Abstract
Acute graft-versus-host disease (aGVHD) still remains one of the life-threatening complications following allogeneic hematopoietic stem cell transplantation (allo-HSCT). Immunomodulation of alloreactive donor T cell responses, as well as cytokine secretion is a potential therapeutic approach for the prevention of aGVHD. The synthetic triterpenoid, CDDO (2-cyano-3, 12-dioxooleana-1, 9-dien-28-oic acid), exhibits potent antitumor activity and has also been shown to mediate anti-inflammatory and immunomodulatory effects. We therefore wanted to assess the effects of CDDO on early lethal aGVHD. In this study, we found that CDDO significantly inhibited in vitro mixed lymphocyte responses and preferentially promoted the apoptosis of proliferating but not resting alloreactive T cells. Using a full major histocompatibility complex (MHC)-disparate murine aGVHD model, we found that the administration of CDDO immediately after transplantation significantly decreased liver pathology as determined by histologic assessment and prolonged survival in mice. Importantly, administration of CDDO did not adversely impair donor myeloid reconstitution as determined by peripheral blood cell count and the extent of donor chimerism. These findings indicate that CDDO has a significant immunomodulatory effects in vitro and on early lethal aGVHD development, particularly affecting the liver, in a murine allo-HSCT model.
Keywords: Allogeneic hematopoietic stem cell transplantation (allo-HSCT), Mixed lymphocyte responses
Introduction
Acute graft-versus-host disease (aGVHD), a result of alloreactive donor T cells in the hematopoietic graft, is a life-threatening complication of allogeneic hematopoietic stem cell transplantation (allo-HSCT). Although donor T cells can mediate graft-versus-tumor (GVT) effects, which improve the outcome of malignant diseases after allo-HSCT treatment, separation of the beneficial GVT from aGVHD is still a dilemma [1]. With aGVHD development, the primary target organs (gut, liver, and skin) are injured by both cellular and inflammatory cytokine effectors. Therefore, immunomodulation of alloreactive donor T cells responses as well cytokine secretion may represent a realistic approach to the prevention of aGVHD [2-4].
The synthetic triterpenoid, 2-cyano-3, 12-dioxooleana-1, 9-dien-28-oic acid (CDDO), is a multifunctional molecule that exerts numerous biologic effects including anti-inflammatory, potent antiproliferative and apoptosis-inducing activities [5]. It has been shown that CDDO is effective in inducing apoptosis in leukemic and multiple myeloma cells in vitro [6,7]. It was reported that CDDO can sensitize leukemia cells to apoptosis involving downregulation of FLIP and sensitization to TRAIL [8]. In addition, as the ligand for the peroxisome proliferator activator receptor-gamma (PPAR-γ), one of the mechanisms by which CDDO can induce apoptosis in tumor cells involves upregulation of the PPAR-γ pathway [9]. Recently, PPAR-γ has also been found to broadly regulate inflammatory responses [10-12]. In a pathogen-driven animal ileitis model, CDDO prevented ileitis development through the global downregulation of inflammatory cytokines and chemokines [13]. Thus, CDDO is an attractive candidate for administration in an allo-HSCT setting because of its direct and indirect antitumor effects, together with its potential immunomodulating properties.
We report here that the CDDO significantly inhibited in vitro mixed lymphocyte responses and preferentially promoted apoptosis in proliferating but not resting alloreactive T cells. Moreover, CDDO administered immediately following murine allo-HSCT resulted in significant reduction of early aGVHD lethality with significant protection of liver pathology, with no observed adverse effects on myeloid recovery and donor chimerism.
Materials and Methods
Animals
Female C57BL/6 (B6, H2b) and BALB/c (H2d) mice were purchased from the Animal Production Area of the National Cancer Institute (Frederick, MD). Animals were kept in specific pathogen-free conditions. All animal protocols were approved and in vivo studies were performed at University of Nevada, Reno. Mice were between 8 and 13 weeks of age at the start of the experiments.
Reagents and Media
CDDO was manufactured through the NIH RAID Program and kindly provided by Drs. E. Sausville and M. Sporn. CDDO was prepared in a vehicle solution of 10% DMSO (Sigma, St Louis, MO), 10% Cremophor EL (Sigma), and 80% Dulbecco's phosphate-buffered saline solution (DPBS) (Mediatech, Herndon, VA) at a concentration of 1.2 mg/mL. Briefly, CDDO was weighed out and dissolved into the DMSO, followed by the addition of the Cremophor EL and DPBS. The solution was then sonicated for 30 minutes, sterile filtered, and stored at 4°C for up to 2 months prior to use. The proteasome inhibitor, bortezomib, was kindly provided by Millennium Pharmaceuticals (Cambridge, MA). Stock bortezomib solution (2.603 × 10−3M) was prepared in Dulbecco phosphate-buffered saline solution (PBS) and stored at −70°C for up to 2 months prior to use. Bortezomib solutions were protected from light at all times. All mitogen assays and mixed lymphocyte reactions (MLRs) were cultured in complete medium containing RPMI 1640 (Cambrex, Walkersville, MD), supplemented with 10% fetal bovine serum (Gemini, Woodland, CA), 10 mM HEPES, 2 mM L-glutamine (Cambrex), 100 U/mL penicillin/streptomycin (Mediatech), 1 mM nonessential amino acids, 1 mM sodium pyruvate, and 5 × 10−5 M 2-ME (Invitrogen, Grand Island, NY).
