNEUROSCI.4667-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Marti-Fabregas J, Romaguera-Ros M, Gomez-Pinedo U, Martinez-Ramirez S, Jimenez-Xarrie E, Marin R, et al. Proliferation in the human ipsilateral sub-ventricular zone after ischemic stroke. Neurology. 2010;74:357–65. doi: 10.1212/WNL.0b013e3181cbccec. [DOI] [PubMed] [Google Scholar]
- 11.Nakayama D, Matsuyama T, Ishibashi-Ueda H, Nakagomi T, Kasahara Y, Hirose H, et al. Injury-induced neural stem/progenitor cells in post-stroke human cerebral cortex. Eur J Neurosci. 2010;31:90–8. doi: 10.1111/j.1460-9568.2009.07043.x. [DOI] [PubMed] [Google Scholar]
- 12.Li L, Harms KM, Ventura PB, Lagace DC, Eisch AJ, Cunningham LA. Focal cerebral ischemia induces a multilineage cytogenic response from adult sub-ventricular zone that is predominantly gliogenic. Glia. 2010;58:1610–9. doi: 10.1002/glia.21033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kernie SG, Parent JM. Forebrain neurogenesis after focal ischemic and traumatic brain injury. Neurobiol Dis. 2010;37:267–74. doi: 10.1016/j.nbd.2009.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Parent JM, Vexler ZS, Gong C, Derugin N, Ferriero DM. Rat forebrain neurogenesis and striatal neuron replacement after focal stroke. Ann Neurol. 2002;52:802–13. doi: 10.1002/ana.10393. [DOI] [PubMed] [Google Scholar]
- 15.Zhang RL, Zhang ZG, Chopp M. Ischemic stroke and neurogenesis in the sub-ventricular zone. Neuropharmacology. 2008;55:345–52. doi: 10.1016/j.neuropharm.2008.05.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ohab JJ, Fleming S, Blesch A, Carmichael ST. A neurovascular niche for neuro-genesis after stroke. J Neurosci. 2006;26:13007–16. doi: 10.1523/JNEUROSCI.4323-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Roitbak T, Li L, Cunningham LA. Neural stem/progenitor cells promote endothelial cell morphogenesis and protect endothelial cells against ischemia via HIF-1alpha-regulated VEGF signaling. J Cereb Blood Flow Metab. 2008 doi: 10.1038/jcbfm.2008.38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Xiong Y, Mahmood A, Chopp M. Angiogenesis, neurogenesis and brain recovery of function following injury. Curr Opin Investig Drugs. 2010;11:298–308. [PMC free article] [PubMed] [Google Scholar]
- 19.Harms KM, Li L, Cunningham LA. Murine neural stem/progenitor cells protect neurons against ischemia by HIF-1alpha-regulated VEGF signaling. PLoS ONE. 2010;5:e9767. doi: 10.1371/journal.pone.0009767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hayashi J, Takagi Y, Fukuda H, Imazato T, Nishimura M, Fujimoto M, et al. Primate embryonic stem cell-derived neuronal progenitors transplanted into ischemic brain. J Cereb Blood Flow Metab. 2006;26:906–14. doi: 10.1038/sj.jcbfm.9600247. [DOI] [PubMed] [Google Scholar]
- 21.Jin K, Mao X, Xie L, Galvan V, Lai B, Wang Y, et al. Transplantation of human neural precursor cells in Matrigel scaffolding improves outcome from focal cerebral ischemia after delayed postischemic treatment in rats. J Cereb Blood Flow Metab. 2010;30:534–44. doi: 10.1038/jcbfm.2009.219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jin K, Mao X, Xie L, Greenberg RB, Peng B, Moore A, et al. Delayed transplantation of human neural precursor cells improves outcome from focal cerebral ischemia in aged rats. Aging cell. 2010;9:1076–83. doi: 10.1111/j.1474-9726.2010.00638.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zhang RL, Chopp M, Roberts C, Jia L, Wei M, Lu M, et al. Ascl1 lineage cells contribute to ischemia-induced neurogenesis and oligodendrogenesis. J Cereb Blood Flow Metab. 