Abstract
Background
Pancreatic cancer (PC) is one of the most deadly forms of cancer in the United States, with an annual incidence to death ratio of 0.92 because of the late stage at diagnosis. Identification of high-risk individuals (HRIs) that would be ideal for screening is needed to identify precursor lesions and small early stage disease. Those with a genetic predisposition have largely been identified, but little is known about those at high-risk for sporadic PC. This study asserts that a high-risk population does exist in sporadic pancreatic adenocarcinoma and proposes simple guidelines for screening.
Methods
A systematic review was conducted of the literature regarding identification of and screening in high-risk groups.
Results
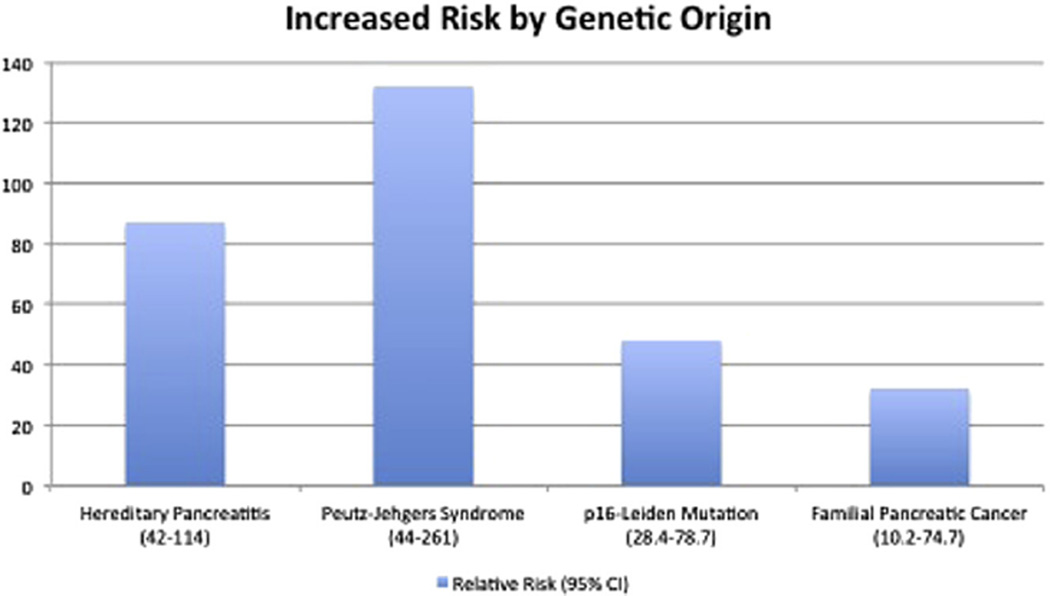
Those with the highest genetic risk of developing PC include those with hereditary pancreatitis (87 times more likely at age 55), Peutz–Jehgers syndrome (132 times more likely at age 50), p16-Leiden mutations (48 times more likely), and familial pancreatic cancer (FPC) kindreds (32 times more likely). Those with the highest risk of developing sporadic PC include those with new-onset diabetes older than 50 y and smoking history.
Conclusions
Given that sporadic PC is the single largest patient population effected with this devastating disease, some form of screening should be initiated. Currently, the medical community does nothing to attempt early detection of PC. However, sufficient evidence now exists to begin a screening protocol in a high-risk cohort, which would be patients with new-onset diabetes older than 50 y and a smoking history.
Keywords: Pancreatic cancer, Screening, Sporadic, Genetic predisposition
1. Introduction
Pancreatic cancer (PC) is projected to be the 10th most common cancer in the United States in 2013, yet it will be the fourth leading cause of cancer death [1]. Because more than 95% of PC is diagnosed at a later stage, the 5-y survival rate remains only 6% [1]. Resection of PC at an early stage represents the greatest potential for long-term survival. A screening program to detect PC at a resectable stage is needed. However, with a low incidence rate of about 1%, a screening program would be beneficial only for HRIs [2].
Many risk factors for PC have been suggested and quantified [3], but no true high-risk sporadic PC population has been definitively identified for a screening program. This is particularly true for sporadic pancreatic adenocarcinoma patients, which make up 90%–95% of PC sufferers [4]. Chronic pancreatitis, diabetes, smoking, and other lifestyle risk factors have been linked to the development of PC [5,6], but currently there is no accepted standard on how to evaluate, screen, or risk stratify these types of high-risk patients. This large patient population of possible sporadic PCs is known, but currently there are no standards to begin to screen and or prevent this carcinogenetic pathway from occurring. One of the reasons for the lack of any screening is that one-third of United States adults (34.9%) are obese, an estimated 42.1 million people, or 18.1% of all adults (aged ≥18 y) smoke (i.e., >20 pack year history), and 25.8 million Americans, 8.3% of the population, have diabetes. This denominator of possible patients, estimated to be 80 million people is significantly greater than the 46,420 estimated new cases of sporadic PCs in 2014. However, the argument for screening is based on the fact that a cumulative risk of two out of three of these factors could be more predictive, and if we do not start with some form of screening then we will not see any overall survival improvement in the next decade.
This study asserts that a true higher risk population does exist in sporadic pancreatic adenocarcinoma.