Mitogen Assay
Naïve unfractionated BALB/c splenocytes were plated in quadruplicate in 96-well plates at a concentration of 2.5 × 105 cells per well, and were treated with 2 μg/mL Concanavalin A (ConA) (Sigma) and various doses of CDDO in complete media. Plates were incubated for 24 hours at 37°C and 5% CO2, and were then pulsed with 1 μCi/well [3H] thymidine (MP Biomedicals, Solon, OH) for an additional 16 hours prior to harvesting and reading on a 1450 Microbeta Trilux scintillation counter (Wallac, Turku, Finland).
In Vitro MLR Cultures
To monitor primary responses, responder cells (BALB/c splenocytes, 2.5 × 105/well) and stimulator cells (30 Gy irradiated B6 splenocytes, 2.5 × 105/well) were plated in 96-well plates in quadruplicate in complete media. The plates were incubated at 37°C with 5% CO2 for 3-7 days, then pulsed with [3H]-thymidine (1 μCi/well) for 16-18 hours prior to harvesting and counting.
MTT Colorimetric Assay
A colorimetric assay using 3-(4,5-dimethylthiazoyl)-2,5-diphenyltetrazolium bromide (MTT) was performed as per the manufacturer's instruction (Roche Diagnostics GmbH, Mannheim, Germany). Briefly, B6 spleen cells (2 × 105cells/well) were plated in 96-well plates with complete media and 2 μg/mL ConA in triplicate in the presence of various concentrations of CDDO or Bortezomib and incubated at 37°C in a 5% humidified CO2 incubator for 48 hours. Then, 10 μL of MTT labeling reagent (5 mg/mL) was added to each well and incubation was continued for a further 4 hours at 37°C. In each well, 100 μL/well of solubilization solution (10% SDS in 0.01 M HCl) was added. After overnight incubation at 37°C in a 5% humidified CO2 incubator, plates were read at 550 nm on a Versamax tunable microplate reader (Molecular Devices Corporation, Sunnyvale, CA). The reference wavelength was 650 nm.
CFSE Labeling and Apoptosis Assessment
BALB/c splenocytes were labeled with 2.5 μM Carboxy-fluorescein diacetate succinimidyl ester (CFSE) (Molecular Probes, Eugene, OR) according to the manufacturer's instructions. CFSE labeled BALB/c splenocytes were plated at a concentration of 2.5 × 105 cells per well along with 2.5 × 105 irradiated (30 Gy) T cell-depleted B6 splenocytes and various concentrations of CDDO. T cells were depleted from B6 splenocytes by incubating with anti-Thy 1.2 mAb (clone 30H-12), followed by incubation with rabbit complement. After 3 days in culture, the cells were harvested and labeled with PE-Cy5 conjugated CD4 and CD8 antibodies and PE Annexin V (BD Pharmingen, San Diego, CA). During flow cytometry analysis, gates were drawn on CD4 or CD8 positive cells, then gated on CFSEhigh/CFSElow cells, and the percentage of Annexin V positive cells determined.
Complete Blood Count (CBC) and Flow Cytometry
Peripheral blood, spleen, and bone marrow samples were collected on day 14 after transplantation. Blood samples were collected in microvette capillary blood collection tubes (Sarstedt, Germany) and the CBC analyzed on a Hemavet 950 (Drew Scientific, Oxford, CT). Donor cell chimerism was determined by flow cytometry. Cells (1 × 106) were labeled with fluorescence, either FITC- or PE-conjugated anti-mouse mAbs (FITC-H2Dd, PE-H2Kb) purchased from BD Biosciences (San Diego, CA) for 2-color flow cytometry. Nonspecific binding was corrected with isotype-matched controls. All results were obtained on a BD FACScan flow cytometer (Becton Dickinson, San Jose, CA).
Cell Preparation
Bone marrow cell (BMC) suspensions were prepared by gently releasing cells from the femurs, tibiae, and backbones into DPBS with a mortar and pestle. Splenocyte preparations were prepared by gently crushing the spleens. Preparations were filtered to remove debris and washed twice in DPBS for injection. Cell counts were performed on a Coulter Z1 cell counter (Coulter Electronics, Hialeah, FL).
In Vivo Studies
B6 recipient mice received myeloablative doses (900 or 950 cGy) total body irradiation (TBI) from a 137Cs source. Irradiation was followed by the infusion of 1.0 × 107 BALB/c BMCs into the tail vein with or without BALB/c splenocytes (SCs; 35-40 × 106 cells) as a source of allogeneic T cells. Recipient B6 mice then received a vehicle control or CDDO intraperitoneally with the dose range (240-480 μg) given daily from days 0 through +1 posttransplantation. Mice were monitored daily. All moribund mice were humanely killed. All experiments were performed at least 3 times with 5 to 13 mice per group.
