Abstract
Objectives
Fracture and secondary caries are the primary reasons for dental restoration failure. The objective of this study was to develop a self-healing composite to heal cracks, while containing dimethylaminohexadecyl methacrylate (DMAHDM) for antibacterial function and nanoparticles of amorphous calcium phosphate (NACP) for remineralization.
Methods
Microcapsules were synthesized with poly(urea-formaldehyde) (PUF) shells containing triethylene glycol dimethacrylate (TEGDMA) and N,N-dihydroxyethyl-p-toluidine (DHEPT) as healing liquid. Composite contained 20 mass% of NACP and 35% glass fillers. In addition, composite contained 0%, 2.5%, 5%, 7.5%, or 10% of microcapsules. A single edge V-notched beam method measured fracture toughness (KIC) and self-healing efficiency. A dental plaque microcosm biofilm model was used to test the antibacterial properties.
Results
Incorporation of microcapsules up to 7.5% into the composite did not adversely affect the mechanical properties (p > 0.1). Successful self-healing was achieved, with KIC recovery of 65–81% (mean ± sd; n = 6) to regain the load-bearing capability after composite fracture. The self-healing DMAHDM-NACP composite displayed a strong antibacterial potency, inhibiting biofilm viability and lactic acid production, and reducing colony-forming units by 3–4 orders of magnitude, compared to control composite without DMAHDM.
Conclusions
A dental composite was developed with triple benefits of self-healing after fracture, antibacterial activity, and remineralization capability for the first time.
Clinical significance
The self-healing, antibacterial and remineralizing composite may be promising for tooth cavity restorations to combat bulk fracture and secondary caries. The method of using triple agents (self-healing microcapsules, DMAHDM, and NACP) may have wide applicability to other dental composites, adhesives, sealants and cements.
Keywords: Self-healing dental composite, microcapsules, polymerizable healing liquid, antibacterial, mechanical properties, fracture toughness recovery
1. Introduction
Dental composites generally consist of a resin matrix with inorganic filler particles for reinforcement.1 Composites are widely used in dental practice as filling materials due to their esthetics and direct-filling capability.2–4 Extensive efforts have been made to significantly improve the composite properties.5–8 Nonetheless, composite restorations are still challenged by two main problems: bulk fracture and secondary caries.9 Studies suggested that fracture was a frequent reason for composite failure, while secondary caries was a common reason for failure after five years of clinical service.10 Replacing the failed restorations accounts for 50–70% of all restorations performed,11 hence replacement dentistry represents a significant financial burden.12 Therefore, it would be highly desirable to develop a new composite with self-healing capability to heal cracks in situ while also possessing a caries-inhibiting capability.
Self-healing polymers were developed to recover the load-bearing capabilities after cracking.13 Self-healing composite with microcapsules containing a healing agent displayed healing efficacy.14 The microcapsules had an intact shell encapsulating a healing liquid inside.15 After incorporation of microcapsules into a polymer matrix, a propagating crack in the matrix would rupture the microcapsules, releasing the healing liquid into the crack planes, thus contacting the catalyst in the matrix, which triggers the polymerization of the healing liquid and autonomously heals the crcak.16 In a previous study,14 dicyclopentadiene (DCPD) was encapsulated in poly(urea-formaldehyde) (PUF) shells. Grubb’s catalyst, a transition metal carbine complex, was incorporated into an epoxy matrix. Polymerization was triggered when a crack ruptured the microspheres, releasing DCPD to react with Grubb’s catalyst, achieving a good self-healing efficiency.14
A self-healing dental composite was developed by incorporating DCPD-containing microcapsules into a resin containing Grubb’s catalyst, in which self-healing achieved a recovery of 57% of the original fracture toughness (KIC) of the composite.17 The incorporation of microcapsules did not significantly affect the virgin mechanical properties of the composite.18 Another study developed polyurethane (PU) shell-based nanocapsules containing triethylene glycol dimethacrylate (TEGDMA); however, no initiator or catalyst was described and no healing results were reported.19 Regarding the use of DCPD and Grubb’s catalyst in the oral environment, biocompatibility would be a potential concern.20 Hence for the use of DCPD and Grubb’s catalyst system in dental applications, DCPD toxicity,21 Grubb’s catalyst toxicity, availability and high cost22 remain as challenges. To date, a self-healing dental composite using a biocompatible healing liquid and catalyst with a demonstrated substantial self-healing efficacy remains to be developed.
Besides the fracture issue, secondary caries is another reason for restoration failures. To combat caries, previous studies developed quaternary ammonium methacrylates (QAMs) to inhibit oral biofilms and reduce acid production.23,24 Furthermore, previous studies incorporated calcium phosphate (CaP) filler particles in composites for remineralization.25,26 However, there has been no report on the development of a self-healing dental composite that also possesses antibacterial and remineralization capabilities.
In the present study, self-healing dental composite was developed with microcapsules containing a healing liquid of TEGDMA with N,N-dihydroxyethyl-p-toluidine (DHEPT) as the tertiary amine accelerator. In the resin matrix, benzoyl peroxide (BPO) was incorporated as the self-healing initiator. TEGDMA, DHEPT, and BPO have been used in dental practice with a history of biocompatibility. The objective of this study was to develop a self-healing composite with substantial self-healing efficacy, while also possessing antibacterial and remineralizing capabilities. It was hypothesized that: (1) Self-healing microcapsules containing TEGDMA-DHEPT healing liquid could be synthesized and incorporated into an antibacterial and remineralizing composite without compromising the original mechanical properties of the composite; (2) The recovery of fracture toughness of this composite would be directly proportional to the microcapsule filling level in the composite; (3) The new composite would exhibit an excellent self-healing efficacy and strong antibacterial activities against dental plaque microcosm biofilms.
