Abstract
Although conditions favoring casein micelle aggregation are well known, factors promoting the dissociation of the casein micelle are not fully understood. It was our objective to investigate the ethanol-induced dissociation of micellar casein as affected by temperature and a wide range of pH, along with the concentrations of calcium and casein. Two different concentrations of casein micelles were dispersed in imidazole buffer with 0 to 80% ethanol (vol/vol) and 2 and 10 mM calcium. Apparent micelle size was determined by dynamic light scattering at 5, 30, and 60°C. In the absence of ethanol, casein precipitation occurred at pH 4.6 in imidazole buffer. Ten to forty percent ethanol promoted casein aggregation (>1,000 nm) and higher temperature (30 and 60°C) enhanced this effect. Higher ethanol concentrations at 50 to 80% induced the dissociation (<40 nm) of the casein micelle upon acidification (pH <5) and alkalization (pH >8) in imidazole buffer. In addition, higher concentrations of casein (0.25 mg/mL) and calcium (20 mM) caused the formation of larger aggregates (>1,000 nm) in the presence of ethanol when comparing with the initial lower concentrations of casein (0.1 mg/mL) and calcium (2 mM). Casein micelle dissociation can be achieved near the isoelectric pH by modifying the solvent composition and temperature.
Keywords: casein micelle, ethanol, particle size, calcium
INTRODUCTION
Milk proteins are fundamental functional constitutes for food manufacturing due to their high nutritional benefits and unique structural and physicochemical properties (Elzoghby et al., 2011; Ye, 2011). As the most abundant protein in bovine milk, caseins (αS1-, αS2-, β-, and κ-CN) are a group of proline-rich, intrinsically disordered phosphoproteins (de Kruif and Holt, 2003). The determination of ethanol stability of milk was initially used as an indirect rapid test to assess thermal stability (Horne and Parker, 1981a). Horne and Parker (1981a,b,c,d, 1982) conducted extensive research on the effects of ethanol on fluid milk stability and demonstrated a sigmoidal pH-dependent behavior within the 6 to 7 pH range (Horne et al., 1986). Furthermore, the subject of ethanol-induced stability of milk is summarized in a review by Horne (2003). Of note, the study of the ethanol-induced aggregation-dissociation of casein micelles is important to understand factors that control micellar stability and to investigate novel casein micelle functionality. Casein micelles have been shown to dissociate with combinations of high temperature (>40°C) and high ethanol concentration (>40%; Zadow, 1993) and this effect is believed to be due to a change in solvent quality as temperature increases (O’Connell et al., 2001). Recent studies have explored the effect of high pressure on the heat- and ethanol-induced changes in milk and Huppertz et al. (2004) showed that the ethanol stability of raw skim milk was reduced by high hydrostatic pressure. Most recently, it was concluded that high ethanol content (>30%) combined with high temperature (>40°C) dissociated the casein micelles but blocked sites available for hydrophobic interaction (Trejo and Harte, 2010).
Micellar aggregation or dissociation into submicellar particles can be achieved by altering environmental factors (e.g., pH, temperature, and ionic strength) that affect micelle stability due to the absence of a rigid 3-dimensional tertiary conformation in casein micelles (Walstra, 1990; Marchin et al., 2007; McMahon and Oommen, 2008; Beliciu and Moraru, 2009). A decrease in pH can induce low temperature-mediated micelle dissociation by decreasing the stability of the κ-CN layer and promoting migration of colloidal calcium phosphate into the serum phase (Huppertz et al., 2004; Yong and Foegeding, 2010). The dissociation of the casein micelle as the pH decreases is temperature dependent. Whereas migration of micellar calcium phosphate to the serum occurs at pH 5.5, and 40% of casein is liberated into the serum at 4°C, little disruption of micelles occurs at 30°C (Dalgleish and Law, 1988), probably due to an increase in hydrophobic interactions. At alkaline pH (10.0), the disruption and disassembly of casein micelles occurred in the absence of solvent and subsequently micelles gradually reassembled by decreasing milk pH to 6.6 (Huppertz et al., 2008).
