Abstract
Objectives
Dentin-composite bond failure is caused by factors including hybrid layer degradation, which in turn can be caused by hydrolysis and enzymatic degradation of the exposed collagen in the dentin. The objectives of this study were to investigate a new antibacterial monomer (dimethylaminododecyl methacrylate, DMADDM) as an inhibitor for matrix metalloproteinases (MMPs), and to determine the effects of DMADDM on both soluble recombinant human MMPs (rhMMPs) and dentin matrix-bound endogenous MMPs.
Methods
Inhibitory effects of DMADDM at six mass% (0.1% to 10%) on soluble rhMMP-8 and rhMMP-9 were measured using a colorimetic assay. Matrix-bound endogenous MMP activity was evaluated in demineralized human dentin. Dentin beams were divided into four groups (n = 10) and incubated in calcium- and zinc-containing media (control medium); or control medium + 0.2% chlorhexidine (CHX); 5% 12-methacryloyloxydodecylpyridinium bromide (MDPB); or 5% DMADDM. Dissolution of dentin collagen peptides was evaluated by mechanical testing in three-point flexure, loss of dentin mass, and a hydroxyproline assay.
Results
Use of 0.1% to 10% DMADDM exhibited a strong concentration-dependent anti-MMP effect, reaching 90% of inhibition on rhMMP-8 and rhMMP-9 at 5% DMADDM concentration. Dentin beams in medium with 5% DMADDM showed 34% decrease in elastic modulus (vs. 73% decrease for control), 3% loss of dry dentin mass (vs. 28% loss for control), and significantly less solubilized hydroxyproline when compared with control (p < 0.05).
Significance
The new antibacterial monomer DMADDM was effective in inhibiting both soluble rhMMPs and matrix-bound human dentin MMPs. These results, together with previous studies showing that adhesives containing DMADDM inhibited biofilms without compromising dentin bond strength, suggest that DMADDM is promising for use in adhesives to prevent collagen degradation in hybrid layer and protect the resin-dentin bond.
Keywords: Dental monomer, quaternary ammonium methacrylate, antibacterial activity, human dentin, matrix metalloproteinase inhibitor, tooth restoration
1. Introduction
Dental resin composites are popular filling materials because of their excellent esthetics, direct-filling capability, and improved load-bearing properties [1–3]. After being bonded to the tooth structure via adhesive [4], it is desirable to have a long-lasting restoration-tooth bonded interface. Although most adhesives exhibit excellent short-term bonding strength, the durability of the bonded interface still remains a challenge [5,6]. Nearly half of all dental restorations fail within 10 years, and replacing them accounts for 50–70% of all restorations performed [7,8]. This is costly, considering that the annual cost for tooth cavity restorations was about $46 billion in 2005 in the United States alone [7]. The cost is increasing rapidly with an aging population, longer life expectancy, and increased tooth retention in seniors [8]. Therefore, there is an urgent need to improve the dentin-resin interfacial bond to reduce failure and increase the longevity of dental restorations [5,6].
The degradation of the hybrid layer at the dentin-adhesive interface was believed to be the primary reason for failure, which was caused by several mechanical and chemical factors, including the hydrolysis and enzymatic degradation of the exposed collagen and the adhesive resin [9]. Water sorption in vivo was inevitable due to the polar ether-linkages and/or hydroxyl groups in adhesive [10], and it may result in hydrolysis of the hydrophilic resin components [11,12]. Recently, it was reported that host-derived matrix metalloproteinases (MMPs) were involved in hybrid layer degradation [13]. MMPs are a group of zinc- and calcium-dependent host-derived proteases and exist in mineralized dentin [14]. They can be released and activated by the acidic etchants of dentin bonding [15], and by lactic acid from oral pathogenic bacteria [15,16]. The activated collagen-bound MMPs and/or non-collagen-bound MMPs may progressively degrade the uncovered collagen fibrils in the bonded dentin. The breakdown of collagen may increase the water content, cause further collagen degradation, and deteriorate the dentin-restoration bond [9,17].
Chlorhexidine (CHX) had MMP inhibitory and anti-enzyme properties [18]. Collagen degradation of demineralized dentin was almost completely inhibited via CHX [19,20]. However, CHX is water-soluble and electrostatically binds to demineralized dentin matrix. When incorporated into adhesive, CHX may diffuse out of the dentin collagen matrix via a competitive desorption mechanism in the presence of other cations, leading to a decrease in its long-term anti-MMP effectiveness [21]. In contrast, the bond strength of an antibacterial adhesive containing 12-methacryloyloxydodecyl-pyridinium bromide (MDPB) did not decrease with time in a one-year period [22]. Another study showed that MDPB was effective in inhibiting both soluble MMPs and matrix-bound dentin MMPs [21]. Recently, a new antibacterial monomer dimethylaminododecyl methacrylate (DMADDM) was synthesized and incorporated into a bonding agent, which showed no reduction in bonding strength from 1 day (d) to 6 months of water-aging [23]. While the dentin shear bond strength of a commercial adhesive control decreased from about 30 MPa at 1 d to 20 MPa at 6 months, that of the DMADDM adhesive stayed at 30 MPa [24]. However, the anti-MMP properties of DMADDM have not been reported.
The objectives of this study were to investigate for the first time the effects of DMADDM on soluble rhMMP-8 and rhMMP-9 and human dentin matrix-bond endogenous MMPs, and on dentin elastic modulus and dentin dissolution and mass loss. It was hypothesized that: (1) DMADDM will have potent inhibitory effects against soluble rhMMP-8 and rhMMP-9 and matrix-bond endogenous MMPs; (2) The use of DMADDM will greatly reduce the elastic modulus loss in demineralized dentin, dentin mass loss, and the dissolution of collagen peptides from dentin, compared to control without DMADDM.
2. Materials and methods
2.1. Synthesis of antibacterial monomer
The synthesis of DMADDM was detailed elsewhere [23,24]. A modified Menschutkin reaction method was used where a tertiary amine group was reacted with an organo-halide. A benefit of this method is that the reaction products are generated at virtually quantitative amounts and require minimal purification [25]. Briefly, 10 mmol of 1-(dimethylamino)docecane (Sigma, St. Louis, MO) and 10 mM of 2-bromoethyl methacrylate (BEMA, Monomer-Polymer and Dajec Labs, Trevose, PA) were combined with 3 g of ethanol in a 20 mL scintillation vial. The vial was stirred at 70 °C for 24 h for the reaction to be completed. Then the solvent was removed via evaporation, yielding DMADDM as a clear, colorless and viscous liquid [23,24]. The reaction and products were verified via Fourier transform infrared spectroscopy (FTIR) in a previous study [25].
2.2. Inhibition of soluble recombinant human matrix metalloproteinases (rhMMPs)
Purified rhMMP-8 (catalog No. 72008), rhMMP-9 (catalog No. 55576-1) and the Sensolyte Generic MMP colorimetric assay kit (catalog No. 72095) were obtained from AnaSpec Inc. (San Jose, CA). MMP-8 is the major collagenase in human dentin [26]. MMP-9 is the most studied gelatinase in dentin [27]. The Sensolyte Generic MMP kit contains a thiopeptide that can be cleaved by MMPs to release a sulfhydryl group. The sulfhydryl group reacts with 5,5′-dithiobis(2-nitrobenzoic acid) to produce a colored reaction product 2-nitro-5-thiobenzoic acid, which can be detected spectrophotometrically at 412 nm [21].
