Abstract
Purpose
To evaluate the ocular surface change and the inflammatory response in a rabbit model of short-term exposure keratopathy.
Methods
Short term exposure keratopathy by continuous eyelid opening was induced in New Zealand white rabbits for up to 4 hours. Ultrasound pachymetry was used to detect central total corneal thickness. In vivo confocal microscopy and impression cytology were performed to evaluate the morphology of ocular surface epithelium and the infiltration of inflammatory cells. Immunohistochemistry for macrophage,neutrophil, CD4(+) T cells, and CD8(+) T cells were performed to classify the inflammatory cells. Scanning electron microscopy(SEM) was performed to detect ocular surface change.The concentrations of IL-8, IL-17, Line and TNF-αwere analyzed by multiplex immunobead assay. TUNEL staining was performed to detect cellular apoptosis.
Results
Significant decrease ofcentral total cornealthickness were found within the first 5 minutes and remained stable thereafter, while there were no changes of corneal epithelial thickness.No significant change of corneal, limbal and conjunctival epithelial morphology was found by in vivo confocal microscopy except the time dependent increase of superficial cellular defects in the central cornea. Impression cytology also demonstrated time dependent increase of sloughing superficial cells of the central cornea. Aggregations ofinflammatory cells were found at 1 hour in the limbal epithelium, 2 hours in the perilimbal conjunctival epithelium, and 3 hours in the peripheral corneal epithelium.In eyes receiving exposure for 4 hours, the infiltration of the inflammatory cells can still be detected at 8 hours after closing eyes.Immunohistochemical study demonstrated the cells to be macrophages, neutrophils, CD4-T cells and CD-8 T cells.SEM demonstrated time-depending increase of intercellular border and sloughing of superficial epithelial cells in corneal surface. Time dependent increase of IL-8, IL-17 and TNF-α in tear was found.TUNEL staining revealed some apoptotic cells in the corneal epithelium and superficial stroma at 3 hours after exposure.
Conclusions
Short term exposure keratopathy can cause significant changes to the ocular surface and inflammatory response. Decrease of central total corneal thickness, aggregation of inflammatory cells, and cornea epithelial cell and superficial keratocyte apoptosis were found no less than 4 hours following the insult.
Introduction
The superficial part of the cornea is a layer of stratified, non-keratinized, non-secretory epithelium, whichrelies on a stable tear film to maintain healthy corneal physiology and optical properties.[1–3]Any pathological conditions causing dry eye disease may result in discomfort, visual impairment, and tear film instability with the potential to damage ocular surface.[4–6]Among the etiologies of dry eye syndrome, exposure keratopathy is uniquely caused by not only an unclosed eyelid, but also incomplete blinking.[7–10]Eyelidclosure and blinking contribute to replenishing and spreading the tear film across the corneal surface and preventing tear film evaporation. Exposure keratopathy can be classified into neurotrophic (cranial nerve V palsy, aneurysm, cerebrovascular accident, multiple sclerosis, tumor, herpes simplex, herpes zoster),[11–13] neuroparalytic (cranial nerve VII palsy),[14] lid malposition(lagophthalmos, proptosis),[15] and iatrogenic event(general or topical anesthesia, ocular procedure or surgery, artificial respiration in anintensive care unit).[16, 17]Exposure keratopathy may lead to chemosis, cornea erosion, corneal melting, infectious keratitis, and even corneal perforation.[18–20] In terminal patients willing to donor corneas, the protection of corneal damage from exposure keratopathy is also important to assure acceptable quality of donor corneas.[21–23]During excimer laser refractive surgery, iatrogenic exposure keratopathy may significantly affect the surgical results.[24, 25]
Although numerous dry eye studies having been conducted, most of the studies focused on dry eye secondary to aqueous tear deficiency,[26–31]or animal model of desiccating stress[32–36]. The role of inflammation on the pathogenesis of aqueous tear deficientdry eyehas drawnresearch attention during the past few years, and thus anti-inflammatory agents have been proposed to treat dry eye syndrome in conjunction with the use of artificial tears.[37, 38]As for evaporative type of dry eye, most of the studies focusing on desiccating stress (with or without inhibition of tear secretion) in mouse eyes which were exposed to a controlled environment chamber with stable humidity and temperature. In those studies, continuous desiccating stress was applied for about 10 hours a day for more than 1 week and the experimental mice can blink their eyes freely. In addition, the exposure to a continuous air draft from a fan placedin front of the cageis usually performed[32–36, 39]. None of the procedures was similar to the pathogenic condition in which patients with exposure keratopathy encountered.It's worthy to building up ananimal model to mimic exposure keratopathy in which theblinking and closure of eyes are impaired,and the ocular surface dried out in natural condition for a period of time. Such experimental condition can be applied to patients with exposure keratopathy, especially for patients in intensive care units or operative rooms if the ocular surface is not adequately protected.
In the current study, we built up a rabbit model of short term exposure keratopathy. In vivo confocal microscopy, immunohistochemistry, ultrasound pachymetry, impression cytology,scanning electron microscopy (SEM) and TUNEL staining were performed to identify the early changes of the cornea, limbus, and conjunctiva after the induction of exposure keratopathy. Our results demonstrated the significant changes of ocular surface in early exposure keratoapthy,which have not been previously reported.
Materials and Methods
Animals models of short-term exposure keratopathy
New Zealand albino rabbits (female, weight, 3.0–3.5 kg; age, 6 months) were used in this study. Use, care, and treatment of all animals followed the regulations of the ARVO Statement for the Use of Animals in Ophthalmic and Vision Research. All experimental procedures were approved by the Committee for Animal Research of the National Taiwan University Hospital. All in vivo experimental procedures (including pachymetry, in vivo confocal microscopy and impression cytology) were performed under general anesthesia induced by the intramuscular injection of ketamine hydrochloride (35 mg/kg) and xylazine hydrochloride (5 mg/kg). The model of exposure keratopathy was modified by other studies of desiccating stress[32–36]. In brief, the experiments were performed in an environmentally controlled room (50–60%humidity, 25 to 28°C). The right eyes of the animals were used for all experiments, and the left eyes were left untreated. Eyelid specula were appliedon the right eyes continuously without adding any lubricants. The interpalpabral fissures were maintained widely open to assure the exposure of central cornea, limbus and perilimbal conjunctivafor following time periods: every 5 minutes in the first 30 minutes and hours 1, 2, 3, and 4. After exposure for 4 hours, eyelid specula were removed and rabbit eyes were maintained closed for 4, 8 and 24 hours for recovery. For immunohistochemistry and SEM, rabbits at different time points (post-exposure for 3 hours for immunohistochemistry and post-exposure 1, 2 and 4 hours for SEM) were euthanized with intravenous injection of 240 mg/kg thiamylal sodium (Shinlin Singseng Pharmaceutical, Taoyuan, Taiwan). Six eyes were included for each time point.
Central total corneal thickness measured by ultrasound pachymetry
To measure the central total corneal thickness, ultrasound pachymetry (OcuScan, Alcon, Fort Worth, TX) was performed on the center of the cornea. To prevent the artifacts caused by topical medications, the cornea remained dry during the examination without adding topical anesthetic medications or lubricants.
Observation of corneal epithelial condition under surgical microscopy
At different time points, topical fluorescein dye was applied on corneal surface, and the severity of any corneal epithelial defect was recorded under surgical microscopy illuminated with cobalt blue light (OPMI Pico I; Carl Zeiss Meditec, Jena, Germany).
