Abstract
Smith Lemli Opitz syndrome (SLOS) is an inherited malformation and mental retardation metabolic disorder with no cure. Mutations in the last enzyme of the cholesterol biosynthetic pathway, 7-dehydrocholesterol reductase (DHCR7), lead to cholesterol insufficiency and accumulation of its dehyrdocholesterol precursors, and contribute to its pathogenesis. The central nervous system (CNS) constitutes a major pathophysiological component of this disorder and remains unamenable to dietary cholesterol therapy due to the impenetrability of the blood brain barrier (BBB). The goal of this study was to restore sterol homeostasis in the CNS. To bypass the BBB, gene therapy using an adeno-associated virus (AAV-8) vector carrying a functional copy of the DHCR7 gene was administered by intrathecal (IT) injection directly into the cerebrospinal fluid of newborn mice. Two months post-treatment, vector DNA and DHCR7 expression was observed in the brain and a corresponding improvement of sterol levels seen in the brain and spinal cord. Interestingly, sterol levels in the peripheral nervous system also showed a similar improvement. This study shows that IT gene therapy can have a positive biochemical effect on sterol homeostasis in the central and peripheral nervous systems in a SLOS animal model. A single dose delivered three days after birth had a sustained effect into adulthood, eight weeks post-treatment. These observations pave the way for further studies to understand the effect of biochemical improvement of sterol levels on neuronal function, to provide a greater understanding of neuronal cholesterol homeostasis, and to develop potential therapies.
Keywords: Smith Lemli Opitz syndrome (SLOS), 7-dehydrocholesterol reductase (DHCR7), Central nervous system (CNS), Intrathecal, AAV, Gene therapy
1. Introduction
Smith-Lemli-Opitz syndrome (SLOS, OMIM 270400. locus 11q13.4) is an inborn error of cholesterol biosynthesis [1], [2], [3]. It is an autosomal recessive disorder with a reported incidence of 1 in 10,000–70,000 and a carrier frequency as high as 1 in 30 in Caucasian populations [3], [4], [5]. Clinical manifestations include physical dysmorphias and cognitive impairments that vary in scope and severity. Several organ systems are affected, particularly the central nervous system (CNS), heart, kidneys, genitalia, and limbs. Typical features include 2–3 toe syndactyly, craniofacial malformations, and growth and developmental delays. Feeding and behavioral abnormalities are also common among SLOS children [1], [6], [7], [8].
SLOS results from mutations in the DHCR7 gene, which encodes 7-dehydrocholesterol reductase (EC 1.3.1.21, 3β-hydroxysterol-Δ7-reductase), the last enzyme in the Kandutsch-Russell pathway of cholesterol biosynthesis, and converts 7-dehydrocholesterol (7-DHC) to cholesterol [2], [3], [9]. The resulting loss of enzyme activity leads to an overall cholesterol deficiency and markedly elevated levels of its precursor, 7-DHC, and in some tissues its isomer, 8-DHC [10]. Severe cases of SLOS resulting in perinatal lethality generally carry DHCR7 null mutations in both alleles while typically affected individuals retain some residual enzyme activity [11], [12], [13]. Diagnosis for SLOS is based on elevated levels of 7-DHC as measured by Gas Chromatography/Mass Spectrometry (GC/MS) in patient serum or plasma, or in the amniotic fluid or chorionic villi samples in prenatal cases [14]. An alternative, non-invasive, prenatal diagnostic test for SLOS relies on the presence of elevated levels of fetal 7- and 8-DHC derived dehydrosteroids in maternal urine [15].
Severely reduced cholesterol is predicted to have dramatic consequences due to its quintessential role in normal cell physiology and in both embryonic and postnatal development. Cholesterol is a major component of cell and organelle membranes and vesicles, lipid rafts, and of the myelin sheath that surrounds the white matter of the brain, central and peripheral nerves. It is an effector of hedgehog signaling, and is a precursor of steroid hormones and bile acids. Its shortage, thus, leads to developmental and neurological consequences contributing to the pathology of SLOS [8], [16], [17], [18]. Further compounding this problem is the build-up of its precursor 7-DHC, which can get incorporated in membranes, altering integral properties such as fluidity, dipole potentials, domain structure, interactions and functioning of resident membrane receptor proteins. 7-DHC is also highly reactive and is rapidly converted to toxic oxysterols, which can exert deleterious effects on cells and tissues [19], [20], [21].
At present, there is no cure for SLOS and efforts are mainly directed towards treating symptoms and correcting the underlying cholesterol deficiency. The current standard for SLOS treatment involves dietary supplementation through high doses of food-based or formulated cholesterol [22]. This form of therapy has limited scope. While some benefits to non-neuronal aspects, such as growth and biochemical parameters are observed, neuronal or cognitive deficits remain unchanged [23]. Reports of positive behavioral outcomes are anecdotal and were not confirmed in a clinical trial [24]. This is expected as the blood brain barrier (BBB) prevents the uptake of lipoprotein cholesterol from the circulation and the brain derives cholesterol primarily by de novo synthesis. Dysmorphias due to prenatal disease onset also remain untreatable unless amenable to surgical intervention. As maternal cholesterol is only available to the fetus during early gestation due to the formation of placental barrier, the presence of self-sustaining cholesterol synthesis in early fetal development is obligatory.
Gene therapy to restore DHCR7 activity offers several potential advantages over conventional dietary cholesterol supplementation [25]. Introduction of a functional copy of the defective gene, DHCR7, should theoretically correct both the cholesterol deficiency and the excess build-up of harmful 7-DHC in successfully transduced cells. The effect of gene therapy may be further extended based on the assumption of free exchange of lipoprotein particles carrying cholesterol and 7-DHC between transduced cells and untransduced cells resulting in a more global reduction of 7-DHC to cholesterol. That is, successfully transduced cells might act as active centers for the overall increase in conversion of 7-DHC to cholesterol. Gene therapy also allows the ability to control timing and to target specific organs for treatment, thus enhancing its effectiveness and scope. Treatment can be administered in adult, early neonatal or even prenatal stages, while administration by various routes and/or using different viral vectors can target various tissue types.