2. Methods
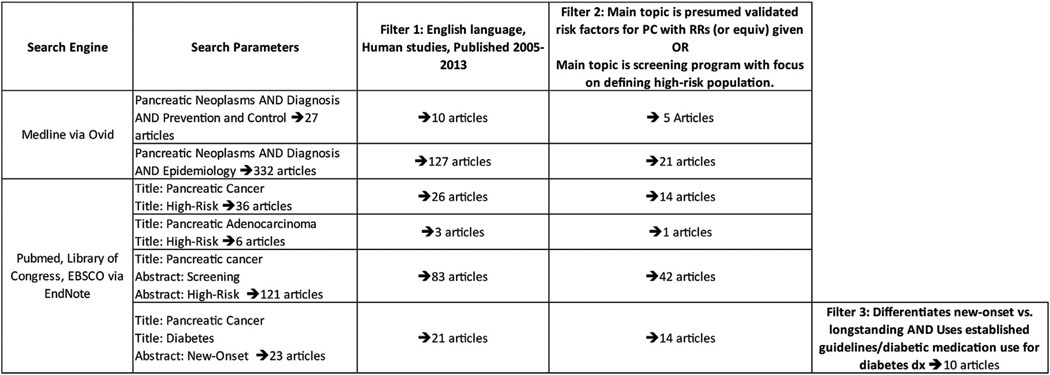
A literature search was conducted using Medline via Ovid (National Institute of Health - National Library of Medicine). Search parameters included: “pancreatic neoplasms and diagnosis and prevention and control” and “pancreatic neoplasms and diagnosis and epidemiology.” A second literature search was conducted using PubMed, Library of Congress, and EBSCO, all via EndNote. Several search parameters were used, including “title” search terms of “PC,” “pancreatic adenocarcinoma,” “high-risk,” and “diabetes,” and “abstract” search terms of “screening,” “high-risk,” and “new-onset” (Fig. 1).
Fig. 1.
Current reported literature for screening PC.
All searches were further restricted to include only English language articles, human studies, and publish dates between 2005 and 2013, to include only current-screening methods and therapies. Further filtering retained only articles that discussed screening methods and/or protocols and/or risk factors for PC.
Articles pertaining to screening were included only if they discussed presumed validated screening methods, with a focus on defining a high-risk population ideal for screening. Articles discussing non-validated or potential screening methods were removed because non-validated methods would not yet be useful for a screening protocol.
Articles pertaining to risk factors were included only if they discussed presumed validated risk factors for PC because many risk factors have been speculated, but not proven, to be significant. Articles that did not include risk ratios (RR), or an equivalent measure, were removed. To reduce the possibility of missed diagnoses, studies pertaining to diabetes were considered only if they stratified risk based on new-onset versus longstanding and used established guidelines (A1c ≥6.5%, fasting plasma glucose ≥126 mg/dL, or oral glucose tolerance test ≥200 mg/dL) or prescription diabetic medication use to determine diabetes status [7].
3. Results
This study defines three separate at-risk groups of the approximately 45,000 patients diagnosed with PC each year: (1) those with known hereditary conditions that predispose an individual to PC, (2) those with familial clustering of PC caused by an unknown genetic mutation (groups 1 and 2 totaling approximately 4500 [8]), and (3) those with clinical predisposition for PC (approximately 19,000 [9,10]).
3.1. Known hereditary conditions causing PC
It is well known that there are several hereditary conditions that predispose an individual to PC. Some confer a greater risk than others (Fig. 2).
Fig. 2.
Increased risk for PC, conferred by genetic risk factors (relative risk compared with general population) [9,14,17,24]. (Color version of the figure is available online.)
An inherited form of chronic pancreatitis, known as hereditary pancreatitis, is one such condition. Hereditary pancreatitis is most frequently caused by a mutation in either the PRSS1/cationic trypsinogen gene or the SPINK1/serine protease inhibitor gene [8]. This condition has been shown to confer an RR of up to 87 (95% confidence interval [CI] 42–114) [11], with a mean diagnostic age of approximately 55 [11,12]. Presenting symptoms of hereditary pancreatitis include attacks of acute pancreatitis in childhood, usually with a family history of the disease [8,12].
Peutz–Jehgerssyndrome(PJS) is caused by a mutation in the STK11/LKB1 gene and confers an increased risk for many different types of cancers, including pancreatic [8,13–15]. A study conducted in 2000 by Giardiello et al. [16] established an RR of 132 (95% CI 44–261), with a mean onset age of 40.8 ± 16.2 y (95% CI 23.9–57.8). Lifetime risks ranging from 3% at age 30 to 11% at age 70 have been reported by Hearle et al. [17], with similar values reported by Lim et al. [18] Presenting symptoms of PJS include gastrointestinal hamartomas and mucocutaneous pigmentations, with a family history of the disease.
Individuals with a CDKN2A/p16 mutation, comprising 1/4th of the population with familial atypical multiple mole melanoma, also have an increased risk of developing PC [8]. This is especially true in those with a specific mutation known as p16-Leiden. RRs as high as 47.8 (95% CI 28.4–78.7) have been reported for p16-Leiden mutations [19]. One study’s findings indicate that 1/6th of individuals with a p16-Leiden mutation will be diagnosed with PC by age 75 [20]. Presenting symptoms of p16-Leiden and/or familial atypical multiple mole melanoma include malignant melanoma and multiple atypical precursor nevi, with a family history of the disease [20].