In vivo confocal microscopy
In vivo confocal microscopy was performed on the right eye of all subjects at different time points with the Heidelberg Retinal Tomography(HRT-3) equipped with a Rostock Corneal Module (RCM) (Heidelberg Engineering GmbH, Heidelberg, Germany). This instrument uses a 60× water-immersion objective lens (Olympus Europa GmbH, Hamburg, Germany) and a 670 nm diode laser as a light source, resulting in an image dimension of 400 × 400 μm2 and a transverse resolution of 1 μm.
Before examination, one drop of vidisic gel (HanBul Pharm Co., Ltd., Seoul, Korea) was applied to the surface of a sterile disposable plastic cap (Tomo-cap, Heidelberg Engineering GmbH, Heidelberg, Germany) on the front lens of the microscope. The surface of the tomo-cap was positioned on the following 3 locations: (1)central cornea, (2)limbus, and (3)bulbar conjunctiva 2 mm from the limbus.In the limbal images, the corneal part shown as the upper 1/3 of the image (Fig 1A) was termed as juxta-limbal cornea, and the conjunctical part shown as the lower 1/3 of the image (Fig 1A) was termed as juxta-limbal conjunctiva in this study.The images were examined layer by layer. The scanning was performed continuously from the anterior surface of the cornea to the posterior surface. At least 6 examinations per eye were performed.
Fig 1. Impression cytology and in vivo confocal microscopy of the limbal epithelium.
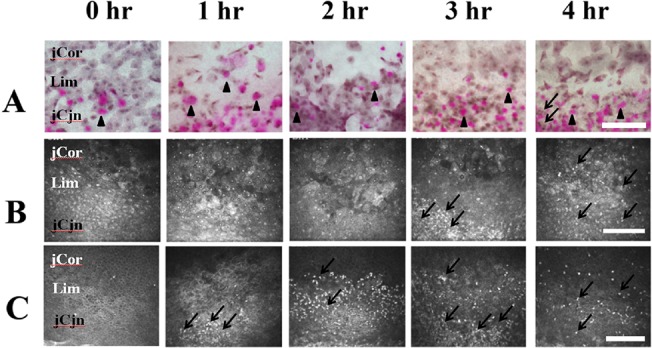
(A) Impression cytological findings. At post exposure 1 to 4 hours, the morphology of the superficial epithelial cells and goblet cells (black arrow heads, the purple dots indicate goblet cells containing mucin which were stained by PAS) attached to the filter papers were similar. At post exposure 4 hours, some inflammatory cells (black arrows) can be found at the juxta-limbal conjunctival site. (B) In vivo confocal microscopic findings of the surface epithelial layer of the limbus. there was no significant change of morphology of superficial cells from post exposure 1 to 4 hours. An aggregation of inflammatory cells (black arrows) can be found atpost exposure 3 hours to 4 hours. (C) In vivo confocal microscopic findings of the basal epithelial layer of the limbus. There was no significant change of basal cell morphology from post exposure 1 to 4 hours. Aggregation of inflammatory cells (black arrows) can be found at post exposure 1 and 2 hours. The inflammatory cells migrated to the juxta-limbal corneal and juxta-limbal conjuncival areas at post-exposure 3 and 4 hours.jCor: juxtal-limbal cornea. Lim: limbus. jCjn: juxta-limbal conjunctiva. hr: Hours after exposure. Bar: 200 um.
Impression cytology
Impression cytology was performed using a mixed cellulose ester membrane filter with a pore size of 0.22 μm (Millipore, MA). The membrane was applied to the following locations of ocular surface, including the central cornea, limbus, and bulbar conjunctiva 2 mm from the limbus. After being removed andfixed with 95% ethanol, all specimens were stained with periodic acid-Schiff (PAS),counterstained with hematoxylin (Sigma Aldrich, St. Louis, MO), and mounted with Permount. Cytology features were observed by Eclipse E800 Nikon Microscope equipped with a Spot Digital Camera and Spot version 1.1 CE software (Diagnostic Instruments, Sterling Heights, MI) at a magnification of ×200.
Immunohistochemistry
Rabbit eyes were cryopreserved, cut into 8-μm sections, air-dried and fixed in 4% paraformaldehyde for 10 min. Sections were then permeabilized with 0.4% Triton X-100, and blocked with bovine serum albumin with bovine serum. The infiltration of macrophageswere evaluate with mouse monoclonal anti-human AM-3K antibody (Cosmo Bio Co. Ltd, Tokyo, Japan).[40–42]The infiltration of neutrophils was demonstrated with mouse anti-rabbit neutrophil antibody (NIMP-R14, ab2557, Abcam,Cambridge, UK). Monocloncal rat anti-mouse neutrophil antibodies (Thermo Fisher Scientific Inc., Waltham, MA, USA) were used to detect the infiltration of CD4- and CD-8 positive T lymphocytesseparately.Terminal deoxynucleotidyl transferase dUTP nick end labeling (TUNEL) staining kit was purchased from Biovision (Milpitas, CA), and thestaining was used to evaluate the apoptosis of ocular surface cells.The staining pattern of the tissue sections was observed by conventional fluorescence microscopy using an Eclipse E800 Nikon Microscope with a VFM Epi-Fluorescence Attachment (Nikon, Melville, NY) equipped with a Spot Digital Camera and Spot version 1.1 CE software (Diagnostic Instruments, Sterling Heights, MI). All experiments were repeated 6 times to ensure consistent results.
Scanning Electron Microscopy (SEM) Observation
At different time points after exposure, the rabbit corneas were obtained after sacrifice. The corneas were carefully excised, fixed in 4% glutaraldehyde in 0.05 M cacodylate buffer for 1 hour, and then postfixed in 1% osmium tetroxide in veronal acetate buffer containing 0.22 M sucrose. The fixed materials were dehydrated through a series of ethanol washes. The corneas were placed in t-butyl alcohol, treated in a freeze-drying apparatus (EIKO ID-2; EIKO, Tokyo, Japan), and then sputter coated with gold using an auto fine coater (JEOL JFC-1600; JEOL, Tokyo, Japan). After processing, the surface of the corneal epithelium was observed by means of a SEM microscope (Hitachi SU8220; Hitachi, Ibaragi, Japan).
Measurement of IL-8, IL-17 and TNF-apha level in tear
To collect tear sample from pre-exposure (exposure 0 hour, control) and post-exposure 4 hours, 30 ul of phosphate-buffered saline was instilled into the inferior fornix. We collected 20 ul of tear fluid and buffered by micropitte at the medical and laterior canthus. To minimize ocular surface irritation, we collected the mixture as soon as possible after phosphate-buffered saline instillation. The fluid was place into a 1.5 mL Eppendorf tube and stored at-70°C until further examination. The concentration of the cytokines was measured using Quantibody Rabbit Cytokine Array (Catalog#: QAL-CYT-1, RayBiotech, Norcross, GA)
Statistical Analysis
For central total corneal thickness and corneal epithelial thickness, experimental data were analyzed using Student’s two tailed t-test. To compare the cytokine level in tears, the data were analyzed using Wilcoxon signed-rank test. All the results were expressed as the mean± standard deviation. The probability of p<0.05 was considered to be statistically significant.
