Abstract
Neural steroids, and the enzymes that produce these hormones, are important for sexual differentiation of the brain during development. Aromatase converts testosterone into oestradiol. 5α-reductase converts testosterone to 5α-dihydrotestosterone and occurs in two isozymes: type 1 (5αR1) and type 2 (5αR2). Each of these enzymes is present in the developing brain in many species, although no work has been done examining expression of all three enzymes in non-avian reptiles with genetic sex determination. In this study, we evaluated mRNA expression of neural aromatase, 5αR1 and 5αR2 on the day of hatching and at day 50 in one such lizard, the green anole. We describe the distribution of these enzymes throughout the brain and quantification of mRNA expression in three regions that control adult sexual behaviours: the preoptic area (POA) and ventromedial amygdala (AMY), which are involved in male displays, as well as the ventromedial hypothalamus, which regulates female receptivity. Younger animals had a greater number (POA) and density (AMY) of 5αR1 mRNA expressing cells. We detected no effects of sex or age on aromatase or 5αR2. Compared to data from adults, the present results support the idea that the green anole forebrain has not completely differentiated by 50 days after hatching and that 5αR1 may play a role in early development of regions important for masculine function.
Keywords: Androgen metabolism, preoptic area, amygdala, ventromedial hypothalamus, Anolis carolinensis, lizard
Introduction
Steroid hormones are involved in the regulation of adult reproductive behaviours (activation), and play key roles in the developing brain (organisation; 1, 2). Local metabolism of these hormones within the brain is in many cases a necessary step in these processes, particularly the conversion of testosterone (T) into oestradiol (E2) via the aromatase enzyme and to 5α-dihydrotestosterone (DHT) by 5α-reductase (5αR; 3, 4).
Brain aromatase is present during development in many species such as Japanese quail, where it is not sexually dimorphic (5, 6) and mice and rats, where aromatase expression and activity in the hypothalamus are higher in males than females (7-9). Furthermore, T treatment during development can increase both expression and activity of neural aromatase in rodents and quail (8, 10-12). Aromatase and/or oestrogenic metabolites during development are required for organising appropriate adult sexual behavior in rodents and Japanese quail (13-16). In developing non-avian reptiles, the role of aromatase has largely been investigated in gonadal rather than brain differentiation, and the majority of this work has been done in those reptiles with temperature-dependent sex determination (e.g. 17-21, but see 22). Among these animals, aromatase is expressed in the developing brain of alligators, leopard geckos, and red-eared slider turtles, and both whole brain activity and mRNA appear to be sexually monomorphic (23-25).
Much less work has been done on 5αR, with none on developing reptiles. Two isozymes exist, 5αR1 and 5αR2. They are differentially expressed during brain development, such that in rodents 5αR1 mRNA levels are higher and consistent across ages, whereas 5αR2 levels show a peak early in development, and then decrease to relatively low adult levels (26, 27). Interestingly, T during development selectively regulates the two isozymes of 5αR, such that 5αR2 expression is increased by T in males only (26) and 5αR1 is not regulated by T (26, 28). Inhibiting 5αR can reduce masculinisation of the brain and/or behavior in male rats and zebra finches (29, 30), while treatment with DHT in females can cause modest masculinisation of the brain in zebra finches (31). Thus, 5αR in the brain may be involved in normal sexual differentiation.
Green anole lizards (Anolis carolinensis) offer an excellent model to examine the expression of 5αR1, 5αR2 and aromatase during development. These lizards are common in the southeastern United States, and adult 5αR2 and aromatase mRNAs are sexually dimorphic in three brain areas critical for reproduction (32, 33): the preoptic area (POA) and ventromedial amygdala (AMY), which are important in male reproductive behaviours, and the ventromedial hypothalamus (VMH), which is important in female behaviours (34). Unmanipulated adult males have a greater number of cells expressing aromatase mRNA in the POA, while females have a greater density of cells expressing aromatase in the AMY and VMH, as well as 5αR2 cells in the VMH (32, 33). In adults, systemic inhibition of aromatase activity in gonadectomised, T-treated animals revealed that E2 production is important for female receptivity and plays a role in sexual motivation in males (35, 36). A similar study documented that 5αR activity (DHT production) is important for the full expression of male sexual behaviors (37). However, a variety of studies in which adult steroid levels have been manipulated indicate that T, rather than its metabolites E2 or 5α-DHT, seems to be the primary activator of male sexual behaviors (34). Thus, both E2 and 5α-DHT appear to have facilitatory roles in anole reproductive behaviors. Additionally, T appears to regulate anole neural aromatase and 5αR such that systemic T treatment in adulthood increases whole brain activities of aromatase and 5αR in males (38), as well as the density of cells expressing 5αR2 mRNA in the female VMH (39). 5αR1 was not detected in the adult hypothalamus, although it is expressed in the brainstem (33). It is currently unknown when sexually dimorphic expression of aromatase and 5αR2 mRNA begins in this species.