2011;31:614–25. doi: 10.1038/jcbfm.2010.134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Meletis K, Barnabe-Heider F, Carlen M, Evergren E, Tomilin N, Shupliakov O, et al. Spinal cord injury reveals multilineage differentiation of ependymal cells. PLoS Biol. 2008;6:e182. doi: 10.1371/journal.pbio.0060182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mazumdar J, O'Brien WT, Johnson RS, La Manna JC, Chavez JC, Klein PS, et al. O2 regulates stem cells through Wnt/beta-catenin signalling. Nat Cell Biol. 2010;12:1007–13. doi: 10.1038/ncb2102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Roitbak T, Surviladze Z, Cunningham LA. Continuous expression of HIF-1alpha in neural stem/progenitor cells. Cell Mol Neurobiol. 2011;31:119–33. doi: 10.1007/s10571-010-9561-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Duncan AW, Rattis FM, Di Mascio LN, Congdon KL, Pazianos G, Zhao C, et al. Integration of Notch and Wnt signaling in hematopoietic stem cell maintenance. Nat Immunol. 2005;6:314–22. doi: 10.1038/ni1164. [DOI] [PubMed] [Google Scholar]
- 28.Gustafsson MV, Zheng X, Pereira T, Gradin K, Jin S, Lundkvist J, et al. Hypoxia requires Notch signaling to maintain the undifferentiated cell state. Dev Cell. 2005;9:617–28. doi: 10.1016/j.devcel.2005.09.010. [DOI] [PubMed] [Google Scholar]
- 29.Kaidi A, Williams AC, Paraskeva C. Interaction between beta-catenin and HIF-1 promotes cellular adaptation to hypoxia. Nat Cell Biol. 2007;9:210–7. doi: 10.1038/ncb1534. [DOI] [PubMed] [Google Scholar]
- 30.Ban HS, Uto Y, Nakamura H. Hypoxia-inducible factor inhibitors: a survey of recent patented compounds (2004–2010) Expert Opin Ther Pat. 2011;21:131–46. doi: 10.1517/13543776.2011.547477. [DOI] [PubMed] [Google Scholar]
- 31.Chen S, Sang N. Histone deacetylase inhibitors: the epigenetic therapeutics that repress hypoxia-inducible factors. J Biomed Biotechnol. 2011;2011:197946. doi: 10.1155/2011/197946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Koh MY, Spivak-Kroizman TR, Powis G. HIF-1alpha and cancer therapy. Recent Results Cancer Res. 2010;180:15–34. doi: 10.1007/978-3-540-78281-0_3. [DOI] [PubMed] [Google Scholar]
- 33.Smirnova NA, RakhmanI, Moroz N, Basso M, Payappilly J, Kazakov S, et al. Utilization of an in vivo reporter for high throughput identification of branched small molecule regulators of hypoxic adaptation. Chem Biol. 2010;17:380–91. doi: 10.1016/j.chembiol.2010.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Semenza GL. Hydroxylation of HIF-1: oxygen sensing at the molecular level. Physiology (Bethesda, MD) 2004;19:176–82. doi: 10.1152/physiol.00001.2004. [DOI] [PubMed] [Google Scholar]
- 35.Semenza GL. Oxygen-dependent regulation of mitochondrial respiration by hypoxia-inducible factor 1. Bio chem J. 2007;405:1–9. doi: 10.1042/BJ20070389. [DOI] [PubMed] [Google Scholar]
- 36.Sharp FR, Bernaudin M. HIF1 and oxygen sensing in the brain. Nat Rev Neurosci. 2004;5:437–48. doi: 10.1038/nrn1408. [DOI] [PubMed] [Google Scholar]
- 37.Patel SA, Simon MC. Biology of hypoxia-inducible factor-2alpha in development and disease. Cell Death Differ. 2008;15:628–34. doi: 10.1038/cdd.2008.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Santilli G, Lamorte G, Carlessi L, Ferrari D, Rota Nodari L, Binda E, et al. Mild hypoxia enhances proliferation and multipotency of human neural stem cells. PLoS ONE. 2010;5:e8575. doi: 10.1371/journal.pone.0008575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Mohyeldin A, Garzon-Muvdi T, Quinones-Hinojosa A. Oxygen in stem cell biology: a critical component of the stem cell niche. Cell Stem Cell. 2010;7:150–61. doi: 10.1016/j.stem.2010.07.007. [DOI] [PubMed] [Google Scholar]