Results
1. Corneal epithelial defects
Corneal epithelial defects (>0.3×0.3 mm2 per defect area) were detected starting 2 hour post-exposure, and time-dependently increased to 4 hours after exposure (Fig 2).
Fig 2. The external eye photography with fluorescein staining revealed grossly detectable epithelial defect(>0.3×0.3 mm2)firstly at 2 hours after exposure.
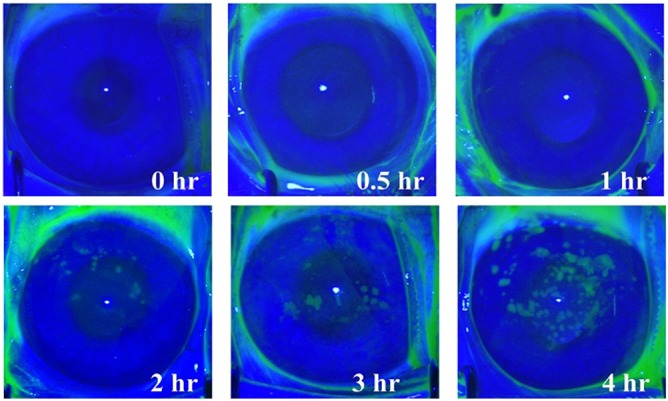
The number of the defect areas increased time-dependently.
2. Change of the central total corneal thickness
The central total corneal thickness, evaluated by ultrasound pachymetry, decreased dramatically from 0 minutes (342.3 ±11.2μm) to 5 minutes (318.7±12.5μm) (P<0.05)post-exposure (Fig 3A). However, the central corneal thickness remained stable from 5 minutes until the end of 4 hours post-exposure (Fig 3A and 3B). Corneal epithelial thickness, evaluated by in vivo confocal microscopy, demonstrated the stable thickness during the entireobservational period (P>0.05) (Fig 3C).
Fig 3. The changes of central total corneal and corneal epithelial thickness after exposure.
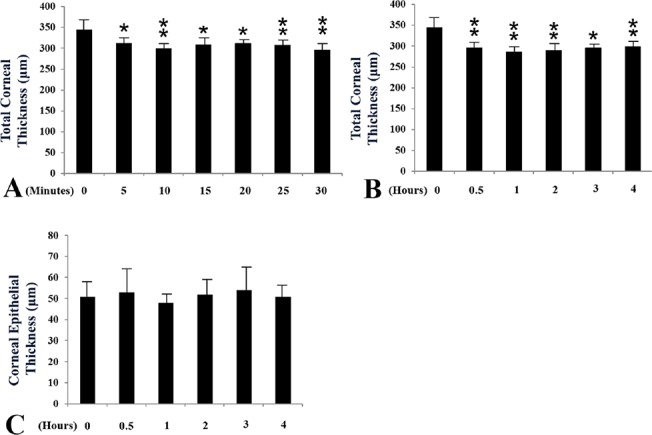
(A,B) The changes of central totalcorneal thickness from post exposure 0 to 30 minutes (A) and 0 to 4 hours (B). (C) The changes of central corneal epithelial thickness from post exposure 0 to 4 hours. * and ** indicated significant differences compared to the pre-exposure group (*: p<0.05; *: p<0.01 by Student’st test).
3.Change of the central cornea
Impression cytology
From 1 hour to the end of 4 hours after exposure to the dessicating stimulus, the number of the superficial corneal epithelial cells attached to the filtering papers progressively increased. However, the cells retained their normal squamous morphology. Furthermore, no inflammatory cells were detected (Fig 4A).
Fig 4. Impression cytology and in vivo confocal microscopy of the central cornea epithelium.
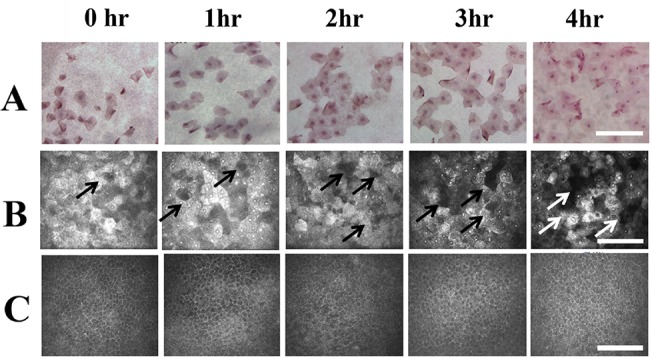
(A) Impression cytological findings. The longer time the cornea exposure, the more surface cells attached to the filtering paper were found. However, the morphology of the detached cells remained similar from post-exposure 1 hour to 4 hours. (B). In vivo confocal microscopic findings of the surface epithelial layer of the central cornea. From 1 hour to 4 hours after exposure, the time dependent increase of cellular disappearance (White and black arrows) was found, which indicated the sloughing of the cell. (C) In vivo confocal microscopic findings of the basal epithelial layer of the central cornea. There was changes of cellular morphology or density from post-exposure 1 to 4 hours. During the observational period, there was no inflammatory cells detected at the superficial or the basal epithelial layers. hr: Hours after exposure. Bar: 200 um.
In vivo confocal microscopy
From 1 hour to 4 hours after exposure, the localized loss of superficial epithelial cells, which indicated the sloughing of the cell, was detected (Fig 4B). However, there was no obvious morphological changes in the superficial epithelial cells during the whole observational period. The morphology and density of corneal basal cells remained stable from the beginning to 4 hours after exposure (Fig 4C). There was no inflammatory cells detected until the end of the experiment.
4. Change of the limbus
Impression cytology
From post-exposure 1 hour to the end of 4 hours, the morphology of the superficial epithelial cells and goblet cells attached to the filter papers remained constant (Fig 1A). Sporadically distributed inflammatory cells were found in the juxta-limbal conjunctiva at 4 hours post exposure (Fig 1A), which is consistent with the finding from in vivo confocal microscopy(Fig 1B).
In vivo confocal microscopy
In the limbus, there was no significant change of morphology of superficial and basal cells from post exposure 1 to 4 hours (Fig 1B and 1C). An aggregation of inflammatory cells was initially detected 1 hour post exposure at the basal limbal epithelial layer, then increased in number and spread to the juxta-limbal basal conjunctiva at post-exposure 2 hours. At post exposure 3 hours, the inflammatory cells at the basal limbal epithelial layer migrated more extensively to the juxta-limbal basal corneal epithelium and juxta-limbal basal conjunctival epithelium, and can be detected at the juxta-limbal conjunctival superficial epitheliums. At post exposure 4 hours, the aggregation phenomenon of the inflammatory cells disappeared, and the cells spread to the junxta-limbal cornea and juxta-limbal conjunctiva in both the superficial and basal epithelial layers (Fig 1B and 1C).
5. Change of the peripheral conjunctiva at 2 mm away from the limbus
Impression cytology
From post-exposure 1 hour to the end of 4 hours, the morphology and density of the conjunctival epithelial cells and goblet cells remained stable. However, numerous inflammatory cells can be detected from at post exposure 3 and 4 hours. (Fig 5A).
Fig 5. Impression cytology and in vivo confocal microscopy of the peripheral conjunctval epithelium at 2 mm from the limbus.