This study examines the patterns of cells expressing 5αR1, 5αR2 and aromatase mRNA in the brain during two periods in development, the day of hatching and on day 50. The two ages include a time prior to (day 0; P0) and after (day 50; P50) males have higher levels of circulating T than females (sex difference occurs by 35 days after hatching; 40). Thus, in addition to our primary goal of determining the general distributions of expression in hatchling and juvenile anoles, these two ages also allow us to begin to assess whether naturally occurring differences T levels between males and females might influence steroid metabolizing enzyme mRNA expression in the developing green anole forebrain.
Methods
Animals
During the breeding season (April), adult males and females were purchased from Charles Sullivan (Nashville, TN). They were group housed with one male and 5 females in each 29-gallon terrarium. These glass tanks contained peat moss as substrate, as well as water dishes, sticks and rocks. To facilitate egg-laying, nest boxes filled with damp peat moss were provided. Ambient temperatures ranged from 28 °C during the day to 19 °C at night (14:10 light/dark cycle). Full spectrum bulbs and heat lamps were provided directly above the cages to allow basking temperatures of 10 °C above ambient. Relative humidity was maintained at about 70%. Animals were fed crickets three times a week and misted daily with water.
Nest boxes were checked daily, and eggs were individually placed in moistened vermiculite (1:1 by mass with dH20) in a cup covered with plastic wrap to maintain moisture. The eggs were incubated at 27 °C until hatching, which took an average of 33 days in this study. Hatchlings were placed in 10-gallon tanks (set up similarly to those used for adults) until experimental use. Up to 10 hatchlings were housed together following unique toe-clipping for identification. They were fed fruit flies and misted with water daily.
All procedures adhered to NIH guidelines and were approved by the Michigan State University Institutional Animal Care and Use Committee.
Tissue Collection
On P0 and P50, lizards were rapidly decapitated and their whole heads collected, similar to (41). The heads were frozen in cold methyl-butane and stored at -80 °C until processing. They were sectioned coronally at 16 μm into four alternate series and thaw mounted onto SuperFrost Plus slides (Fisher Scientific; Hampton, NH). The slides were stored at −80 °C with dessicant until further processing.
Gonadal sex of the hatchlings was determined through visual inspection at the time of euthanasia. In addition, torsos were collected and fixed in Bouin's solution (9% formaldehyde, 0.9% saturated picric acid, 5% glacial acetic acid). They were then dehydrated and embedded in paraffin. The torsos were sectioned at 10 μm, stained with hematoxylin and eosin, and sex was confirmed via microscopic examination of the gonads.