2. Materials and methods
2.1. Synthesis of self-healing microcapsules
Microcapsules were prepared by in situ polymerization of formaldehyde and urea following a previous study.27 Briefly, DHEPT (Sigma-Aldrich, St. Louis, MO) at 1% (all mass fractions) was added to TEGDMA monomer (Esstech, Essington, PA). At room temperature, 50 mL of distilled water and 13 mL of a 2.5% aqueous solution of ethylene-maleic anhydride (EMA) copolymer (Sigma-Aldrich) were mixed in a 250 mL round-bottom glass flask. 27 The flask was suspended in a water bath on a hotplate (Isotemp, Fisher Scientific, Pittsburg, PA). The EMA solution was used as a surfactant to form an “oil-in-water” emulsion (“oil” being the TEGDMA-DHEPT). Under agitation by a magnetic stir bar (diameter = 7.8 mm, length = 50 mm, Fisher Scientific) at 300 rpm, the shell-forming material urea (1.25 g), ammonium chloride (0.125 g) and resorcinol (0.125 g) (Sigma-Aldrich) were added into the solution. Resorcinol was added in the reaction of shell formation to enhance the rigidity of the shell.28 The pH was adjusted to 3.5 via drop-wise addition of 1 M sodium hydroxide solution. Then, the agitation rate was increased to 400 rpm, and 30 mL of the TEGDMA-DHEPT liquid was added into the flask. A stabilized emulsion of fine TEGDMA-DHEPT droplets was formed after 10 min of agitation. Then, 3.15 g of a 37% aqueous solution of formaldehyde (Sigma-Aldrich) was added, and the flask was sealed with aluminum foil to prevent evaporation. The temperature of the water bath was raised to 55 °C and the shell material was isothermally polymerized for 4 hours (h) under continuous agitation.28 In this process, ammonium chloride catalyzed the reaction of urea with formaldehyde to form PUF at the oil-water interface to develop the shell.28 The microcapsules thus obtained were rinsed with water and acetone repeatedly, vacuum-filtered, and air-dried for 24 h in a hood. The microcapsules were examined by optical microscope (TE2000-S, Nikon, Japan) and their sizes were measured with an image analysis software (Nis-Elements BR2.30, Nikon). The microcapsules were also examined with scanning electronic microscopy (SEM, Quanta 200, FEI, Hillsboro, OR). The microcapsules were spread onto an adhesive tape and sputter-coated with gold prior to SEM examination.
2.2. Synthesis of calcium phosphate nanoparticles and DMAHDM
Nanoparticles of amorphous calcium phosphate (NACP) were prepared by a spray-drying technique.25 Briefly, calcium carbonate (CaCO3, Fisher, Fair Lawn, NJ) and dicalcium phosphate anhydrous (CaHPO4, Baker Chemical, Phillipsburg, NJ) were dissolved into an acetic acid solution to obtain Ca and P ionic concentrations of 8 mmol/L and 5.333 mmol/L, respectively, yielding a Ca/P molar ratio of 1.5. Then the solution was sprayed into a heated chamber of a spray dryer, and an electrostatic precipitator (AirQuality, Minneapolis, MN) was used to collect the dried particles. This method produced NACP with a mean particle size of 116 nm.29
The synthesis of dimethylaminododecyl methacrylate (DMAHDM) was recently described.30 Briefly, 10 mmol of 2-(dimethy-lamino) ethyl methacrylate (DMAEMA, Sigma, St. Louis, MO) and 10 mmol of 1-bromohexadecane (BHD, TCI America, Portland, OR) were dissolved in 3 g of ethanol and allowed to react at 70 °C for 24 h under continuous agitation. After the reaction was completed, the ethanol was removed via evaporation. This yielded DMAHDM as a clear, colorless, and viscous liquid.30
2.3. Fabrication of composite specimens
Bisphenol A glycidyl dimethacrylate (BisGMA) and TEGDMA (Esstech) were mixed at 1:1 mass ratio to form the resin matrix.26 The monomer mixture was rendered light-curable by adding 1% of photo-initiator phenyl-bis(2,4,6-trimethylbenzoyl) phosphine oxide (BAPO, Sigma-Aldrich).31 Then 0.5% by mass of benzoyl peroxide (BPO) (Sigma-Aldrich) was dissolved in the mixture. BPO serves as an initiator to react with the DHEPT in the healing liquid to trigger the polymerization. DMAHDM was added into the BisGMA-TEGDMA resin at 10% by mass, following a previous study.26 The resin matrix was filled with 20% NACP for Ca and P ion release and remineralization,25,29,31 and 35% glass fillers for mechanical reinforcement.26 The glass particles were barium boroaluminosilicate with a median particle size of 1.45 μm (Caulk/Dentsply, Milford, DE) which were silanized with 4% 3-methacryloxypropyltrimethoxysilane and 2% n-propylamine.29 This composite resin was then mixed with microcapsules at microcapsule mass fractions of 0%, 2.5%, 5%, 7.5% and 10%. Microcapsule mass fractions > 10% were not included because they decreased the virgin mechanical properties of the composite.
2.4. Flexural testing
For flexural test, each composite paste was placed in a 2 × 2 × 25 mm mold and photo-cured (Triad 2000, Dentsply, York, PA) for 1 min on each open side of the mold. 26 The specimens were demolded and stored in distilled water at 37 °C for 24 h prior to testing. For KIC testing, a single edge V-notched beam (SEVNB) method was used.32,33 A notch with a depth of approximately 500 μm was machined into the specimen using a thin diamond blade with a 150 μm thickness (Buehler, Lake Bluff, IL).32 Then a diamond polishing paste of 3 μm was placed into the notch tip, and a new razor blade was used to cut the notch further to a total depth of about 700–800 μm, yielding a relatively sharp notch tip.32 The notch length was measured using an optical microscope (TE2000-S, Nikon) on both sides of the specimen, and then the notch length was averaged. The specimens were incubated in 37 °C in water for 24 h prior to testing.
Specimens without notches were used to measure flexural strength and elastic modulus. A computer-controlled Universal Testing Machine (5500R, MTS, Cary, NC) was used to fracture the specimens in three-point flexure with a span of 10 mm and a crosshead speed of 1 mm/min.25,26 Flexural strength S = 3PmaxL/(2bh2), where P max is the load-at-failure, L is span, b is width and h is thickness. Elastic modulus E = (P/d)(L3/[4bh3]), where load P divided by displacement d is the slope in the linear elastic region of the load-displacement curve.25,26 Six specimens were tested for each group.