DMADDM was dissolved in deionized water at DMADDM/(DMADDM + water) = 0.1%, 1%, 2.5%, 5%, 7.5% and 10% by mass. The thiopeptolide substrate solution was diluted to 0.2 mM with the supplied assay buffer in a 1:50 volume ratio. The assay was performed in a 96-well plate using four replicate wells for each DMADDM solution. For example, for rhMMP-9, each well contained 2 μL of rhMMP-9 (19.6 ng/well), 10 μL of a DMADDM solution at various concentrations (0.1, 1, 2.5, 5, 7.5 and 10 mg of DMADDM per well, respectively), and 50 μL of thiopeptolide substrate solution. Additional buffer was added to achieve a total of 100 μL for each well. As this resulted in a 10-fold dilution of the anti-MMP agent DMADDM, the DMADDM solutions at the aforementioned concentrations were prepared to 10 times their designated final concentrations. The DMADDM solutions were pre-incubated with each MMP enzyme for 20 min to prevent a burst of uninhibited enzyme activity, based on a previous study [21].
The control groups included: (1) a positive control containing rhMMP only without anti-MMP agent; (2) an inhibitor control containing rhMMP and 10 μL of the 20 μM GM6001, a known MMP inhibitor supplied in the assay kit by the manufacturer; (3) a test compound control containing assay buffer and the GM6001 or the DMADDM solution at each concentration to be tested; (4) a substrate control containing assay buffer. Additional assay buffer was added to bring the total volume of all control wells to 50 μL prior to adding 50μL of the thiopeptolide substrate solution to obtain the 100 μL/well.
The reagents were mixed by shaking the plate gently for 30 s. Then they were read kinetically at every 10 min for 60 min by measuring the absorbance at 412 nm using a plate reader (SpectraMax M5, Molecular Devices, Sunnyvale, CA) [21]. Background absorbance was determined using the mean absorbance readings from the “substrate control” wells and subtracted from the readings of other wells containing the thiopeptolide substrate [21].
The potency of MMP inhibition by the proprietary MMP inhibitor GM6001 (inhibitor control) and the DMADDM at six concentrations was calculated as the percentage of the adjusted absorbance of the positive control [21]. Percentage of MMP inhibition = 1 -(absorbance of test compound group - absorbance of test compound control)/(absorbance of the positive control - absorbance of substrate control). The test compound control was used for subtraction of background absorbance from the compound group. The substrate control was used for subtraction of background absorbance from the positive control group.
For statistical analysis, as the normality and homoscedasticity assumptions of the data appeared to be valid, the percentages of inhibition in the six groups (for the aforementioned six DMADDM concentrations) were analyzed using one-way ANOVA on ranks and Tukey’s multiple comparison tests at α = 0.05.
2.3. Preparation and incubation of demineralized human dentin beams
Extracted human third molars were collected with approval by the University of Maryland. The teeth were stored at 4 °C in 0.9% NaCl supplemented with 0.02% sodium azide to prevent bacterial growth and used within 1 month after extraction. For each tooth, the enamel and superficial dentin were removed by horizontal sectioning at 1 mm below the central fissures using a water-cooled cutting saw (Isomet, Buehler, Lake Bluff, IL). A 1-mm thick dentin disk consisting of midcoronal dentin was prepared from each tooth. Two dentin beams of 6 × 2 × 1 mm were sectioned from the middle of each disk under water cooling [21]. Prior to demineralization, for each dentin beam, a small dimple was made at the end of one of the two 6 × 2 mm surfaces to allow subsequent measurements to be performed on the same surface. The beams were submerged in 10% phosphoric acid for 18 h to completely demineralize the dentin [21]. Absence of residual mineral was confirmed using digital radiography. Then, the elastic modulus of each demineralized dentin beam was determined using three-point flexure (Section 2.4), and this is termed the elastic modulus at 0 d.
Demineralized dentin beams were randomized into four groups for aging in four types of solutions (n = 10). A calcium- and zinc-containing complete storage medium was used which contained 5 mM HEPES, 2.5 mM CaCl2.H2O, 0.05 mM ZnCl2, and 0.3 mM NaN3 (adjusted to pH 7.4 with 1M HCl), and this is referred to as control medium. The four solutions were: control medium; control medium plus 5% DMADDM; control medium plus 0.2% CHX (Sigma); and control medium plus 5% MDPB. MDPB powder was kindly provided by Kuraray Medical Inc. (Tokyo, Japan), included here since it was a well-known and potent quaternary ammonium methacrylate. For calculation of the mass%, for example, DMADDM/(DMADDM + control medium) = 5%. The 5% DMADDM was based on Section 2.2 which showed that the inhibition of rhMMP-8 and rhMMP-9 in the 5% DMADDM group was excellent and exceeded 90%. Furthermore, the result of our previous study showed that an adhesive containing 5% DMADDM had strong antibacterial activity after being cured [23]. Each demineralized dentin beam was placed in a polypropylene tube containing 1 mL of one of the four solutions. The sealed tubes were incubated in a shaker at 60 cycles/min and 37 °C for 30 d [21]. Elastic modulus of dentin beams were determined using three-point flexure (Section 2.4), and this is termed the elastic modulus at 30 d.
2.4. Elastic modulus of demineralized dentin beams
Elastic modulus of demineralized dentin beams were measured using three-point exure with a span of 4 mm, suitable for testing small-sized human tooth samples. A universal testing machine (model V1000, John Chatillon & Sons, Greensboro, NC) was used with a 1 N load-cell (Transducer Techniques, Temecula, CA). Each beam was loaded to a maximum displacement of 200 μm at a rate of 60 μm/min, which was determined in preliminary study to be suitable for measuring elastic modulus of demineralized dentin in the elastic region. After reaching maximum displacement, the load was reduced to 0 N within 15 s [28]. The dentin beam was taken out of the immersion; it remained saturated with solution and wet during testing. Elastic modulus (E) was calculated as: E = mL3/4bd3, where m is slope of load-displacement curve, L is span, b is dentin beam width, and d is dentin beam thickness [28]. As this test used relatively small dentin beams, the elastic modulus was approximate, to determine the ranking of elastic modulus of dentin for the different treatment groups [28].
The data were analyzed using a two factor repeated measures analysis of variance to examine the effects of the four solutions and the repeated factor storage time (0 d vs. 30 d), and the interaction of the two variables on elastic modulus. Pair-wise multiple comparisons were performed using the Tukey’s test. Statistical significance was set at α = 0.05.