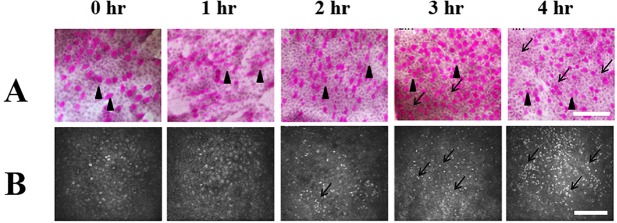
(A) Impression cytological findings. At post exposure 1 to 4 hours, the morphology of the superficial conjunctival epithelial cells and goblet cells (black arrow heads, the purple dots indicate goblet cells containing mucin which were stained by PAS)attached to the filter papers were similar. Abundant inflammatory cells were found from at post exposure 3 and 4 hours ((black arrows). (B) In vivo confocal microscopic findings of the surface epithelial layer of the conjunctiva. There was no significant change of morphology of superficial cells from post exposure 1 to 4 hours. No goblet cells shown by impression cytology was clearly identified. A time dependent increase of inflammatory cells (black arrows) can be found at post exposure 2, 3 and 4 hours. hr: Hours after exposure.Bar: 200 um.
In vivo confocal microscopy
From 1 hour to the end of 4 hours post exposure, the number and morphology of the superficial conjunctival cells remained constant. However, time dependent increase of inflammatory cells were found from post exposure 2 to 4hours (Fig 5B).
6. Change of the inflammatory reaction during the recovery process
In vivo confocal microscopy showed time dependent change of the inflammatory cellular infiltrate at the recovery phase after exposure for 4 hours (Fig 6). The strong infiltration of the inflammatory cells was still detected at 4 hours and 8 hours after termination of the eye exposure (Fig 6A and 6B). At 24 hours after the termination of eye exposure, only minimal infiltration of the inflammatory cells was detected (Fig 6C).
Fig 6. Time dependent change of inflammatory cellular infiltration after termination of eye exposure by in vivo confocal microscopy.
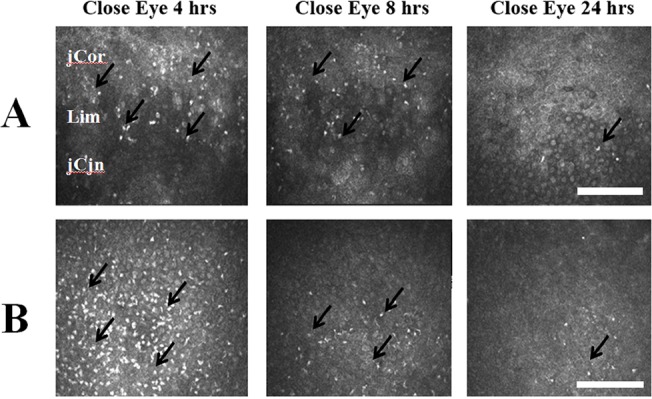
The eye closure (termination of eye exposure) was performed on eyes undergoing exposure for 4 hours.(A) Detecting on the limbus. After termination of eye exposure for 4 hours and 8 hours, still abundant inflammatory cells can be detect. After termination for 24 hours, only minimal inflammatory cells can be traced. (B) Detecting on the peripheral conjunctval epithelium at 2 mm from the limbus. After termination of eye exposure for 4 hours, abundant inflammatory cells can be detect. Time dependent decrease of inflammatory cells was found at 8 hours and 24 hours after termination of the eye exposure.Cor: juxtal-limbal cornea. Lim: limbus. jCjn: juxta-limbal conjunctiva. hr: Hours after exposure. Black arrows: infiltration of inflammatory cells. Bar: 200 um.
7. Immunohistochemistry and TUNEL staining
Macrophages, neutrophils, CD4(+) T-cellsand CD8(+) T-cells were found on the epithelial layers and subepithelial stromal layers of the limbus, peri-limbal cornea, and perilimbal conjunctivaat post-exposure 3 hours (Fig 7). The result is consistent withinflammatory cell infiltration detected by in vivo confocal microscopy.The epithelial layer of central cornea, showed positive TUNEL staining at post exposure 3 hours (Fig 8) and 4 hours (data not shown).
Fig 7. Immunohistochemical staining at the limbus at post-exposure 3 hours.
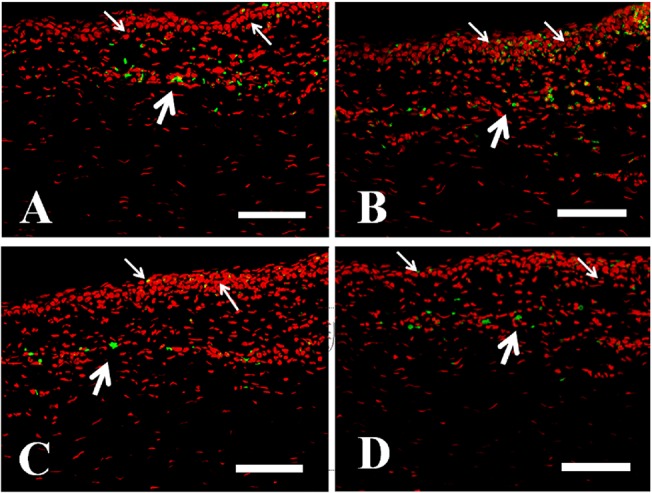
(A) AM3K cells, which indicated macrophages, can be found in the epithelial layer (white thin arrows) and the superficial stroma (white thick stroma). (B) Neutrophils can be found in the epithelial layer (white thin arrows) and the superficial stroma (white thick stroma). (C)CD4 (+) cells, which indicated T helper cells, can be found in the epithelial layer (white thin arrows) and the superficial stroma (white thick stroma). (D) CD 8(+) cells, which indicated T killer cells, can be found in the epithelial layer (white thin arrows) and the superficial stroma (white thick stroma). Green: the staining of macrophages, neutrophils, CD4+ and CD8+ T cells. Red: PI for staining of nucleus. Scale bar: 50 um.
Fig 8. TUNEL staining for the detection of apoptotic cells in the central cornea.
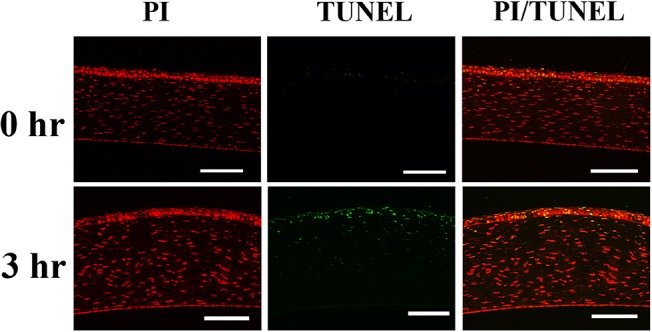
At post exposure 3 hours, the TUNEL stain positive cells can be detected in the corneal epithelium and superficial keratocytes. Green: TUNEL, which stains apoptotic cells. Red: PI, which stains nucleus. Scale bar: 100 um. hr: Hours after exposure.
8. Scanning Electron Microscopy (SEM) observation
At different time points after exposure, the SEM showed time-dependent increase of intercellular gaps in corneal superficial epithelial layer (Fig 9). At post-exposure 1 hour, no obvious superficial epithelial sloughing, or increased intercellular gaps was found (Fig 9A). At post-exposure 2 hours, the increased intercellular gaps can be clearly seen (Fig 9B). At post-exposure 4 hours, sloughing of superficial epithelial cells were observed (Fig 9C)
Fig 9. SEM examination on corneal epithelial cells at different time points after exposure (A) At post-exposure 1 hour, no obvious superficial epithelial sloughing, or increased intercellular gaps was found. (B) At post-exposure 2 hours, the increased intercellular gaps can be clearly seen. However, no obvious epithelial sloughing can be detected. (C) At post-exposure 4 hours, sloughing of superficial epithelial cells were observed.