2.5. Measurement of KIC and self-healing efficiency
The notched specimens were used to measure the KIC values. Specimens were tested in three-point fixture with the notch on the tensile side, and the middle loading pin was aligned with the notch via the use of a magnifier (3×). KIC was calculated following an established SEVNB method.33 This yielded the original virgin KIC of the specimen, KIC-virgin.
In order to test the self-healing efficacy, immediately following specimen fracture, the two halves of the specimen were placed back into the mold to ensure a good contact of the two fractured planes. This would be similar to the case of a resin-based restoration in a tooth cavity, where the cracked restoration would stay in the tooth cavity to allow the released healing liquid to heal the crack. The healing of a completed cracked specimen would be a more rigorous test than the healing of a partial or small crack in the specimen. Because the fracture ruptured the microcapsules in the composite, the released TEGDMA-DHEPT from the microcapsules would react with the BPO in the resin matrix. This would cause the polymerization of the released liquid to heal and bond the two cracked planes into one cohesive specimen. Specimens were incubated in a humidor at 37 °C for 24 h. The notch length of the healed specimen was again measured, and made sure that it was the same as the virgin notch length of the same specimen. Then the healed bar was tested again using the same flexural method to measure the KIC. This yielded the healed KIC value, KIC-healed. The self-healing efficiency (η) was assessed following a previous study as:34
2.6. Dental plaque microcosm biofilm live/dead assay
The cover of a sterile 96-well plate was used as molds to make composite disk specimens for the antibacterial experiments, following a previous study.30 Each composite paste was placed into the dent, covered with a mylar strip and photo-cured for 30 s to form a disk of approximately 8 mm in diameter and 0.5 mm in thickness.30 The composite with the same BisGMA-TEGDMA resin but without DMAHDM served as a control for antibacterial experiments, which was filled with the same 20% NACP and 35% glass fillers. All the cured composite disks were immersed in distilled water at 37 °C for 1 d. Disks were sterilized with ethylene oxide (Anprolene AN 74i, Andersen, Haw River, NC) and de-gassed following the manufacturer’s instructions.
The dental plaque microcosm biofilm model using human saliva was approved by the University of Maryland. Whole human saliva was used as an inoculum to provide multi-species biofilms consisting of organisms found in the oral cavity. To represent the diverse bacterial populations, saliva from ten healthy individuals was collected and combined for the experiments, following a previous study.35 Saliva was collected from adult donors who had natural dentition without active caries or periopathology, and without the use of antibiotics within the last 3 months. The donors did not brush teeth for 24 h and abstained from food/drink intake for 2 h prior to donating saliva. Stimulated saliva was collected during parafilm gum chewing and was kept on ice. The saliva was combined and diluted in sterile glycerol to a concentration of 70%, then stored at −80 °C for subsequent use.36
The saliva-glycerol stock with 1:50 final dilution was added into the growth medium as inoculum. The McBain artificial saliva growth medium was used that consisted of: mucin (type II, porcine, gastric), 2.5 g/L; bacteriological peptone, 2.0 g/L; tryptone, 2.0 g/L; yeast extract, 1.0 g/L; NaCl, 0.35 g/L; KCl, 0.2 g/L; CaCl2, 0.2 g/L; cysteine hydrochloride, 0.1 g/L; hemin, 0.001 g/L; vitamin K1, 0.0002 g/L, at pH7.37 A composite disk was placed into each well of 24-well plates filled with 1.5 mL of inoculum, and incubated in 5% CO2 at 37 °C for 8 h. Then, the disks were transferred to new 24-well plates filled with fresh medium and incubated for 16 h. After 16 h, the disks were moved to new 24-well plates with fresh medium and incubated for 24 h. This totaled 48 h of incubation, which was shown to form microcosm biofilms on resin disks.26
Disks with 48 h biofilms were rinsed with phosphate-buffered saline and then stained using the Bac Light live/dead bacterial viability kit (Molecular Probes, Eugene, OR). Live bacteria were stained to produce a green fluorescence, and bacteria with compromised membranes were stained to yield a red fluorescence. Specimens were examined with an inverted epifluorescence microscope (Eclipse TE2000-S, Nikon, Japan).26
2.7. Biofilm metabolic assay
The MTT (3-[4,5-Dimethylthiazol-2-yl]-2,5-diphenyltetrazolium bromide) assay is a colorimetric assay that measures the enzymatic reduction of MTT, a yellow tetrazole, to formazan.26 Disks with 48 h biofilms were transferred to a new 24-well plate, then 1 mL of MTT dye (0.5 mg/mL MTT in phosphate-buffered saline) was added to each well and incubated at 37 °C in 5% CO2 for 1 h. During this process, the metabolically active bacteria reduced the MTT, a yellow tetrazole, to purple formazan. After 1 h, the disks were transferred to another 24-well plate, 1 mL of dimethyl sulphoxide (DMSO) was added to dissolve the formazan crystals, and the plate was incubated for 20 min with gentle mixing at room temperature in the dark. Then, 200 mL of the DMSO solution from each well was moved to a 96-well plate, and the absorbance at 540 nm was measured via a microplate reader (SpectraMax M5, Molecular Devices, Sunnyvale, CA). A higher absorbance indicates a higher formazan concentration, showing a higher metabolic activity in the biofilm on the disk.26
2.8. Biofilm lactic acid production measurement
Disks with 48 h biofilms were rinsed in cysteine peptone water (CPW) to remove loose bacteria. Each disk was placed in a new 24-well plate filled with 1.5 mL of buffered peptone water (BPW) supplemented with 0.2% sucrose.26 Disks with biofilms were incubated at 5% CO2 and 37 °C for 3 h to allow the biofilms to produce acid.26 Then, the BPW solutions were stored for lactate analysis. Lactate concentrations in the BPW solutions were measured using an enzymatic (lactate dehydrogenase) method.26 The microplate reader (SpectraMax M5) was used to measure the absorbance at 340 nm for the collected BPW solutions. Standard curves were prepared using a lactic acid standard (Supelco Analytical, Bellefonte, PA) as in a previous study.26
2.9. Biofilm colony-forming unit (CFU) counts
Composite disks with 2-day biofilms were transferred into tubes with 2 mL CPW, and the biofilms were harvested by sonication and vortexing (Fisher, Pittsburgh, PA).38,39 Three types of agar plates were used to measure the CFU counts to assess the microorganism viability. First, tryptic soy blood agar culture plates were used to determine total microorganisms.37 Second, mitis salivarius agar (MSA) culture plates containing 15% sucrose were used to determine total streptococci.40 This is because MSA contains selective agents including crystal violet, potassium tellurite and trypan blue, which inhibit most Gram-negative bacilli and most Gram-positive bacteria except streptococci, thus enabling the streptococci to grow.40 Third, cariogenic mutans streptococci is known to be resistant to bacitracin, and this property is used to isolate mutans streptococci from the highly heterogeneous oral microflora.37 Therefore, MSA agar culture plates plus 0.2 units of bacitracin per mL was used to determine mutans streptococci. 37 The bacterial suspensions were serially diluted, spread onto agar plates and incubated at 37 °C in 5% CO2 for 24 h.38,39 The number of colonies that grew was counted and used, along with the dilution factor, to calculate the CFU counts on each composite disk.38,39
2.10. Statistical analysis
One-way and two-way analyses-of-variance (ANOVA) were performed to detect the significant effects of the variables. Tukey’s multiple comparison was used to compare the data at p of 0.05.