We have previously shown that systemic treatment of SLOS model mice with recombinant adeno-associated virus (AAV) vectors carrying human DHCR7 (hDHCR7) cDNA provided positive biochemical and physiological outcomes [25], [26]. Effectiveness of treatment was assessed by measuring the 7-DHC/cholesterol (7-DHC/C) ratio in serum and tissues of treated and untreated SLOS mice, and their normal counterparts by analytical GC/MS methods. This ratio is typically elevated in untreated mice and very low in normal mice due to negligible amounts of 7-DHC. Gene therapy partially normalized this ratio in treated animals. Treated mice also showed improved growth rates as compared to untreated mice. Treatment was more effective when administered in neonates versus post-weaning juveniles. Transgene expression was detected in the target tissue, liver, a principle cholesterolgenic site, and the outcome was sustained for several weeks following a single dose. While positive biochemical effects as measured by reduced 7-DHC/cholesterol ratio were observed in the serum and liver of systemically treated SLOS mice, no effect on sterol levels were observed in the central or peripheral nervous tissues, i.e. the brain or sciatic nerves. Like cholesterol itself, AAV-DHCR7 particles did not effectively cross the blood brain barrier (BBB) from systemic circulation.
The objective of this study was to target gene therapy to the nervous system, particularly to the CNS, and restore sterol levels in the brain. Previous studies in lysosomal storage disease models have shown that intrathecal (IT) injection of AAV vectors into the cerebrospinal fluid (CSF) of affected mice was able to circumvent the BBB [27], [28]. A single injection resulted in widespread and long-lasting expression of the therapeutic enzyme in the brain and a marked reduction in storage bodies. To deliver DHCR7 directly to the brain, AAV type 8 vectors carrying a human DHCR7 cDNA were administered via IT injection into newborn SLOS mice. Treatment was administered in the neonatal stage as this corresponds to a period of rapid nervous system expansion and myelination, and dramatically elevated brain cholesterol synthesis. Cholesterol turnover in adult brain is very slow (4–6 months in mice) making intervention at a later stage likely to be less effective.
This study demonstrates that AAV vectors administrated via the IT route are able to deliver functional copies of DHCR7 to the CNS, a tissue that has proved therapeutically challenging so far. More surprisingly, IT administration also had a positive effect on sterols in peripheral nerves, specifically the sciatic nerves. The ability to normalize cholesterol levels in nervous tissues, particularly the brain, the most cholesterol-rich of all tissues, should have dramatic and far-reaching implications affecting overall organ function, cognition and behavior.
2. Methods
2.1. Animal husbandry
Animal work conformed to NIH guidelines and was approved by the Institutional Animal Care and Use Committee. The animals were maintained in an AALAC certified facility and fed normal, cholesterol-free chow (Teklad irradiated rodent diet 2918: Harlan, Madison, WI).
2.2. Generation of experimental animals
Use of mutant mice and their breeding protocol was as previously described [29]. In brief, our study animals were generated by crossing two separate mouse models of SLOS: heterozygous null mutant females carrying a partial deletion of Dhcr7 (Δ/+) [30], and homozygous mutant males, carrying a hypomorphic point mutation (T93M) [31]. Both models were on FVB genetic backgrounds that had been backcrossed for several generations (> 14) to minimize extraneous genetic heterogeneity. Mice were genotyped using toe clippings two days after birth. Δ/T93M mice, which exhibit the most severe, yet viable phenotype of the disease [31], were injected the following day with vector (treated group) or saline (control group). The Δ/T93M mice exhibit classic SLOS symptoms, such as elevated 7-DHC/C ratios in the serum and tissue, decreased size compared to normal (T93M/+) littermates, and sporadic syndactyly. Littermates having the Δ/T93M genotype were assigned to each experimental group (AAV-treated and saline-treated). Males and females were included in both groups; no apparent correlation has been noted between gender and 7-DHC/C ratios [25].
2.3. Preparation of AAV2/8 Vectors
Vector construction, production and purification were as described previously [25]. hDHCR7 cDNA was cloned into the EcoRI site of the pV4.1c plasmid, which contained a CMV promoter/enhancer and AAV2 inverted terminal repeats. A woodchuck hepatitis virus post-translational regulatory element (WPRE) was included at the 3′ untranslated end of the DHCR7 to increase translational utility. AAV2/8-DHCR7 particles were produced by triple-transfecting HEK293 cells with plasmid pV4.1c-DHCR7, an AAV2/8 packaging plasmid, containing an AAV2 rep gene fused to an AAV8 cap gene [32], and a plasmid containing adenovirus helper genes [33]. DNA packaged in capsid was purified by Optiprep Gradient (Iodixanol) and 2 cesium chloride gradient centrifugations [34]. Finally, the viral vector was dialyzed against normal saline and the titer in vector genomes (vg) per ml was established by quantitative PCR (AAV-DHCR7 titer: 5.8 × 1012 vg/ml).
2.4. Treatment of SLOS mice and tissue collection
Experiments were conducted to examine the delivery of IT administered AAV2/8 packaged DHCR7 cDNA and the effect on sterol levels in the CNS. Three day old Δ/T93M SLOS mouse pups were anesthetized with isofluorane and intrathecally injected with a single 30 μl dose of either AAV-DHCR7 or saline. Injections were performed using a 30-gauge needle, pumped at a controlled rate of 10 μl/min between the vertebrae in the lumbar region of the neonatal pups. Green dye was mixed with the vector and saline as a visual aid to monitor the injection process. A successful injection resulted in the dye appearing only in the brain and spinal cord. Mice were then allowed to recover on a heat pad and then returned to their mothers. Mice were sacrificed eight weeks post injection, and brain, spinal cord, sciatic nerve and liver harvested. All organs were immediately flash frozen in liquid nitrogen and stored at − 80 °C until needed.
2.5. Analysis of cholesterol and dehydrocholesterol by GC/MS
For sterol extraction, tissues were first powdered in liquid nitrogen to ensure homogenous sampling. An aliquot (10–50 mg) of powdered tissue, or a single sciatic nerve was used for sterol extraction as previously described (25, 26). An internal standard (stigmasterol) was added to the samples, which were then saponified. Afterwards, the samples were extracted and derivatized with N,O-Bis (tri-methylsilyl)trifluoroacetamide (BSTFA) to form the trimethylsilyl (TMS) derivatives. The derivatives were solvated in cyclohaxane and transferred to autosampler vials for analysis by GC/MS. To prevent conversion of 7-DHC to previtamin-D3, all tubes were protected from light by foil covers, and the entire procedure conducted under reduced lighting conditions. Analysis was as previously described using an Agilent 7890A/5975C Gas Chromatography/Mass Spectrometry instrument with a DB1 column [25], [29], [35]. Calibration curves were prepared daily for each analytical batch by combining increasing amounts of the analytes with a fixed amount of stigmasterol, followed by derivatization and solvation in cyclohexane.