Histology
The murine GVHD target tissues (liver, colon, and small intestine) were fixed in 10% formalin, embedded in paraffin, sectioned, and stained with hematoxylin and eosin. Tissue sections were evaluated by a pathologist and GVHD changes were graded in coded fashion. For liver specimens we used the pathologic grading system reported by Li et al [14] with modifications. Liver GVHD was graded based on degree of hepatocyte apoptosis, venulitis, bile duct inflammation, and lobular hepatitis. If there was a discordance in severity of these parameters, the lesion was graded based on the highest degree. Score 0 was given when no apoptotic hepatocytes were seen, no mononuclear cells were found in or surrounding the terminal hepatic vein and within bile ducts, and no lobular inflammatory foci were found. Score 1 was given when rare apoptotic hepatocytes were seen, <25% of the portal tract was occupied by inflammatory cells, only a few mononuclear cells were found in or surrounding the terminal hepatic vein, and <10 lobular inflammatory foci were found in a 100 × field. Score 2 was given when occasional apoptotic hepatocytes were seen, 25%-50% of the portal tract was occupied by inflammatory cells, mononuclear cells surrounded the vein without forming a confluent layer, and 10-20 lobular inflammatory foci were found in a 100 × field. Score 3 was given when frequent apoptotic hepatocytes were seen, 50%-75% of the portal tract was occupied by inflammatory cells, a single confluent layer of mononuclear cells surrounded the vein, and 20-30 lobular inflammatory foci were found in a 100 × field. Score 4 was given when numerous apoptotic hepatocytes were seen, >50% of the portal tract was occupied by inflammatory cells, the cellular infiltrate obscured the vein wall, and >30 lobular inflammatory foci were found in a 100 × field. A similar grading system was developed for intestinal specimens. Intestinal GVHD was graded based on degree of individual crypt cell necrosis, goblet cell depletion, cryptitis, crypt abscesses, distortion of crypt architecture including crypt dropout, loss of villi in the small intestine, and sloughing of the colonic mucosa. Score 0 was given when no individual crypt cell necrosis was seen and normal intestinal architecture was preserved. One polymorphonucleated leukocyte (PMNL) per 5 or more epithelial cells was considered a normal feature. Score 1 was given when rare individual crypt cell necrosis was seen in the background of preserved normal intestinal architecture. Score 2 was given when occasional individual crypt cell necrosis was seen, focal areas with more than 1 PMNL per 5 epithelial cells (cryptitis) without crypt abscess formation were seen in the background of preserved intestinal architecture. Score 3 was given when frequent individual necrotic crypt cells were seen, areas with more than 1 PMNL per 5 epithelial cells (cryptitis) with focal crypt abscess formation were seen in the background of preserved intestinal architecture. Score 4 was given when numerous necrotic individual crypt cells were seen, prominent areas of cryptitis and crypt abscess, crypt dropout, loss of villi (in small intestine), confluent destruction of glands, loss of mucosa with formation of granulation tissue and pseudomembrane were detected.
Cytokine Analysis
Blood samples were obtained on day + 5 after transplantation. Serum levels of tumor necrosis factor alpha (TNF-α) were determined by ELISA (R&D Systems, Minneapolis, MN) as per manufacturer's instructions.
Statistics
Survival data were plotted by the Kaplan-Meier method and analyzed by the log-rank test. T cell proliferation was evaluated with the one-way ANOVA and Dunnett's Multiple Comparison Test. CBC and flow cytometry data was analyzed with the unpaired Student's t test with the Welch correction. Pathology scores were evaluated with the Mann-Whitney test. P-value <.05 in a 2-tailed analysis was considered significant.
Results
CDDO Can Inhibit Mitogen or Alloantigen-induced T cell Proliferation In Vitro in a Dose-Dependent Manner
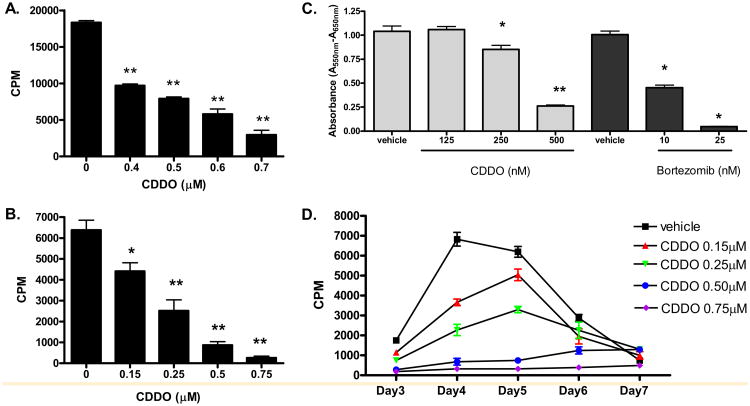
CDDO has been demonstrated to have potent antiproliferative and anti-inflammatory activities [13,15,16]. Therefore, we first assessed the effects of CDDO on murine T cell response to either mitogen or alloantigen in vitro. The results demonstrate that CDDO can significantly inhibit proliferative T cell responses to the mitogen ConA (Figure 1A; one-way ANOVA, P < 0.01) or alloantigen in the MLR in a dose-dependent manner and at concentrations as low as 0.15 μM (Figure 1B and D). We then compared the efficacy of CDDO with the proteasome inhibitor, bortezomib, on T cell response to the mitogen (Figure 1C). Bortezomib was far superior to CDDO on T cell growth inhibition at the concentrations tested. Therefore, CDDO was capable of inhibiting mitogen stimulated and allogeneic MLR-induced murine T cell proliferation in vitro, although it is not as potent when compared to the proteasome inhibitor bortezomib.
Figure 1.