- 40.Jang YY, Sharkis SJ. A low level of reactive oxygen species selects for primitive hematopoietic stem cells that may reside in the low-oxygenic niche. Blood. 2007;110:3056–63. doi: 10.1182/blood-2007-05-087759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Takubo K, Goda N, Yamada W, Iriuchishima H, Ikeda E, Kubota Y, et al. Regulation of the HIF-1alpha level is essential for hematopoietic stem cells. Cell Stem Cell. 2010;7:391–402. doi: 10.1016/j.stem.2010.06.020. [DOI] [PubMed] [Google Scholar]
- 42.Li L, Candelario KM, Thomas K, Cunningham LA. Hypoxia inducible factor-1 alpha regulates the NSPC response to cerebral ischemia. in preparation. [Google Scholar]
- 43.Stroka DM, Burkhardt T, Desbaillets I, Wenger RH, Neil DA, Bauer C, et al. HIF-1 is expressed in normoxic tissue and displays an organ-specific regulation under systemic hypoxia. FASEB J. 2001;15:2445–53. doi: 10.1096/fj.01-0125com. [DOI] [PubMed] [Google Scholar]
- 44.Isaacs JS, Jung YJ, Mimnaugh EG, Martinez A, Cuttitta F, Neckers LM. Hsp90 regulatesavon Hippel–Lindau-independenthypoxia-inducible factor-1 alpha-degradative pathway. J Biol Chem. 2002;277:29936–44. doi: 10.1074/jbc.M204733200. [DOI] [PubMed] [Google Scholar]
- 45.Liu YV, Baek JH, Zhang H, Diez R, Cole RN, Semenza GL. RACK1 competes with HSP90 for binding to HIF-1alpha and is required for O(2)-independent and HSP90 inhibitor-induced degradation of HIF-1alpha. Mol Cell. 2007;25:207–17. doi: 10.1016/j.molcel.2007.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Liu YV, Semenza GL. RACK1 vs HSP90: competition for HIF-1 alpha degradation vs. stabilization. Cell Cycle. 2007;6:656–9. doi: 10.4161/cc.6.6.3981. [DOI] [PubMed] [Google Scholar]
- 47.Xiong L, Zhao T, Huang X, Liu ZH, Zhao H, Li MM, et al. Heat shock protein 90 is involved in regulation of hypoxia-driven proliferation of embryonic neural stem/progenitor cells. Cell Stress Chaperones. 2009;14:183–92. doi: 10.1007/s12192-008-0071-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Majmundar AJ, Wong WJ, Simon MC. Hypoxia-inducible factors and the response to hypoxic stress. Mol Cell. 2010;40:294–309. doi: 10.1016/j.molcel.2010.09.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Robel S, Berninger B, Gotz M. The stem cell potential of glia: lessons from reactive gliosis. Nat Rev Neurosci. 2011;12:88–104. doi: 10.1038/nrn2978. [DOI] [PubMed] [Google Scholar]
- 50.Zhao T, Zhang CP, Liu ZH, Wu LY, Huang X, Wu HT, et al. Hypoxia-driven proliferation of embryonic neural stem/progenitor cells – role of hypoxia-inducible transcription factor-1alpha. FEBS J. 2008;275:1824–34. doi: 10.1111/j.1742-4658.2008.06340.x. [DOI] [PubMed] [Google Scholar]
- 51.Panchision DM. The role of oxygen in regulating neural stem cells in development and disease. J Cell Physiol. 2009;220:562–8. doi: 10.1002/jcp.21812. [DOI] [PubMed] [Google Scholar]
- 52.Vieira HL, Alves PM, Vercelli A. Modulation of neuronal stem cell differentiation by hypoxia and reactive oxygen species. Prog Neurobiol. 2011;93:444–55. doi: 10.1016/j.pneurobio.2011.01.007. [DOI] [PubMed] [Google Scholar]
- 53.Zhang K, Zhu L, Fan M. Oxygen, a key factor regulating cell behavior during neurogenesis and cerebral diseases. Front Mol Neurosci. 2011;4:5. doi: 10.3389/fnmol.2011.00005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Keith B, Simon MC. Hypoxia-inducible factors, stem cells, and cancer. Cell. 2007;129:465–72. doi: 10.1016/j.cell.2007.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Simon MC, Keith B. The role of oxygen availability in embryonic development and stem cell function. Nat Rev. 2008;9:285–96. doi: 10.1038/nrm2354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Massouh M, Saghatelyan A. De-routing neuronal precursors in the adult brain to sites of injury: role of the vasculature. Neuropharmacology. 2010;58:877–83. doi: 10.1016/j.neuropharm.2009.12.021. [DOI] [PubMed] [Google Scholar]