2.6. Nucleic acid extraction and analysis
DNA and RNA were simultaneously extracted from a small sample of powdered brain using the Qiagen Allprep DNA/RNA Mini Kit (Qiagen). Presence of DHCR7 DNA was confirmed by polymerase chain reaction (PCR) from brain DNA preparations using a commercial primer pair specific for human hDHCR7 cDNA (Origene) followed by gel electroporesis. Transgene expression was detected by reverse transcription (RT) of brain RNA using oligo dT primer followed by PCR using the hDHCR7 primer pair. Transgene copy number was calculated by quantitative PCR (qPCR) using the standard curve method and measuring florescence of SYBR green bound to amplified hDHCR7 DNA. A standard PCR protocol was used for all primers: 95 °C for 10 min; 40 cycles of 95 °C for 10 s, 60 °C for 15 s and 72 °C for 10 s; followed by 95 °C for 5 s, 65 °C for 1 min, and 97 °C continuous to generate the melting curve. The conserved mouse housekeeping gene, fatty acid binding protein (Fabpi, NC_000069.6), which is present at a single copy per genome, served as an internal control. Transgene expression was quantified by RT-qPCR using the hDHCR7 primer pair and ΔCt calculated relative to levels of mouse glyceraldehyde 3-phosphate dehydrogenase (GAPDH, NC_000072.6) mRNA, which served as the internal control. Reaction conditions for hDHCR7 and mGAPDH were optimized to give comparable efficiencies, 110% for hDHCR7 and 93% for mGAPDH. Transgene expression levels are represented as 2-ΔCt where ΔCt = (Ct (hDHCR7)-Ct (mGAPDH)). All quantitative real-time PCR analyses were performed using an ABI 7900 instrument and accompanying software as per manufacturer's instructions.
2.7. Statistical analysis
Time-course data for tissue sterol ratios in SLOS mice are represented as means from 6 animals at each time point, and variation is represented as ± standard error of the mean (SEM). For evaluating the effect of AAV-DHCR7 compared to saline on the sterol ratio in SLOS mice, unpaired analysis using the non-parametric Mann–Whitney test was performed as the data did not meet the criteria for a normal distribution. P-values were based on two-tailed analysis and values lower than 0.05 were taken as significant.
3. Results
3.1. Sterol levels in the central and peripheral nervous system
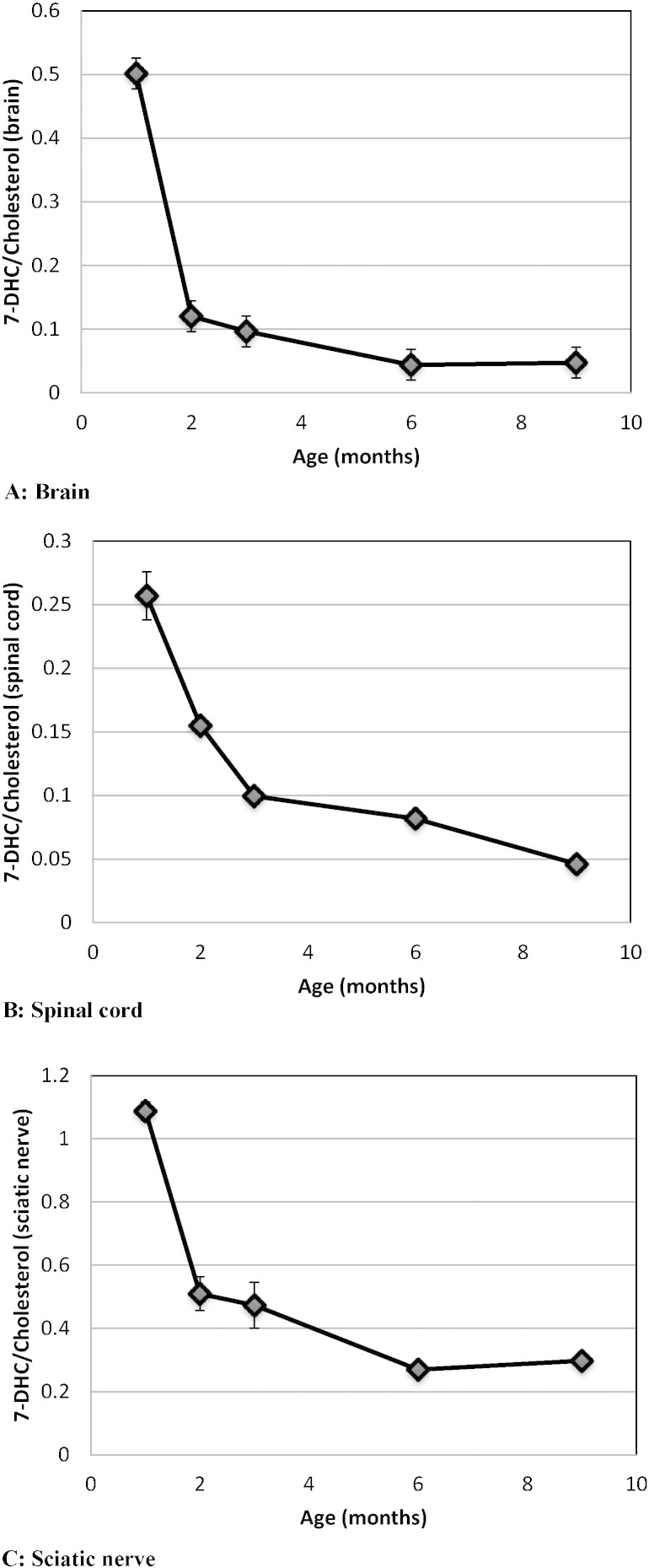
Cholesterol biosynthesis is clearly very important for brain and nervous system development. It plays a fundamental role in neurotransmission as an integral component of synaptic vesicles and myelin. Brain cholesterol synthesis is known to spike in the perinatal phase to meet the demand for growth and myelination. We have previously shown in a Δ/T93M SLOS mouse model that the 7-DHC/cholesterol ratio in the brain is very high during the first 4 postnatal weeks, being the highest at birth, and gradually approaches normal levels by 8–12 months [35]. These results in brain were obtained from mice with a mixed genetic background. Subsequent backcrossing to obtain a uniform C57BL/6 background resulted in dramatically reduced viability of SLOS mice making further experimentation challenging. Viability was restored by backcrossing the SLOS mutations into a homogenous FVB/N background as described in the methods section. Information of cholesterol homeostasis in the peripheral nervous system (PNS), also a heavily myelinated tissue, and its status in SLOS cases is largely unexplored. Therefore, using Δ/T93M mice with a FVB/N background, we characterized age related changes in cholesterol insufficiency and 7-DHC accumulation in the CNS (brain and spinal cord) and the PNS (sciatic nerves).