2.5. Loss of mass versus immersion time for demineralized dentin
Demineralized dentin beams at 0 d were placed in uncapped polypropylene tubes and placed in a container with anhydrous calcium sulfate (Drierite, Hammond Drierite, Xenio, OH), to be desiccated until a constant weight was reached. The dry mass of each beam was measured to an accuracy of 0.1 mg via an analytical balance (AB204, Mettler Toledo, Switzerland). After the measurement, each dried and shrunken dentin beam was placed in its original polypropylene tube with incubation medium. This completely rehydrated the dried beam, and reversibly recovered its original dimensions [28]. After 30 d, the beam was dried again as described above, and the dry mass was again measured. Percentage of mass loss in dentin = (dry mass at 0 d – dry mass at 30 d)/dry mass at 0 d. The data were analyzed using Kruskal-Wallis one-way ANOVA and Dunn’s multiple comparison tests at α = 0.05.
2.6. Dissolution of collagen peptides from demineralized dentin beams
When the demineralized dentin beams were incubated in the calcium and zinc-containing medium, the activated matrix-bound MMPs slowly digested the demineralized collagen and released the solubilized peptide fragments [29]. A hydroxyproline assay (Sigma) was used to evaluate the collagen degradation. At 30 d, 100 μL of the medium was collected from each vial and transferred to a labeled polypropylene tube. An aliquot of 100 μL concentrated hydrochloric acid (HCl, 12 M) was added into the tube and the mixture was hydrolyzed at 120 °C for 3 hours. Then, 5 mg of activated charcoal was added, mixed and centrifuged at 13 kg for 2 minutes. An aliquot of 10 μL supernatant was transferred to a 96 well plate. At the same time, hydroxyproline standards for colorimetric detection were prepared by diluting 10 μL of the 1 mg/mL hydroxyproline standard solution with 90 μL of water to prepare a 0.1 mg/mL standard solution. An amount of 0.1 mg/mL hydroxyproline standard solution, at 0, 2, 4, 6, 8, or 10 μL, was added into a 96 well plate, generating corresponding standards of 0 (blank), 0.2, 0.4, 0.6, 0.8, and 1.0 μg/well. The plate was placed in a 60 °C oven to evaporate all the wells. Then, 100 μL Chloramine T/Oxidation Buffer Mixture was added to each sample well and standard well. After incubation at room temperature for 5 minutes, 100 μL 4-(dimethylamino) benzaldehyde (DMAB) reagent was added to each sample well and standard well, and incubated for 90 minutes at 60 °C. Then the absorbance was measured at 560 nm using the plate reader. The background for the assay was the value obtained for the 0 (blank) hydroxyproline standard. The blank value was subtracted from all the readings. The amount of hydroxyproline (μg/mL) was determined from a pre-established calibration curve derived from a linear regression equation of the absorbance of hydroxyproline against the known concentrations of hydroxyproline in the standards, following the manufacturer’s instructions for the hydroxyproline assay.
The resulting amount of hydroxyproline was used to estimate the percentage of solubilized collagen fragments in the medium. This estimate was based on the assumption that 90% of the dry mass of demineralized dentin consisted of type I collagen according to a previous study [30], and that the dentin collagen contained 9.6 mass% of hydroxyproline [31]. Hence, the total hydroxyproline mass in the dentin beam at 0 d = dentin dry mass at 0 d × 0.9 × 0.096. Therefore, the percentage of solubilized collagen at 30 d = the measured mass of solubilized hydroxyproline at 30 d/the total hydroxyproline mass in the dentin beam at 0 d = the measured mass of solubilized hydroxyproline at 30 d/(dentin dry mass at 0 d × 0.9 × 0.096). However, in order to compare with previous study [28], the dissolved collagen from the demineralized dentin beam was expressed as μg of solubilized hydroxyproline per mg dry mass of demineralized dentin at 0 d. The amounts of dissolved collagen from the demineralized dentin beams in the four groups were evaluated using Kruskal-Wallis one-way ANOVA and Dunn’s multiple comparison tests at α = 0.05.
3. Results
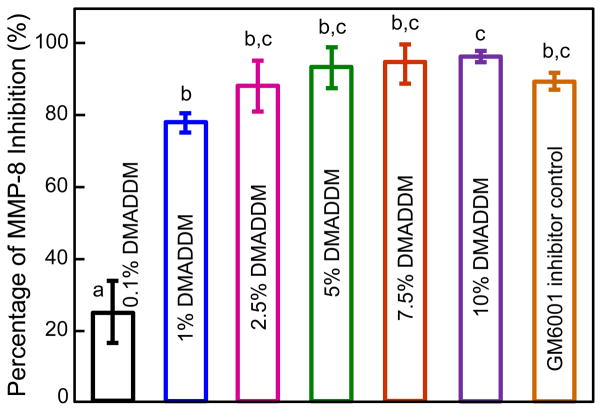
The anti-MMP-8 results of DMADDM are plotted in Fig. 1 (mean ± sd; n = 4). The percentage of rhMMP-8 inhibition by GM6001 inhibitor control was 90.4 ± 2.7 %. When soluble rhMMP-8 was incubated with increasing concentrations of DMADDM, the enzyme was increasingly inhibited. At 5% DMADDM, rhMMP-8 inhibition by DMADDM matched that of GM6001 inhibitor control. At 7.5% and 10% DMADDM, the rhMMP-8 inhibition was 95.2% and 97.0%, respectively, which exceeded that of GM6001 inhibitor.
Figure 1.
Anti-MMP-8 effect of antibacterial monomer DMADDM. When soluble rhMMP-8 was incubated with increasing concentrations of DMADDM, the enzyme was increasingly inhibited, reaching 90% inhibition at 5% DMADDM. Values (mean ± sd; n = 4) with dissimilar letters are significantly different from each other (p < 0.05).
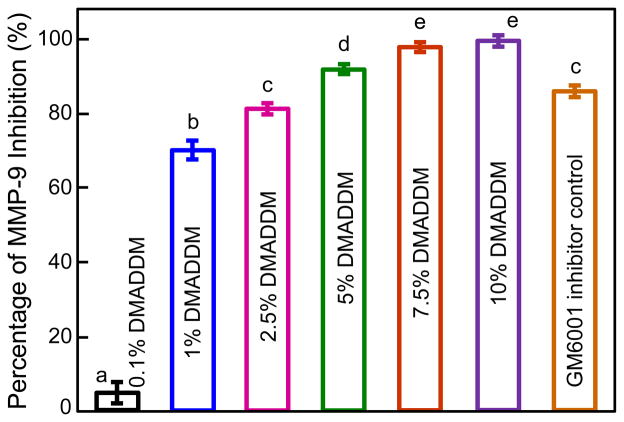
The anti-MMP-9 results are plotted in Fig. 2 (mean ± sd; n = 4). The percentage of rhMMP-9 inhibition by GM6001 inhibitor was 85.35 ± 1.3 %. rhMMP-9 was increasingly inhibited with increasing DMADDM concentration. The percentages of rhMMP-9 inhibition by DMADDM were 4.4 ± 3.6%, 70.4 ± 3.0%, 81.5 ± 1.2%, 92.4 ± 0.7%, 98.2 ± 1.3% and 99.2 ± 1.2%, at 0.1%, 1%, 2.5%, 5%, 7.5% and 10% of DMADDM, respectively.
Figure 2.
Anti-MMP-9 effect of DMADDM. Soluble rhMMP-9 was increasingly inhibited with increased concentrations of DMADDM, reaching 90% inhibition at 5% DMADDM. Values (mean ± sd; n = 4) with dissimilar letters are significantly different from each other (p < 0.05).