CDDO inhibits the mitogen and alloantigen-induced T cell proliferation. (A) BALB/c splenocytes were exposed to ConA (2 (μg/mL), plus 4 different concentrations of CDDO or vehicle and cultured for 2 days as described in Materials and Methods. T cell proliferation was significantly inhibited in a dose-dependent manner in the presence of ≥0.4 μM CDDO. (B, D) BALB/c splenocytes were cultured with irradiated B6 splenocytes at a 1:1 ratio for 4 days (B) or 3-7 days (D), then pulsed with [3H] thymidine for 16 hours prior to harvesting as described in Materials and Methods. T cell proliferation was significantly inhibited in a dose-dependent manner in the presence of ≥0.15 μM CDDO (significant differences compared with vehicle control, *P < 0.05 and **P < 0.01). Results from 1 of 4 independent experiments are presented. (C) B6 splenocytes (2 × 105 cells/well) were exposed to ConA (2 μg/mL), with different concentrations of CDDO, bortezomib, or individual vehicles. Plates were incubated at 37°C and 5% CO2 for 48 hours, then an MTT assay was performed. T cell proliferation was significantly inhibited in the presence of 250 and 500 nM CDDO (P < 0.05 and P < 0.01, respectively) and in the presence of 10 and 25 nM bortezomib (P < 0.05) compared to their vehicle controls (ANOVA with Dunnett's posttest comparison).
CDDO Selectively Induces Higher Apoptosis in Proliferating But Not Resting T Cells In Vitro
We next assessed the mechanism by which CDDO significantly suppressed allogeneic MLR-induced murine T cell alloreactivity in vitro. CSFE dye dilution was used for assessment of cell division and annexin V binding was used as a marker of apoptosis. The results demonstrate that the addition of CDDO into the MLR resulted in significant increases in apoptosis of alloreactive CD4+ and CD8+ T cells (one-way ANOVA, P < 0.05). Of interest, predominate apoptotic cells were in the CSFElo population (Figure 2A and B), suggesting that the proliferating T cells were primarily targeted. These results indicate that CDDO is capable of suppressing alloreactive T cell responses, resulting from, in part, the selective depletion of alloreactive T cells.
Figure 2.
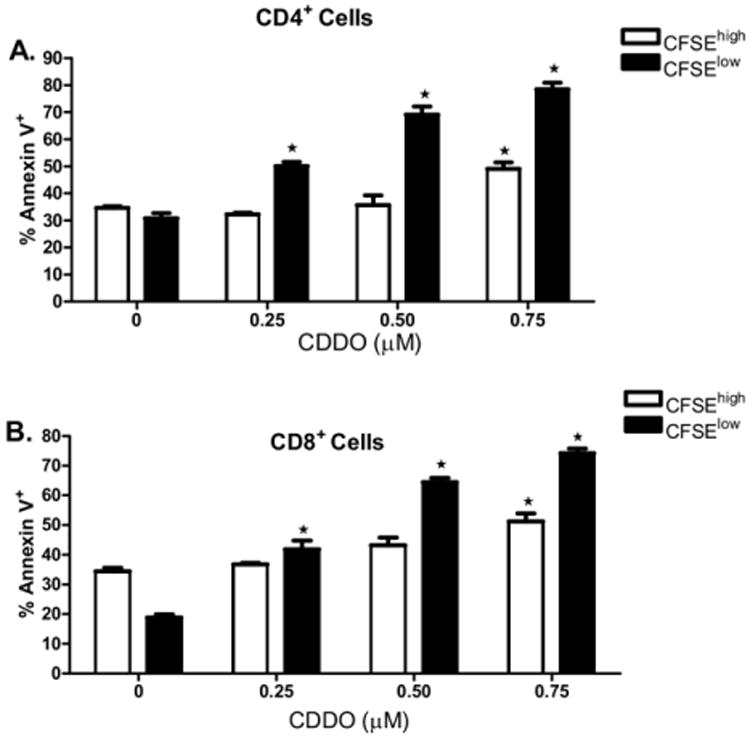
Proliferating, and not resting, T cells are highly sensitive to CDDO-mediated apoptosis. CFSE-labeled BALB/c splenocytes were incubated with irradiated T cell-depleted B6 splenocytes at a 1:1 ratio with a dose range of CDDO as described in Materials and Methods. After 3 days in culture, cells were harvested and analyzed by flow cytometry. Cells were first gated on CD4 or CD8 expression, then gated on CFSEhigh or CFSElow staining, and analyzed for annexin V binding in proliferating or resting T cells. (A) Proportionally greater increases in annexin V binding were observed on proliferating (CFSElow) compared with nonalloreactive (CFSEhigh) CD4+ T cells with exposure to 0.25 μM, 0.5 μM, and 0.75 μM CDDO. (B) Proportionally greater increases in annexin V binding were observed on proliferating (CFSElow) compared with nonalloreactive (CFSEhigh) CD8+ T cells with exposure to 0.25 μM, 0.5 μM, and 0.75 μM CDDO. *Significant differences of annexin V binding on CDDO-exposed T cells compared with control (P < 0.05). Results from 1 of 3 independent experiments are presented.