In line with our earlier studies, the 7-DHC/cholesterol ratio in brain was high at 1 month, declined dramatically by 2 months and then continued to decline more slowly for several more months (Fig. 1A). Spinal cord and sciatic nerve showed similar patterns over time (Fig. 1B and C). Interestingly, however, the sterol ratios in sciatic nerve were 2–4 times higher than the ratios in brain or spinal cord. These results imply that therapeutic intervention should be early and should target both the CNS and PNS.
Fig. 1.
Kinetics of 7-DHC/cholesterol in the brain (A), spinal cord (B) and sciatic nerve (C) of SLOS mice. For each time point, the ratio is an average of 6 animals and the bars represent the standard error of the mean (SEM). A higher 7-DHC/cholesterol ratio is indicative of a greater biochemical imbalance. This ratio is very low (close to zero) in normal mice and does not change with age.
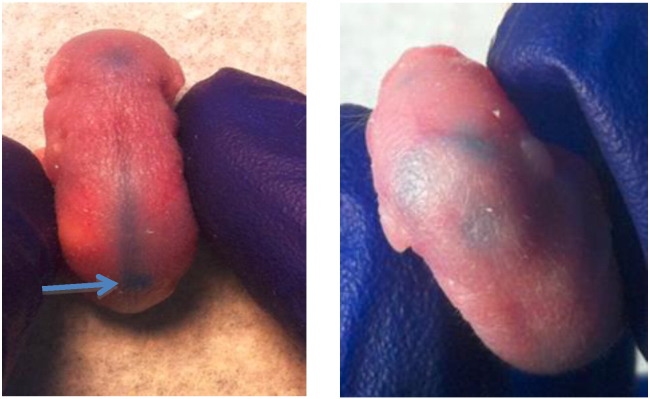
3.2. Intrathecal injection in neonatal mice
All experiments were performed using SLOS mice with the Δ/T93M genotype in a FVB/N genetic background. Anesthetized 3 day old mice were administered AAV-DHCR7 or saline via injection between the vertebrae in the lumbar region directly into the CSF. Successful IT injection was confirmed by the accumulation of tracking dye in the CNS space, i.e. spinal cord and brain (Fig. 2). This is indicative of successful delivery of vector to the CNS. These mice were allowed to recover and tissues collected for analysis 8 weeks post-injection.
Fig. 2.
IT injection into the CSF in a 3 day old mouse pup. Point of injection is indicated and delivery of dye to the spinal cord and brain is observed.
3.3. Delivery of viral DNA and mRNA expression of the transgene hDHCR7 in the brain
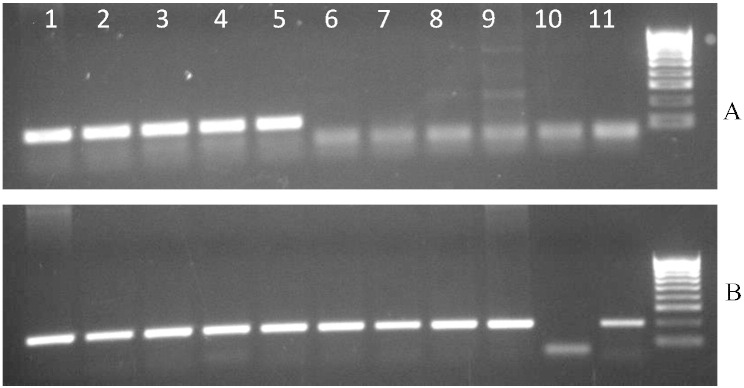
To determine effectiveness of AAV vector delivery via IT injection into the brain, presence of the hDHCR7 transgene was confirmed in brain DNA preparations by PCR analysis using sequence specific primers. As seen in Fig. 3A (lanes 1–5) only animals treated with vector particles show the presence of a 123 bp DNA band corresponding to the PCR product for the human DHCR7 cDNA in brain DNA preparations. This band is absent in the brain DNA of saline-treated animals (lanes 6–10). Brain DNA of all animals show presence of the reference nuclear gene (Fig. 3B). These results confirm that IT administration of AAV vectors was successful in delivering DHCR7 cDNA to the CNS, and that the presence of transgenes persisted for at least 8 weeks post-treatment.
Fig. 3.
Detection of human DHCR7 DNA in brain 8-week after IT delivery of AAV vectors. (A) The hDHCR7 specific band (123 bp) is seen only in IT injected animals (lanes 1–5), and is absent in the saline injected counterparts (lanes 6–10). Lane 11 represents a non-template control. (B) All brain DNA preparations show the presence of the reference nuclear gene Fabpi (the order of lanes is the same as in A except that lanes 10 and 11 are reversed).
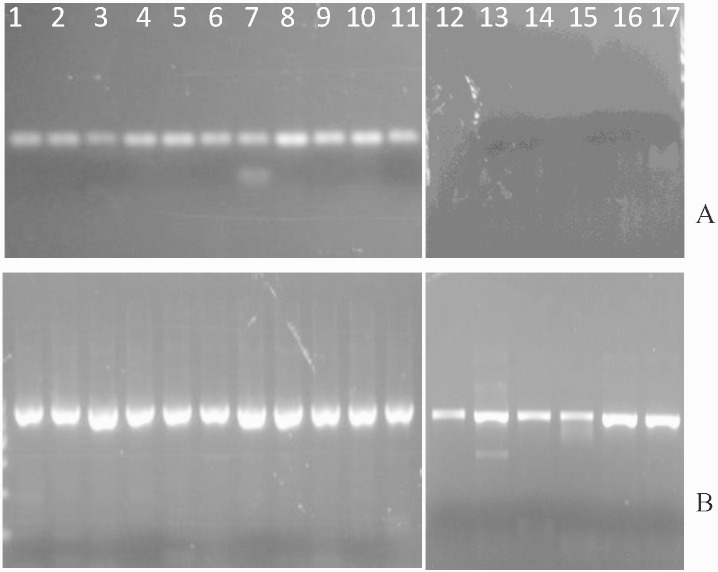
To determine if the delivered DHCR7 transgene is expressed in the brain, RNA isolated from vector and saline treated brain tissue was converted to cDNA via reverse transcription (RT) and subjected to PCR analysis. Fig. 4 A shows the presence or absence of the 123 bp PCR product specific to hDHCR7 cDNA. Expression of hDHCR7 mRNA was seen in the brains of all vector treated mice (lanes 1–11). In contrast, preparations from saline-treated animals showed no DHCR7-specific amplification product (lanes 12–17). All samples showed mRNA expression of the mouse GAPDH reference gene, indicative of successful RT reaction (Fig. 4B).