In situ hybridisation
5αR1, 5αR2 and aromatase were cloned and linearized previously (32, 33). Sense (SP6 for 5αR1 and 5αR2; T7 for aromatase) and antisense (T7 for 5αR1 and 5αR2; T3 for aromatase) probes were transcribed using the Digoxigenin RNA Labeling Kit per manufacturer's instructions (Roche Diagnostics; Indianapolis, IN). Probes were cleaned using a G50 Sephadex bead column and stored at −80 °C until use. For each gene, one set of slides from each animal was used for the antisense reaction. As controls, another set of slides from one animal from each group was used for the sense reaction. Slides were thawed and then fixed for 10 min in 4% paraformaldehyde in 0.1M phosphate buffered saline (PBS; pH 7.4). They were treated with 0.25% acetic anhydrase in triethanolamine-HCl with 0.9% NaCl buffer (pH 8.0). Slides then incubated overnight at 55 °C with 200 ng/ml (5αR1and aromatase) or 100 ng/ml (5αR2) probe in hybridisation buffer (50% formamide, 4× 3M NaCl and 0.4M Na Citrate [SSC], 1× Denhardt's solution, 200 μg/ml fish sperm DNA, 10% dextran sulfate, 20 mM dithiothreitol, 250 μg/ml tRNA, 2 mM EDTA, and 0.1% Tween-20). The next day, slides were rinsed in 2× SSC and 0.2× SSC at 60 °C. They were then treated with 0.9% H2O2 in maleic acid buffer (pH 7.5) with 0.1% Tween-20 (MABT) for 30 min. The slides were incubated in a blocking solution of 5% normal sheep serum (Jackson Immuno Research; West Grove, PA) in MABT for 30 min, and were treated with 0.5 μl/ml Anti-Digoxigenin-AP Fab fragments (Roche) in MABT. This antibody produced no labelling in reactions in which the antisense and sense probes were eliminated. After two hours, the color reaction was conducted by incubating the slides with 4.5 μl/ml NBT and 3.5 μl/ml BCIP (Roche) in 0.1M Tris-HCl and 0.1M NaCl (pH 9.5). The reaction was monitored so that the slides incubated with the antisense probe showed distinct reaction product within the cytoplasm of individual cells with an absence of labelling on the sense-treated slides (about 10 minutes for each gene; Fig. 1). The reaction was stopped with 1M Tris and 0.5M EDTA (pH 8.0). Slides were dehydrated with increasing concentrations of ethanol and treated with Citrisolv (Fisher Scientific). They were coverslipped with VectaMount mounting medium (Vector Laboratories; Burlingame, CA) and allowed to dry.
Figure 1.
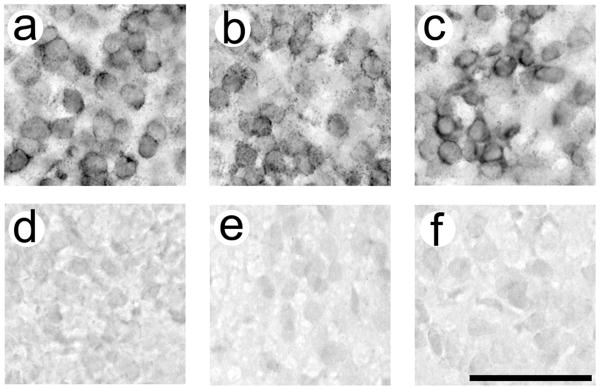
Validation of in situ hybridization procedures. Aromatase is depicted in (a) and (d), 5αR1 in (b) and (e), and 5αR2 in (c) and (f). Tissue treated with antisense probes (a-c) show dark cytoplasmic labelling, whereas tissue treated with sense probes (d-f) show no labeling. All pictures are from the VMH. Antisense and sense labelling for each gene is depicted from the same individual. P50 females are depicted in (a), (c), (d), and (f). A P50 male is depicted in (b) and (e). Scale bar = 50 μm.
Stereological analysis
The slides were examined under brightfield illumination using Stereo Investigator software (MicroBrightfield, Inc.; Williston, VT) following Cohen and Wade (32, 33) by a user blind to experimental group. Estimates of total counts of cells positive for each gene were determined in antisense-labelled tissue using the Optical Fractionator function. After tracing the outline of a brain region (as defined by gene expression) in each tissue section in which it existed, the software placed a grid over each area (POA: 100×100 μm2, AMY: 40×40 μm2, VMH: 80×80 μm2) and sampling sites (30×30 μm2) were placed randomly within the defined region. The software calculated a volume for the brain region, and estimated the total number of positive cell based on the overall size of the region and the samples in which manual counts were taken. A Gundersen coefficient of error at or below 0.1 was confirmed to ensure accurate estimates of cell count. The density of positive cells was determined by dividing the estimated cell count by the calculated volume for the region.
Nissl analysis
Due to sex and age differences in enzyme expression in the POA and AMY (see Results), one series of sections from the animals was stained with thionin. The number of total cells, cell density and volume of the POA and AMY were analyzed using the stereological procedures described above.
Statistical Analysis
Analysis of each gene and brain region was conducted separately using data averaged from the two sides of the brain within each individual. Two-way ANOVAs were performed on the means of individuals to determine the effects of sex and age on the cells expressing mRNA of 5αR1, 5αR2 and aromatase in the POA and VMH. Interactions were broken down with Bonferroni-corrected t-tests as appropriate. Final sample sizes ranged from 4 to 8 animals per group and are included in the figures.