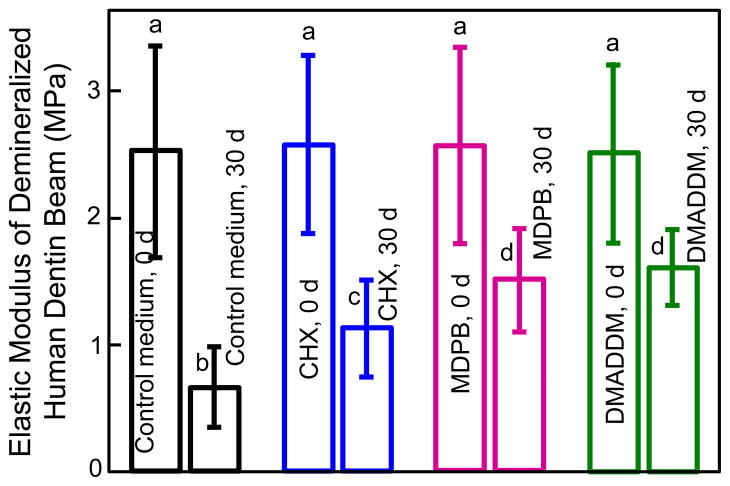
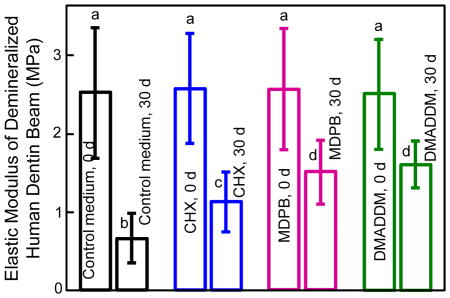
Elastic modulus results of demineralized dentin beams at 0 d and after 30 d immersion are plotted in Fig. 3 (mean ± sd; n = 10). Demineralized dentin beams of the four groups at 0 d had similar elastic modulus as expected (p > 0.1). At 30 d, the elastic modulus decreased significantly for each group (p < 0.05). Furthermore, the elastic modulus at 30 d varied significantly among the groups (p < 0.05). Dentin immersed in control medium for 30 d had the greatest lost in elastic modulus, losing 72% of the initial elastic modulus. Dentin immersed in CHX solution lost 52% of the elastic modulus, vs. 35% loss in MDPB solution, and 34% loss in DMADDM solution. The solution type and immersion time both had significant effects on elastic modulus (p < 0.05), and there was a significant interaction between these two variables (p < 0.05).
Figure 3.
Changes in elastic modulus of demineralized human dentin beams before and after 30 days of incubation in control medium, and medium containing 0.2% CHX, 5% MDPB, or 5% DMADDM. Values (mean ± sd; n = 10) with dissimilar letters are significantly different from each other (p < 0.05).
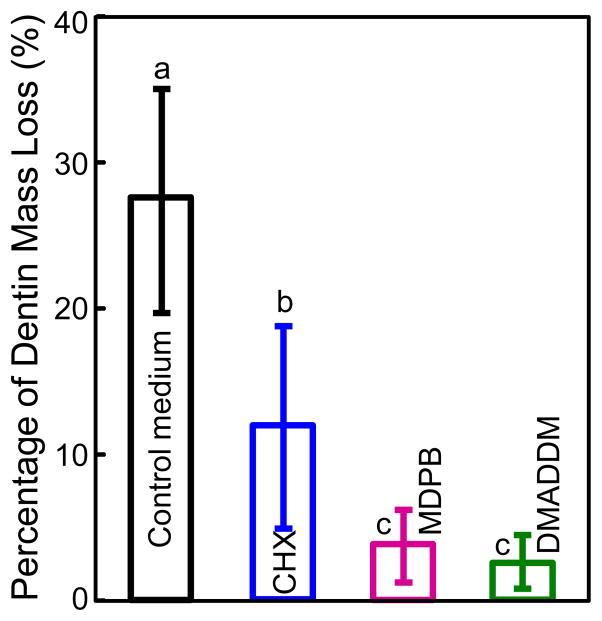
The percentage of dentin mass loss at 30 d is plotted in Fig. 4 (mean ± sd; n = 10). Dentin incubated in control medium without any inhibitor lost 28% of the dry mass. Dentin mass losses of the other three groups were significantly less than the control group (p < 0.05), yielding a loss of 12% for CHX group, 4% for MDPB group, and 3% for DMADDM group.
Figure 4.
Loss of mass of demineralized dentin beams in 30-day incubation in control medium, or medium containing 0.2% CHX, 5% MDPB, or 5% DMADDM. Loss of dentin mass = (dry mass at 0 d - dry mass at 30 d)/dry mass at 0 d. Values (mean ± sd; n = 10) with dissimilar letters are significantly different from each other (p < 0.05).
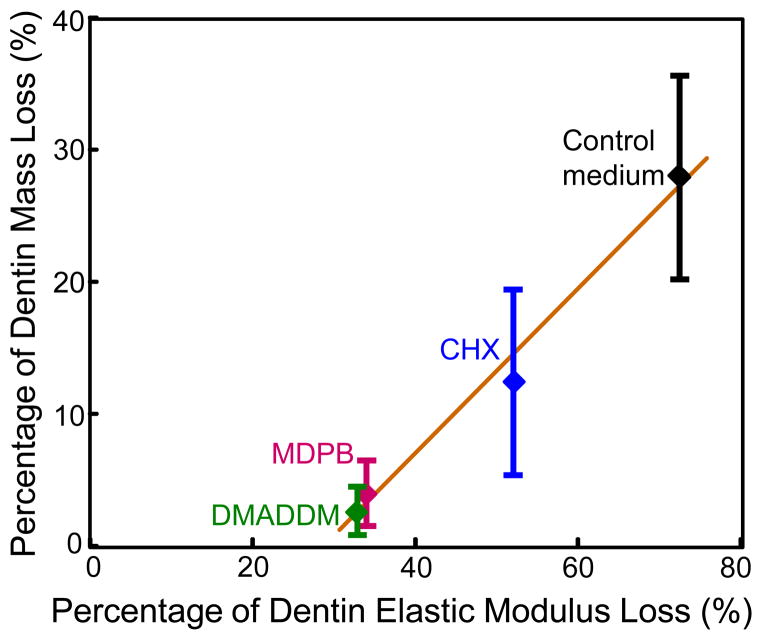
The relationship between elastic modulus loss and dentin mass loss is shown in Fig. 5 (mean ± sd; n = 10). Dentin in control medium had the greatest loss in mass, and the greatest loss in elastic modulus. Dentin in DMADDM solution had the least loss in mass, and the least loss in elastic modulus. The loss of elastic modulus was linearly proportional to dentin mass loss at 30 d, with a correlation coefficient of 0.9869. These results showed that the loss of elastic modulus of dentin and the loss of dentin mass confirmed each other, indicating that dentin dissolution was responsible for loss of elastic modulus.
Figure 5.
Relationship between the loss of elastic modulus and the loss of mass for demineralized dentin (mean ± sd; n = 10). The loss of elastic modulus X was linearly proportional to the loss of dentin mass Y. R is the correlation coefficient.