The effect of calcium concentration, temperature, and pH on casein micelle stability in the presence of various concentrations of ethanol remains unknown. The objectives of this study were to determine aggregation and dissociation of casein micelles (casein micelle mapping) as affected by ethanol concentration, calcium concentration, casein concentration, pH, and temperature in a buffer solution. Potential applications of ethanol-induced modifications of casein micelle include textural and stability enhancements of dairy products (Ausar et al., 2005) and the use of casein micelles in cosmetic, pharmaceutical, and biomedical applications (Mattiasson et al., 1998; de Kruif and Tuinier, 2001; Roux et al., 2002; Livney, 2010; Elzoghby et al., 2011; Matalanis et al., 2011; Neethirajan and Jayas, 2011).
MATERIALS AND METHODS
Micellar casein powder was purchased from American Casein Corporation (Burlington, NJ) and used throughout the study. Micellar casein is free-flowing powder obtained by UF of milk without acidification. The typical composition is 86% protein, 1.5% fat, 6.0% ash, 4% carbohydrates, and 5% moisture content. Twenty millimolar Imidazole buffer (Fisher Chemicals, Fair Lawn, NJ) containing 20 or 2 mM CaCl2 (Fisher Chemicals, Fair Lawn, NJ) was mixed with absolute ethanol (99.5%; Acros Organics, Fair Lawn, NJ) to yield ethanol concentrations from 0 to 80% (vol/vol; 10% increments). The pH of buffer/ethanol mixtures was gently adjusted using 2 N HCl or NaOH (Fisher Chemicals) from 3 to 10. Casein was diluted with the imidazole/ethanol mixture at 0.25 and 0.1 mg/mL, respectively. Afterward, each sample was stored overnight at 4°C and analyzed for particle size at 5, 30, and 60°C, respectively. Two independent replicate samples were performed.
Particle size analysis was conducted by photon correlation spectroscopy using a DelsaNano C particle size analyzer (165° angle, 1.34 refractive index, 50-μm pinhole; Beckman Coulter Inc., Atlanta, GA). Calibration was checked with the standard sample provided by Beckman Coulter Inc. and native casein micelles from raw skim milk diluted in protein-free milk serum at atmospheric pressure and a pH of approximately 7.0. Samples continuously flowed through a quartz measuring cell and the temperature was controlled by immersion of a heating/cooling coil connected to a controlled temperature water bath (Isotemp 3006S; Fisher Scientific, Pittsburgh, PA). The pH of the sample was modified using 2 N HCl or NaOH (Fisher Chemicals) and monitored by direct immersion of a pH electrode (UB-10; Denver Instrument Co., Denver, CO) into the sample reservoir. Nitrogen gas was used to prevent condensation in the measuring cell at 4°C. The particle size (casein micelle apparent diameter, D3,4) was calculated as the average of 70 autocorrelation functions. All measurements were conducted in 2 replicates. Ethanol versus pH versus particle size contour plots were constructed using SigmaPlot software (version 12; Systat Software Inc., San Jose, CA). The upper limit for particle diameter in the contour plot was set at 10 μm, as detailed information on aggregates in the micron scale was not considered of interest.
For each temperature, experiments were analyzed as factorials of 2 factors, factor pH at 9 levels (3, 4, 4.5, 5, 6, 7, 8, 9, and 10), and factor ethanol at 9 levels (0, 10, 20, 30, 40, 50, 60, 70, and 80%), in a completely randomized block design with 2 replications. Because a single source of casein powder was used throughout the experiments, the coefficient of variation of the variable under study (micelle diameter) was <4%, and the effect of pH and ethanol content was highly significant (P < 0.001).