Hereditary pancreatitis, PJS, and p16 mutations represent the known genetic conditions that cause the greatest increased risk for PC. Diagnosis of these diseases can be made by clinical recognition of the symptoms or by genetic testing.
There are other mutations, however, that increase risk to a lesser extent. These include hereditary breast and ovarian cancer syndrome caused by BRCA1 and BRCA2 mutations. Among these mutations, BRCA2 represents the higher risk group, with RRs ranging from 2.13 (95% CI 0.36–7.03) to 6.6 (95% CI 1.9–23) [21,22] and one reported odds ratio (OR) of 12.8 (95% CI 3.7–44.2) in a population with the 6174delT mutation [23]. It has been suggested that BRCA2 mutations with a family history of PC may cause an even greater increased risk; however, this has not yet been proven. Additionally, if BRCA2 mutations do cause a very high familial risk of PC, this population will be captured in the familial clustering cohort. Findings are mixed regarding BRCA1 mutations, with the RR ranging from 0–3 [8,24]. Those with mutations causing hereditary nonpolyposis colorectal cancer, also known as Lynch syndrome, have a standard incidence ration of 0–8.6 [8]. Lynch syndrome is caused by mutations in MLH1, MSH2, MSH6, or PMS2 genes [24]. The strongest clinical symptom of BRCA mutations and Lynch syndrome is a family history of breast and/or ovarian cancer and colon cancer, respectively [8]. Genetic testing is necessary for definitive diagnosis.
Familial adenomatous polyposis (mutations in the APC gene), cystic fibrosis (mutations in the CFTR gene), mutations in the PALB2 gene, and the ABO gene have been implicated in PC. Further studies are needed to establish these as significant risk factors [8,25].
3.2. Familial clustering of PC
Familial clustering of PC is defined as familial pancreatic cancer (FPC) and is considered to occur when ≥2 first-degree blood relatives (FDR) in one family have a PC diagnosis. FPC is thought to be caused by an unknown autosomal dominant gene [8]. RR values are crudely based on the number of individuals in one family who have a PC diagnosis—the risk increasing with an increasing number of affected relatives. One study found that for an individual with ≥3 FDRs with PC, the RR is 32 (95% CI 10.4–74.7), and with 2 FDRs, RR is 6.4 (95% CI 1.8–16.4) [26]. Age of onset in FPC families has been equivocal, with some studies showing a decreased age-of-onset from the standard population and others showing no difference [8].
A more accurate risk assessment can be provided by PancPro, which was developed in 2007 to generate precise risk values based on more detailed histories [27]. Available at no cost through the CancerGene software package (http://www4.utsouthwestern.edu/breasthealth/cagene/) [28], several studies have shown it to yield an accurate assessment of PC risk, even in the absence of knowing the exact genetic cause [27,29]. Use of PancPro to determine risk in FPC is preferred over calculation based on number of affected relatives. Two consensus summits for PC have suggested risk cutoffs of 5- and 10-fold for classification as “high-risk” [2,30]. However, a more appropriate middle ground was suggested by Leonardi et al. [29], who found a PancPro risk of >7% to be indicative of a high-risk for PC in FPC kindreds. A recent study performed by Zubarik et al. [31] looked at patients who had a FDR diagnosed with PC and screened these eligible patients with a CA 19-9 level and endoscopic ultrasound. A total of 546 patients were enrolled, CA 19-9 was elevated in 27 patients (4.9%, 95% CI, 3.2%–7.1%). Neoplastic or malignant findings were detected in five patients (0.9%, 95% CI, 0.3%–2.1%), and pancreatic adenocarcinoma in 1 patient (0.2%, 95% CI, 0.005%–1.02%). They concluded that potentially early stage PC can be identified by screening patients.
3.3. Clinical predisposition for pancreatic cancer
Several clinical conditions have been implicated as risk factors for PC. These include diabetes, obesity, smoking, heavy drinking, and chronic pancreatitis [8,25]. ORs for these conditions vary, with few studies deeming them major risk factors, individually. However, when one differentiates between new-onset diabetes and longstanding diabetes, the risk for PC increases [9]. New-onset diabetes occurring ≤2 y before a PC diagnosis is referred to as PC-induced diabetes mellitus [32]. It is now an accepted premise that new-onset diabetes can be an early manifestation of PC [32,33], and its diagnosis can precede PC symptoms by up to 36 mo [9,34]. This represents a window of opportunity for the early diagnosis of sporadic PC. Because only 1% of patients with new-onset diabetes (approximately 19,000 of 1.9 million) will be diagnosed with PC within 2 y, distinguishing characteristics are necessary to determine those who do have PC [9,10,35].
3.3.1. New-onset diabetes with advanced age
Late-onset diabetes refers to new-onset diabetes in a more advanced age (Table 1). Several studies have asserted that this represents a significant risk factor for PC, more so than simply new-onset diabetes [9,34,35]. No definition of “advanced age” has been established, though cutoffs have ranged from 50 y [9] to 65 y [35]. Chari et al. [9] calculated an observed-to-expected ratio of 7.94 (95% CI 4.70–12.55) in new-onset diabetes patients aged older than 50 y. Mizuno et al. [34] calculated an adjusted OR of 1.12 (95% CI 1.03–1.24) for onset age of diabetes, per year, beginning at age 55. Using these reports and other reports in the literature, we are able to estimate a standardized mortality ratio of 1.4, or a 40% increase in PC risk after the age of 50 y.