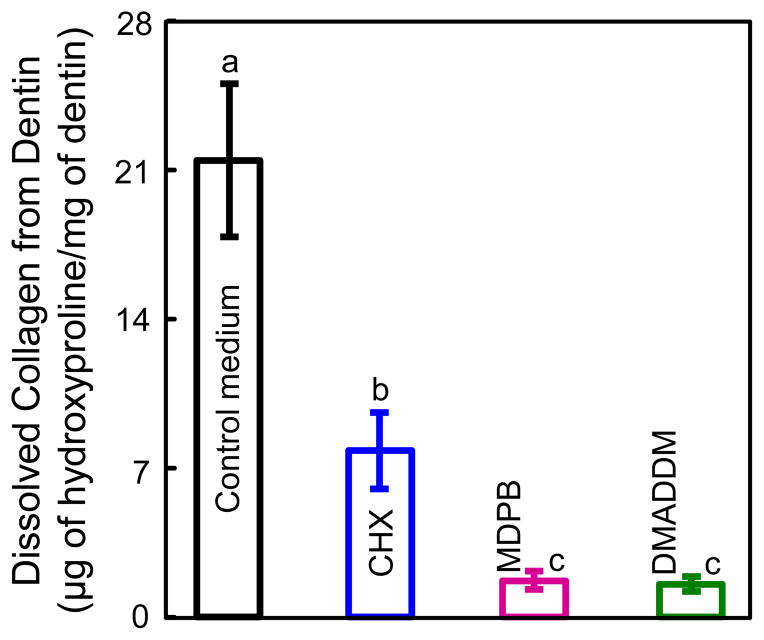
The dissolution of collagen peptides determined by hydroxyproline analysis is shown in Fig. 6 (mean ± sd; n = 10). The control medium group had 21.76 μg of hydroxyproline dissolution per 1 mg dry weight of collagen matrix, significantly higher than those for other groups (7.83 μg for CHX group, 1.64 μg for MDPB group, and 1.50 μg for DMADDM group) (p < 0.05). MDPB and DMADDM groups had similar hydroxyproline amounts (p > 0.05).
Figure 6.
The amount of dissolved collagen from demineralized dentin beams in 30-d aging (mean ± sd; n = 10). The Y-axis = the measured mass (μg) of dissolved hydroxyproline at 30 d/dentin dry mass (mg) at 0 d. Four mediums were tested in the 30-d incubation: Control medium, control medium + 0.2% CHX, control medium + 5% MDPB, control medium + 5% DMADDM. Values with dissimilar letters are significantly different from each other (p < 0.05).
4. Discussion
DMADDM was recently synthesized and incorporated into adhesive and composite, which achieved potent antibacterial activities [23,32]. Recent studies on CHX, benzalkonium chloride and MDPB indicated possible anti-MMP effects of antibacterial agents containing cationic groups [19,21,33]. In the present study, DMADDM was examined as a potential inhibitor of collagen-bound MMPs and non-collagen-bound MMPs. The results substantiated the two hypotheses and showed for the first time that: (1) DMADDM had potent inhibitory effects against the activity of soluble MMPs and matrix-bond endogenous MMPs; and (2) the use of DMADDM minimized the decrease in elastic modulus, dentin mass loss, and dissolution of collagen peptides of demineralized human dentin, compared to control without DMADDM.
Dentin is a biological composite of apatite crystallites in a collagen matrix with a fluid-filled tubular structure [34]. The bonding of this heterogeneous and intrinsically wet substrate to a composite restoration depends on the penetration of adhesive into the conditioned dentin to create micromechanical interlocks. It is unlikely for resin monomers to displace all the water around the collagen fibrils and within the interfibrillar spaces [35]. Hence, there is likely a layer of residual water over the collagen fibrils that can prevent intimate contact between the resin and the collagen fibrils. Furthermore, the discrepancy between dentin demineralization and adhesive infiltration within the water-saturated dentin can cause incompletely-impregnated zones along the bottom of the hybrid layer [36–38]. The poorly-encapsulated collagen could be hydrolyzed by the endogenous MMPs [13,27,39], thereby compromising the durability of the dentin-restoration bond.
MMPs are calcium- and zinc-dependent endopeptidases, which contribute to the breakdown of collagen matrices in dental caries [14] and periodontal diseases [40]. During dentin bonding, the mildly-acidic adhesive resin components [15,41] could activate MMPs (MMP-2, -8, -9) [15,26,42], thus causing degradation of collagen fibrils. Collagens can be degraded by interstitial collagenases, which include MMP-1, MMP-8, and MMP-13, resulting in the release of 3/4-length to 1/4-length peptides [14]. These peptides lose the triple-helical conformation and can then be further degraded by the gelatinases MMP-2 and MMP-9 [14].
MMP-8 and MMP-9 were chosen as representative collagenases and gelatinaes, respectively, to evaluate the anti-MMP effect of DMADDM in this study. Since previous studies used 5% of DMADDM in adhesive [23,24], the MMPs-inhibitory effect was tested in the neighborhood of 5% DMADDM to mimic adhesive application. The concentrations above 5% (7.5% and 10%) could mimic the situation after solvent evaporation from the bonding agent; the concentrations of 0.1%, 1% and 2.5% could approximate the gradient dilation of adhesive by dentin tubular fluid and residual water in the hybrid layer. The rhMMP-8 and rhMMP-9 inactivation reached ≥ 90% at DMADDM concentrations of ≥ 5%, even greater than that of the MMP inhibitor GM6001. MMP-8 and -9 are multi-domain enzymes containing at least a prodomain and a catalytic domain [43]. The protease domain has one catalytic zinc, one structural zinc and three calcium ions. The catalytic zinc coordinates with three histidines. One of the three histidine molecules is adjacent to a glutamic acid residue that contains a negative charge [21]. The cationic quaternary ammonium group of a QAM may alter the configuration of the catalytic site of the MMP via electrostatic binding to the negative charge of glutamic acid residue. This QAM-MMP complex would make MMPs unable to hydrolyze the specific peptide bond of collagen [21], thus preserving the collagen in the hybrid layer and protecting the dentin-composite bond.
The efficiency of DMADDM against MMP-8 and MMP-9 was different. MMP-9 was relatively more difficult to be inhibited by DMADDM, especially at concentrations lower than 5%. Compared with the structure of MMP-8, the gelatinase MMP-9 has a gelatin-binding domain in the catalytic domain, which contains three fibronectin-type II repeats [14]. This fibronectin-like domain may hinder the approaching of DMADDM to the catalytic domain, thus hindering the inactivation of MMP-9. With the increase of DMADDM concentration to 7.5% and 10%, the increased positive charge density likely became strong enough to overcome the spatial obstacle, thereby effectively altering the configuration of the catalytic site electrostatically and inhibiting the MMP-9.