RESULTS AND DISCUSSION
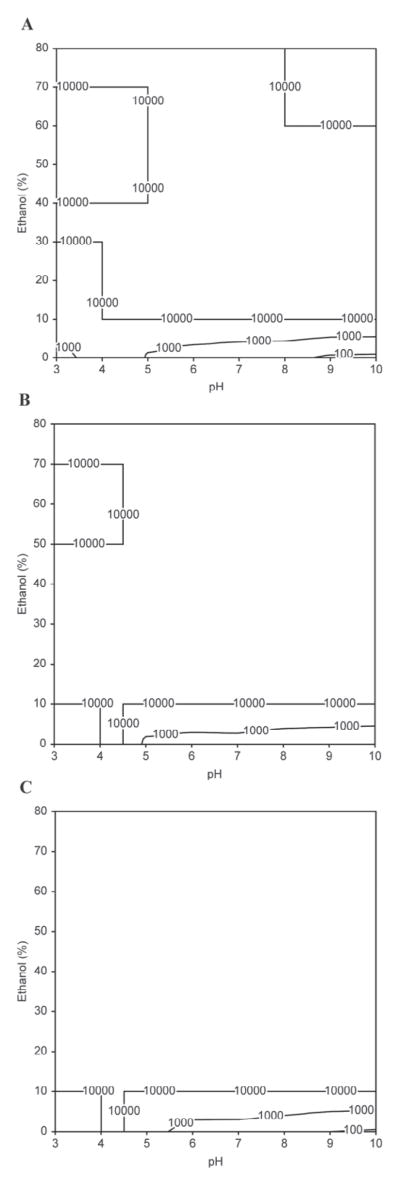
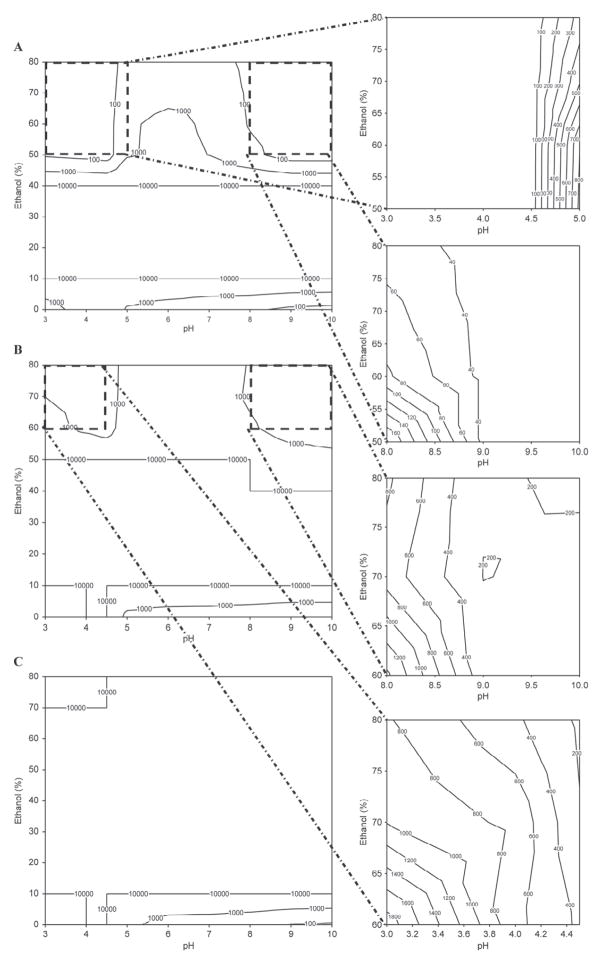
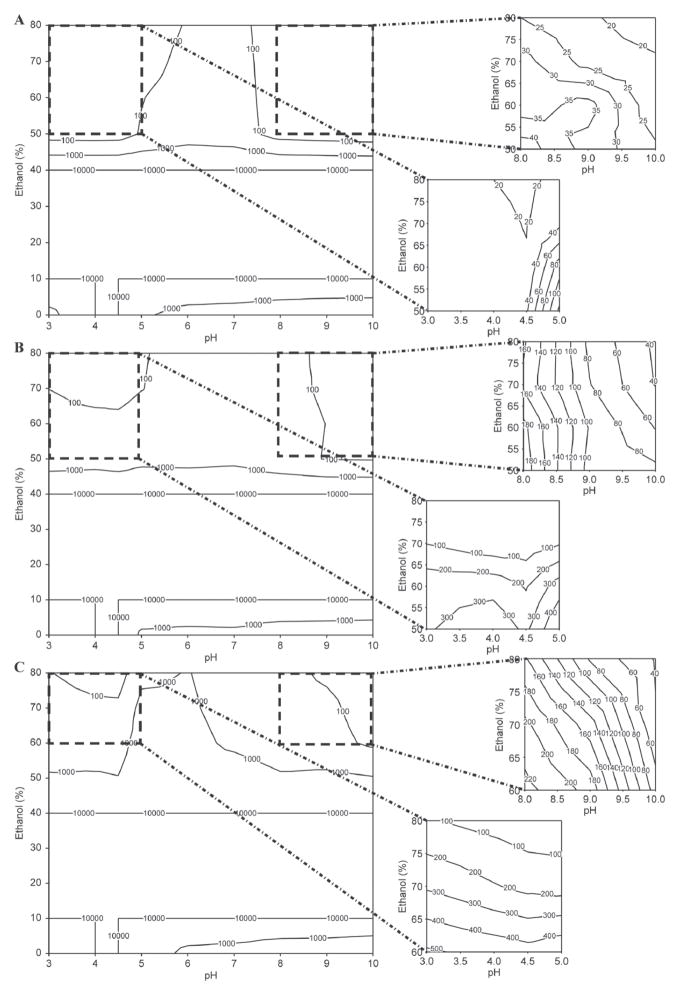
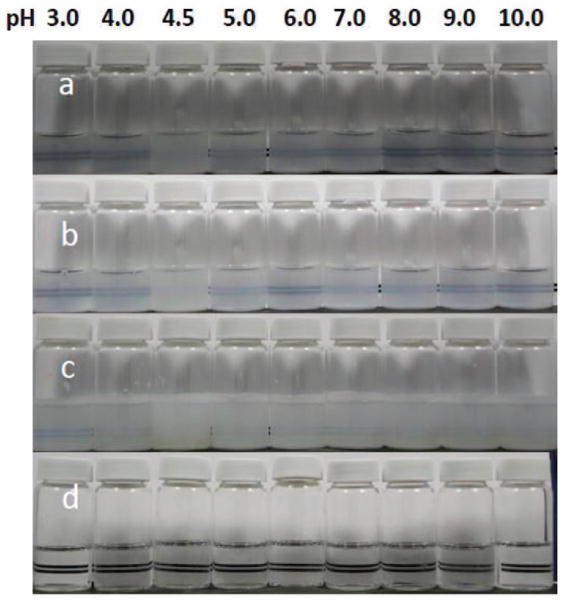
In the absence of ethanol or at a low ethanol concentration (<10%), casein micelles aggregated at their isoelectric point regardless of other factors including temperature, calcium concentration, and initial casein concentration (Figures 1A, 2A, 3A, and 4a). This was expected, as the reduction in micelle surface charge causes the collapse of the κ-CN layer as pH approaches approximately 4.6 (de Kruif and Tuinier, 2001) initiating aggregation-coalescence of unstabilized micelles into submicron-sized aggregates. The casein micelles formed larger aggregates as the ethanol concentration was promoted to 20 to 40% (Figures 4b and 4c). This behavior was more evident for samples with higher casein concentration (0.25 mg/mL; Figures 1C, 2C, and 3C). Large-sized aggregation was observed as the pH reached the 5.0 to 6.0 range (Figure 4). However, casein micelle aggregates gradually decreased to the submicron range above 40 to 50% ethanol concentration, and exhibited pH-dependent behavior that was different from that of casein micelles in milk. Above 50% ethanol, the behavior of casein micelles was pH, temperature, and calcium and casein concentration dependent (Figures 1–3). Dissociation of casein micelles below 40 nm was observed at pH <4.5, regardless of calcium concentration when temperature was maintained at higher temperature 60°C at the 50 to 80% ethanol concentration level (Figure 3). Of note, the particle sizes of casein micelles decreased with the increase in pH above 8 in the presence of ethanol, consistent with the results of some earlier studies reported in the literature without the use of ethanol (Vaia et al., 2006; Zhong et al., 2007; Liu and Guo, 2008), as the structure of casein micelles is loose and dissociated at high pH (Liu and Guo, 2008).
Figure 1.
Contour plots depicting the relationships between pH, ethanol concentration, and casein micelle apparent diameter (D3,4, nm) in the presence of 20 mM imidazole at 5°C with (A) 2 mM CaCl2 and 0.1 mg of casein/mL, (B) 20 mM CaCl2 and 0.1 mg of casein/mL, (C) 2 mM CaCl2 and 0.25 mg of casein/mL. Data points represent the modal distribution of particle size at the peak frequency distribution. The upper limit for contour plots is 10 μm.
Figure 2.
Contour plots depicting the relationships between pH, ethanol concentration, and casein micelle apparent diameter (D3,4, nm) in the presence of 20 mM imidazole at 30°C with (A) 2 mM CaCl2 and 0.1 mg of casein/mL, (B) 20 mM CaCl2 and 0.1 mg of casein/mL, (C) 2 mM CaCl2 and 0.25 mg of casein/mL. Data points represent the modal distribution of particle size at the peak frequency distribution. The upper limit for contour plots is 10 μm.
Figure 3.