Fig. 4.
Expression of mRNA in brain. RNA extracts from brains of AAV-DHCR7 treated (lanes 1–11) and saline treated (lanes 12–17) mice were tested for hDHCR7 mRNA (A) and mouse GAPDH mRNA (B). Non-reverse transcriptase treated samples showed the absence of hDHCR7 or GAPDH specific PCR product confirming that the amplification products result from mRNA derived cDNA rather than from the presence of contaminating DNA (data not shown).
These results confirmed that IT injection with AAV vectors was able to deliver a functional copy of hDHCR7 to the brain. Furthermore, the transgene was capable of expression for at least 8 weeks post-treatment.
3.4. Sterol levels in the CNS after IT injection with AAV-DHCR7
Accumulation of 7-DHC, generally accompanied by cholesterol insufficiency in the serum, plasma and tissues, is the characteristic biomarker of SLOS. The 7-DHC/C ratio is used as an indicator of biochemical severity and is elevated in SLOS mice (Δ/T93M), whereas this ratio is close to zero in normal animals (+/+, Δ/+ or T93M/+). The goal of IT administered gene therapy was to minimize this ratio in the CNS, which so far has remained unresponsive to systemic gene therapy efforts or dietary cholesterol supplementation. To determine whether the IT delivery of hDHCR7 via AAV vector indeed was able to normalize the reduction of dehydrocholesterol to cholesterol, sterol levels were measured by GC/MS in the CNS tissues of SLOS mice eight weeks post-treatment. Unlike the serum and liver, the CNS tissues showed substantially increased levels of 8-DHC in addition to7-DHC. Thus, the ratio of total DHC (7- and 8-DHC)/cholesterol was measured to estimate effectiveness of treatment. Other potentially affected sterols, desmosterol and 7-dehydrodesmosterol, were close to background levels in the nervous tissues and were not quantified.
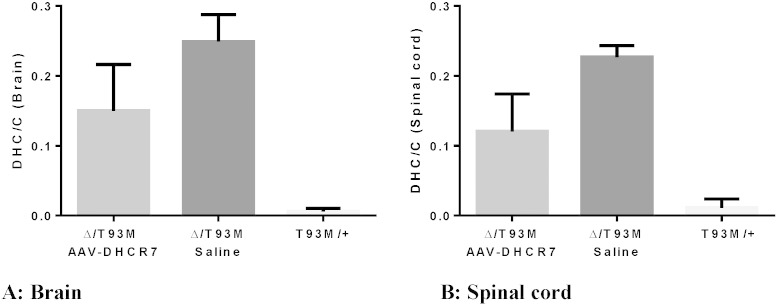
Results for the treated and untreated SLOS mice are shown in Fig. 5. Sterol analysis shows that in both CNS tissues, the brain and spinal cord, the DHC/cholesterol (DHC/C) ratio was significantly improved. Although IT gene therapy partially restored sterol levels in the CNS, ratios were still greater than in normal mice. Reduction of DHC/C was ~ 40% in both brain (P = 0.0275) and spinal cord (P = 0.0027) of treated versus control SLOS mice. These results show that IT injection of vector into the CSF of neonatal SLOS mice improved sterol levels in the CNS implying that functional DHCR7 was expressed in these tissues.
Fig. 5.
Partial normalization of cholesterol metabolism. DHC/cholesterol in the brain (A) and spinal cord (B) of treated (n = 11) and untreated (n = 5) SLOS mice and their normal (T93M/+) littermates (n = 6). SLOS mice were treated IT with 30 μl of AAV-DHCR7 or saline at 3 days of age and sterol analysis by GC/MS was performed at 8 weeks post-treatment. DHC is the sum of 7-DHC and 8-DHC. Statistical analyses were performed using the non-parametric Mann–Whitney test.
3.5. hDHCR7 Transgene copy number and mRNA expression levels correlate with DHC/cholesterol ratio
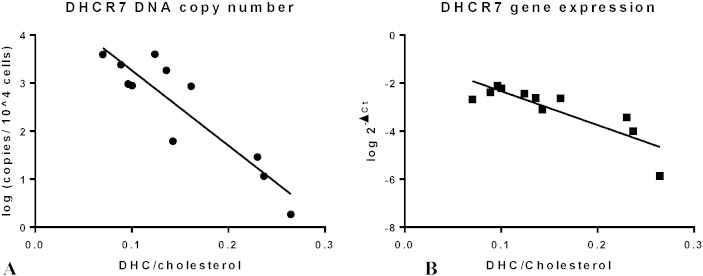
Although we injected a constant dose of vector to each newborn, the delivered dose to the CNS was variable. That is, some injections were more successful than others in delivering vector (and dye) into the IT space. We therefore compared the successfully delivered dose to the effect on sterol metabolism in brain. Quantitative PCR analysis was performed to determine transgene copy number in the brain using the standard curve method and mouse nuclear gene Fabpi for normalization. Expression of hDHCR7 mRNA was measured using ΔCt relative quantification analysis by comparing expression to the mouse reference mRNA, GAPDH. Both transgene copy number and relative mRNA expression of hDHCR7 showed a significant negative correlation with DHC/cholesterol ratio (Fig. 6). This indicated that brains with more hDHCR7 DNA copies had higher expression of hDHCR7 mRNA and lower DHC/cholesterol ratios.
Fig. 6.
Correlation of DHC/C ratios with hDHCR7 DNA and mRNA in brain. (A) Transgene (hDHCR7) copy number versus DHC/C shows an exponential relationship with a correlation coefficient of r2 = 0.82. (B) Relative hDHCR7 mRNA levels expressed as 2− ΔCt (Ct(hDHCR7)− Ct(mGAPDH)) versus DHC/cholesterol similarly shows an exponential relationship with a correlation coefficient of r2 = 0.73.