Next, demineralized dentin beams were used as a clinically relevant model of acid etched dentin to evaluate the inhibitory effect of DMADDM on collagen-bound MMPs in a complex molecularly cross-talking environment. Demineralization of dentin exposed not only the collagen and the substance of endogenous enzymes, but also the non-collagenous proteins such as MMPs, cysteine cathepsins, endogenous tissue inhibitors of metalloproteinases (TIMPs), and the small integrin-binding ligand, N-linked glycoprotein family (SIBLING family). The non-collagenous proteins could interact with each other and form a network to regulate endogenous proteases. Some MMPs are activated by other members of the MMP family or other proteases like cysteine cathepsins. TIMPs can inhibit the activity of MMPs. In addition, the SIBLING molecules can form specific complexes with different MMPs [44] to activate the latent form by promoting structural changes, or restore MMP activity previously-inhibited due to binding to a specific TIMP [44]. Therefore, it is essential to test the MMP inhibition of DMADDM against the native substrate of demineralized human dentin where a complex proteases-regulation network exists.
Based on the results of previous antibacterial studies [24,45] and the inhibitory effects on soluble MMPs in the present study, 5% DMADDM was effective against oral pathogenic bacteria [24,45] and soluble MMPs. Hence, 5% DMADDM was selected to test the effect on endogenous bound MMPs in demineralized dentin. CHX and MDPB were shown to exhibit an inhibitory effect on the endogenous collagenolytic activity in dentin [21,46], hence they were chosen as controls. Comparing the elastic modulus losses after aging for 30 d, the highest elastic modulus was found in the group treated with DMADDM and MDPB, whereas the lowest elastic modulus was obtained in control group. Furthermore, the amount of the elastic modulus loss was lineally proportional to the dentin mass loss. However, the extent of elastic modulus loss was greater than the extent of dentin mass loss. This indicates that a small amount of collagen degradation could trigger a relatively large elastic modulus loss in dentin and degrade the bonding performance.
A relatively lower concentration of 0.2% CHX was used, because in a previous study on a CHX-incorporated primer, at higher CHX content (e.g., 2%), the resin polymerization of the bonding agent was adversely interfered [47]. Furthermore, water-soluble inhibitors such as CHX will slowly leach out from the resin-dentin interface and lose its MMP-inhibitory effect over time. Indeed, a recent study showed that the protective effect of CHX on the bonded interface decreased in 9 months, and severe degradation occurred in the hybrid layer at 18 months [48]. Unlike CHX, the new antibacterial monomer DMADDM contains a methacryloyl group and can be copolymerized in adhesive resin, thus likely providing a durable MMP-inhibitory effect. Incorporation of QAMs into adhesives was an effective strategy to achieving long-term antibacterial efficacy. A previous study tested the antibacterial effect of a photo-cured QAM-containing resin after water-aging from 1 d to 6 months, and showed a strong anti-biofilm potency that did not decrease with time [23]. The positive charges were suggested to be the reason for both antibacterial effect [49] and anti-MMP effect [21]. The long-lasting antibacterial effect of the cured QAM-containing resin indicates the existence of cationic quaternary ammonium groups in the cured resin, which was confirmed by the positive charges on the cured QAM-containing resin surface [50]. Therefore, the positive charges of the cured DMADDM-containing resin are expected to continue to inhibit the residual active MMPs in the hybrid layer. Additional studies are needed to further validate the anti-MMP effect of the DMADDM-containing bonding agent after curing.
A polymerizable anti-MMP monomer is promising for use in bioactive dental resins such as adhesives, cements, bases, liners and sealants. The anti-MMP monomer can be incorporated into the primer or bonding agent of a dental adhesive system, or into a resin cement to obtain a more stabilized and longer-lasting bond at the resin-dentin interface. When such an adhesive or resin cement is applied to dentin, before polymerization, free monomers can inhibit both soluble and collagen-bound MMPs. After polymerization, the immobilized anti-MMP component will likely continue to inhibit the residual active MMPs in the hybrid layer. It was recently demonstrated that DMADDM had much lower cytotoxicity than BisGMA, a common monomer in dental materials [51]. Taken together, these results suggest that DMADDM is promising for use in therapeutic adhesives not only to combat a variety of oral pathogens and cariogenic biofilms as shown in previous studies [23,24], but also to inhibit MMPs and enhance the dentin-resin bonding durability.
5. Conclusion
A new monomer DMADDM which had been shown to be strongly-antibacterial and inhibited biofilm acids in recent studies, was tested here as an inhibitor against human dentin MMPs for its potential to combat degradation of dentin collagen and the dentin-resin bond. DMADDM exhibited a strong anti-MMP effect, reaching more than 90% inhibition on rhMMP-8 and rhMMP-9 with 5% or more DMADDM. Furthermore, the use of DMADDM preserved the mechanical stiffness and hindered the dissolution of demineralized human dentin. Therefore, DMADDM is promising for use in bonding agents to prevent dentin collagen degradation in the hybrid layer and prolong the dentin-restoration bond.
Highlights.
Dentin-composite bond failure is caused by factors including hybrid layer degradation, which in turn can be caused by hydrolysis and enzymatic degradation of the exposed collagen in the dentin. The objectives of this study were to investigate a new antibacterial monomer (dimethylaminododecyl methacrylate, DMADDM) as an inhibitor for matrix metalloproteinases (MMPs), and to determine the effects of DMADDM on both soluble recombinant human MMPs (rhMMPs) and dentin matrix-bound endogenous MMPs. The new antibacterial monomer DMADDM was effective in inhibiting both soluble rhMMPs and matrix-bound human dentin MMPs. DMADDM is promising for use in adhesives to prevent collagen degradation in hybrid layer and protect the resin-dentin bond. The figure below plots the changes in elastic modulus of demineralized human dentin beams before and after 30 days of incubation in control medium, and in medium containing 0.2% CHX, or 5% MDPB, or 5% DMADDM. Values (mean ± sd; n = 10) with dissimilar letters are significantly different from each other (p < 0.05). Antibacterial monomer DMADDM can inhibit MMPs and protect dentin.