Contour plots depicting the relationships between pH, ethanol concentration, and casein micelle apparent diameter (D3,4, nm) in the presence of 20 mM imidazole at 60°C with (A) 2 mM CaCl2 and 0.1 mg of casein/mL, (B) 20 mM CaCl2 and 0.1 mg of casein/mL, (C) 2 mM CaCl2 and 0.25 mg of casein/mL. Data points represent the modal distribution of particle size at the peak frequency distribution. The upper limit for contour plots is 10 μm.
Figure 4.
Visual images of 0.1 mg of casein micelle/mL in the presence of 20 mM imidazole and 2 mM CaCl2 at 60°C with (a) 0% ethanol, (b) 10% ethanol, (c) 30% ethanol, or (d) 50% ethanol. Color version available in the online PDF.
The molecular mechanism of how ethanol affects the protein quaternary structure is still not fully elucidated. Whereas initial studies found that ethanol interacts with polar amino acid side chains in BSA (Lubas et al., 1979), Avdulov et al. (1996) showed that binding was primarily explained by ethanol methyl groups interacting with hydrophobic pockets in the protein. Colloidal calcium phosphate may also explain ethanol-induced dissociation of the casein micelle. Because ethanol is less polar than water, it lowers the dielectric constant of the medium, potentiating electrostatic interactions that play a key role in stabilizing the casein micelle. The increased solubility of colloidal calcium phosphate during acidification was also a contributor to micellar dissociation. The loss of this micellar electrostatic glue may promote casein dissociation at elevated concentrations of ethanol and low pH.
In Figures 1B, 2B, and 3B, the size of the aggregation increased with the increase of the calcium concentration, regardless of other factors (2 vs. 20 mM). The milk salts calcium and phosphate have an important role in the structure of casein. The addition of calcium enhanced the rennet coagulation of milk because it neutralized negatively charged residues on the surface of casein, thereby causing the increase in the aggregation of casein micelles (Lucey and Fox, 1993; Choi et al., 2007). In addition, the particle-stabilizing properties of the hairy layer of κ-CN surrounding the casein micelles and calcium-sensitive β-CN were closely associated with the calcium concentration (Choi et al., 2007; Portnaya et al., 2008).
To determine the effect of casein concentration on the particle size of casein micelles at high ethanol concentration at a broad range of pH, the concentration of casein was increased upon 0.25 mg/mL (2.5-fold higher than the previous sample at 0.1 mg/mL). Other conditions remained the same. The particle sizes of samples with higher casein concentration were increased, forming larger aggregates than the previous samples, regardless of pH value (Figures 1C, 2C, and 3C). This finding expands the potential application of dissociated casein micelle solutions, as protein concentration will greatly affect the physical properties of the final product.
It is important to note that experiments were run in a tightly controlled system exhibiting low experimental error. However, as several factors affect the structure and function of casein micelles (e.g., season and processing condition), it is probable that other sources of casein or milk powder show variations in their response to ethanol, pH, and temperature. Despite this, our own exploratory tests showed that caseins from various sources showed similar patterns of dissociation-aggregation in response to the same physical factors.
CONCLUSIONS
The combined effects of the concentrations of calcium and casein, along with processing temperature in the presence of different concentrations of ethanol, contributed in obtaining a wide range of particle sizes of the casein micelle. It appears that 2 distinct phenomena occur within the micelle: one that facilitates aggregation and one that induces dissociation. In fact, it is well known that caseins precipitate at their isoelectric point of 4.6 and protein aggregation is induced in the presence of ethanol. However, acidification (pH <5) or alkalization (pH >8), or both, in the presence of elevated ethanol concentrations (>50%) induces micelle dissociation at higher temperature (30 and 60°C). In addition, higher concentrations of soluble calcium and casein lead to larger aggregates in the presence of ethanol. Although the dissociative effects of the micelles is not fully clear, casein micelles can be dissociated at low and high pH, high ethanol concentrations, and moderate to high temperature combinations. It is hypothesized that the presence of a nonpolar solvent, such as ethanol, promotes the solubility of the otherwise insoluble proteins during acidification or alkalization through hydrophobic interactions of methyl groups with hydrophobic amino acids. These findings indicate that it is possible to achieve targeted casein micelle size populations based on a combination of pH, temperature, and ethanol concentrations.
Acknowledgments
This project was supported by award number R21HD065170 from the Eunice Kennedy Shriver National Institute of Child Health & Human Development (Rockville, MD). The content is solely the responsibility of the authors and does not necessarily represent the official views of the Eunice Kennedy Shriver National Institute of Child Health & Human Development or the National Institutes of Health (Bethesda, MD). This research was also supported by the Dairy Research Institute (Rosemont, IL).