- 57.Pellerin L, Magistretti PJ. Neuroenergetics: calling upon astrocytes to satisfy hungry neurons. Neuroscientist. 2004;10:53–62. doi: 10.1177/1073858403260159. [DOI] [PubMed] [Google Scholar]
- 58.Chung S, Arrell DK, Faustino RS, Terzic A, Dzeja PP. Glycolytic network restructuring integraltothe energetics of embryonic stem cell cardiac differentiation. J Mol Cell Cardiol. 2010;48:725–34. doi: 10.1016/j.yjmcc.2009.12.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Kang J, Shakya A, Tantin D. Stem cells, stress, metabolism and cancer:adrama in two Octs. Trends Biochem Sci. 2009;34:491–9. doi: 10.1016/j.tibs.2009.06.003. [DOI] [PubMed] [Google Scholar]
- 60.Lonergan T, Bavister B, Brenner C. Mitochondria in stem cells. Mitochondrion. 2007;7:289–96. doi: 10.1016/j.mito.2007.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Lonergan T, Brenner C, Bavister B. Differentiation-related changes in mito-chondrial properties as indicators of stem cell competence. J Cell Physiol. 2006;208:149–53. doi: 10.1002/jcp.20641. [DOI] [PubMed] [Google Scholar]
- 62.Prigione A, Adjaye J. Modulation of mitochondrial biogenesis and bioenergetic metabolism upon in vitro and in vivo differentiation of human ES and iPS cells. Int J Dev Biol. 2010;54:1729–41. doi: 10.1387/ijdb.103198ap. [DOI] [PubMed] [Google Scholar]
- 63.Prigione A, Fauler B, Lurz R, Lehrach H, Adjaye J. The senescence-related mitochondrial/oxidative stress pathway is repressed in human induced pluripotent stem cells. Stem Cells. 2010;28:721–33. doi: 10.1002/stem.404. [DOI] [PubMed] [Google Scholar]
- 64.Seyfried TN, Shelton LM. Cancer as a metabolic disease. Nutr Metab (Lond) 2010;7:7. doi: 10.1186/1743-7075-7-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Puceat M. Role of Rac-GTPase and reactive oxygen species in cardiac differentiation of stem cells. Antiox Redox Signal. 2005;7:1435–9. doi: 10.1089/ars.2005.7.1435. [DOI] [PubMed] [Google Scholar]
- 66.Tsatmali M, Walcott EC, Makarenkova H, Crossin KL. Reactive oxygen species modulate the differentiation of neurons in clonal cortical cultures. Mol Cell Neurosci. 2006;33:345–57. doi: 10.1016/j.mcn.2006.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Candelario KM, Shuttleworth CW, Cunningham LA. Metabolic properties of neural stem/progenitor cells isolated from embryonic and adult brain. in preparation. [Google Scholar]
- 68.Vander Heiden MG, Cantley LC, Thompson CB. Understanding the Warburg effect: the metabolic requirements of cell proliferation. Science. 2009;324:1029–33. doi: 10.1126/science.1160809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Manalo DJ, Rowan A, Lavoie T, Natarajan L, Kelly BD, Ye SQ, et al. Transcriptional regulation of vascular endothelial cell responses to hypoxia by HIF-1. Blood. 2005;105:659–69. doi: 10.1182/blood-2004-07-2958. [DOI] [PubMed] [Google Scholar]
- 70.Covello KL, Simon MC. HIFs, hypoxia, and vascular development. Curr Top Dev Biol. 2004;62:37–54. doi: 10.1016/S0070-2153(04)62002-3. [DOI] [PubMed] [Google Scholar]