3. Results
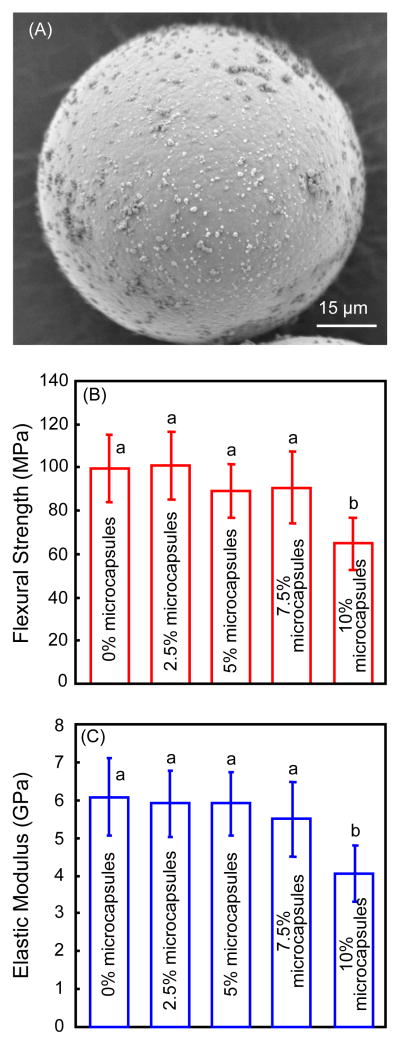
The microcapsule diameters were measured (mean ± sd; n = 200) to be 70 ± 24 μm. SEM image of a representative microcapsule was shown in Fig. 1(A). The microcapsules had numerous PUF nanoparticles attaching to a smooth shell surface to form a rough shell. The smooth shell surface appeared to be dense without voids or pores. These microcapsules were incorporated into composite specimens. The mechanical properties of the composites are plotted in Fig. 1(B) for flexural strength, and (C) for elastic modulus (mean ± sd; n = 6). There was no significant difference among the groups with microcapsule mass fraction from 0% to 7.5% (p > 0.1). However, the mechanical properties at 10% microcapsules were significantly lower than the other groups (p < 0.05).
Figure 1.
Self-healing microcapsule incorporation into dental composite. (A) Representative SEM image of a microcapsule, (B) flexural strength and (C) elastic modulus of composite containing microcapsules at different mass fractions (mean ± sd; n = 6). The 0% refers to composite containing 0% microcapsules; 2.5% refers to composite containing 2.5% microcapsules, and so on. In each plot, values with dissimilar letters are significantly different from each other (p < 0.05).
Fig. 2 plots (A) the virgin and healed KIC, and (B) the self-healing efficiency (mean ± sd; n = 6). For KIC-virgin, there was no significant difference from 0% to 7.5% microcapsules (p > 0.1). However, further increasing the microcapsule mass fraction to 10% reduced the KIC-virgin, from 1.16 MPa·m1/2 at 0% to 0.91 MPa·m1/2 at 10% microcapsules (p < 0.05). The KIC-healed significantly increased from no healing at 0% microcapsules to maximum healing at 7.5% and 10% microcapsules (p < 0.05). The self-healing efficiency is plotted in (B). A KIC recovery of 65%–81% was achieved for composites with microcapsule mass fractions at 7.5% and 10% microcapsules.
Figure 2.
Self-healing of composite as a function of microcapsule mass fraction. (A) Virgin and healed fracture toughness KIC, and (B) self-healing efficiency for KIC (mean ± sd; n = 6). The 0% refers to composite containing 0% microcapsules; 2.5% refers to composite containing 2.5% microcapsules, and so on. In each plot, values with dissimilar letters are significantly different from each other (p < 0.05).
Typical fracture surfaces of composite containing 7.5% microcapsules are shown in Fig. 3: (A) virgin fracture surface, and (B) fractured, healed and re-fractured surface. The virgin fracture surface showed fractured microcapsules imbedded in the composite matrix. In (B), the fracture surface contained polymer films (arrows) indicating that the healing liquid was released and polymerized, consistent with the recovery of the load-bearing capability after fracture in Fig. 2.
Figure 3.
Typical SEM images of the fractured surfaces of composite containing 7.5% microcapsules. (A) Virgin fracture surface of composite, and (B) the healed and re-fractured surface of composite. Arrows indicate examples of polymer films of the released and polymerized healing liquid.