Table 1. Risk for pancreatic cancer in new-onset diabetes with advanced age.
New-onset diabetes and advanced age
| Study | Study population | Findings (95% CI) |
|---|---|---|
| Chari et al. 2005 [7] | New-onset DM >50 y-old | RR = 7.94 (4.70–2.55) |
| New-onset DM 50–70 y-old | RR = 5.23 (1.70–12.20) | |
| New-onset DM ≥70 y-old | RR = 9.91 (5.26–16.96) | |
| Hart et al. 2011 [36] | New-onset DM with PC | Mean age at PC dx = 76.4 ± 6.8 |
| New-onset DM without PC | Mean age at index date = 71.7 ± 9.7 | |
| Lee et al. 2012 [32] | New-onset DM with PC | Mean age at PC dx = 61.3 ± 10.2 |
| New-onset DM without PC | Mean age at index date = 55.8 ± 10.3 | |
| Aggarwal et al. 2012 [33] | New-onset DM with PC | Median age at PC dx = 76 (range 39–90) |
| Mizuno et al. 2013 [34] | New-onset DM ≥55 y-old | OR*, per year over age 55 = 1.12 (1.03–1.24) |
| Subjects with new-onset DM, <55 y-old, with PC | 0/19 patients | |
| Subjects with new-onset DM, ≥55 y-old, with PC | 7/21 patients |
DM = diabetes mellitus; dx = diagnosis.
Bold values demonstrate populations that are at significant risk of pancreatic cancer.
Multivariate analysis.
3.3.2. New-onset diabetes with weight loss
Age of onset is one of the characteristics that has been suggested to help distinguish between PC-induced diabetes mellitus and standard type II diabetes (Table 2).Weight loss also appears to be a consistent predictive factor of PC, with seven of the reviewed studies finding an association [9,32–37]. Though the only OR presented was a multiple logistic regression analysis yielding an OR of 1.68 (95% CI 1.46–1.93) [35], Mizuno et al., Hart et al., and Pannala et al. all reported weight loss or decreased body mass index preceding PC diagnosis by means of 12, 13, and 12 mo, respectively. Aggarwal et al. reported a median weight loss of 2.2 kg (range (−36) to 7.4) from baseline to time of diabetes diagnosis. Lee et al. reported a mean weight loss of 4.1 ± 4.7 kg in those with new-onset diabetes and PC, compared with −0.4 ± 1.5 kg in those with new-onset diabetes without PC. Hart et al. reported a mean weight loss of −2.1 ± 3.8 kg at diabetes diagnosis in those who progressed to PC.
Table 2. Risk for PC in new-onset diabetes with weight loss.
New-onset diabetes and weight loss
| Study | Study population | Mean weight change (kg ± SD) |
|---|---|---|
| Hart et al. 2011 [36] | New-onset DM with PC | At DM dx = (−2.1 ± 3.8) |
| New-onset DM without PC | At DM dx = +1.4 ± 4.7 | |
| New-onset DM with PC | At PC dx = (−8.3 ± 8.3) | |
| New-onset DM without PC | At PC dx = (−0.8 ± 4.8) | |
| Lee et al. 2012 [32] | New-onset DM with PC | (−4.1 ± 4.7) |
| New-onset DM without PC | (−0.4 ± 1.5) | |
| Aggarwal et al. 2012 [33] | New-onset DM with PC | At DM dx = (−2.2) (range [(−36) to 7.4])* |
| Longstanding DM with PC | At DM dx = +2.5 (range [(−2) to 16]* |
DM = diabetes mellitus; dx = diagnosis; SD = standard deviation.
Bold values demonstrate populations that are at significant risk of pancreatic cancer.
Median weight change.
3.3.3. Smoking status
Smoking, alone, has been shown to cause a slightly increased risk for PC (Table 3) [38]. It has also been shown to have a synergistic effect with several other risk factors, including hereditary pancreatitis (2.1-fold increased risk compared with non-smokers with hereditary pancreatitis) [39], CDKN2A mutations (hazard ratio = 25.8) [40], and FPC (standard incidence ration = 19.2) [26]. In the new-onset diabetes population, one conditional logistic analysis yielded an OR of 5.84 (95% CI 0.62–55.43) in smokers [9]. Another yielded an adjusted OR of 4.77 (95% CI 2.71–8.38) [41].
Table 3. Risk for PC in smokers with other PC risk factors.
Risk factor + smoking
| Study | Study population | Findings (95% CI) |
|---|---|---|
| Hereditary pancreatitis + smoking | ||
| Lowenfels et al. 2001 [36] | HP with smoking versus HP without smoking | AOR* = 2.1 (0.7–6.1) |
| CDKN2A + smoking | ||
| McWilliams et al. [37] | CDKN2A mutation + current smoker | HR = 25.8 |
| FPC + smoking | ||
| Klein et al. 2004 [24] | FPC kindred with at least one FDR with PC + ever smoker | SIR = 19.2 (7.7–39.5) |
| FPC kindred with at least one FDR with PC + never smoker | SIR = 6.25 (1.70–16.0) | |
| New-onset diabetes + smoking | ||
| Chari et al. 2005 [7] | New-onset DM + smoking | OR† = 5.84 (0.62–55.43) |
| Ben et al. [38] | New-onset DM + smoking | AOR‡ = 4.77 (2.71–8.38) |
| New-onset DM without smoking | AOR‡ = 4.39 (3.28–5.89) |
DM = diabetes mellitus; HP = hereditary pancreatitis; HR = hazard ratio; SIR = standard incidence ration.