- 71.Iyer NV, Kotch LE, Agani F, Leung SW, Laughner E, Wenger RH, et al. Cellular and developmental control of O2 homeostasis by hypoxia-inducible factor 1 alpha. Genes Dev. 1998;12:149–62. doi: 10.1101/gad.12.2.149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Tomita S, Ueno M, Sakamoto M, Kitahama Y, Ueki M, Maekawa N, et al. Defective brain development in mice lacking the Hif-1alpha gene in neural cells. Mol Cell Biol. 2003;23:6739–49. doi: 10.1128/MCB.23.19.6739-6749.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Ferrara N, Gerber HP, Le Couter J. The biology of VEGF and its receptors. Nat Med. 2003;9:669–76. doi: 10.1038/nm0603-669. [DOI] [PubMed] [Google Scholar]
- 74.Jin K, Zhu Y, Sun Y, Mao XO, Xie L, Greenberg DA. Vascular endothelial growth factor (VEGF) stimulates neurogenesis in vitro and in vivo. Proc Natl Acad Sci U S A. 2002;99:11946–50. doi: 10.1073/pnas.182296499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Sun Y, Jin K, Xie L, Childs J, Mao XO, Logvinova A, et al. VEGF-induced neuroprotection, neurogenesis, and angiogenesis after focal cerebral ischemia. J Clin Invest. 2003;111:1843–51. doi: 10.1172/JCI17977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Wang Y, Jin K, Mao XO, Xie L, Banwait S, Marti HH, et al. VEGF-overexpressing transgenic mice show enhanced post-ischemic neurogenesis and neuromigration. J Neurosci Res. 2007;85:740–7. doi: 10.1002/jnr.21169. [DOI] [PubMed] [Google Scholar]
- 77.Goldberg JS, Hirschi KK. Diverse roles of the vasculature within the neural stem cell niche. Regen Med. 2009;4:879–97. doi: 10.2217/rme.09.61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Madri JA. Modeling the neurovascular niche: implications for recovery from CNS injury. J Physiol Pharmacol. 2009;60(Suppl. 4):95–104. [PubMed] [Google Scholar]
- 79.Tavazoie M, Van der Veken L, Silva-Vargas V, Louissaint M, Colonna L, Zaidi B, et al. A specialized vascular niche for adult neural stem cells. Cell Stem Cell. 2008;3:279–88. doi: 10.1016/j.stem.2008.07.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Mirzadeh Z, Merkle FT, Soriano-Navarro M, Garcia-Verdugo JM, Alvarez-Buylla A. Neural stem cells confer unique pinwheel architecture to the ventricular surface in neurogenic regions of the adult brain. Cell Stem Cell. 2008;3:265–78. doi: 10.1016/j.stem.2008.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Kokovay E, Goderie S, Wang Y, Lotz S, Lin G, Sun Y, et al. Adult SVZ lineage cells home to and leave the vascular niche via differential responses to SDF1/CXCR4 signaling. Cell Stem Cell. 2010;7:163–73. doi: 10.1016/j.stem.2010.05.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Shen Q, Goderie SK, Jin L, Karanth N, Sun Y, Abramova N, et al. Endothelial cells stimulate self-renewal and expand neurogenesis of neural stem cells. Science. 2004;304:1338–40. doi: 10.1126/science.1095505. [DOI] [PubMed] [Google Scholar]
- 83.Robin AM, Zhang ZG, Wang L, Zhang RL, Katakowski M, Zhang L, et al. Stromal cell-derived factor 1alpha mediates neural progenitor cell motility after focal cerebral ischemia. J Cereb Blood Flow Metab. 2006;26:125–34. doi: 10.1038/sj.jcbfm.9600172. [DOI] [PubMed] [Google Scholar]
- 84.Thored P, Arvidsson A, Cacci E, Ahlenius H, Kallur T, Darsalia V, et al. Persistent production of neurons from adult brain stem cells during recovery after stroke. Stem Cells. 2006;24:739–47. doi: 10.1634/stemcells.2005-0281. [DOI] [PubMed] [Google Scholar]
- 85.Barkho BZ, Munoz AE, Li X, Li L, Cunningham LA, Zhao X. Endogenous matrix metalloproteinase (MMP)-3 and MMP-9 promote the differentiation and migration of adult neural progenitor cells in response to chemokines. Stem Cells. 2008;26:3139–49. doi: 10.1634/stemcells.2008-0519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Liu H, Xue W, Ge G, Luo X, Li Y, Xiang H, et al. Hypoxic preconditioning advances CXCR4 and CXCR7 expression by activating HIF-1alpha in MSCs. Biochem Biophys Res Commun. 2010;401:509–15. doi: 10.1016/j.bbrc.2010.09.076. [DOI] [PubMed] [Google Scholar]