Due to tissue damage from processing, individual sections from AMYs in some P0 animals were incomplete. We therefore could not obtain a total estimate of cells expressing each of the enzymes in the AMY of that age group. Instead, we conducted a stereological analysis on sections from P0 animals as they were available (using identical procedures as above), and the software calculated the number of cells and the volume of the AMY in each section analyzed. Density estimates were obtained for each section from these values and averages for each individual were included in statistical analyses. This procedure was also used for all P50 animals in order to compare densities per section across ages. Two-way ANOVAs were conducted on the density per section to determine the effects of sex and age. Because the AMY was intact in a greater number of the P50 animals, we were able to obtain estimates of the total number of cells expressing each of the three genes, and therefore also used t-tests to compare the effects of sex on the number of cells and density of cells expressing all three enzymes at that age.
Results
Distribution of 5αR1, 5αR2 and aromatase
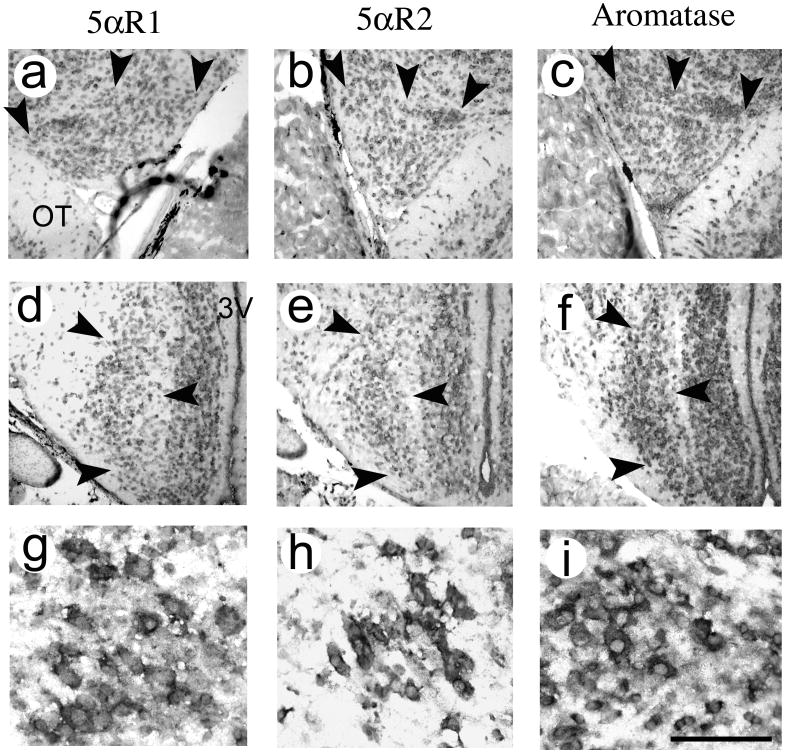
The mRNA of each of the three enzymes, 5αR1, 5αR2 and aromatase, was expressed in discrete regions throughout the brain (Fig. 2). 5αR2 and aromatase were similarly distributed in the developing anole brain as in adults (32, 33). 5αR1, on the other hand, was more widely expressed in the forebrain of developing anoles than in adulthood (32). Its distribution appeared similar to that of 5αR2 in both development and adulthood. Overall, 5αR1 mRNA expression was generally diminished compared to aromatase in the forebrain, with the lowest levels present in the nucleus accumbens and septum, and no expression detected in the bed nucleus of the stria terminalis (Table 2). 5αR2 mRNA expression was greatest in the anterior dorsal ventricular ridge (ADVR) and dorsal cortex, as well as a few brainstem motonuclei. Aromatase mRNA was greatest in the VMH, AMY and the ADVR.
Figure 2.