Bold values demonstrate populations that are at significant risk of pancreatic cancer.
AOR age- and sex-adjusted OR.
OR conditional logistic regression analysis.
AOR odds ratio adjusted for age, history of diabetes, family history of PC, heavy alcohol consumption, and smoking status; logistic regression analysis.
Therefore, patients with new-onset diabetes after the age of 50 y, who have a past history of at least five pack year history of smoking, currently represent the highest risk population for sporadic PC.
4. Discussion
Screening in the general population is not appropriate, as only a small portion (1.3%) of the population will develop PC [2]. However, PC is an exceptionally dismal diagnosis, with 5-y survival of only 6% [1]. It is well established that early detection is the only hope for long-term survival and that a screening protocol is needed for HRIs. The first step in establishing such a protocol is to provide a complete definition of a HRI. Previous definitions have included only those with known genetic or familial predispositions, which encompasses only 5%–10% of PC patients [4,42]. Extensive research within this population is being conducted by Johns Hopkins University with The National Familial Pancreas Tumor Registry [43]. However, screening that is restricted to this population, alone, leaves more than 40,000 patients with a near 0% chance of survival [1]. A definition of high-risk that attempts to encompass the remaining 90%–95% of PC sufferers, those with sporadic PC, must be established.
Patients with new-onset diabetes represent the greatest potential for identifying this group at an early stage. Indeed, studies show that diabetes prevalence in the PC population reaches 40%–64% [44–47], with up to 88% being new-onset [47,48]. In contrast, the most recent Center for Disease Control data report that only 11.3% of the United States population aged older 20 y has diabetes [10], and World Health Organization data show a worldwide prevalence of only 9.8% and 9.2% in adult men and women, respectively [49]. PC-induced diabetes is widely believed to be a symptom of PC that can precede the standard symptoms (jaundice, cachexia, and so forth) by up to 3 y [9,32,34]. These findings open up a window for detection of early stage PC. Much research has attempted to find clinical features or serum biomarkers that distinguish between PC-induced diabetes and standard type 2 diabetes. Although a serum biomarker with a high sensitivity and specificity would be ideal, no such test has been validated, when used alone. Currently, new-onset diabetes with advanced age and/or new-onset diabetes with weight loss represent our greatest ability to distinguish the two.
Regarding late-onset diabetes, Mizuno et al. [34] has conducted perhaps the most extensive study, focusing on age of diabetes onset as a risk factor for PC. In this study, 0% of the patients aged younger than 55 y developed PC within 2 y of the diabetes diagnosis. In contrast, 33% of those aged ≥55 y were diagnosed with PC within 2 y of the diabetes diagnosis. This supports the finding by Chari et al. [9] that new-onset diabetes in those over 50 y confers a nearly 8-fold risk compared with the general population. No age cutoff to define “late-onset” diabetes has been established [34]. However, the aforementioned results suggest that new-onset diabetes at 50 y would be an appropriate definition.
Regarding new-onset diabetes with weight loss, Hart et al. [36] present the most definitive evidence, finding that 59% (17 of 29) of patients with PC-induced diabetes had lost weight in the 1–2 y before diabetes diagnosis versus weight gain in 56% (24 of 43) of patients with type 2 diabetes. Pannala et al. [37] found increased severity of diabetes with significant reductions in body mass index, particularly in the 12 mo before PC diagnosis, before the onset of cachexia. Sah et al. [32] supported this finding by proposing a mechanism between PC and adipose tissue that would explain the weight loss, separate from and before the muscle wasting associated with cachexia. Other studies have observed weight loss, as well, though not directly setting out to [33–35,46]. Identification of this group of individuals will require annual screening for diabetes and accurate records of weight, both of which are most appropriate in the primary care setting.
Because lifetime risk for PC is low, screening is appropriate only for those with the highest risk for PC. This includes FPC kindreds with ≥7-fold risk, hereditary pancreatitis and PJS patients, and p16-Leiden mutation carriers. This also includes individuals with new-onset diabetes aged older than 50 y or with weight loss, capturing a large piece of the sporadic PC population. These groups see an elevated risk of at least 7-fold and up to 132-fold. When compared with the levels of risk that warrant screening for other types of cancer, screening in these high-risk groups should certainly be warranted, as well. For example, biennial mammograms are suggested for all women aged older than 40 y, who see a lifetime breast cancer risk of 12.29% [50,51]. Likewise, the average lifetime risk of colon cancer is 4.82%, and screening is recommended for all aged older than 50 y [50,51]. As such, screening for PC in HRIs should no longer be unheeded, especially considering the absolute necessity of early detection in PC.
The limitations of this study include the fact that the conditions discussed are rare. This includes not only the risk factors for PC, but PC itself. Therefore, the data used are based on relatively small subject populations and a small number of studies. Additionally, the suggestions for screening are based mainly on elevated risk for PC, not proven efficacy of screening. This is especially true in the diabetes high-risk group, as no research program has been conducted to test efficacy of screening in this population. These limitations highlight the need for more studies to be conducted in this area.