The self-healing composite also possessed antibacterial function, as shown in Fig. 4. Representative live/dead staining images are shown in (A) control composite without DMAHDM, and (B) composite containing 7.5% microcapsules and 10% DMAHDM in the resin matrix. Since this composite contained 20% NACP, 35% glass and 7.5% microcapsules, the resin mass fraction was 37.5%, hence the DMAHDM mass fraction in the overall composite was 3.75%. Live bacteria were stained green, while dead bacteria were stained red. The control composite had continuous green coverage as the biofilm was primarily alive. The composite with DMAHDM showed mostly red staining, indicating a strong antibacterial activity. Other composites with DMAHDM had similar live/dead images. The MTT assay results are plotted in (C), and the lactic acid production of biofilms is plotted in (D) (mean ± sd; n = 6). Biofilms on the control composite without DMAHDM had relatively high metabolic activity and acid production. In contrast, biofilms on composites containing DMAHDM had much lower metabolic activity and acid production (p < 0.05). Their metabolic activity was about 1/25 that of the control, and their lactic acid production was nearly 1/100 that of the control.
Figure 4.
Dental plaque microcosm biofilm growth on composites. (A) and (B) Representative live/dead images of dental plaque microcosm biofilms on control composite and that with DMAHDM, respectively. Live bacteria were stained green, and dead bacteria were stained red. Live and dead bacteria that were close to each other yielded yellow/orange colors. (C) MTT metabolic activity, and (D) lactic acid production of biofilms (mean±sd; n = 6). In each plot, values with dissimilar letters are significantly different from each other (p < 0.05).
The CFU of biofilms grown for 2 days on composites is plotted in Fig. 5: (A) total microorganisms, (B) total streptococci, and (C) mutans streptococci (mean ± sd; n = 6). Control composite had the highest CFU. The composites containing DMAHDM, regardless of microcapsule mass fractions, reduced the biofilm CFU by 3–4 orders of magnitude compared to control composite without DMAHDM (p < 0.05).
Figure 5.
Colony-forming unit (CFU) counts of biofilms grown for 2 days on composites. (A) Total microorganisms, (B) total streptococci, and (C) mutans streptococci (mean ± sd; n = 6). In each plot, values with dissimilar letters are significantly different from each other (p < 0.05).
4. Discussion
The present study developed the first self-healing and antibacterial dental composite with NACP for remineralization. Mechanical properties are important for composite in load-bearing restorations.41 Incorporation of microcapsules into the composite to achieve self-healing ability must not sacrifice the original mechanical properties of the composite. Previous studies showed that the incorporation of microcapsules was accompanied with significant improvements in tensile strength42 and flexural strength of the polymer matrix.43 However, another study reported a continuous reduction in the strength of the resin with increasing microcapsule content.44 While these previous studies tested resins without filler particles, the present study tested a composite filled with 35% of glass fillers and 20% of NACP. The flexural strength and elastic modulus of the composite were not significantly reduced by the addition of up to 7.5% of microcapsules. Regarding KIC, a previous study showed that addition of DCPD-filled PUF microcapsules into epoxy yielded an increase in the virgin KIC.44 However, another study added 5% of microcapsules into a resin composite containing 55% of glass fillers, and showed that the virgin KIC of the composite did not change significantly.17 This is consistent with the present study, which showed that the virgin KIC of the composite did not decrease or increase significantly from 0% to 7.5 % of microcapsules.
The present study tested the TEGDMA-DHEPT healing liquid with BPO as the self-healing initiator in the resin of the NACP-DMAHDM composite, achieving substantial self-healing efficacy and recovery of load-bearing capability. The in situ curing of the reactive TEGDMA was triggered by the free-radical initiation which was caused by the tertiary amine activator DHEPT and initiator BPO. Future study should investigate the possibility of the leakage of the uncured TEGDMA and DHEPT. The present study showed polymer films on the fractured, healed and re-fractured surfaces, which confirmed microcapsule rupture and in situ polymerization of the healing liquid, consistent with the recovery in KIC. It was the formation of these polymer films that halted the crack propagation, bonded the cracked planes together and healed the specimen.45 The recovery of 65% in KIC for the NACP-DMAHDM composite with 7.5% of microcapsules is consistent with previous studies reporting a 57% recovery of the original fracture toughness17 and 75% recovery in toughness.14 This indicates that the remineralization agent (NACP) and antibacterial agent (DMAHDM) can be incorporated into a self-healing composite, while still achieving self-healing efficacy similar to the reported values where remineralization fillers and antibacterial monomers were not used. The present study tested the healing of the two half specimens after complete fracture. Further study is needed to investigate the self-healing efficacy during the propagation of the crack to stop the propagating crack and heal the crack planes together.
Secondary caries currently limits the lifetime of resin-based composite restorations.11,12,46 Hence it is highly desirable to inhibit tooth decay clinically.47 It was documented that bacteria would colonize resin surfaces and form biofilms, and the biofilms would produce acids leading to caries.48 A complex microbial community forms in vivo on tooth surfaces, and dental plaque microcosm biofilms cover teeth and dental restorative materials.49 Resin composites were shown to accumulate more biofilms and plaque than other restorative materials.50,51 Therefore, it would be beneficial to incorporate antibacterial agents into dental composite to inhibit bacteria and combat caries.52–54 DMAHDM was a recently-developed QAM with an alkyl chain length of 16, showing a strong antibacterial effect.30 The antibacterial property of quaternary ammonium salts arises from their positively-charged quaternary amine N+, which can attract the negatively-charged cell membrane of bacteria, disrupt membranes and cause cytoplasmic leakage.55,56 It was suggested that long-chained quaternary ammonium compounds could insert into bacterial membranes like a needle, leading to disruption of the bacteria.57 In the present study, all the composites containing DMAHDM inhibited the metabolic activity and lactic acid production of the biofilms, and reduced the CFU by 3–4 log. This was achieved with microcapsule mass fraction varying from 0 to 10%, indicating that microcapsule incorporation did not significantly interfere with the antibacterial effect. Further study is needed to investigate whether the DMAHDM in the self-healing composite would exert a long-term and durable antibacterial effect. A previous study on another QAM showed that, due to QAM copolymerization with the resin matrix forming a covalent bonding, the antibacterial effect was long-lasting and not lost over time in water-aging treatment.58
Besides the self-healing ability and antibacterial activity, the novel composite had the third benefit of Ca and P ions release for remineralization. While the present study focused on the synthesis of microcapsules and the testing of self-healing and antibacterial properties, previous studies investigated Ca and P ion release of NACP composite.25,29,59,60 Studies showed that the NACP composite was “smart” and could increase the release of Ca and P ions at a cariogenic low pH, when these ions would be most needed to combat caries.29 In addition, the NACP composite neutralized acid challenges by increasing the cariogenic pH to a safe level.25 Furthermore, the NACP composite remineralized enamel lesions in vitro,59 and inhibited caries formation in a human in situ model.60 Further studies are needed to investigate the effects of NACP in the self-healing composite on caries-inhibition.