CDDO Administration Reduces Acute GVHD Lethality in Mice
After observing that CDDO can significantly inhibit alloreactivity in vitro, we then proceeded to examine the effects of CDDO in vivo on murine aGVHD induction and progression. In these studies, after myeloablative pretransplantation conditioning with TBI, recipient B6 (H2b) mice received 10 million BMCs with or without 35-40 million SCs (as a source of alloreactive T cells) from fully major histocompatibility complex (MHC)-mismatched BALB/c (H2d) donors. Then, a vehicle control or CDDO was administered intraperitoneally to the recipients immediately following transplantation for 2 days. We have found that this regimen resulted in the greatest aGVHD protection compared with more prolonged treatment with CDDO (data not shown). No morbidity was observed in the mice that received allo-HSCT with BMCs alone followed by treatment with CDDO or vehicle control (Figure 3A). Among the lethally irradiated mice that underwent transplantation with BMCs and SCs, the mice that received the vehicle control succumbed rapidly to early aGVHD. In marked contrast, the mice that received CDDO exhibited delayed development of aGVHD and significant improvement in survival (Figure 3A, P < 0.001). Treatment with CDDO led to significant decreases in the serum TNF-α production that occurs in mice on day 5 after myeloablative conditioning and allo-HSCT (Figure 3B, P < 0.05). In addition, whereas both groups of the mice receiving BMCs and SCs showed significant weight loss from aGVHD, the mice treated with CDDO did not experience as severe of a weight loss early posttransplantation as the vehicle-treated mice (Figure 3C, P < 0.001). These results demonstrate that early administration of CDDO is capable of reducing the severity of early, lethal aGVHD after allo-HSCT in mice correlating with the reduction of serum TNF-α levels.
Figure 3.
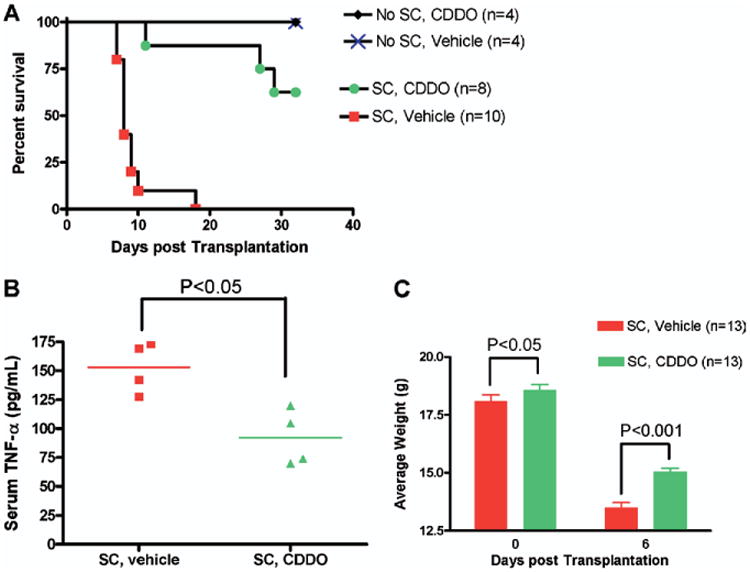
CDDO administration immediately after transplantation results in delayed development of GVHD and significant improvement in survival. B6 (H2b) mice received TBI (900 cGy) followed by infusion of 10 × 106 10 BALB/c (H2d) BMC and 35 × 106 BALB/c SC. Mice were treated with vehicle control or CDDO (240 μg/dose) twice a day intraperitoneally on day 0 and day +1 for a total of 4 doses. (A) Administration of CDDO protected mice from early GVHD mortality. Significant improvement in survival were observed in CDDO-treated mice (●) compared with GVHD control (vehicle control-treated) mice (■; P < .001). Results from 1 of 3 independent experiments are presented. (B) Significant decrease in serum TNF-α levels of mice on day +5 post-BMT in mice that received CDDO compared to mice treated with vehicle. (C) Significant protection from GVHD associated weight loss on day 6 post-BMT in mice that received CDDO. Mean body weights from 1 of 3 independent experiments are presented.
CDDO Administration Results in Decreased Hepatic Injury But Not Intestinal Injury in Mice with GVHD
We next examined the effects of CDDO administration on target organ injury during aGVHD. Samples of liver and gut were harvested on day +8 after allo-HSCT and evaluated for GVHD-associated histopathologic changes. Histopathologic assessment revealed that significant decreases in liver damage occurred in mice following CDDO administration immediately after transplantation compared with vehicle control administration (Figure 4A, 4E and 4F). Surprisingly, CDDO administration did not significantly reduce the gastrointestinal tract damage when compared with mice that received the vehicle control (Figure 4B, 4C and 4D). These results suggested that the reduction of aGVHD lethality in mice is associated with decreases in liver lesions and that CDDO apparently is specific for the liver with regards to GVHD protection.
Figure 4.