5. Conclusions
The definition of a high-risk population for PC should include those with hereditary pancreatitis or PJS, carriers of p16-Leiden mutations, and FPC patients with a ≥7-fold risk determined by PancPro. Additionally, those with new-onset diabetes aged older than 50 y and/or with weight loss should be included. Smoking in any one of these groups should be considered a significant added risk. This definition of HRIs will encompass those at the greatest risk for PC, including those with sporadic PC—a group that has largely been ignored in screening discussions but that is in the greatest need of recognition. Although knowledge regarding PC is far from complete, we have reached a time when standing by should no longer be an option. A screening protocol must be officially established, accepted, and put into practice to use what we do know to attempt to help those who will fall prey to this deadly disease. The first step of screening should fall on the primary care physician to recognize those at high risk. This screening program could include HRIs biannually using magnetic resonance imaging and/or magnetic resonance cholangiopancreatography for a fixed period to time (i.e., between 3 and 5 y) to better assess their baseline parenchyma and their potential risk of disease based on the presence or absence of abnormalities. This screening would not be for the life of the patient, but just at the time of what is believed to be the high-risk time interval (i.e., aged >50 y, with new-onset diabetes). Clearly, established cost profile would have to be evaluated and current-screening guidelines from the U.S. Preventive Services Task Force would have to be changed before this type of screening could be implemented.
Acknowledgment
This research is supported by grant R25-CA-134283 from the National Cancer Institute.
Footnotes
Authors’ contributions: E.B. and R.C.G.M. contributed to the conception and design, analysis and interpretation, data collection, and writing the article. R.C.G.M. did the critical revision of the article and obtaining of the funding.
Each author certifies that he/she has made a direct and substantial contribution to the work reported in the article by participating in each of the following three areas: (1) conceiving and designing the study; or collecting the data; or analyzing and interpreting the data; (2) writing the article or providing critical revisions that are important for the intellectual content; and (3) approving the final version of the article.
Disclosure
The authors reported no proprietary or commercial interest in any product mentioned or concept discussed in the article.
REFERENCES
- 1.Siegel R, Naishadham D, Jemal A. Cancer statistics, 2013. CA Cancer J Clin. 2013;63:11. doi: 10.3322/caac.21166. [DOI] [PubMed] [Google Scholar]
- 2.Canto MI, Harinck F, Hruban RH, et al. International Cancer of the Pancreas Screening (CAPS) consortium summit on the management of patients with increased risk for familial pancreatic cancer. Gut. 2013;62:339. doi: 10.1136/gutjnl-2012-303108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hocevar BA, Kamendulis LM, Pu X, et al. Contribution of environment and genetics to pancreatic cancer susceptibility. PloS One. 2014;9:e90052. doi: 10.1371/journal.pone.0090052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Klein AP. Identifying people at a high risk of developing pancreatic cancer. Nat Rev Cancer. 2013;13:66. doi: 10.1038/nrc3420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Maisonneuve P, Lowenfels AB. Epidemiology of pancreatic cancer: an update. Dig Dis. 2010;28:645. doi: 10.1159/000320068. [DOI] [PubMed] [Google Scholar]
- 6.Raimondi S, Maisonneuve P, Lowenfels AB. Epidemiology of pancreatic cancer: an overview. Nat Rev Gastroenterol Hepatol. 2009;6:699. doi: 10.1038/nrgastro.2009.177. [DOI] [PubMed] [Google Scholar]
- 7.Lee KF, Wagner LK, Lee YE, Suh JH, Lee SR. The impact-absorbing effects of facial fractures in closed-head injuries. An analysis of 210 patients. J. Neurosurg. 1987;66:542. doi: 10.3171/jns.1987.66.4.0542. [DOI] [PubMed] [Google Scholar]
- 8.Klein AP. Genetic susceptibility to pancreatic cancer. Mol Carcinog. 2012;51:14. doi: 10.1002/mc.20855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chari ST, Leibson CL, Rabe KG, Ransom J, de Andrade M, Petersen GM. Probability of pancreatic cancer following diabetes: a population-based study. Gastroenterology. 2005;129:504. doi: 10.1053/j.gastro.2005.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Prevention CDCa. Atlanta, GA: National Institute of Health; 2011. National diabetes fact sheet: national estimates and general information on diabetes and prediabetes in the United States, 2011. [Google Scholar]