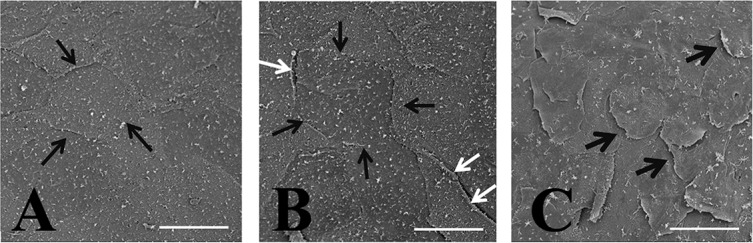
Black thin arrow: intercellular borders. White arrows: the increased intercellular borders with mild detachment of the epithelial cells from underlying layers. Black thick arrows: Sloughing of the superficial epithelial cells from the underlying layers. Bar: 50 um”
9. Expression of IL-8, IL-17 and TNF-αlevel in tear
Table 1 demonstrated the expression level of tear cytokines, including IL-8, IL-17 and TNF-αin different exposure conditions. The tear IL-8, level was 71.3±10.3 pg/ml,381.5±109.1pg/ml and 631.1±147.5 pg/ml in the control (exposure 0 hour), exposure 2 hours and exposure 4 hours groups, respectively. The tear IL-17 level was 356.2±43.4 pg/ml, 342.6±51.6 pg/ml, and 643.5±159.7pg/ml in the control, exposure 2 hours and exposure 4 hours groups, respectively. The tear TNF-αlevel was 901.5±104.5 pg/ml, 1496.2±284.5 pg/ml and 2120.3+718.7 pg/ml in the control, exposure 2 hours and exposure 4 hours groups, respectively. A significant time-dependent change of these three cytokines in tear was found.
Table 1. Tear cytokines in different exposure groups.
IL = interleukin; TNF = tumor necrosis factor. pvalues were Wilcoxon signed-rank test.
| Post-exposure 0 hour | Post-exposure 2 hours | Post-exposure 4 hours | Post-exposure 0 hour vs. 2 hours (p Value) | Post-exposure 0 hourr vs. 4 hours (p Value) | Post-exposure 2 hour vs. 4 hours (p Value) | |
|---|---|---|---|---|---|---|
| IL-8 pg/mL | 71.3±10.3 | 381.5±109.1 | 631.1±147.5 | 0.031* | 0.031* | 0.031* |
| IL-17 pg/mL | 356.2±43.4 | 342.6±51.6 | 643.5±159.7 | 0.22 | 0.031* | 0.031* |
| TNF-α pg/mL | 901.5±104.5 | 1496.2±284.5 | 2120.3+718.7 | 0.063 | 0.031* | 0.063 |
*p <0.05
Discussion
Dry eye disease is an important clinical condition that involveschanges of tear film composition, ocular surface damage,corneal dehydration and inflammation.[43–48] In this animal model of short-term desiccation stress (exposure keratopathy), we found that significant corneal epithelial defects(>0.3×0.3 mm2per defect area) occurred 2 hours afterexposure, but the central totalcorneal thickness decreased significantly as early as 5 minutes after the insult.Normal corneal stromais composed of layers of well-arranged collagen fibers, and needs to be maintained at approximately78% water content. The intact corneal epithelium has cell-cell tight junctions, and provides the main barrier to prevent corneal stromal dehydration or overhydration.[2, 3]Our findings by in vivo confocal microscopic and SEM revealed the time dependent exfoliation of corneal epithelial cells. The corneal dehydration may occurearlier than epithelial exfoliation and grossly-observable epithelial defect can be detected. The TUNEL staining results demonstrated that corneal epithelial cells underwent apoptosis at 3 hours after air exposure. Although further experiments are needed to confirm the observation, the disruption of the cellular pathophysiology after the induction of short term exposure keratoapthy, which lead to a thinning of corneal stroma, seemed to occurred much earlier.The rapid dehydration of corneal stroma under the induction of exposure keratoapthy underscores the necessity of protecting the corneal surface under any circumstance, and is extremely important for patients undergoingrefractive surgery.Inadequateprotection of the corneal surface from desiccation during surgery for only a limited time period may cause changes incorneal stromal thickness, and thus result inovercorrection after the surgery.[24, 25, 49] Interestingly, our in vivo confocal microscopic results revealed no significant changes in corneal epithelial thickness during the observation process, even though the surface cellswere exfoliated. Therefore, the reduction of the central total cornea thickness was predominantlycaused by dehydration of the corneal stroma instead of corneal epithelial layer.
We also observed the limbal and conjunctival surface, and found that the limbal and conjunctival cells seem to be more resistant to desiccation than corneal cells. The density and morphology of limbal epithelial cells, conjunctival epithelial cells and goblet cells appearsto be relativelytable during the observation periods. We previously demonstrated achange inconjunctival epithelial cells for patients with exposure keratopathy due to Graves' ophthamopathy.[50]Patients with Graves' ophthamopathy suffered from more severe bulbar conjunctival damage and inflammation with the superior site than the temporal site. In a mouse model of desiccation stress,Yoon et al.[51, 52] showed that conjunctival goblet density significantly decreased after 5 days without observing the short-term changes.[52]Our findings demonstrate that goblet cells may exhibitresistance to short-term exposure keratoapthy, which has seldom been emphasized before.
In patients with aqueous deficiency dry eye, the role of inflammation has long been recognized. Immunopathologic changes in the conjunctival epithelium of aqueous tear deficiency dry eye patients includes inflammatory cell infiltration, increased expression of immune activation and adhesion molecules, apoptotic markers, matrix metalloproteinases, inflammatory cytokines, T-helper type 1 (Th-1)attracting chemokines and their receptors.[53–58]However, there are limited results showing the role of inflammation in ocular surface after the induction of exposure keratoapthy.We found thatinflammatory cell infiltration, including AM3K, pan-T, CD4 and CD8 positive cells, occurred as early as 1 hours after exposure. The cells appeared first in the limbal basal epithelial layer, and then migrated time and space-sequentially into the superficial layer of limbal epithelium, the juxtal-limbal corneal and juxta-limbal conjunctival epithelial layer, and the peripheral conjunctiva around 2 mm away from the limbus. At the recovery phase after termination of eye exposure for 4 hours, the significant infiltration of the inflammatory cells can still be detected on limbus and juxta-limbal conjunctiva for at least 8 hours. Such finding implied the slow recovery of the inflammatory response after termination of eye exposure. Our in vivo confocal microscopic and SEM findings also demonstrated a time depended recovery of corneal surface epithelial cells after termination of eye exposure (data not shown).In general, the in vivo confocal microscopic findings revealed more information than impression cytologic findings, since the latter only collected the superficial layer of the ocular surfaceafter mechanical peeling. The results from impression cytology may only provide limited information.Our results pointed out the origin of the inflammatory cells is from limbal vessels instead of the tear film, and the infiltration of inflammatory cells reached perilimbal corneal epithelium no less than 4 hours after exposure. Therefore, in the clinical practice, we must be vigilantwith regard to cornealprotection in patients with exposure keratopathy, especially forpotential corneal donors.The infiltration of inflammatory cells in exposure keratopathy is important forclinical differentiation between infectious and non-infectious keratitis. The proper diagnosis willinfluence the treatment strategy, and the decision of qualifying potential donor corneasfor further transplantation. Since the sterile infiltration of the inflammatory cells may mimic infectious keratitis, the decision of initiating antibiotic treatment in patients, and the qualification of donor corneas without microbiological proofwould be difficult.