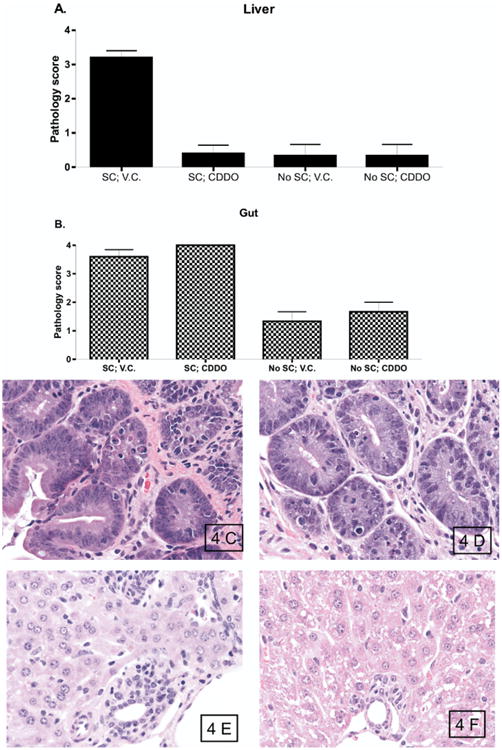
CDDO administration results in decreased hepatic injury but not intestinal injury in mice with GVHD. B6 (H2b) mice received TBI (950 cGy), followed by infusion of 10 × 106 BALB/c (H2d)BMC and 35 × 106 BALB/c SC. Mice were treated with vehicle control or CDDO (240 μg/dose) three times a day intraperitoneally on day 0 and day +1 for a total of 2 doses. (A, B). Pathology scores in the liver (A) and gut (B) of mice that received BMC alone or with splenocytes, followed by administration of vehicle control or CDDO. (C, D) Photomicrographs of representative gut tissue sections. Mice that received BMC with spleen cells followed by vehicle control (C) and mice that received BMC with spleen cells followed by CDDO (D) demonstrated similar prominent tissue damage with numerous apoptotic bodies. (E, F). Photomicrographs of representative liver tissue sections. Mice that received BMC with spleen cells followed by vehicle control (E) demonstrated expensive GVHD with bile duct proliferation, vasculitis, lobular hepatitis, and numerous apoptotic cells. In contrast, mice that received BMC with spleen cells followed by CDDO (F) demonstrated preserved portal triad architecture, no vasculitis, no lobular hepatitis, and virtually no apoptotic cells. H&E staining, original magnification ×500.
CDDO Administration Does Not Adversely Affect Donor Engraftment and Peripheral Blood Recovery in allo-HSCT Mice
We then assessed the effects of CDDO administration on donor engraftment and peripheral blood recovery in allo-HSCT mice. Mice were analyzed at day +14 after transplant for myeloid reconstitution and donor engraftment. We found CDDO administration had no significant effect on peripheral white blood cell (WBC) or platelet (PLT) recovery post-allo-HSCT compared with mice that received vehicle control (Figure 5A). Likewise, no significant differences in donor cell chimerism were observed in mice that received CDDO post-allo-HSCT (Figure 5B). These results demonstrated that CDDO administration to recipients of allo-HSCT does not impair donor engraftment or myeloid reconstitution.
Figure 5.
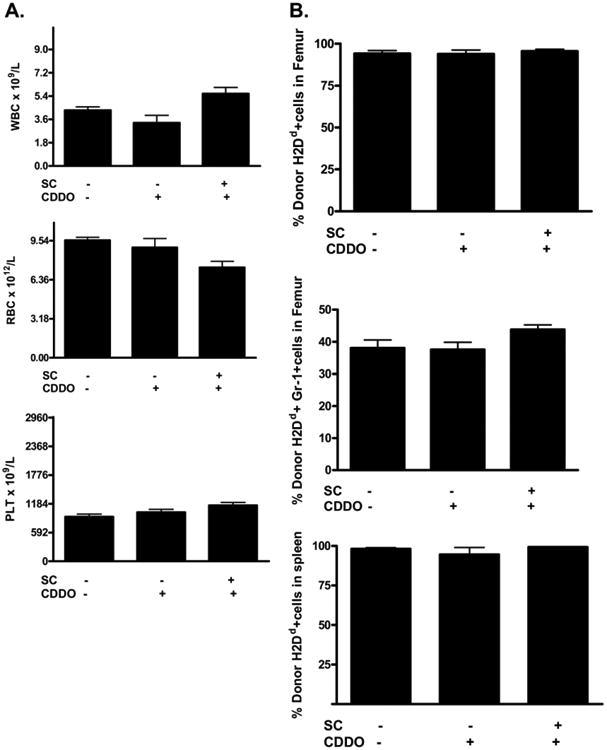
CDDO administration does not adversely affect donor engraftment. B6 (H2b) mice received TBI (900 cGy) followed by infusion of 10 × 106 BALB/c (H2d) BMC and 40 × 106 BALB/c SC. Mice were treated with vehicle control or CDDO (240 μg/dose) twice a day intraperitoneally on day 0 and day +1 for a total of 4 doses. Blood, bone marrow, and spleen were collected and analyzed for engraftment of donor cells on day 14 posttransplantation (4-6 mice/group). (A) CBC parameters were not significantly affected by CDDO treatment in mice. (B) The frequency of donor cells in the bone marrow and spleen following allogeneic BMT are not affected by CDDO administration. Results from 1 of 2 independent experiments are presented.
Discussion
The use of allo-HSCT in cancer treatment has been seriously hampered by the occurrence of aGVHD and cancer relapse. Therefore, assessment of agents with known antineoplastic activity that also possess antiproliferative and anti-inflammatory activity may be useful in both the prevention of aGVHD and cancer relapse. In this study, we have examined the use of the synthetic triterpenoid, CDDO, as a new therapeutic to decrease alloantigen-induced T response and reduce early aGVHD lethality. The number of alloreactive T cells plays a critical role in the induction and development of aGVHD [1,3,4]. The data presented here demonstrate that CDDO can inhibit alloantigen-induced T cell proliferation in vitro, in part by selectively targeting alloreactive T cells. In vivo data further confirmed that treatment of mice with CDDO immediately following allo-HSCT led to significant improvement in survival from early aGVHD.