Distribution of 5αR1 (a, d, g), 5αR2 (b, e, h) and aromatase (c, f, i) mRNA expressing cells among selected brain areas. The AMY is depicted in (a-c; a is a picture from the left, while b and c are both from the right side of the brain) and the VMH is depicted in (d-f). The glossopharyngeal portion of the nucleus ambiguous and the ventral motor nucleus of the facial nerve is depicted in (g-i). Interestingly, the ventricular layer of the 3V shows labelling for all three genes, similar to aromatase expression in teleost fish (e.g. 59). Arrows indicate the boundary of the brain areas. Pictures were taken from P50 males except (f) and (g), which were from P50 females. OT = optic tract, 3V = third ventricle. Scale bar = 100 μm in (a-f), 50 μm in (g-i).
Table 2.
Relative intensities of 5αR1, 5αR2 and aromatase mRNA expression. High intensity of staining, with dark labelling almost filling the cytoplasm is indicated by (+++). Medium intensity of staining (++) was defined as lighter labelling but also filling the cytoplasm. Light staining intensity (+) was defined as labelling that was still less intense and did not fill the entire cytoplasm. No detectable labelling is indicated by (-). Patterns were consistent between ages and sexes. Regions were based on (56-58).
| Brain Area | 5αR1 | 5αR2 | Aromatase |
|---|---|---|---|
| Anterior dorsal ventricular ridge | ++ | +++ | +++ |
| Dorsal cortex | ++ | +++ | ++ |
| Nucleus accumbens | + | ++ | + |
| Bed nucleus of the stria terminalis | - | + | + |
| Preoptic area | ++ | ++ | ++ |
| Ventromedial amygdala | ++ | ++ | +++ |
| Septum | + | + | + |
| Ventromedial hypothalamus | ++ | ++ | +++ |
| Torus semicircularis | ++ | ++ | ++ |
| Oculomotor nucleus (III) | ++ | ++ | ++ |
| Trigeminal motor regions (V) | +++ | +++ | - |
| Trochlear nucleus (IV) | ++ | ++ | ++ |
| Nucleus ambiguus (IX) and ventral motor nucleus of the facial nerve (VIImv) | ++ | ++ | + |
| Nucleus ambiguus X | ++ | +++ | - |
| Spinal accessory and hypoglossal nuclei (XI/XII) | ++ | ++ | - |
5αR1
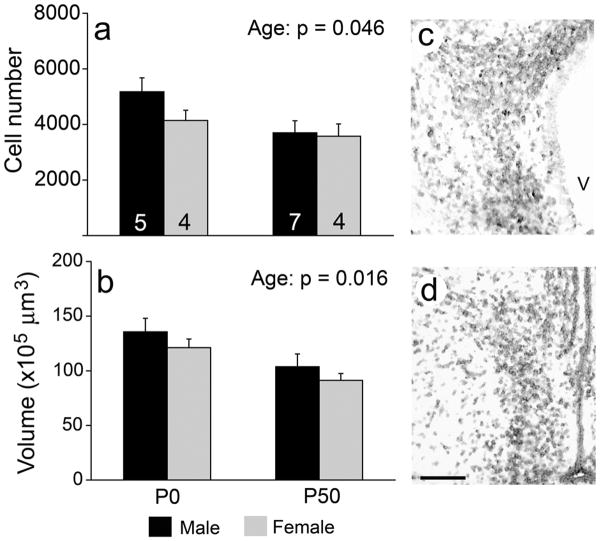
In the POA, a greater number of 5αR1 expressing cells was detected at P0 than P50 (F = 4.69, p = 0.046; Fig. 3). P0 animals also had a larger volume of the region as defined by 5αR1 mRNA expression than P50 animals (F = 7.19, p = 0.016; Fig. 3). No other effects were detected on these variables (all F < 1.53, p > 0.235). We saw no effects of sex, age, or an interaction on the density of 5αR1 expressing cells (all F < 1.34, p > 0.263; Table 3). In Nissl-stained tissue, no significant effects were detected on the number and density of cells overall, or on the volume of the region (all F < 3.31, p > 0.089; Table 4), suggesting that the differences in cell number and volume of this brain region are based on changes in 5αR1 expression within existing cells.
Figure 3.
5αR1 mRNA expression in the POA. Animals at P0 had a greater total number of cells (a) and volume of the region (b) than at P50. A P0 male is shown in (c) and a P50 male in (d). Scale bar = 100 μm. V = 3rd ventricle. Sample sizes are indicated in (a).
Table 3.