References
- Ausar SF, I, Bianco D, Castagna LF, Alasino RV, Narambuena CF, Leiva EPM, Beltramo DM. Reversible precipitation of casein micelles with a cationic hydroxyethylcellulose. J Agric Food Chem. 2005;53:9031–9038. doi: 10.1021/jf050766o. [DOI] [PubMed] [Google Scholar]
- Avdulov NA, Chochina SV, Daragan VA, Schroeder F, Mayo KH, Wood WG. Direct binding of ethanol to bovine serum albumin: A fluorescent and 13C NMR multiplet relaxation study. Biochemistry. 1996;35:340–347. doi: 10.1021/bi9513416. [DOI] [PubMed] [Google Scholar]
- Beliciu CM, Moraru CI. Effect of solvent and temperature on the size distribution of casein micelles measured by dynamic light scattering. J Dairy Sci. 2009;92:1829–1839. doi: 10.3168/jds.2008-1467. [DOI] [PubMed] [Google Scholar]
- Choi J, Horne DS, Lucey JA. Effect of insoluble calcium concentration on rennet coagulation properties of milk. J Dairy Sci. 2007;90:2612–2623. doi: 10.3168/jds.2006-814. [DOI] [PubMed] [Google Scholar]
- Dalgleish DG, Law AJR. pH-induced dissociation of casein micelles. 1. Analysis of liberated caseins. J Dairy Res. 1988;55:529–538. [Google Scholar]
- de Kruif CG, Holt C. Casein micelle structure, functions and interactions. In: Fox PF, McSweeney PLH, editors. Advanced Dairy Chemistry. 3. Vol. 1. Kluwer Academic; New York, NY: 2003. pp. 233–276. [Google Scholar]
- de Kruif CG, Tuinier R. Polysaccharide protein interactions. Food Hydrocoll. 2001;15:555–563. [Google Scholar]
- Elzoghby AO, El-Fotoh WSA, Elgindy NA. Casein-based formulations as promising controlled release drug delivery systems. J Control Release. 2011;153:206–216. doi: 10.1016/j.jconrel.2011.02.010. [DOI] [PubMed] [Google Scholar]
- Horne DS. Ethanol stability. In: Fox PF, McSweeney PLH, editors. Advanced Dairy Chemistry. 3. Vol. 1. Kluwer Academic; New York, NY: 2003. pp. 975–999. [Google Scholar]
- Horne DS, Parker TG. Factors affecting the ethanol stability of bovine milk: I. Effect of serum phase components. J Dairy Res. 1981a;48:273–284. [Google Scholar]
- Horne DS, Parker TG. Factors affecting the ethanol stability of bovine milk. II. The origin of the pH transition. J Dairy Res. 1981b;48:285–291. [Google Scholar]
- Horne DS, Parker TG. Factors affecting the ethanol stability of bovine milk. III. Substitution of ethanol by other organic solvents. Int J Biol Macromol. 1981c;3:399–402. [Google Scholar]
- Horne DS, Parker TG. Factors affecting the ethanol stability of bovine milk: IV. Effect of forewarming. J Dairy Res. 1981d;48:405–415. [Google Scholar]
- Horne DS, Parker TG. Factors affecting the ethanol stability of bovine milk: V. Effects of chemical modification of milk protein. J Dairy Res. 1982;49:449–457. [Google Scholar]
- Horne DS, Parker TG, Donnelly WJ, Davies DT. Factors affecting the ethanol stability of bovine skim milk: VII. Lactational and compositional effects. J Dairy Res. 1986;53:407–417. [Google Scholar]
- Huppertz T, Grosman S, Fox PF, Kelly AL. Heat and ethanol stabilities of high-pressure-treated bovine milk. Int Dairy J. 2004;14:125–133. [Google Scholar]
- Huppertz T, Vaia B, Smiddy MA. Reformation of casein particles from alkaline-disrupted casein micelles. J Dairy Res. 2008;75:44–47. doi: 10.1017/S0022029907002956. [DOI] [PubMed] [Google Scholar]
- Liu Y, Guo R. pH-dependent structures and properties of casein micelles. Biophys Chem. 2008;136:67–73. doi: 10.1016/j.bpc.2008.03.012. [DOI] [PubMed] [Google Scholar]
- Livney YD. Milk proteins as vehicles for bioactives. Curr Opin Colloid Interface Sci. 2010;15:73–83. [Google Scholar]