The mechanism for the modulation of allo-reactive T cell response and reduction of GVHD lethality by CDDO is not definitively known, in part, resulting from the multifunctional properties of CDDO. One is that inhibiting alloreactive T cell response may be attributable to inhibitory effects of nuclear factor kappa B (NF-κB) as CDDO has recently been demonstrated to inhibit NF-κB on tumor cell lines [17]. Another mechanism may be by the induction of immunosuppressive cytokines. Transforming growth factor beta (TGF-β) has been shown to attenuate aGVHD [18,19]. CDDO has been demonstrated to induce TGF-β production by intraepithelial lymphocytes to prevent ileitis through the global downregulation of inflammatory cytokines and chemokines [13]. As a ligand for the PPAR-γ [9,20], CDDO may also modulate immune responses and inhibit T cell proliferation. Dose-dependent inhibition of T cell proliferation by CDDO in an MLR may suggest the reduction of the allo-reactive response is from direct cytotoxicity on proliferating T cells.
As shown in Figure 3, CDDO-dependent reduction of GVHD lethality can be observed when CDDO is administered within a dose range of 240-480 μg daily from days 0 through +1 posttransplantation, indicating that the development of GVHD can be delayed by CDDO when administered immediately after allo-HSCT. We further found that extended administration of CDDO while aGVHD was ongoing did not lead to increased protection of GVHD (data not shown). This is consistent with other agents such as proteasome inhibitors, which also were found to be protective only if given immediately after allo-HSCT [21,22]. In this murine GVHD model, lethal TBI has a moderate conditioning-related gastrointestinal toxicity [23], and it has been demonstrated that inhibition of NF-κB can exacerbate gut injury in other models while preventing systemic inflammation [24]. In this setting, extended treatment with CDDO may maximize the apoptotic potential of cellular and inflammatory cytokine mediated allo-reactive response to gut damage. It may possibly explain our finding of decreased hepatic histopathology but not intestinal histopathology in these mice with aGVHD after treatment with CDDO. It is also possible that CDDO can affect chemokine production responsible for donor allo-reactive T cells homing to the liver.
In summary, the data presented here demonstrate that CDDO significantly inhibits allo-reactive T cell responses in MLR cultures, and when administered immediately following murine allo-HSCT, results in significant inhibition of early aGVHD with no deleterious effects on donor engraftment. Besides a direct antitumor effect, CDDO has also been reported to be able to sensitize leukemia cells to apoptosis through downregulation of FLIP and sensitization to TRAIL. Therefore, future studies will determine whether CDDO can reduce aGVHD lethality with increased graft-versus-tumor effect in tumor-bearing recipients.
Acknowledgments
We thank Dr. Ruth Gault, Qing Zhou, William H. D. Hallett, Kory Alderson, and Milad Motarjemi for assisting in the preparation of the manuscript and helpful discussions.
This work was supported by grants R01 CA102282 and 2P30 CA16672.
References
- 1.Fowler DH. Shared biology of GVHD and GVT effects: potential methods of separation. Crit Rev Oncol Hematol. 2006;57:225–244. doi: 10.1016/j.critrevonc.2005.07.001. [DOI] [PubMed] [Google Scholar]
- 2.Murphy GF, Korngold R. Significance of selectively targeted apoptotic rete cells in graft-versus-host disease. Biol Blood Marrow Transplant. 2004;10:357–365. doi: 10.1016/j.bbmt.2004.02.005. [DOI] [PubMed] [Google Scholar]
- 3.Blazar BR, Murphy WJ. Bone marrow transplantation and approaches to avoid graft-versus-host disease (GVHD) Philos Trans R Soc Lond B Biol Sci. 2005;360:1747–1767. doi: 10.1098/rstb.2005.1701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ferrara JL, Reddy P. Pathophysiology of graft-versus-host disease. Semin Hematol. 2006;43:3–10. doi: 10.1053/j.seminhematol.2005.09.001. [DOI] [PubMed] [Google Scholar]
- 5.Ray DM, Morse KM, Hilchey SP, et al. The novel triterpenoid 2-cyano-3,12-dioxooleana-1,9-dien-28-oic acid (CDDO) induces apoptosis of human diffuse large B-cell lymphoma cells through a peroxisome proliferator-activated receptor gamma-independent pathway. Exp Hematol. 2006;34:1201–1210. doi: 10.1016/j.exphem.2006.04.026. [DOI] [PubMed] [Google Scholar]
- 6.Konopleva M, Tsao T, Estrov Z, et al. The synthetic triterpenoid 2-cyano-3,12-dioxooleana-1,9-dien-28-oic acid induces caspase-dependent and -independent apoptosis in acute myelogenous leukemia. Cancer Res. 2004;64:7927–7935. doi: 10.1158/0008-5472.CAN-03-2402. [DOI] [PubMed] [Google Scholar]