- 87.Wang X, Li C, Chen Y, Hao Y, Zhou W, Chen C, et al. Hypoxia enhances CXCR4 expression favoring microglia migration via HIF-1alpha activation. Biochem Biophys Res Commun. 2008;371:283–8. doi: 10.1016/j.bbrc.2008.04.055. [DOI] [PubMed] [Google Scholar]
- 88.Ingraham CA, Park GC, Makarenkova HP, Crossin KL. Matrix metallopro-teinase (MMP)-9 induced by Wnt signaling increases the proliferation and migration of embryonic neural stem cells at low O2 levels. J Biol Chem. 2011;286:17649–57. doi: 10.1074/jbc.M111.229427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Alexson TO, Hitoshi S, Coles BL, Bernstein A, van der Kooy D. Notch signaling is required to maintain all neural stem cell populations – irrespective of spatial or temporal niche. Dev Neurosci. 2006;28:34–48. doi: 10.1159/000090751. [DOI] [PubMed] [Google Scholar]
- 90.Mizutani K, Yoon K, Dang L, Tokunaga A, Gaiano N. Differential Notch signalling distinguishes neural stem cells from intermediate progenitors. Nature. 2007;449:351–5. doi: 10.1038/nature06090. [DOI] [PubMed] [Google Scholar]
- 91.Breunig JJ, Silbereis J, Vaccarino FM, Sestan N, Rakic P. Notch regulates cell fate and dendrite morphology of newborn neurons in the postnatal dentate gyrus. Proc Natl Acad Sci U S A. 2007;104:20558–63. doi: 10.1073/pnas.0710156104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Ables JL, Decarolis NA, Johnson MA, Rivera PD, Gao Z, Cooper DC, et al. Notch1 is required for maintenance of the reservoir of adult hippocampal stem cells. J Neurosci. 2010;30:10484–92. doi: 10.1523/JNEUROSCI.4721-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Carlen M, Meletis K, Goritz C, Darsalia V, Evergren E, Tanigaki K, et al. Fore-brain ependymal cells are Notch-dependent and generate neuroblasts and astrocytes after stroke. Nat Neurosci. 2009;12:259–67. doi: 10.1038/nn.2268. [DOI] [PubMed] [Google Scholar]
- 94.Pistollato F, Abbadi S, Rampazzo E, Viola G, Della Puppa A, Cavallini L, et al. Hypoxia and succinate antagonize 2-deoxyglucose effects on glioblastoma. Biochem Pharmacol. 2010;80:1517–27. doi: 10.1016/j.bcp.2010.08.003. [DOI] [PubMed] [Google Scholar]
- 95.Adachi K, Mirzadeh Z, Sakaguchi M, Yamashita T, Nikolcheva T, Gotoh Y, et al. Beta-catenin signaling promotes proliferation of progenitor cells in the adult mouse subventricular zone. Stem Cells. 2007;25:2827–36. doi: 10.1634/stemcells.2007-0177. [DOI] [PubMed] [Google Scholar]
- 96.Li G, Pleasure SJ. Morphogenesis of the dentate gyrus: what we are learning from mouse mutants. Dev Neurosci. 2005;27:93–9. doi: 10.1159/000085980. [DOI] [PubMed] [Google Scholar]
- 97.Lie DC, Colamarino SA, Song HJ, Desire L, Mira H, Consiglio A, et al. Wnt signalling regulates adult hippocampal neurogenesis. Nature. 2005;437:1370–5. doi: 10.1038/nature04108. [DOI] [PubMed] [Google Scholar]
- 98.Morris DC, Zhang ZG, Wang Y, Zhang RL, Gregg S, Liu XS, et al. Wnt expression in the adult rat subventricular zone after stroke. Neurosci Lett. 2007;418:170–4. doi: 10.1016/j.neulet.2007.03.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Gao Z, Ure K, Ables JL, Lagace DC, Nave KA, Goebbels S, et al. Neurod1 is essential for the survival and maturation ofadult-born neurons. Nat Neurosci. 2009;12:1090–2. doi: 10.1038/nn.2385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Kuwabara T, Hsieh J, Muotri A, Yeo G, Warashina M, Lie DC, et al. Wnt-mediated activation of Neuro D1 and retro-elements during adult neurogenesis. Nat Neurosci. 2009;12:1097–105. doi: 10.1038/nn.2360. [DOI] [PMC free article] [PubMed] [Google Scholar]