3.6. Effect on sterol levels in sciatic nerves of IT-treated neonatal mice
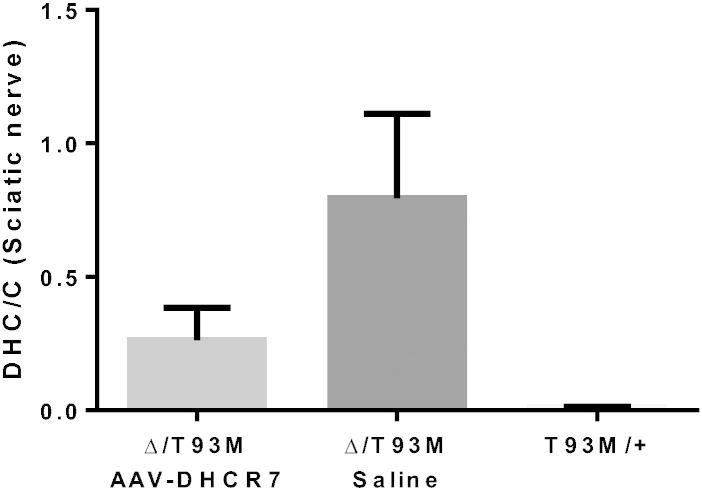
PNS myelination is not well characterized in SLOS, and the effect of dietary cholesterol supplementation, or gene therapy with a functional DHCR7 gene is unknown. In separate experiments, efforts to treat the PNS by intravenous (systemic) administration of AAV-DHCR7 to neonatal mice were unsuccessful (data not shown). Therefore, following the IT administration of AAV-DHCR7 to newborn mice, sterol levels were also measured in the sciatic nerves, and DHC/cholesterol ratios were compared to ratios in sham-treated and normal mice. Sterol ratios in the sciatic nerves of (Δ/T93M) SLOS mice showed an even more dramatic improvement than in brain and spinal cord (Fig. 7). While still higher than normal mice, the vector-treated mice showed ~ 60% reduction in DHC/cholesterol ratio as compared to their saline-treated counterparts (P = 0.0087). Thus, IT administration of AAV-DHCR7 resulted in partial normalization of sterol levels in the PNS as well as in the CNS.
Fig. 7.
Partial normalization of cholesterol metabolism in sciatic nerve. Ratios of DHC/cholesterol in the sciatic nerve of SLOS mice treated IT with AAV-DHCR7 (n = 11), or saline (n = 5) and their normal littermates (n = 6) were compared. Tissues were collected 8 weeks after treatment of 3-day old mice, DHC is the sum of 7-DHC and 8-DHC, and the non-parametric Mann–Whitney test was used for statistical analysis.
In spite of the relatively large effect on sterol metabolism, the presence of the hDHCR7 cDNA or mRNA in sciatic nerve preparations was not reliably detected. This could be attributed to low amounts of total DNA and RNA obtained due to the small amount of starting tissue (single sciatic nerve samples were extracted and each weighed ~ 2–4 mg). In these samples the nuclear reference gene Fabpi and the reference mRNA GAPDH were near the limits of detection, and hDHCR7 sequences were not significantly above background.
3.7. Lack of systemic effect on sterol metabolism following IT administration
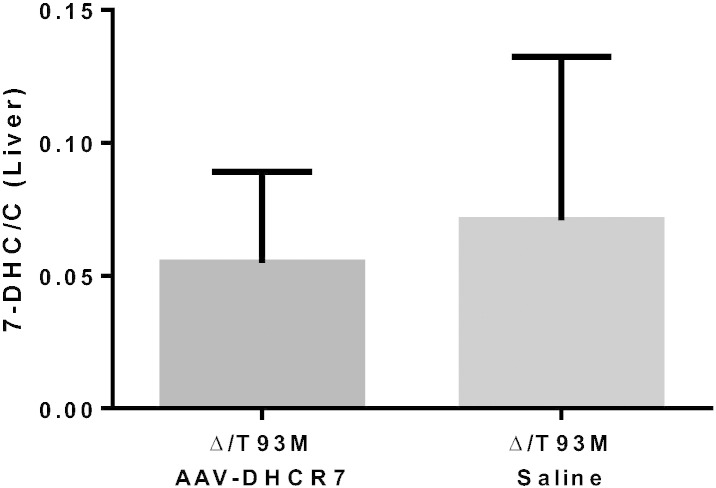
Although AAV vector was delivered directly into the CSF via IT injection, we wanted to assess the effect on sterol ratios in peripheral tissues, such as the liver, which is a target for AAV-DHCR7 that has entered the circulatory system [26]. Fig. 8 shows that while the sterol ratios in the treated animals were slightly lower than their untreated counterparts, the effect was not significant (P = 0.89). Thus, although a low level of systemic distribution following IT administration of vector was possible, the effect on normalization of sterol ratios was mainly restricted to the central and peripheral nervous systems.
Fig. 8.
Sterol analysis in the liver of IT-treated SLOS mice. The 7-DHC/cholesterol ratio remains unchanged in AAV-DHCR7 (n = 9) versus saline (n = 5) at 8 weeks post-treatment. As 8-DHC levels are negligible in the liver, only 7-DHC levels were measured. Statistical analysis was performed using the non-parametric Mann–Whitney test.
4. Discussion
It has been well-established, based on previous studies from our laboratory, that gene transfer using a functional copy of hDHCR7 has the potential to partially normalize sterol levels in SLOS model mice [25], [26]. Furthermore, biochemical improvement was accompanied by physiological benefits. These studies were based on intravenous administration of AAV vectors; noted improvements were systemic, including liver and serum, but did not extend to the brain. Neither the viral vectors nor resulting cholesterol in circulation were able to alter sterol levels in the CNS due to the impenetrability posed by the BBB. SLOS, however, has substantial neurological consequences in addition to systemic malfunctions. Thus, the goal of this study was to extend the scope of gene transfer by targeting the CNS of SLOS mice and assess the effect on sterol levels.
In animals, the brain represents a separate major cholesterolergenic site in addition to the liver [18], [22]. Thus, cholesterol biosynthesis in this organ is very high, especially in the early post-natal period, to meet the demands for membrane and myelin formation that are required for nervous system expansion and development. Consequently, as observed by the 7-DHC/C ratio, cholesterol imbalance is particularly high in both the central and peripheral neuronal tissues of the Δ/T93M SLOS model mice (Fig. 1). To circumvent the BBB with the aim of normalizing sterol levels in the neuronal tissue, AAV-2/8 vector carrying functional hDHCR7 was administered directly into the CSF of newborn SLOS mice via IT injection. This resulted in a dramatic and significant shift towards normalization of sterol levels in the CNS (brain and spinal cord) as assessed by reduction in the DHC/C ratio by GC/MS analysis. The presence of viral DNA as well as expression of hDHCR7 mRNA confirmed the sustained effect of the transgene several weeks post-injection and established an apparent dose–response relationship.