5. Conclusions
A novel dental composite was developed with triple benefits of self-healing, antibacterial and remineralization capabilities. The original properties of the composite including flexural strength, elastic modulus and fracture toughness were not adversely affected when microcapsules were added up to 7.5%. Self-healing was achieved with 65–81% recovery in the virgin fracture toughness, thereby recovering the load-bearing capability of a cracked dental composite. The self-healing corresponded to the formation of polymer films on re-fractured surfaces indicating the polymerization of the healing liquid in the crack planes. Furthermore, the self-healing composite displayed a strong antibacterial effect against dental plaque microcosm biofilms, inhibiting biofilm viability and lactic acid production, and reducing biofilm CFU by 3–4 log. These results, together with recent studies showing NACP with acid neutralization, lesion remineralization and inhibition of secondary caries in a human in situ model, indicate the promise of developing a composite addressing both the fracture issue (self-healing) and the caries issue (antibacterial and remineralization). The self-healing NACP-DMAHDM composite may be promising for tooth cavity restorations to combat bulk fracture and secondary caries. The method of using triple agents (self-healing microcapsules, NACP, DMAHDM) may have applicability to other composites, adhesives, cements and sealants in restorative and preventive dentistry.
Acknowledgments
This study was supported by NIH R01 DE17974 (HX) and a Seed Grant (HX) from the University of Maryland School of Dentistry.
References
- 1.Lynch CD. Successful posterior composites. London: Quintessence Publishing Co; 2008. [Google Scholar]
- 2.Bayne SC, Thompson JY, Swift EJ, Stamatiades P, Wilkerson M. A characterization of first-generation flowable composites. Journal of the American Dental Association. 1998;129:567–77. doi: 10.14219/jada.archive.1998.0274. [DOI] [PubMed] [Google Scholar]
- 3.Drummond JL. Degradation, fatigue, and failure of resin dental composite materials. Journal of Dental Research. 2008;87:710–9. doi: 10.1177/154405910808700802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ferracane JL. Resin composite - State of the art. Dental Materials. 2011;27:29–38. doi: 10.1016/j.dental.2010.10.020. [DOI] [PubMed] [Google Scholar]
- 5.Lim BS, Ferracane JL, Sakaguchi RL, Condon JR. Reduction of polymerization contraction stress for dental composites by two-step light-activation. Dental Materials. 2002;18:436–44. doi: 10.1016/s0109-5641(01)00066-5. [DOI] [PubMed] [Google Scholar]
- 6.Lu H, Stansbury JW, Bowman CN. Impact of curing protocol on conversion and shrinkage stress. Journal of Dental Research. 2005;84:822–6. doi: 10.1177/154405910508400908. [DOI] [PubMed] [Google Scholar]
- 7.Xu X, Ling L, Wang R, Burgess JO. Formation and characterization of a novel fluoride-releasing dental composite. Dental Materials. 2006;22:1014–23. doi: 10.1016/j.dental.2005.11.027. [DOI] [PubMed] [Google Scholar]
- 8.Milward PJ, Adusei GO, Lynch CD. Improving some selected properties of dental polyacid-modified composite resins. Dental Materials. 2011;27:997–1002. doi: 10.1016/j.dental.2011.06.006. [DOI] [PubMed] [Google Scholar]
- 9.Demarco FF, Correa MB, Cenci MS, Moraes RR, Opdam NJM. Longevity of posterior composite restorations: not only a matter of materials. Dental Materials. 2012;28:87–101. doi: 10.1016/j.dental.2011.09.003. [DOI] [PubMed] [Google Scholar]
- 10.Brunthaler A, Konig F, Lucas T, Sperr W, Schedle A. Longevity of direct resin composite restorations in posterior teeth. Clinical Oral Investigation. 2003;7:63–70. doi: 10.1007/s00784-003-0206-7. [DOI] [PubMed] [Google Scholar]
- 11.Bagramian RA, Garcia-Godoy F, Volpe AR. The global increase in dental caries. A pending public health crisis. American Journal of Dentistry. 2009;22:3–8. [PubMed] [Google Scholar]
- 12.Deligeorgi V, Mjor IA, Wilson NH. An overview of reasons for the placement and replacement of restorations. Primary Dental Care. 2001;8:5–11. doi: 10.1308/135576101771799335. [DOI] [PubMed] [Google Scholar]
- 13.Yuan YC, Yin T, Rong MZ, Zhang MQ. Self healing in polymers and polymer composites. Concepts, realization and outlook: A review. Express Polymer Letters. 2008;2:238–50. [Google Scholar]
- 14.White SR, Sottos NR, Geubelle PH, Moore JS, Kessler MR, Sriram SR, Brown EN, Viswanathan S. Autonomic healing of polymer composites. Nature. 2001;409:794–7. doi: 10.1038/35057232. [DOI] [PubMed] [Google Scholar]
- 15.Yuan L, Liang G, Xie JQ, Li L, Guo J. Preparation and characterization of poly (urea-formaldehyde) microcapsules filled with epoxy resins. Polymer. 2006;47:5338–49. [Google Scholar]
- 16.Esser-Kahn AP, Odom SA, Sottos NR, White SR, Moore JS. Triggered release from polymer capsules. Macromolecules. 2011;44:5539–53. [Google Scholar]
- 17.Wertzberger BE, Steere JT, Pfeifer RM, Nensel MA, Latta MA, Gross SM. Physical characterization of a self-healing dental restorative material. Journal of Applied Polymer Science. 2010;118:428–34. [Google Scholar]