- 7.Ikeda T, Nakata Y, Kimura F, et al. Induction of redox imbalance and apoptosis in multiple myeloma cells by the novel triterpenoid 2-cyano-3,12-dioxoolean-1,9-dien-28-oic acid. Mol Cancer Ther. 2004;3:39–45. [PubMed] [Google Scholar]
- 8.Suh WS, Kim YS, Schimmer AD, et al. Synthetic triterpenoids activate a pathway for apoptosis in AML cells involving down-regulation of FLIP and sensitization to TRAIL. Leukemia. 2003;17:2122–2129. doi: 10.1038/sj.leu.2403112. [DOI] [PubMed] [Google Scholar]
- 9.Chintharlapalli S, Papineni S, Konopleva M, et al. 2-Cyano-3,12-dioxoolean-1,9-dien-28-oic acid and related compounds inhibit growth of colon cancer cells through peroxisome proliferator-activated receptor gamma-dependent and -independent pathways. Mol Pharmacol. 2005;68:119–128. doi: 10.1124/mol.105.011437. [DOI] [PubMed] [Google Scholar]
- 10.Nakajima A, Wada K, Miki H, et al. Endogenous PPAR gamma mediates anti-inflammatory activity in murine ischemia-reperfusion injury. Gastroenterology. 2001;120:460–469. doi: 10.1053/gast.2001.21191. [DOI] [PubMed] [Google Scholar]
- 11.Schmidt S, Moric E, Schmidt M, et al. Anti-inflammatory and antiproliferative actions of PPAR-gamma agonists on T lymphocytes derived from MS patients. J Leukoc Biol. 2004;75:478–485. doi: 10.1189/jlb.0803402. [DOI] [PubMed] [Google Scholar]
- 12.Rollins MD, Sudarshan S, Firpo MA, et al. Anti-inflammatory effects of PPAR-gamma agonists directly correlate with PPAR-gamma expression during acute pancreatitis. J Gastrointest Surg. 2006;10:1120–1130. doi: 10.1016/j.gassur.2006.04.027. [DOI] [PubMed] [Google Scholar]
- 13.Minns LA, Buzoni-Gatel D, Ely KH, et al. A novel triterpenoid induces transforming growth factor beta production by intra-epithelial lymphocytes to prevent ileitis. Gastroenterology. 2004;127:119–126. doi: 10.1053/j.gastro.2004.03.053. [DOI] [PubMed] [Google Scholar]
- 14.Li J, Helm K, Howell CD. Contributions of donor CD4 and CD8 cells to liver injury during murine graft-versus-host disease. Transplantation. 1996;62:1621–1628. doi: 10.1097/00007890-199612150-00016. [DOI] [PubMed] [Google Scholar]
- 15.Suh N, Wang Y, Honda T, et al. A novel synthetic oleanane triterpenoid, 2-cyano-3,12-dioxoolean-1,9-dien-28-oic acid, with potent differentiating, antiproliferative, and anti-inflammatory activity. Cancer Res. 1999;59:336–341. [PubMed] [Google Scholar]
- 16.Honda T, Rounds BV, Gribble GW, et al. Design and synthesis of 2-cyano-3,12-dioxoolean-1,9-dien-28-oic acid, a novel and highly active inhibitor of nitric oxide production in mouse macrophages. Bioorg Med Chem Lett. 1998;8:2711–2714. doi: 10.1016/s0960-894x(98)00479-x. [DOI] [PubMed] [Google Scholar]
- 17.Stadheim TA, Suh N, Ganju N, et al. The novel triterpenoid 2-cyano-3,12-dioxooleana-1,9-dien-28-oic acid (CDDO) potently enhances apoptosis induced by tumor necrosis factor in human leukemia cells. J Biol Chem. 2002;277:16448–16455. doi: 10.1074/jbc.M108974200. [DOI] [PubMed] [Google Scholar]
- 18.Banovic T, MacDonald KP, Morris ES, et al. TGF-beta in allogeneic stem cell transplantation: friend or foe? Blood. 2005;106:2206–2214. doi: 10.1182/blood-2005-01-0062. [DOI] [PubMed] [Google Scholar]
- 19.Kleinclauss F, Perruche S, Masson E, et al. Intravenous apoptotic spleen cell infusion induces a TGF-beta-dependent regulatory T-cell expansion. Cell Death Differ. 2006;13:41–52. doi: 10.1038/sj.cdd.4401699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Place AE, Suh N, Williams CR, et al. The novel synthetic triter-penoid, CDDO-imidazolide, inhibits inflammatory response and tumor growth in vivo. Clin Cancer Res. 2003;9:2798–2806. [PubMed] [Google Scholar]
- 21.Vodanovic-Jankovic S, Hari P, Jacobs P, et al. NF-kappaB as a target for the prevention of graft-versus-host disease: comparative efficacy of bortezomib and PS-1145. Blood. 2006;107:827–834. doi: 10.1182/blood-2005-05-1820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sun K, Wilkins DE, Anver MR, et al. Differential effects of proteasome inhibition by bortezomib on murine acute graft-versus-host disease (GVHD): delayed administration of bortezomib results in increased GVHD-dependent gastrointestinal toxicity. Blood. 2005;106:3293–3299. doi: 10.1182/blood-2004-11-4526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hill GR, Crawford JM, Cooke KR, et al. Total body irradiation and acute graft-versus-host disease: the role of gastrointestinal damage and inflammatory cytokines. Blood. 1997;90:3204–3213. [PubMed] [Google Scholar]
- 24.Chen LW, Egan L, Li ZW, et al. The two faces of IKK and NF-kappaB inhibition: prevention of systemic inflammation but increased local injury following intestinal ischemia-reperfusion. Nat Med. 2003;9:575–581. doi: 10.1038/nm849. [DOI] [PubMed] [Google Scholar]