Interestingly, a similar effect towards normalization of sterol levels was also observed in the sciatic nerves, which represent the PNS. Like the brain, sciatic nerve was resistant to systemic treatment, but responsive to IT administration of AAV-DHCR7. This suggests transport of either the vector itself or cholesterol, along the nerve path distal to the site of injection.
4.1. Conclusions
Our results show for the first time that delivering a functional copy of the DHCR7 gene is able to at least partially restore sterol levels in the CNS in a SLOS mouse model. This is an important consideration as neuronal and cognitive deficits are severe in SLOS, and dietary cholesterol supplementation, the present form of therapy for these patients, is unable to restore these deficits. While complete normalization of sterol levels to those observed in unaffected mice was not achieved, even partial correction may provide tangible, physiological improvements [26]. These experiments provide an initial basis to assess several parameters relevant to CNS biochemistry and function. The IT approach to vector administration is minimally invasive and based on results in non-human primates is likely to translate to humans [36]. Further studies employing IT gene transfer in SLOS model mice are needed to evaluate the improvement, if any, in structural and cognitive parameters, as well as to establish a critical level of biochemical normalization necessary to provide significant functional correction.
Conflict of interest
The authors have no conflict of interest to disclose.
Acknowledgments
We respectfully acknowledge the assistance of Saurin Kadakia and Sean Sun. for help with intrathecal injections. This work was supported by the US National Institutes of Health Grant R01HD053036 to C. Shackleton and G. Watson.
References
- 1.Smith D.W., Lemli L., Opitz J.M. A newly recognized syndrome of multiple congenital anomalies. J. Pediatr. 1964;64:210–217. doi: 10.1016/s0022-3476(64)80264-x. [DOI] [PubMed] [Google Scholar]
- 2.Irons M., Elias E.R., Salen G., Tint G.S., Batta A.K. Defective cholesterol biosynthesis in Smith–Lemli–Opitz syndrome. Lancet. 1993;341:1414. doi: 10.1016/0140-6736(93)90983-n. [DOI] [PubMed] [Google Scholar]
- 3.Tint G.S., Irons M., Elias E.R., Batta A.K., Frieden R., Chen T.S., Salen G. Defective cholesterol biosynthesis associated with the Smith–Lemli–Opitz syndrome. N. Engl. J. Med. 1994;330:107–113. doi: 10.1056/NEJM199401133300205. [DOI] [PubMed] [Google Scholar]
- 4.Battaile K.P., Battaile B.C., Merkens L.S., Maslen C.L., Steiner R.D. Carrier frequency of the common mutation IVS8-1G > C in DHCR7 and estimate of the expected incidence of Smith–Lemli–Opitz syndrome. Mol Genet Metab. 2001;72:67–71. doi: 10.1006/mgme.2000.3103. [DOI] [PubMed] [Google Scholar]
- 5.Cross J.L., Iben J., Simpson C.L., Thurm A., Swedo S., Tierney E., Bailey-Wilson J.E., Biesecker L.G., Porter F.D., Wassif C.A. Determination of the allelic frequency in Smith–Lemli–Opitz syndrome by analysis of massively parallel sequencing data sets. Clin. Genet. 2014;87:570–575. doi: 10.1111/cge.12425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ryan A.K., Bartlett K., Clayton P., Eaton S., Mills L., Donnai D., Winter R.M., Burn J. Smith–Lemli–Opitz syndrome: a variable clinical and biochemical phenotype. J. Med. Genet. 1998;35:558–565. doi: 10.1136/jmg.35.7.558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cunniff C., Kratz L.E., Moser A., Natowicz M.R., Kelley R.I. Clinical and biochemical spectrum of patients with RSH/Smith–Lemli–Opitz syndrome and abnormal cholesterol metabolism. J. Am. J. Med. Genet. 1997;68:263–269. [PubMed] [Google Scholar]
- 8.Porter F.D., Herman G.E. Malformation syndromes caused by disorders of cholesterol synthesis. J. Lipid Res. 2011;52:6–34. doi: 10.1194/jlr.R009548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kandutsch A.A., Russell A.E. Preputial gland tumor sterols. 3. A metabolic pathway from lanosterol to cholesterol. J. Biol. Chem. 1960;235:2256–2261. [PubMed] [Google Scholar]
- 10.Batta A.K., Tint G.S., Shefer S., Abuelo D., Salen G. Identification of 8-dehydrocholesterol (cholesta-5,8-dien-3 beta-ol) in patients with Smith–Lemli–Opitz syndrome. J. Lipid Res. 1995;36:705–713. [PubMed] [Google Scholar]
- 11.Opitz J.M., Penchaszadeh V.B., Holt M.C., Spano L.M. Smith–Lemli–Opitz (RSH) syndrome bibliography. Am. J. Med. Genet. 1987;28:745–750. doi: 10.1002/ajmg.1320280324. [DOI] [PubMed] [Google Scholar]
- 12.Cunniff C., Kratz L.E., Moser A., Natowicz M.R., Kelley R.I. Clinical and biochemical spectrum of patients with RSH/Smith–Lemli–Opitz syndrome and abnormal cholesterol metabolism. Am. J. Med. Genet. 1997;68:263–269. [PubMed] [Google Scholar]
- 13.Kelley R.I. RSH/Smith–Lemli–Opitz syndrome: mutations and metabolic morphogenesis. Am. J. Hum. Genet. 1998;63:322–326. doi: 10.1086/301987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kelley R.I. Diagnosis of Smith–Lemli–Opitz syndrome by gas chromatography/mass spectrometry of 7-dehydrocholesterol in plasma, amniotic fluid and cultured skin fibroblasts. Clin. Chim. Acta. 1995;236:45–58. doi: 10.1016/0009-8981(95)06038-4. [DOI] [PubMed] [Google Scholar]
- 15.Shackleton C.H., Marcos J., Palomaki G.E., Craig W.Y., Kelley R.I., Kratz L.E., Haddow J.E. Dehydrosteroid measurements in maternal urine or serum for the prenatal diagnosis of Smith–Lemli–Opitz syndrome (SLOS) Am. J. Med. Genet. A. 2007 Sep 15;143A(18):2129–2136. doi: 10.1002/ajmg.a.31901. [DOI] [PubMed] [Google Scholar]