Our immunohistochemical results demonstrated the inflammatory cells that infiltrated after the induction of exposure keratopathy were AM3K(+), Pan-T(+), CD4(+) and CD8(+) cells. T helper cellshavelong been known to play a key role in aqueous tear deficiency. However, to the best of our knowledge, the role of macrophages/neutrophils and T killer cells in dry eye has not been reported previously. It has been recognized that the inflammatory mediators associated with aqueous tear deficiency dry eye included Th1-related cytokines (e.g INF-alpha), Th17-related cytokines, chemokines and theirreceptors, metalloproteinase, and secretory phospholipases (e.g IL-1β, IL-6, IFN-γ and TNF-α) and MMPs.[57, 59–63]We analyzed the tear cytokines (IL-8, IL-17 and TNF-α), and found a time dependent increase of these three cytokines after exposure, which was correlated to the time dependent change of the inflammatory cellular infiltration revealed by in vivo confocal microscopy. IL-8 is a potential neutrophil chemotactic/activation factor. It is a primer inflammatory cytokine secreted responding to proinflammatory stimuli in many cell types, including macrophages, T-cells, neutrophils, monocytes, fibroblasts, and endothelial cells. [64] IL-8 is also secreted from corneal epithelial cells and retinal cells. [65] IL-17 is produced by T helper type lymphocyte, and has been studied as a possible connection between inflammation and disruption of the corneal barrier after desiccating stress. [58, 66–67] TNF-α is a pleiotropic cytokine that has multiple proinflammatory and costimulatory effects on a broad range of cell types. [68]Ji YW, et al demonstrated that neutralization of ocular surface TNF-α reduces ocular surface and lacrimal gland inflammation induced by in vivo dry eye.[69] Our result demonstrated a mixture of cytokines involved in the exposure keratopathy. It is important to further identify the detailed expression of the inflammatory cytokines, and the difference between aqueous tear deficiency and exposure keratoapthy.
There are some limitations of this study.First of all, initially we attemptedto mimic evaporative dry eye, a chronic pathophysiology process, the study design more closely approximates acute pathophysiology process of exposure keratopathy found in intensive care unit patients, or those under general anaesthesia. In those patients, poor eyelid closure and decreased blink reflex for more than several hours or even several days may cause conjunctival hyperemia, mucopurelent secretion, corneal staining, corneal filaments and infectious keratitis,[16, 17, 70] which are different from the signs and symptoms of other patients with evaporative dry eye experience.[71] Second, in vivoconfocal microscopy only revealed morphological changes in the observed cells and structures. Immunohistochemistry and other methods may be needed for further identification the changes of cellular micro-structure of the ocular surface cells. Third, the rabbit model is different from the human corneas for have no Bowman's layer. The hematologic profile is also not similar between these two species. However, our study is still valuablefrom several aspects. Weperformed a multimodal evaluation of the ocular surface corneal and conjunctival changes in exposure keratopathy, and provide information that has not been previously reported.
In conclusion, this study provides a successful model of exposure keratopathy.We found changesin central totalcorneal thickness, morphological changesto epithelial cells,inflammatory cell infiltration on ocular surface and change of several tear cytokines no less than 4 hours after exposure. These finding pointed out the vulnerability of ocular surface under a short-term exposure keratopathy.
Acknowledgments
We appreciated Yi-Chun Cheng and Chien-Tzu Peng, Ching-Yi Chen for their technologic supports.
Data Availability
All relevant data are within the paper.
Funding Statement
Supported, in part, by the National Taiwan University Hospital Plan Asia One and the Department of Medical Research at the NTUH, the Jointed Research Grant between National Taiwan University Hospital and Min-Sheng General Hospital 100001, and Grant NSC 103-2314-B-002-074-MY3 from the Ministry of Science and Technology, Taiwan.
References
- 1. Shan H, Min D (2010) Prevention of exposure keratopathy in intensive care unit. International journal of ophthalmology 3: 346–348. 10.3980/j.issn.2222-3959.2010.04.16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Freegard TJ (1997) The physical basis of transparency of the normal cornea. Eye (Lond) 11 (Pt 4): 465–471. [DOI] [PubMed] [Google Scholar]
- 3. Maurice D (1984) The cornea and the sclera. The Eye 1: 1–158. [Google Scholar]
- 4. Moss SE, Klein R, Klein BE (2004) Incidence of dry eye in an older population. Arch Ophthalmol 122: 369–373. [DOI] [PubMed] [Google Scholar]
- 5. Ang RT, Dartt DA, Tsubota K (2001) Dry eye after refractive surgery. Curr Opin Ophthalmol 12: 318–322. [DOI] [PubMed] [Google Scholar]
- 6. Danjo Y, Lee M, Horimoto K, Hamano T (1994) Ocular surface damage and tear lactoferrin in dry eye syndrome. Acta Ophthalmol (Copenh) 72: 433–437. [DOI] [PubMed] [Google Scholar]
- 7. Gire A, Kwok A, Marx DP (2013) PROSE treatment for lagophthalmos and exposure keratopathy. Ophthal Plast Reconstr Surg 29: e38–40. 10.1097/IOP.0b013e3182674069 [DOI] [PubMed] [Google Scholar]
- 8. Collins M, Seeto R, Campbell L, Ross M (1989) Blinking and corneal sensitivity. Acta Ophthalmol (Copenh) 67: 525–531. [DOI] [PubMed] [Google Scholar]
- 9. Tsubota K, Nakamori K (1995) Effects of ocular surface area and blink rate on tear dynamics. Arch Ophthalmol 113: 155–158. [DOI] [PubMed] [Google Scholar]
- 10. Jester JV, Rife L, Nii D, Luttrull JK, Wilson L, Smith RE. (1982) In vivo biomicroscopy and photography of meibomian glands in a rabbit model of meibomian gland dysfunction. Invest Ophthalmol Vis Sci 22: 660–667. [PubMed] [Google Scholar]
- 11. Bonini S, Rama P, Olzi D, Lambiase A (2003) Neurotrophic keratitis. Eye (Lond) 17: 989–995. [DOI] [PubMed] [Google Scholar]
- 12. Cobo LM (1988) Corneal complications of herpes zoster ophthalmicus. Prevention and treatment. Cornea 7: 50–56. [PubMed] [Google Scholar]
- 13. Shaikh S, Ta CN (2002) Evaluation and management of herpes zoster ophthalmicus. American family physician 66: 1723–1730. [PubMed] [Google Scholar]