Acknowledgments
We thank Drs. Joseph M. Antonucci, Nancy J. Lin and Sheng Lin-Gibson of the National Institute of Standards and Technology (NIST) for fruitful discussions. This study was supported by NIH R01DE17974 (HX), National Natural Science Foundation of China 81100772 (FL), and a bridge funding and a seed grant from the University of Maryland School of Dentistry (HX).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Watts DC, Marouf AS, Al-Hindi AM. Photo-polymerization shrinkage-stress kinetics in resin-composites: methods development. Dental Materials. 2003;19:1–11. doi: 10.1016/s0109-5641(02)00123-9. [DOI] [PubMed] [Google Scholar]
- 2.Drummond JL. Degradation, fatigue, and failure of resin dental composite materials. Journal of Dental Research. 2008;87:710–9. doi: 10.1177/154405910808700802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ferracane JL. Resin composite--state of the art. Dental Materials. 2011;27:29–38. doi: 10.1016/j.dental.2010.10.020. [DOI] [PubMed] [Google Scholar]
- 4.Garcia-Godoy F, Kramer N, Feilzer AJ, Frankenberger R. Long-term degradation of enamel and dentin bonds: 6-year results in vitro vs. in vivo. Dental Materials. 2010;26:1113–8. doi: 10.1016/j.dental.2010.07.012. [DOI] [PubMed] [Google Scholar]
- 5.Pashley DH, Tay FR, Breschi L, Tjäderhane L, Carvalho RM, Carrilho M, Tezvergil-Mutluay A. State of the art etch-and-rinse adhesives. Dental Materials. 2011;27:1–16. doi: 10.1016/j.dental.2010.10.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Liu Y, Tjäderhane L, Breschi L, Mazzoni A, Li N, Mao J, Pashley DH, Tay FR. Limitations in bonding to dentin and experimental strategies to prevent bond degradation. Journal of Dental Research. 2011;90:953–68. doi: 10.1177/0022034510391799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Deligeorgi V, Mjor IA, Wilson NH. An overview of reasons for the placement and replacement of restorations. Primary Dental Care. 2001;8:5–11. doi: 10.1308/135576101771799335. [DOI] [PubMed] [Google Scholar]
- 8.Frost PM. An audit on the placement and replacement of restorations in a general dental practice. Primary Dental Care. 2002;9:31–6. doi: 10.1308/135576102322547548. [DOI] [PubMed] [Google Scholar]
- 9.Manuja N, Nagpal R, Pandit IK. Dental adhesion: mechanism, techniques and durability. Journal of Clinical Pediatric Dentistry. 2012;36:223–34. [PubMed] [Google Scholar]
- 10.Sideridou I, Tserki V, Papanastasiou G. Study of water sorption, solubility and modulus of elasticity of light-cured dimethacrylate-based dental resins. Biomaterials. 2003;24:655–65. doi: 10.1016/s0142-9612(02)00380-0. [DOI] [PubMed] [Google Scholar]
- 11.Tay FR, Pashley DH. Water treeing--a potential mechanism for degradation of dentin adhesives. American Journal of Dentistry. 2003;16:6–12. [PubMed] [Google Scholar]
- 12.De Munck J, Van Landuyt K, Peumans M, Poitevin A, Lambrechts P, Braem M, Van Meerbeek B. A critical review of the durability of adhesion to tooth tissue: methods and results. Journal of Dental Research. 2005;84:118–32. doi: 10.1177/154405910508400204. [DOI] [PubMed] [Google Scholar]
- 13.Pashley DH, Tay FR, Yiu C, Hashimoto M, Breschi L, Carvalho RM, Ito S. Collagen degradation by host-derived enzymes during aging. Journal of Dental Research. 2004;83:216–21. doi: 10.1177/154405910408300306. [DOI] [PubMed] [Google Scholar]
- 14.Chaussain-Miller C, Fioretti F, Goldberg M, Menashi S. The role of matrix metalloproteinases (MMPs) in human caries. Journal of Dental Research. 2006;85:22–32. doi: 10.1177/154405910608500104. [DOI] [PubMed] [Google Scholar]
- 15.Nishitani Y, Yoshiyama M, Wadgaonkar B, Breschi L, Mannello F, Mazzoni A, Carvalho RM, Tjäderhane L, Tay FR, Pashley DH. Activation of gelatinolytic/collagenolytic activity in dentin by self-etching adhesives. European Journal of Oral Sciences. 2006;114:160–6. doi: 10.1111/j.1600-0722.2006.00342.x. [DOI] [PubMed] [Google Scholar]
- 16.Tjäderhane L, Larjava H, Sorsa T, Uitto VJ, Larmas M, Salo T. The activation and function of host matrix metalloproteinases in dentin matrix breakdown in caries lesions. Journal of Dental Research. 1998;77:1622–9. doi: 10.1177/00220345980770081001. [DOI] [PubMed] [Google Scholar]
- 17.Spencer P, Wang Y. Adhesive phase separation at the dentin interface under wet bonding conditions. Journal of Biomedical Materials Research. 2002;62:447–56. doi: 10.1002/jbm.10364. [DOI] [PubMed] [Google Scholar]
- 18.Gendron R, Grenier D, Sorsa T, Mayrand D. Inhibition of the activities of matrix metalloproteinases 2, 8, and 9 by chlorhexidine. Clinical and Diagnostic Laboratory Immunology. 1999;6:437–9. doi: 10.1128/cdli.6.3.437-439.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Breschi L, Mazzoni A, Nato F, Carrilho M, Visintini E, Tjäderhane L, Ruggeri A, Jr, Tay FR, de Dorigo ES, Pashley DH. Chlorhexidine stabilizes the adhesive interface: a 2-year in vitro study. Dental Materials. 2010;26:320–5. doi: 10.1016/j.dental.2009.11.153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Osorio R, Yamauti M, Osorio E, Ruiz-Requena ME, Pashley D, Tay F, Toledano M. Effect of dentin etching and chlorhexidine application on metalloproteinase-mediated collagen degradation. European Journal of Oral Sciences. 2011;119:79–85. doi: 10.1111/j.1600-0722.2010.00789.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tezvergil-Mutluay A, Agee KA, Uchiyama T, Imazato S, Mutluay MM, Cadenaro M, Breschi L, Nishitani Y, Tay FR, Pashley DH. The inhibitory effects of quaternary ammonium methacrylates on soluble and matrix-bound MMPs. Journal of Dental Research. 2011;90:535–40. doi: 10.1177/0022034510389472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Donmez N, Belli S, Pashley DH, Tay FR. Ultrastructural correlates of in vivo/in vitro bond degradation in self-etch adhesives. Journal of Dental Research. 2005;84:355–9. doi: 10.1177/154405910508400412. [DOI] [PubMed] [Google Scholar]
- 23.Zhang K, Cheng L, Wu EJ, Weir MD, Bai Y, Xu HH. Effect of water-aging on dentin bond strength and anti-biofilm activity of bonding agent containing antibacterial monomer dimethylaminododecyl methacrylate. Journal of Dentistry. 2013;41:504–13. doi: 10.1016/j.jdent.2013.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cheng L, Weir MD, Zhang K, Arola DD, Zhou X, Xu HH. Dental primer and adhesive containing a new antibacterial quaternary ammonium monomer dimethylaminododecyl methacrylate. Journal of Dentistry. 2013;41:345–55. doi: 10.1016/j.jdent.2013.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Antonucci JM, Zeiger DN, Tang K, Lin-Gibson S, Fowler BO, Lin NJ. Synthesis and characterization of dimethacrylates containing quaternary ammonium functionalities for dental applications. Dental Materials. 2012;28:219–28. doi: 10.1016/j.dental.2011.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sulkala M, Tervahartiala T, Sorsa T, Larmas M, Salo T, Tjäderhane L. Matrix metalloproteinase-8 (MMP-8) is the major collagenase in human dentin. Archives of OralBiology. 2007;52:121–7. doi: 10.1016/j.archoralbio.2006.08.009. [DOI] [PubMed] [Google Scholar]