- 11.Rebours V, Boutron-Ruault MC, Schnee M, et al. Risk of pancreatic adenocarcinoma in patients with hereditary pancreatitis: a national exhaustive series. Am J Gastroenterol. 2008;103:111. doi: 10.1111/j.1572-0241.2007.01597.x. [DOI] [PubMed] [Google Scholar]
- 12.Lowenfels AB, Maisonneuve P, Whitcomb DC. Risk factors for cancer in hereditary pancreatitis. International Hereditary Pancreatitis Study Group. Med Clin North Am. 2000;84:565. doi: 10.1016/s0025-7125(05)70240-6. [DOI] [PubMed] [Google Scholar]
- 13.Hemminki A, Markie D, Tomlinson I, et al. A serine/threonine kinase gene defective in Peutz-Jeghers syndrome. Nature. 1998;391:184. doi: 10.1038/34432. [DOI] [PubMed] [Google Scholar]
- 14.Jenne DE, Reimann H, Nezu J, et al. Peutz-Jeghers syndrome is caused by mutations in a novel serine threonine kinase. Nat Genet. 1998;18:38. doi: 10.1038/ng0198-38. [DOI] [PubMed] [Google Scholar]
- 15.Canto MI. Strategies for screening for pancreatic adenocarcinoma in high-risk patients. Semin Oncol. 2007;34:295. doi: 10.1053/j.seminoncol.2007.05.008. [DOI] [PubMed] [Google Scholar]
- 16.Giardiello FM, Brensinger JD, Tersmette AC, et al. Very high risk of cancer in familial Peutz-Jeghers syndrome. Gastroenterology. 2000;119:1447. doi: 10.1053/gast.2000.20228. [DOI] [PubMed] [Google Scholar]
- 17.Hearle N, Schumacher V, Menko FH, et al. Frequency and spectrum of cancers in the Peutz-Jeghers syndrome. Clin Cancer Res: Official J Am Assoc Cancer Res. 2006;12:3209. doi: 10.1158/1078-0432.CCR-06-0083. [DOI] [PubMed] [Google Scholar]
- 18.Lim W, Olschwang S, Keller JJ, et al. Relative frequency and morphology of cancers in STK11 mutation carriers. Gastroenterology. 2004;126:1788. doi: 10.1053/j.gastro.2004.03.014. [DOI] [PubMed] [Google Scholar]
- 19.de Snoo FA, Bishop DT, Bergman W, et al. Increased risk of cancer other than melanoma in CDKN2A founder mutation (p16-Leiden)-positive melanoma families. Clin Cancer Res: Official J Am Assoc Cancer Res. 2008;14:7151. doi: 10.1158/1078-0432.CCR-08-0403. [DOI] [PubMed] [Google Scholar]
- 20.Vasen HF, Gruis NA, Frants RR, van Der Velden PA, Hille ET, Bergman W. Risk of developing pancreatic cancer in families with familial atypical multiple mole melanoma associated with a specific 19 deletion of p16 (p16-Leiden) Int J Cancer. 2000;87:809. [PubMed] [Google Scholar]
- 21.Iqbal J, Ragone A, Lubinski J, et al. The incidence of pancreatic cancer in BRCA1 and BRCA2 mutation carriers. Br J Cancer. 2012;107:2005. doi: 10.1038/bjc.2012.483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Risch HA, McLaughlin JR, Cole DE, et al. Population BRCA1 and BRCA2 mutation frequencies and cancer penetrances: a kin-cohort study in Ontario, Canada. J Natl Cancer Inst. 2006;98:1694. doi: 10.1093/jnci/djj465. [DOI] [PubMed] [Google Scholar]
- 23.Figer A, Irmin L, Geva R, et al. The rate of the 6174delT founder Jewish mutation in BRCA2 in patients with non-colonic gastrointestinal tract tumours in Israel. Br J Cancer. 2001;84:478. doi: 10.1054/bjoc.2000.1605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Axilbund JE, Wiley EA. Genetic testing by cancer site: pancreas. Cancer J. 2012;18:350. doi: 10.1097/PPO.0b013e3182624694. [DOI] [PubMed] [Google Scholar]
- 25.Poruk KE, Firpo MA, Adler DG, Mulvihill SJ. Screening for pancreatic cancer: why, how, and who? Ann Surg. 2013;257:17. doi: 10.1097/SLA.0b013e31825ffbfb. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Klein AP, Brune KA, Petersen GM, et al. Prospective risk of pancreatic cancer in familial pancreatic cancer kindreds. Cancer Res. 2004;64:2634. doi: 10.1158/0008-5472.can-03-3823. [DOI] [PubMed] [Google Scholar]
- 27.Wang W, Chen S, Brune KA, Hruban RH, Parmigiani G, Klein AP. PancPRO: risk assessment for individuals with a family history of pancreatic cancer. Clin Oncol: Official J Am Soc Clin Oncol. 2007;25:1417. doi: 10.1200/JCO.2006.09.2452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chu D, Kohlmann W, Adler DG. Identification and screening of individuals at increased risk for pancreatic cancer with emphasis on known environmental and genetic factors and hereditary syndromes. JOP: J pancreas. 2010;11:203. [PubMed] [Google Scholar]
- 29.Leonardi G, Marchi S, Falconi M, et al. “PancPro” as a tool for selecting families eligible for pancreatic cancer screening: an Italian study of incident cases. Dig Liver Dis: Official J Ital Soc Gastroenterol Ital Assoc Study Liver. 2012;44:585. doi: 10.1016/j.dld.2011.12.019. [DOI] [PubMed] [Google Scholar]
- 30.Brand RE, Lerch MM, Rubinstein WS, et al. Advances in counselling and surveillance of patients at risk for pancreatic cancer. Gut. 2007;56:1460. doi: 10.1136/gut.2006.108456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zubarik R, Gordon SR, Lidofsky SD, et al. Screening for pancreatic cancer in a high-risk population with serum CA 19-9 and targeted EUS: a feasibility study. Gastrointest Endosc. 2011;74:87. doi: 10.1016/j.gie.2011.03.1235. [DOI] [PubMed] [Google Scholar]