- 14. Elner VM, Mauffray RO, Fante RG, Harris M, Morton AD, Hassan AS. (2003) Comprehensive midfacial elevation for ocular complications of facial nerve palsy. Archives of facial plastic surgery 5: 427–433. [DOI] [PubMed] [Google Scholar]
- 15. Asman P (2003) Ophthalmological evaluation in thyroid-associated ophthalmopathy. Acta Ophthalmol Scand 81: 437–448. [DOI] [PubMed] [Google Scholar]
- 16. Grixti A, Sadri M, Edgar J,Datta AV (2012) Common Ocular Surface Disorders in Patients in Intensive Care Units. The Ocular Surface 10: 26–42. 10.1016/j.jtos.2011.10.001 [DOI] [PubMed] [Google Scholar]
- 17. Jammal H, Khader Y, Shihadeh W, Ababneh L, Aljizawi G, AlQasem A. (2012) Exposure keratopathy in sedated and ventilated patients. J Crit Care 27: 537–541. 10.1016/j.jcrc.2012.02.005 [DOI] [PubMed] [Google Scholar]
- 18. Dinakaran S (2002) Exposure keratopathy following phacoemulsification under local anaesthesia. Eur J Ophthalmol 12: 331–332. [DOI] [PubMed] [Google Scholar]
- 19. Foster KW, Roberts D, Goodwin CR, Huang CC (2005) Surgical pearl: Preventing perioperative exposure keratopathy. J Am Acad Dermatol 53: 707–708. [DOI] [PubMed] [Google Scholar]
- 20. Ezra DG, Lewis G, Healy M,Coombes A (2005) Preventing exposure keratopathy in the critically ill: a prospective study comparing eye care regimes. Br J Ophthalmol 89: 1068–1069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. van der Wekken RJ, Torn E, Ros FE, Haas LE (2013) A red eye on the intensive care unit. Exposure keratopathy with corneal abrasion secondary to lagophthalmos due to chemosis. Neth J Med 71: 204–207. [PubMed] [Google Scholar]
- 22. Lenart SB, Garrity JA (2000) Eye care for patients receiving neuromuscular blocking agents or propofol during mechanical ventilation. Am J Crit Care 9: 188–191. [PubMed] [Google Scholar]
- 23. McHugh J, Alexander P, Kalhoro A, Ionides A (2008) Screening for ocular surface disease in the intensive care unit. Eye (Lond) 22: 1465–1468. [DOI] [PubMed] [Google Scholar]
- 24. Joo CK, Kim TG (1999) Corneal perforation during laser in situ keratomileusis. J Cataract Refract Surg 25: 1165–1167. [DOI] [PubMed] [Google Scholar]
- 25. Buratto L, Ferrari M (1997) Indications, techniques, results, limits, and complications of laser in situ keratomileusis. Curr Opin Ophthalmol 8: 59–66. [DOI] [PubMed] [Google Scholar]
- 26. Pflugfelder SC, Wilhelmus KR, Osato MS, Matoba AY, Font RL (1986) The autoimmune nature of aqueous tear deficiency. Ophthalmology 93: 1513–1517. [DOI] [PubMed] [Google Scholar]
- 27. Pflugfelder SC, Huang AJ, Feuer W, Chuchovski PT, Pereira IC, Tseng SC. (1990) Conjunctival cytologic features of primary Sjogren's syndrome. Ophthalmology 97: 985–991. [DOI] [PubMed] [Google Scholar]
- 28. Jones DT, Monroy D, Ji Z, Pflugfelder SC (1998) Alterations of ocular surface gene expression in Sjogren's syndrome. Adv Exp Med Biol 438: 533–536. [DOI] [PubMed] [Google Scholar]
- 29. Yuan Y, Wang J, Chen Q, Tao A, Shen M, Shousha MA (2010) Reduced tear meniscus dynamics in dry eye patients with aqueous tear deficiency. Am J Ophthalmol 149: 932–938 e931. 10.1016/j.ajo.2010.01.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Shen M, Li J, Wang J, Ma H, Cai C, Tao A, et al. (2009) Upper and lower tear menisci in the diagnosis of dry eye. Invest Ophthalmol Vis Sci 50: 2722–2726. 10.1167/iovs.08-2704 [DOI] [PubMed] [Google Scholar]
- 31. Moore JE, Graham JE, Goodall EA, Dartt DA, Leccisotti A, McGilligan VE, et al. (2009) Concordance between common dry eye diagnostic tests. Br J Ophthalmol 93: 66–72. 10.1136/bjo.2007.131722 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Yeh S, Song XJ, Farley W, Li DQ, Stern ME, Pflugfelder SC. (2003) Apoptosis of ocular surface cells in experimentally induced dry eye. Investigative ophthalmology & visual science 44: 124–129. [DOI] [PubMed] [Google Scholar]
- 33. Dursun D, Wang M, Monroy D, Li DQ, Lokeshwar BL, Stern ME, et al. (2002) A mouse model of keratoconjunctivitis sicca. Investigative ophthalmology & visual science 43: 632–638. [PubMed] [Google Scholar]
- 34. El Annan J, Chauhan SK, Ecoiffier T, Zhang Q, Saban DR, Dana R (2009) Characterization of effector T cells in dry eye disease. Investigative ophthalmology & visual science 50: 3802–3807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Chen Y, Chauhan SK, Lee HS, Stevenson W, Schaumburg CS, Sadrai Z, et al. (2013) Effect of desiccating environmental stress versus systemic muscarinic AChR blockade on dry eye immunopathogenesis. Investigative ophthalmology & visual science 54: 2457–2464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Barabino S, Shen L, Chen L, Rashid S, Rolando M, Dana MR. (2005) The controlled-environment chamber: a new mouse model of dry eye. Investigative ophthalmology & visual science 46: 2766–2771. [DOI] [PubMed] [Google Scholar]
- 37. Bron AJ, Tomlinson A, Foulks GN, Pepose JS, Baudouin C, Geerling G, et al. (2014) Rethinking Dry Eye Disease: A Perspective on Clinical Implications. The Ocular Surface 12: S1–S31. 10.1016/j.jtos.2014.02.002 [DOI] [PubMed] [Google Scholar]
- 38. Johnson ME, Murphy PJ (2004) Changes in the tear film and ocular surface from dry eye syndrome. Prog Retin Eye Res 23: 449–474. [DOI] [PubMed] [Google Scholar]
- 39. Khanal S, Tomlinson A, Diaper CJ (2009) Tear physiology of aqueous deficiency and evaporative dry eye. Optom Vis Sci 86: 1235–1240. 10.1097/OPX.0b013e3181bc63cc [DOI] [PubMed] [Google Scholar]
- 40. Chen WL, Chen YM, Chu HS, Lin CT, Chow LP, Chen CT, et al. (2014) Mechanisms controlling the effects of bevacizumab (avastin) on the inhibition of early but not late formed corneal neovascularization. PLoS One 9: e94205 10.1371/journal.pone.0094205 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Komohara Y, Hirahara J, Horikawa T, Kawamura K, Kiyota E, Sakashita N, et al. (2006) AM-3K, an anti-macrophage antibody, recognizes CD163, a molecule associated with an anti-inflammatory macrophage phenotype. J Histochem Cytochem 54: 763–771. [DOI] [PubMed] [Google Scholar]
- 42. Zeng L, Takeya M, Takahashi K (1996) AM-3K, a novel monoclonal antibody specific for tissue macrophages and its application to pathological investigation. J Pathol 178: 207–214. [DOI] [PubMed] [Google Scholar]