- Lubas B, Soltysik-Rasek M, Leśniewska I. Proton nuclear magnetic resonance study of the association of monovalent and divalent alcohols with bovine serum albumin. Biochemistry. 1979;18:4943–4951. doi: 10.1021/bi00589a024. [DOI] [PubMed] [Google Scholar]
- Lucey JA, Fox PF. Importance of calcium and phosphate in cheese manufacture: A review. J Dairy Sci. 1993;76:1714–1724. [Google Scholar]
- Marchin S, Putaux JL, Pignon F, Léonil J. Effects of the environmental factors on the casein micelle structure studied by cryo transmission electron microscopy and small-angle x-ray scattering/ultrasmall-angle x-ray scattering. J Chem Phys. 2007;126:045101. doi: 10.1063/1.2409933. [DOI] [PubMed] [Google Scholar]
- Matalanis A, Jones OG, McClements DJ. Structured biopolymer-based delivery systems for encapsulation, protection, and release of lipophilic compounds. Food Hydrocoll. 2011;25:1865–1880. [Google Scholar]
- Mattiasson B, Kumar A, Galaev IY. Affinity precipitation of proteins: Design criteria for an efficient polymer. J Mol Recognit. 1998;11:211–216. doi: 10.1002/(SICI)1099-1352(199812)11:1/6<211::AID-JMR425>3.0.CO;2-Y. [DOI] [PubMed] [Google Scholar]
- McMahon DJ, Oommen BS. Supramolecular structure of the casein micelle. J Dairy Sci. 2008;91:1709–1721. doi: 10.3168/jds.2007-0819. [DOI] [PubMed] [Google Scholar]
- Neethirajan S, Jayas DS. Nanotechnology for the food and bioprocessing industries. Food Bioprocess Technol. 2011;4:39–47. doi: 10.1007/s11947-010-0328-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Connell JE, Kelly AL, Fox PF, de Kruif KG. Mechanism for the ethanol-dependent heat-induced dissociation of casein micelles. J Agric Food Chem. 2001;49:4424–4428. doi: 10.1021/jf001479h. [DOI] [PubMed] [Google Scholar]
- Portnaya I, Ben-Shoshan E, Cogan U, Khalfin R, Fass D, Ramon O, Danino D. Self-assembly of bovine β-casein below the isoelectric pH. J Agric Food Chem. 2008;56:2192–2198. doi: 10.1021/jf072630r. [DOI] [PubMed] [Google Scholar]
- Roux E, Francis M, Winnik FM, Leroux JC. Polymer based pH-sensitive carriers as a means to improve the cytoplasmic delivery of drugs. Int J Pharm. 2002;242:25–36. doi: 10.1016/s0378-5173(02)00183-7. [DOI] [PubMed] [Google Scholar]
- Trejo R, Harte F. The effect of ethanol and heat on the functional hydrophobicity of casein micelles. J Dairy Sci. 2010;93:2338–2343. doi: 10.3168/jds.2009-2918. [DOI] [PubMed] [Google Scholar]
- Vaia B, Smiddy MA, Kelly AL, Huppertz T. Solvent-mediated disruption of bovine casein micelles at alkaline pH. J Agric Food Chem. 2006;54:8288–8293. doi: 10.1021/jf061417c. [DOI] [PubMed] [Google Scholar]
- Walstra P. On the stability of casein micelles. J Dairy Sci. 1990;73:1965–1979. [Google Scholar]
- Ye AQ. Functional properties of milk protein concentrates: Emulsifying properties, adsorption and stability of emulsions. Int Dairy J. 2011;21:14–20. [Google Scholar]
- Yong YH, Foegeding EA. Caseins: Utilizing molecular chaperone properties to control protein aggregation in foods. J Agric Food Chem. 2010;58:685–693. doi: 10.1021/jf903072g. [DOI] [PubMed] [Google Scholar]
- Zadow JG. Alcohol-mediated temperature-induced reversible dissociation of the casein micelle in milk. Aust J Dairy Technol. 1993;48:78–81. [Google Scholar]
- Zhong Q, Daubert CR, Velev OD. Physicochemical variables affecting the rheology and microstructure of rennet casein gels. J Agric Food Chem. 2007;55:2688–2697. doi: 10.1021/jf0625914. [DOI] [PubMed] [Google Scholar]