Average total number and density (×102 cells/mm3) of cells expressing 5αR1 (A), 5αR2 (B), and aromatase (C) mRNA in the three forebrain regions analyzed. Standard errors are in parentheses. Values that were not statistically significant are listed here and those associated with significant effects are depicted in the figures.
| POA | AMY | VMH | ||
|---|---|---|---|---|
| A. 5αR1 | Number | Fig. 3 | Fig. 4 | 3,114 (118) |
| Density | 3,698 (144) | Fig. 4 | 2,748 (119) | |
| B. 5αR2 | Number | 6,098 (307) | P50 only: 2,185 (157) | 3,460 (205) |
| Density | 5,271 (216) | Both ages*: 3,388 (115) | 3,174 (140) | |
| C. Aromatase | Number | 8779 (500) | P50 only: 3,198 (228) | 5,186 (343) |
| Density | 6,371 (190) | Both ages*: 5,535 (277) | 3,949 (219) |
Density per section. The density of cells in the total area is similar (see text).
Table 4.
Mean estimated total number and density (per mm3, × 102) of cells, and brain region volume (μm3, × 102), determined in Nissl-stained tissue for the entire preoptic area (POA; standard error in parentheses). Average density per section (cells per mm3) is indicated for the ventromedial amygdala (AMY; standard error in parentheses). No significant differences were detected; n = 4 – 8.
| P0 | P50 | ||||
|---|---|---|---|---|---|
| Male | Female | Male | Female | ||
| POA | Number | 9,277 (976) | 9,172 (676) | 11,516 (1,492) | 10,129 (569) |
| Density | 8,228 (416) | 7,934 (357) | 9,462 (1,014) | 8,922 (576) | |
| Volume | 111,977 (9,375) | 115,908 (8,294) | 121,569 (7,839) | 113,804 (2,684) | |
|
| |||||
| AMY | Density per Section | 63,683 (7,101) | 58,286 (7,173) | 69,465 (3,222) | 78,656 (8,846) |
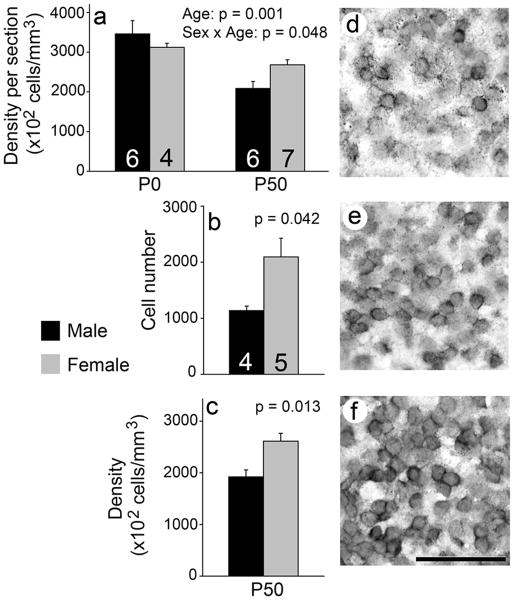
In the AMY, the number and density of 5αR1 expressing cells at P50 was greater in females than males (number: t = 2.48, p = 0.042; density: t = 3.30, p = 0.013; Fig. 4b, c). There was no effect of sex in the volume of P50 animals (t = 1.46, p = 0.188; data not shown). Analysis of thionin-stained tissue revealed no effects of sex on cell number, density or region volume in P50 animals (all t < 0.89, p > 0.396; data not shown). The density per section of 5αR1 expressing cells in the AMY was greater at P0 than P50 (F = 16.94, p = 0.001; Fig. 4a). We saw no main effect of sex (F = 0.33, p = 0.48), but did detect an interaction between sex and age (F = 4.49, p = 0.048) on the density per section. This value was increased in males at P0 compared to P50 (t = 3.64, p = 0.005, α = 0.0125). Further, we detected a trend such that P50 females had a greater density per section than P50 males (t = -2.78, p = 0.018, α = 0.0125). No other effects of age or sex were detected (t < 2.33, p > 0.045, α = 0.0125). We also detected no significant effects on the density per section in Nissl stained tissue (all F < 3.83, p > 0.068; Table 4).
Figure 4.