- 43. Pflugfelder SC, Corrales RM, de Paiva CS (2013) T helper cytokines in dry eye disease. Exp Eye Res 117: 118–125. 10.1016/j.exer.2013.08.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Barabino S, Chen Y, Chauhan S, Dana R (2012) Ocular surface immunity: homeostatic mechanisms and their disruption in dry eye disease. Prog Retin Eye Res 31: 271–285. 10.1016/j.preteyeres.2012.02.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Stevenson W, Chauhan SK, Dana R (2012) Dry eye disease: an immune-mediated ocular surface disorder. Arch Ophthalmol 130: 90–100. 10.1001/archophthalmol.2011.364 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Tomlinson A, Hair M, McFadyen A (2013) Statistical approaches to assessing single and multiple outcome measures in dry eye therapy and diagnosis. Ocul Surf 11: 267–284. 10.1016/j.jtos.2013.05.002 [DOI] [PubMed] [Google Scholar]
- 47. Barrera MJ, Bahamondes V, Sepulveda D, Quest AF, Castro I, Cortés J, et al. (2013) Sjogren's syndrome and the epithelial target: a comprehensive review. J Autoimmun 42: 7–18. 10.1016/j.jaut.2013.02.001 [DOI] [PubMed] [Google Scholar]
- 48. McGinnigle S, Naroo SA, Eperjesi F (2012) Evaluation of dry eye. Surv Ophthalmol 57: 293–316. 10.1016/j.survophthal.2011.11.003 [DOI] [PubMed] [Google Scholar]
- 49. Feltham MH, Stapleton F (2002) The effect of water content on the 193 nm excimer laser ablation. Clin Experiment Ophthalmol 30: 99–103. [DOI] [PubMed] [Google Scholar]
- 50. Wei YH, Chen WL, Hu FR, Liao SL (2013) In vivo confocal microscopy of bulbar conjunctiva in patients with Graves' ophthalmopathy. J Formos Med Assoc. 13: 358–366 [DOI] [PubMed] [Google Scholar]
- 51. Leinowitz H, Waring G (1998) Corneal Disorders: Clinical diagnosis and management, 2ed. WB Saunders Company: 2–31. [Google Scholar]
- 52. Yoon KC, Ahn KY, Choi W, Li Z, Choi JS, Lee SH, et al. (2011) Tear production and ocular surface changes in experimental dry eye after elimination of desiccating stress. Invest Ophthalmol Vis Sci 52: 7267–7273. 10.1167/iovs.11-7231 [DOI] [PubMed] [Google Scholar]
- 53. Stern ME, Pflugfelder SC (2004) Inflammation in dry eye. Ocul Surf 2: 124–130. [DOI] [PubMed] [Google Scholar]
- 54. Pflugfelder SC, de Paiva CS, Li DQ, Stern ME (2008) Epithelial-immune cell interaction in dry eye. Cornea 27 Suppl 1: S9–11. 10.1097/ICO.0b013e31817f4075 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Pflugfelder SC, Stern ME, Symposium P (2009) Immunoregulation on the ocular surface: 2nd Cullen Symposium. Ocul Surf 7: 67–77. [DOI] [PubMed] [Google Scholar]
- 56. Yoon KC, De Paiva CS, Qi H, Chen Z, Farley WJ, Li DQ, et al. (2007) Expression of Th-1 chemokines and chemokine receptors on the ocular surface of C57BL/6 mice: effects of desiccating stress. Invest Ophthalmol Vis Sci 48: 2561–2569. [DOI] [PubMed] [Google Scholar]
- 57. Yoon KC, Park CS, You IC, Choi HJ, Lee KH, Im SK, et al. (2010) Expression of CXCL9, -10, -11, and CXCR3 in the tear film and ocular surface of patients with dry eye syndrome. Invest Ophthalmol Vis Sci 51: 643–650. 10.1167/iovs.09-3425 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Lee SY, Han SJ, Nam SM, Yoon SC, Ahn JM, Kim TI, et al. (2013) Analysis of tear cytokines and clinical correlations in Sjogren syndrome dry eye patients and non-Sjogren syndrome dry eye patients. Am J Ophthalmol 156: 247–253 e241. 10.1016/j.ajo.2013.04.003 [DOI] [PubMed] [Google Scholar]
- 59. Lam H, Bleiden L, de Paiva CS, Farley W, Stern ME, Pflugfelder SC. (2009) Tear cytokine profiles in dysfunctional tear syndrome. American journal of ophthalmology 147: 198–205 e191. 10.1016/j.ajo.2008.08.032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Ellenberg D, Azar DT, Hallak JA, Tobaigy F, Han KY, Jain S, et al. (2010) Novel aspects of corneal angiogenic and lymphangiogenic privilege. Progress in retinal and eye research 29: 208–248. 10.1016/j.preteyeres.2010.01.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. De Paiva CS, Chotikavanich S, Pangelinan SB, Pitcher JD 3rd, Fang B, Zheng X, et al. (2009) IL-17 disrupts corneal barrier following desiccating stress. Mucosal immunology 2: 243–253. 10.1038/mi.2009.5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Corrales RM, Villarreal A, Farley W, Stern ME, Li DQ, Pflugfelder SC. (2007) Strain-related cytokine profiles on the murine ocular surface in response to desiccating stress. Cornea 26: 579–584. [DOI] [PubMed] [Google Scholar]
- 63. Boehm N, Riechardt AI, Wiegand M, Pfeiffer N, Grus FH (2011) Proinflammatory cytokine profiling of tears from dry eye patients by means of antibody microarrays. Invest Ophthalmol Vis Sci 52: 7725–7730. 10.1167/iovs.11-7266 [DOI] [PubMed] [Google Scholar]
- 64. Liesegang TJ. (1997) Contact lens-related microbial keratitis: part I: epidemiology. Cornea 16:125–131 [PubMed] [Google Scholar]
- 65. Rao NA. (2004) Uveitis and other intraocular inflammations In: Yanoff M, Duker JS, editors. Ophthalmology. 2nd ed. Philadelphia: Mosby; pp 1105–238. [Google Scholar]
- 66. Chauhan SK, El Annan J, Ecoiffier T, Goyal S, Zhang Q, Saban DR, et al. (2009) Autoimmunity in dry eye is due to resistance of Th17 to Treg suppression. J Immunol 182:1247–1252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Zheng X, Bian F, Ma P, De Paiva CS, Stern M, Pflugfelder SC, et al. (2010) Induction of Th17 differentiation by corneal epithelial-derived cytokines. J Cell Physiol 222:95–102. 10.1002/jcp.21926 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Aggarwal BB. (2003)Signalling pathways of the TNF superfamily: a double-edged sword. Nat Rev Immunol 3: 745–756 [DOI] [PubMed] [Google Scholar]
- 69. Ji YW, Byun YJ, Choi W, Jeong E, Kim JS, Noh H, et al. (2013) Neutralization of ocular surface TNF-α reduces ocular surface and lacrimal gland inflammation induced by in vivo dry eye. Invest Ophthalmol Vis Sci 54:7557–7566. 10.1167/iovs.12-11515 [DOI] [PubMed] [Google Scholar]
- 70. Saritas TB, Bozkurt B, Simsek B, Cakmak Z, Ozdemir M, Yosunkaya A (2013) Ocular surface disorders in intensive care unit patients. TheScientificWorldJournal 2013: 182038 10.1155/2013/182038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Pereira MV, Gloria AL (2010) Lagophthalmos. Seminars in ophthalmology 25: 72–78. 10.3109/08820538.2010.488578 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All relevant data are within the paper.