- 27.Mazzoni A, Mannello F, Tay FR, Tonti GA, Papa S, Mazzotti G, Di Lenarda R, Pashley DH, Breschi L. Zymographic analysis and characterization of MMP-2 and -9 forms in human sound dentin. Journal of Dental Research. 2007;86:436–40. doi: 10.1177/154405910708600509. [DOI] [PubMed] [Google Scholar]
- 28.Tezvergil-Mutluay A, Agee KA, Hoshika T, Tay FR, Pashley DH. The inhibitory effect of polyvinylphosphonic acid on functional matrix metalloproteinase activities in human demineralized dentin. Acta Biomaterialia. 2010;6:4136–42. doi: 10.1016/j.actbio.2010.05.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Carrilho MR, Tay FR, Donnelly AM, Agee KA, Tjäderhane L, Mazzoni A, Breschi L, Foulger S, Pashley DH. Host-derived loss of dentin matrix stiffness associated with solubilization of collagen. Journal of Biomedical Materials Research Part B: Applied Biomaterials. 2009;90:373–80. doi: 10.1002/jbm.b.31295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lindhe A. Noncollagenous proteins and proteoglycans in dentinogenesis. In: Linde A, editor. Dentin and Dentinogenesis. II. Boca RatonFL: CRC Press; 1984. p. 56. [Google Scholar]
- 31.Butler WT. Dentin collagen, chemical structure and role in mineralization. In: Linde A, editor. Dentin and Dentinogenesis. II. Boca Raton FL: CRC Press; 2000. p. 40. [Google Scholar]
- 32.Zhou C, Weir MD, Zhang K, Deng D, Cheng L, Xu HH. Synthesis of new antibacterial quaternary ammonium monomer for incorporation into CaP nanocomposite. Dental Materials. 2013;29:859–70. doi: 10.1016/j.dental.2013.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tezvergil-Mutluay A, Mutluay MM, Gu LS, Zhang K, Agee KA, Carvalho RM, Manso A, Carrilho M, Tay FR, Breschi L, Suh BI, Pashley DH. The anti-MMP activity of benzalkonium chloride. Journal of Dentistry. 2011;39:57–64. doi: 10.1016/j.jdent.2010.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Pashley DH, Carvalho RM. Dentine permeability and dentine adhesion. Journal ofDentistry. 1997;25:355–72. doi: 10.1016/s0300-5712(96)00057-7. [DOI] [PubMed] [Google Scholar]
- 35.Guo X, Spencer P, Wang Y, Ye Q, Yao X, Williams K. Effects of a solubility enhancer on penetration of hydrophobic component in model adhesives into wet demineralized dentin. Dental Materials. 2007;23:1473–81. doi: 10.1016/j.dental.2006.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hashimoto M, Ohno H, Sano H, Kaga M, Oguchi H. In vitro degradation of resin-dentin bonds analyzed by microtensile bond test, scanning and transmission electron microscopy. Biomaterials. 2003;24:3795–803. doi: 10.1016/s0142-9612(03)00262-x. [DOI] [PubMed] [Google Scholar]
- 37.Wang Y, Spencer P. Quantifying adhesive penetration in adhesive/dentin interface using confocal Raman microspectroscopy. Journal of Biomedical Materials Research. 2002;59:46–55. doi: 10.1002/jbm.1215. [DOI] [PubMed] [Google Scholar]
- 38.Breschi L, Mazzoni A, Ruggeri A, Cadenaro M, Di Lenarda R, De Stefano Dorigo E. Dental adhesion review: aging and stability of the bonded interface. Dental Materials. 2008;24:90–101. doi: 10.1016/j.dental.2007.02.009. [DOI] [PubMed] [Google Scholar]
- 39.Mazzoni A, Pashley DH, Nishitani Y, Breschi L, Mannello F, Tjäderhane L, Toledano M, Pashley EL, Tay FR. Reactivation of inactivated endogenous proteolytic activities in phosphoric acid-etched dentine by etch-and-rinse adhesives. Biomaterials. 2006;27:4470–6. doi: 10.1016/j.biomaterials.2006.01.040. [DOI] [PubMed] [Google Scholar]
- 40.Hannas AR, Pereira JC, Granjeiro JM, Tjäderhane L. The role of matrix metalloproteinases in the oral environment. Acta Odontologica Scandinavica. 2007;65:1–13. doi: 10.1080/00016350600963640. [DOI] [PubMed] [Google Scholar]
- 41.Tay FR, Pashley DH, Loushine RJ, Weller RN, Monticelli F, Osorio R. Self-etching adhesives increase collagenolytic activity in radicular dentin. Journal of Endodontics. 2006;32:862–8. doi: 10.1016/j.joen.2006.04.005. [DOI] [PubMed] [Google Scholar]
- 42.Martin-De Las Heras S, Valenzuela A, Overall CM. The matrix metalloproteinase gelatinase A in human dentine. Archives of Oral Biology. 2000;45:757–65. doi: 10.1016/s0003-9969(00)00052-2. [DOI] [PubMed] [Google Scholar]
- 43.Visse R, Nagase H. Matrix metalloproteinases and tissue inhibitors of metalloproteinases: structure, function, and biochemistry. Circulation Research. 2003;92:827–39. doi: 10.1161/01.RES.0000070112.80711.3D. [DOI] [PubMed] [Google Scholar]
- 44.Fedarko NS, Jain A, Karadag A, Fisher LW. Three small integrin binding ligand N-linked glycoproteins (SIBLINGs) bind and activate specific matrix metalloproteinases. FASEB Journal. 2004;18:734–6. doi: 10.1096/fj.03-0966fje. [DOI] [PubMed] [Google Scholar]
- 45.Li F, Weir MD, Fouad AF, Xu HH. Time-kill behaviour against eight bacterial species and cytotoxicity of antibacterial monomers. Journal of Dentistry. 2013;41:881–91. doi: 10.1016/j.jdent.2013.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Carrilho MR, Geraldeli S, Tay F, de Goes MF, Carvalho RM, Tjäderhane L, Reis AF, Hebling J, Mazzoni A, Breschi L, Pashley D. In vivo preservation of the hybrid layer by chlorhexidine. Journal of Dental Research. 2007;86:529–33. doi: 10.1177/154405910708600608. [DOI] [PubMed] [Google Scholar]
- 47.Hiraishi N, Yiu CK, King NM, Tay FR. Effect of chlorhexidine incorporation into a self-etching primer on dentine bond strength of a luting cement. Journal of Dentistry. 2010;38:496–502. doi: 10.1016/j.jdent.2010.03.005. [DOI] [PubMed] [Google Scholar]
- 48.Sadek FT, Braga RR, Muench A, Liu Y, Pashley DH, Tay FR. Ethanol wet-bonding challenges current anti-degradation strategy. Journal of Dental Research. 2010;89:1499–504. doi: 10.1177/0022034510385240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Buffet-Bataillon S, Tattevin P, Bonnaure-Mallet M, Jolivet-Gougeon A. Emergence of resistance to antibacterial agents: the role of quaternary ammonium compounds - a critical review. International Journal of Antimicrobial Agents. 2012;39:381–9. doi: 10.1016/j.ijantimicag.2012.01.011. [DOI] [PubMed] [Google Scholar]
- 50.Li F, Weir MD, Chen J, Xu HH. Effect of charge density of bonding agent containing a new quaternary ammonium methacrylate on antibacterial and bonding properties. Dental Materials. 2014;30:433–41. doi: 10.1016/j.dental.2014.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Li F, Weir MD, Xu HH. Effects of quaternary ammonium chain length on antibacterial bonding agents. Journal of Dental Research. 2013;92:932–8. doi: 10.1177/0022034513502053. [DOI] [PMC free article] [PubMed] [Google Scholar]