- 32.Sah RP, Nagpal SJ, Mukhopadhyay D, Chari ST. New insights into pancreatic cancer-induced paraneoplastic diabetes. Nat Rev Gastroenterol Hepatol. 2013;10:423. doi: 10.1038/nrgastro.2013.49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Aggarwal G, Rabe KG, Petersen GM, Chari ST. New-onset diabetes in pancreatic cancer: a study in the primary care setting. Pancreatology. 2012;12:156. doi: 10.1016/j.pan.2012.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mizuno S, Nakai Y, Isayama H, et al. Risk factors and early signs of pancreatic cancer in diabetes: screening strategy based on diabetes onset age. J Gastroenterol. 2013;48:238. doi: 10.1007/s00535-012-0622-z. [DOI] [PubMed] [Google Scholar]
- 35.Lee JH, Kim SA, Park HY, et al. New-onset diabetes patients need pancreatic cancer screening? J Clin Gastroenterol. 2012;46:e58. doi: 10.1097/MCG.0b013e318238348c. [DOI] [PubMed] [Google Scholar]
- 36.Hart PA, Kamada P, Rabe KG, et al. Weight loss precedes cancer-specific symptoms in pancreatic cancer-associated diabetes mellitus. Pancreas. 2011;40:768. doi: 10.1097/MPA.0b013e318220816a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Pannala R, Leibson CL, Rabe KG, et al. Temporal association of changes in fasting blood glucose and body mass index with diagnosis of pancreatic cancer. Am J Gastroenterol. 2009;104:2318. doi: 10.1038/ajg.2009.253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Iodice S, Gandini S, Maisonneuve P, Lowenfels AB. Tobacco and the risk of pancreatic cancer: a review and meta-analysis. Langenbeck’s Arch Surg/Deutsche Gesellschaft Chirurgie. 2008;393:535. doi: 10.1007/s00423-007-0266-2. [DOI] [PubMed] [Google Scholar]
- 39.Lowenfels AB, Maisonneuve P, Whitcomb DC, Lerch MM, DiMagno EP. Cigarette smoking as a risk factor for pancreatic cancer in patients with hereditary pancreatitis. JAMA. 2001;286:169. doi: 10.1001/jama.286.2.169. [DOI] [PubMed] [Google Scholar]
- 40.McWilliams RR, Wieben ED, Rabe KG, et al. Prevalence of CDKN2A mutations in pancreatic cancer patients: implications for genetic counseling. Eur J Hum Genet. 2011;19:472. doi: 10.1038/ejhg.2010.198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ben Q, Cai Q, Li Z, et al. The relationship between new-onset diabetes mellitus and pancreatic cancer risk: a case-control study. Eur J Cancer. 2011;47:248. doi: 10.1016/j.ejca.2010.07.010. [DOI] [PubMed] [Google Scholar]
- 42.Lynch HT, Smyrk T, Kern SE, et al. Familial pancreatic cancer: a review. Semin Oncol. 1996;23:251. [PubMed] [Google Scholar]
- 43.Lee Y, Fujita H, Yamana H, Kakegawa T. Factors affecting leakage following esophageal anastomosis. Surg Today. 1994;24:24. doi: 10.1007/BF01676880. [DOI] [PubMed] [Google Scholar]
- 44.Permert J, Larsson J, Fruin AB, et al. Islet hormone secretion in pancreatic cancer patients with diabetes. Pancreas. 1997;15:60. doi: 10.1097/00006676-199707000-00009. [DOI] [PubMed] [Google Scholar]
- 45.Cersosimo E, Pisters PW, Pesola G, McDermott K, Bajorunas D, Brennan MF. Insulin secretion and action in patients with pancreatic cancer. Cancer. 1991;67:486. doi: 10.1002/1097-0142(19910115)67:2<486::aid-cncr2820670228>3.0.co;2-1. [DOI] [PubMed] [Google Scholar]
- 46.Chari ST, Leibson CL, Rabe KG, et al. Pancreatic cancer-associated diabetes mellitus: prevalence and temporal association with diagnosis of cancer. Gastroenterology. 2008;134:95. doi: 10.1053/j.gastro.2007.10.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Pannala R, Leirness JB, Bamlet WR, Basu A, Petersen GM, Chari ST. Prevalence and clinical profile of pancreatic cancer-associated diabetes mellitus. Gastroenterology. 2008;134:981. doi: 10.1053/j.gastro.2008.01.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Chari ST, Klee GG, Miller LJ, Raimondo M, DiMagno EP. Islet amyloid polypeptide is not a satisfactory marker for detecting pancreatic cancer. Gastroenterology. 2001;121:640. doi: 10.1053/gast.2001.27210. [DOI] [PubMed] [Google Scholar]
- 49.Danaei G, Finucane MM, Lu Y, et al. National, regional, and global trends in fasting plasma glucose and diabetes prevalence since 1980: systematic analysis of health examination surveys and epidemiological studies with 370 country-years and 2.7 million participants. Lancet. 2011;378:31. doi: 10.1016/S0140-6736(11)60679-X. [DOI] [PubMed] [Google Scholar]
- 50.Lam L, Krementz E, McGinness C, Godfrey R. Melanoma of the clavicular region: multimodal treatmen. Arch. Surg. 2001;136:1054. doi: 10.1001/archsurg.136.9.1054. [DOI] [PubMed] [Google Scholar]
- 51.Howlader N, Noone AM, Krapcho M, et al. SEER Cancer Statistics Review. National Cancer Institute; 1975–2010. Based on November 2012 SEER data submission, posted to the SEER web site, April 2013. [Google Scholar]