- 18.Then S, Neon GS, kasim NH. Performance of melamine modified urea-formaldehyde microcapsules in a dental host material. Journal of Applied Polymer Science. 2011;122:2557–62. [Google Scholar]
- 19.Ouyang X, Huang X, Pan Q, Zuo C, Huang C, Yang X, Zhao Y. Synthesis and characterization of triethylene glycol dimethacrylate nanocapsules used in a self-healing bonding resin. Journal of Dentistry. 2011;39:825–33. doi: 10.1016/j.jdent.2011.09.001. [DOI] [PubMed] [Google Scholar]
- 20.Jandt KD, Sigusch BW. Future perspectives of resin-based dental materials. Dental Materials. 2009;25:1001–6. doi: 10.1016/j.dental.2009.02.009. [DOI] [PubMed] [Google Scholar]
- 21.Bevan C, Snellings WM, Dodd DE, Egan GF. Subchronic toxicity study of dicyclopentadiene vapor in rats. Toxicology and Industrial Health. 1992;8:353–67. [PubMed] [Google Scholar]
- 22.Caruso MM, Delafuente DA, Ho V, Sottos NR, Moore JS, White SR. Solvent-promoted self-healing epoxy materials. Macromolecules. 2007;40:8830–2. [Google Scholar]
- 23.Imazato S, Torii M, Tsuchitani Y, McCabe JF, Russell RR. Incorporation of bacterial inhibitor into resin composite. Journal of Dental Research. 1994;73:1437–43. doi: 10.1177/00220345940730080701. [DOI] [PubMed] [Google Scholar]
- 24.Gong SQ, Niu LN, Kemp LK, Yiu CK, Ryou H, Qi YP, Blizzard JD, Nikonov S, Brackett MG, Messer RL, Wu CD, Mao J, Bryan Brister L, Rueggeberg FA, Arola DD, Pashley DH, Tay FR. Quaternary ammonium silane-functionalized, methacrylate resin composition with antimicrobial activities and self-repair potential. Acta Biomaterialia. 2012;8:3270–82. doi: 10.1016/j.actbio.2012.05.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Moreau JL, Sun L, Chow LC, Xu HH. Mechanical and acid neutralizing properties and inhibition of bacterial growth of amorphous calcium phosphate dental nanocomposite. Journal of Biomedical Materials Research Part B: Applied Biomaterials. 2011;98:80–8. doi: 10.1002/jbm.b.31834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zhou C, Weir MD, Zhang K, Deng D, Cheng L, Xu HH. Synthesis of new antibacterial quaternary ammonium monomer for incorporation into CaP nanocomposite. Dental Materials. 2013;29:859–70. doi: 10.1016/j.dental.2013.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Blaiszik BJ, Caruso MM, McIlroy DA, Moore JS, White SR, Sottos NR. Microcapsules filled with reactive solutions for self-healing materials. Polymer. 2009;50:990–7. [Google Scholar]
- 28.Brown EN, Kessler MR, Sottos NR, White SR. In situ poly(urea-formaldehyde) microencapsulation of dicyclopentadiene. Journal of Microencapsulation. 2003;20:719–30. doi: 10.1080/0265204031000154160. [DOI] [PubMed] [Google Scholar]
- 29.Xu HH, Moreau JL, Sun L, Chow LC. Nanocomposite containing amorphous calcium phosphate nanoparticles for caries inhibition. Dental Materials. 2011;27:762–9. doi: 10.1016/j.dental.2011.03.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Li F, Weir MD, Xu HH. Effects of quaternary ammonium chain length on antibacterial bonding agents. Journal of Dental Research. 2013;92:932–8. doi: 10.1177/0022034513502053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Melo MA, Cheng L, Weir MD, Hsia RC, Rodrigues LK, Xu HH. Novel dental adhesive containing antibacterial agents and calcium phosphate nanoparticles. Journal of Biomedical Materials Research Part B: Applied Biomaterials. 2013;101:620–9. doi: 10.1002/jbm.b.32864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Xu HH, Quinn JB, Giuseppetti AA. Wear and mechanical properties of nano-silica-fused whisker composites. Journal of Dental Research. 2004;83:930–5. doi: 10.1177/154405910408301208. [DOI] [PubMed] [Google Scholar]
- 33.Kuebler J. VAMAS Report No. 37, TWA3, ESIS Report D2-99, EMPA, CH-8600. Duebendorf, Switzerland: 1999. Fracture toughness of ceramics using the SEVNB method; Round robin. [Google Scholar]
- 34.Wool RP, O’Connor KM. A theory of crack healing in polymers. Journal of Applied Physics. 1981;52:5953–63. [Google Scholar]
- 35.Pratten J, Wilson M, Spratt DA. Characterization of in vitro oral bacterial biofilms by traditional and molecular methods. Oral Microbiology and Immunology. 2003;18:45–9. doi: 10.1034/j.1399-302x.2003.180107.x. [DOI] [PubMed] [Google Scholar]
- 36.Cheng L, Exterkate RA, Zhou X, Li J, ten Cate JM. Effect of Galla chinensis on growth and metabolism of microcosm biofilms. Caries Research. 2011;45:87–92. doi: 10.1159/000324084. [DOI] [PubMed] [Google Scholar]
- 37.McBain AJ, Sissons C, Ledder RG, Sreenivasan PK, De Vizio W, Gilbert P. Development and characterization of a simple perfused oral microcosm. Journal of Applied Microbiology. 2005;98:624–34. doi: 10.1111/j.1365-2672.2004.02483.x. [DOI] [PubMed] [Google Scholar]
- 38.Cheng L, Weir MD, Zhang K, Wu EJ, Xu SM, Zhou X, Xu HH. Dental plaque microcosm biofilm behavior on calcium phosphate nanocomposite with quaternary ammonium. Dental Materials. 2012;28:853–62. doi: 10.1016/j.dental.2012.04.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Cheng L, Weir MD, Zhang K, Xu SM, Chen Q, Zhou X, Xu HH. Antibacterial nanocomposite with calcium phosphate and quaternary ammonium. Journal of Dental Research. 2012;91:460–6. doi: 10.1177/0022034512440579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lima JP, Sampaio de Melo MA, Borges FM, Teixeira AH, Steiner-Oliveira C, Nobre Dos Santos M, Rodrigues LK, Zanin IC. Evaluation of the antimicrobial effect of photodynamic antimicrobial therapy in an in situ model of dentine caries. European Journal of Oral Sciences. 2009;117:568–74. doi: 10.1111/j.1600-0722.2009.00662.x. [DOI] [PubMed] [Google Scholar]