5αR1 mRNA expression in the AMY. A main effect of age was detected on the density of cells – labelling was increased at P0 compared to P50; a sex × age interaction also existed (a). Among P50 animals, females had a greater number (b) and overall density (c) of 5αR1 positive cells than males. A P50 male is shown in (d), a P50 female in (e), and a P0 male in (f). Scale bar = 50 μm. Sample sizes are indicated in (a) and (b). (c) has the same sample sizes as (b).
In the VMH, there were no effects of sex, age, or interactions in the number and density of 5αR1 expressing cells, and the volume of the region as defined by 5αR1 expression was equivalent across groups (all F < 3.62, p > 0.07; Table 3).
5αR2
In the POA, we found no effects of sex, age, or interactions in the number or density of 5αR2 expressing cells (all F < 2.61, p > 0.122; Table 3). However, the volume of the region, as defined by 5αR2 mRNA, was larger in P0 than P50 animals (data not shown; F = 9.52, p = 0.006). No other effects were detected on the volume of the region, as defined by 5αR2 expression (all F < 1.41, p > 0.250).
At P50, no effects of sex were detected on the number or density of cells, or the volume of the AMY (t < 0.68, p = 0.508; Table 3). Parallel results were detected on the average density per section of the cells for all groups; there were no effects of sex, age, or interactions on the density of 5αR2 expressing cells (all F < 2.88, p > 0.106).
No significant effects were detected in the VMH (all F < 1.21, p > 0.285; Table 3).
Aromatase
In the POA, the number and density of aromatase positive cells, as well as the volume of the region defined by aromatase mRNA labelling, were statistically equivalent between the sexes and ages (all F < 3.42, p > 0.08; Table 3).
In the AMY, no effects of sex among P50 animals were detected on the number or density of cells expressing aromatase mRNA, or the volume of the region (all t < 1.25, p > 0.240; Table 3). In the analysis that included both ages, no effects of sex or age, or interaction between the variables were detected on the average density per section (all F < 0.25, p > 0.626).
We also found no significant main effects of sex or age, or any interactions on the number or density of cells expressing aromatase, or on volume of the VMH as determined with labelling of aromatase mRNA expressing cells (all F < 2.84, p > 0.109; Table 3).
Discussion
The present experiment demonstrates that 5αR1, 5αR2 and aromatase mRNAs are expressed in the developing anole lizard forebrain and that expression of these enzymes changes between the day of and 50 days after hatching. The three genes are discussed individually below with context from other species.
5α-reductase
Much less information is available from the literature on the expression of the two forms of 5αR than on aromatase. However, it appears that 5αR2 has a higher affinity for substrate in general than does 5αR1 (reviewed in 4). No sex differences in the developing brain have been detected in the expression of the two isozymes or overall 5αR activity in various species, including South African clawed frogs, Japanese quail, mice, and rats (27, 28, 42, 43). In the present experiment, both 5αR1 and 5αR2 are expressed in specific regions throughout the developing anole brain, including forebrain limbic areas that regulate the display of sexual behaviours in adulthood. Previous work in adult animals has shown that 5αR2 mRNA is expressed across the brain while 5αR1 mRNA is only detected in the brainstem (32). These results suggest that forebrain expression of 5αR1 is decreased in adulthood and therefore could play a specific role in the development of these regions in anoles and perhaps reptiles more broadly. This pattern differs from rodents in which 5αR1 is widely expressed in the brain in adults and 5αR2, with higher expression in females than males, is expressed in the forebrain during development - opposite of what we have found in lizards (44, 45). Based on patterns of expression, it has been hypothesized that, in rodents 5αR2 is important for masculinisation and defeminisation of the brain and 5αR1 is important for breaking down excess T from the brain (26, 46). It is likely that these enzymes also play similar roles in anoles, although we suggest that, based on expression patterns in anoles, 5αR1 plays a role in development whereas 5αR2 may be more important for modulating T levels in the brain. Additional work is needed to determine the role of 5αR1 during development, but based on the present information it appears that 5αR1 and 2 may have been co-opted for opposite functions in lizards and rodents.