- 16.Korade Z., Kenworthy A.K. Lipid rafts, cholesterol, and the brain. Neuropharmacology. 2008;55:1265–1273. doi: 10.1016/j.neuropharm.2008.02.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Orth M., Bellosta S. Cholesterol: its regulation and role in central nervous system disorders. Cholesterol. 2012;2012:1–19. doi: 10.1155/2012/292598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Vance J.E. Dysregulation of cholesterol balance in the brain: contribution to neurodegenerative diseases. Dis. Model. Mech. 2012;5:746–755. doi: 10.1242/dmm.010124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Murphy R.C., Johnson K.M. Cholesterol, reactive oxygen species, and the formation of biologically active mediators. J. Biol. Chem. 2008;283:15521–15525. doi: 10.1074/jbc.R700049200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gelzo M., Granato G., Albano F., Arcucci A., Dello Russo A., De Vendittis E., Ruocco M.R., Corso G. Evaluation of cytotoxic effects of 7-dehydrocholesterol on melanoma cells. Free Radic. Biol. Med. 2014;70:129–140. doi: 10.1016/j.freeradbiomed.2014.02.013. [DOI] [PubMed] [Google Scholar]
- 21.Xu L., Porter N.A. Free radical oxidation of cholesterol and its precursors: implications in cholesterol biosynthesis disorders. Free Radic. Res. 2014;9:1–15. doi: 10.3109/10715762.2014.985219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Svoboda M.D., Christie J.M., Eroglu Y., Freeman K.A., Steiner R.D. Treatment of Smith–Lemli–Opitz syndrome and other sterol disorders. Am. J. Med. Genet. C Semin. Med. Genet. 2012;160C:285–294. doi: 10.1002/ajmg.c.31347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bianconi S.E., Cross J.L., Wassif C.A., Porter F.D. Pathogenesis, Epidemiology, Diagnosis and Clinical Aspects of Smith–Lemli–Opitz Syndrome. Expert Opin. Orphan Drugs. 2015;3:267–280. doi: 10.1517/21678707.2015.1014472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tierney E., Conley S.K., Goodwin H., Porter F.D. Analysis of short-term behavioral effects of dietary cholesterol supplementation in Smith–Lemli–Opitz syndrome. Am. J. Med. Genet. A. 2010;152A:91–95. doi: 10.1002/ajmg.a.33148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Matabosch X., Ying L., Serra M., Wassif C.A., Porter F.D., Shackleton C., Watson G. Increasing cholesterol synthesis in 7-dehydrosterol reductase (DHCR7) deficient mouse models through gene transfer. J. Steroid Biochem. Mol. Biol. 2010;122:303–309. doi: 10.1016/j.jsbmb.2010.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ying L., Matabosch X., Serra M., Watson B., Shackleton C., Watson G. Biochemical and physiological improvement in a mouse model of Smith–Lemli–Opitz Syndrome (SLOS) following gene transfer with AAV vectors. Mol. Genet. Metab. Rep. 2014;1:103–113. doi: 10.1016/j.ymgmr.2014.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Watson G., Bastacky J., Belichenko P., Buddhikot M., Jungles S., Vellard M., Mobley W.C., Kakkis E. Intrathecal administration of AAV vectors for the treatment of lysosomal storage in the brains of MPS I mice. Gene Ther. 2006;13:917–925. doi: 10.1038/sj.gt.3302735. [DOI] [PubMed] [Google Scholar]
- 28.Elliger S.S., Elliger C.A., Aguilar C.P., Raju N.R., Watson G.L. Elimination of lysosomal storage in brains of MPS VII mice treated by intrathecal administration of an adeno-associated virus vector. Gene Ther. 1999;6:1175–1178. doi: 10.1038/sj.gt.3300931. [DOI] [PubMed] [Google Scholar]
- 29.Serra M., Matabosch X., Ying L., Watson G., Shackleton C. Hair and skin sterols in normal mice and those with deficient dehydrosterol reductase (DHCR7), the enzyme associated with Smith–Lemli–Opitz syndrome. J. Steroid Biochem. Mol. Biol. 2010;122:318–325. doi: 10.1016/j.jsbmb.2010.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wassif C.A., Zhu P., Kratz L., Krakowiak P.A., Battaile K.P., Weight F.F., Grinberg A., Steiner R.D., Nwokoro N.A., Kelley R.I., Stewart R.R., Porter F.D. Biochemical, phenotypic and neurophysiological characterization of a genetic mouse model of RSH/Smith–Lemli–Opitz syndrome. Hum. Mol. Genet. 2001;10:555–564. doi: 10.1093/hmg/10.6.555. [DOI] [PubMed] [Google Scholar]
- 31.Correa-Cerro L.S., Wassif C.A., Kratz L., Miller G.F., Munasinghe J.P., Grinberg A., Fliesler S.J., Porter F.D. Development and characterization of a hypomorphic Smith–Lemli–Opitz syndrome mouse model and efficacy of simvastatin therapy. Hum. Mol. Genet. 2006;15:839–851. doi: 10.1093/hmg/ddl003. [DOI] [PubMed] [Google Scholar]
- 32.Gao G.P., Alvira M.R., Wang L., Calcedo R., Johnston J., Wilson J.M. Novel adeno-associated viruses from rhesus monkeys as vectors for human gene therapy. Proc. Natl. Acad. Sci. U. S. A. 2002;99:11854–11859. doi: 10.1073/pnas.182412299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Matsushita T., Elliger S., Elliger C., Podsakoff G., Villarreal L., Kurtzman G.J., Iwaki Y., Colosi P. Adeno-associated virus vectors can be efficiently produced without helper virus. Gene Ther. 1998;5:938–945. doi: 10.1038/sj.gt.3300680. [DOI] [PubMed] [Google Scholar]
- 34.Zolotukhin S., Byrne B.J., Mason E., Zolotukhin I., Potter M., Chesnut K., Summerford C., Samulski R.J., Muzyczka N. Recombinant adeno-associated virus purification using novel methods improves infectious titer and yield. Gene Ther. 1999;6:973–985. doi: 10.1038/sj.gt.3300938. [DOI] [PubMed] [Google Scholar]
- 35.Marcos J., Shackleton C.H., Buddhikot M.M., Porter F.D., Watson G.L. Cholesterol biosynthesis from birth to adulthood in a mouse model for 7-dehydrosterol reductase deficiency (Smith–Lemli–Opitz syndrome) Steroids. 2007;72:802–808. doi: 10.1016/j.steroids.2007.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Salegio E.A., Streeter H., Dube N., Hadaczek P., Samaranch L., Kells A.P., San Sebastian W., Zhai Y., Bringas J., Xu T., Forsayeth J., Bankiewicz K.S. Distribution of nanoparticles throughout the cerebral cortex of rodents and non-human primates: implications for gene and drug therapy. Front. Neuroanat. 2014 Mar 17;8:9. doi: 10.3389/fnana.2014.00009. [DOI] [PMC free article] [PubMed] [Google Scholar]