- 41.Lie N, Bucuta S, Draenert M. Bulk-fill resin-based composites: an in vitro assessment of their mechanical performance. Operative Dentistry. 2013;38:618–25. doi: 10.2341/12-395-L. [DOI] [PubMed] [Google Scholar]
- 42.Li Q, Siddaramaiah, Kim NH, Hui D, Lee JH. Effects of dual component microcapsules of resin and curing agent on the self-healing efficiency of epoxy. Composites Part B: Engineering. 2013;55:79–85. [Google Scholar]
- 43.Yuan L, Huang S, Gu A, Liang G, Chen F, Hu Y, Nutt S. A cyanate ester/microcapsule system with low cure temperature and self-healing capacity. Composites Science and Technology. 2013;87:111–7. [Google Scholar]
- 44.Brown EN, White SR, Sottos NR. Microcapsule induced toughening in a self-healing polymer composite. Journal of Materials Science. 2004;39:1703–10. [Google Scholar]
- 45.Jin H, Miller GM, Sottos NR, White SR. Fracture and fatigue response of a self-healing epoxy adhesive. Polymer. 2011;52:1628–34. [Google Scholar]
- 46.Lynch CD, Frazier KB, McConnell RJ, Blum IR, Wilson NH. Minimally invasive management of dental caries: contemporary teaching of posterior resin-based composite placement in U.S. and Canadian dental schools. Journal of the American Dental Association. 2011;142:612–20. doi: 10.14219/jada.archive.2011.0243. [DOI] [PubMed] [Google Scholar]
- 47.Imazato S, Tay FR, Kaneshiro AV, Takahashi Y, Ebisu S. An in vivo evaluation of bonding ability of comprehensive antibacterial adhesive system incorporating MDPB. Dental Materials. 2007;23:170–6. doi: 10.1016/j.dental.2006.01.005. [DOI] [PubMed] [Google Scholar]
- 48.Featherstone JD. The continuum of dental caries-evidence for a dynamic disease process. Journal of Dental Research. 2004;83:C39–42. doi: 10.1177/154405910408301s08. [DOI] [PubMed] [Google Scholar]
- 49.Tanner J, Robinson C, Soderling E, Vallittu P. Early plaque formation on fibre-reinforced composites in vivo. Clinical Oral Investigations. 2005;9:154–60. doi: 10.1007/s00784-005-0317-4. [DOI] [PubMed] [Google Scholar]
- 50.Svanberg M, Mjör IA, Orstavik D. Mutans streptococci in plaque from margins of amalgam, composite, and glass-ionomer restorations. Journal of Dental Reseach. 1990;69:861–4. doi: 10.1177/00220345900690030601. [DOI] [PubMed] [Google Scholar]
- 51.Beyth N, Domb AJ, Weiss E. An in vitro quantitative antibacterial analysis of amalgam and composite resins. Journal of Dentistry. 2007;35:201–6. doi: 10.1016/j.jdent.2006.07.009. [DOI] [PubMed] [Google Scholar]
- 52.Imazato S, Kinomoto Y, Tarumi H, Ebisu S, Tay FR. Antibacterial activity and bonding characteristics of an adhesive resin containing antibacterial monomer MDPB. Dental Materials. 2003;19:313–9. doi: 10.1016/s0109-5641(02)00060-x. [DOI] [PubMed] [Google Scholar]
- 53.Imazato S. Review: antibacterial properties of resin composites and dentin bonding systems. Dental Materials. 2003;19:449–57. doi: 10.1016/s0109-5641(02)00102-1. [DOI] [PubMed] [Google Scholar]
- 54.Imazato S. Bioactive restorative materials with antibacterial effects: new dimension of innovation in restorative dentistry. Dental Materials Journal. 2009;28:11–9. doi: 10.4012/dmj.28.11. [DOI] [PubMed] [Google Scholar]
- 55.Imazato S, Kuramoto A, Takahashi Y, Ebisu S, Peters MC. In vitro antibacterial effects of the dentin primer of Clearfil Protect Bond. Dental Materials. 2006;22:527–32. doi: 10.1016/j.dental.2005.05.009. [DOI] [PubMed] [Google Scholar]
- 56.Zhang W, Luo XJ, Niu LN, Liu SY, Zhu WC, Epasinghe J, Chen L, Li GH, Huang C, Mao J, Pashley DH, Tay FR. One-pot synthesis of antibacterial monomers with dual biocidal modes. Journal of Dentistry. 2014;42:1078–95. doi: 10.1016/j.jdent.2014.06.001. [DOI] [PubMed] [Google Scholar]
- 57.Murata H, Koepsel RR, Matyjaszewski K, Russell AJ. Permanent, non-leaching antibacterial surface-2:How high density cationic surfaces kill bacterial cells. Biomaterials. 2007;28:4870–9. doi: 10.1016/j.biomaterials.2007.06.012. [DOI] [PubMed] [Google Scholar]
- 58.Zhang K, Cheng L, Wu EJ, Weir MD, Bai Y, Xu HH. Effect of water-aging on dentin bond strength and anti-biofilm activity of bonding agent containing antibacterial monomer dimethylaminododecyl methacrylate. Journal of Dentistry. 2013;41:504–13. doi: 10.1016/j.jdent.2013.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Weir MD, Chow LC, Xu HH. Remineralization of demineralized enamel via calcium phosphate nanocomposite. Journal of Dental Research. 2012;91:979–84. doi: 10.1177/0022034512458288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Melo MA, Weir MD, Rodrigues LK, Xu HH. Novel calcium phosphate nanocomposite with caries-inhibition in a human in situ model. Dental Materials. 2013;29:231–40. doi: 10.1016/j.dental.2012.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]