A number of sex and age differences in the green anole POA and AMY were detected in 5αR1 mRNA expression. For example, more cells expressed 5αR1 in the POA of P0 than in P50 animals. Because there is no difference in the total number of cells in the POA at these two ages (based on Nissl analysis), it appears that expression of 5αR1 decreases within existing cells as the animals get older. This decrease in 5αR1 with age is consistent with the lack of expression of 5αR1 in the adult POA (32). The pattern suggests that 5αR1 could play a role in the organisation of the structure and/or function of the POA. In the AMY, the density of labelled cells per section also decreases between P0 and P50. Similar to the POA, the total number of cells in the AMY is consistent across this period, suggesting that expression within cells decreases. Additionally, we detected sex differences such that P50 females had more cells expressing 5αR1, as well as a greater density of those cells than males, supporting the idea that 5αR1 might play a role in female development in this area. DHT production in the AMY may be important for females, although it is difficult to speculate on a specific function. Perhaps more relevant is that 5αR also reduces progesterone (to 5α-dihydroprogesterone) with a higher affinity than the enzyme has for T (4). In adult anoles, progesterone synergizes with E2 in females to increase receptivity, and works with T to increase male reproductive behaviours (47, 48). By reducing progesterone, 5αR may act to regulate the action of progesterone, as well as the actions of androgens. Although it is currently unknown whether progesterone plays a role in anole development, the present data suggest that more work needs to be done to examine this idea. Another possibility is that 5αR indirectly affects E2 signalling. DHT, through the action of 3-β-hydroxysteroid dehydrogenase, is metabolised into 3-β-androstanediol (3β-diol). 3β-diol then can activate oestrogen receptor β (ERβ; reviewed in 49). All three of the regions we examined in this study do express ERβ in adulthood (50). Thus, it is possible that 5αR1 is metabolising both T and progesterone, and metabolites of DHT may be acting on ERs to influence anole neural development.
Unlike 5αR1, 5αR2 expression in developing anoles was equivalent across the two ages and between the sexes. This is consistent with work from many other species (see above) and in adult anoles. For example, no sex differences in expression of 5αR2 exist in either the POA or VMH, although there is a female-biased difference in the adult AMY (32). Thus, it is likely that anoles have not completely differentiated by 50 days after hatching and 5αR2 has not begun to differentiate in the AMY.
Aromatase
Aromatase mRNA in developing green anoles was not different between the sexes in the POA, AMY, or VMH. This contrasts with adults of this species in which males have a greater number of aromatase expressing cells in the POA than females, and females have an increased density of aromatase positive cells in the AMY and VMH than males (33). The fact that these sex differences were not detected in the present study suggests that the juvenile anoles at the ages that we sampled had not yet differentiated, at least in terms of aromatase expression. Future work examining later ages (with a greater sex difference in T) might indicate the predicted sex difference. Another possible explanation of these results is that T does not regulate aromatase expression in the POA, AMY and VMH. In adults, whole brain aromatase activity is increased by T treatment in BS males, but the same manipulation does not affect the number or density of aromatase mRNA expressing cells in these regions (38, 39). Regardless, the pattern we observed in the current experiment was largely consistent with other species. Although work from some vertebrates has demonstrated sex differences in brain aromatase activity during development (e.g. mice; 51), studies in rats, frogs, and fish have indicated no changes in aromatase activity between developing males and females (27, 52, 53). In both rodents and birds (Japanese quail), neural aromatization of T during development is important for the organisation of both adult male and female reproductive behaviors (15, 54, 55). Similarly, neural aromatase may also be important for anole development, although more work is necessary to determine the extent of the role of aromatase in reptilian brain maturation.
Conclusion
The present data indicate that 5αR1 mRNA expression decreases as the animals get older, and suggests that this isozyme may be more important in the forebrain during development than adulthood because it appears to be absent in mature animals. In addition, the greater number and density of 5αR1 expressing cells in 50-day-old females compared to males supports the possibility that increased T in juvenile males results in deceased expression of this enzyme compared to females. This idea warrants further investigation. In contrast to data from 5αR1, aromatase and 5αR2 are distributed similarly across two ages during development in the green anole brain and do not differ between the sexes at those ages. Thus, 5αR2 and aromatase are probably not significantly affected by naturally occurring sex differences in circulating T of 50 day-old hatchlings. Currently, not much is known about the distribution of the individual isozymes of 5αR across species, and our work raises some important questions. For example, although the timing of 5αR1 and 2 expression in lizards is opposite that of rodents, these enzymes likely perform similar functions in both groups. More work needs to be done to determine the role of these enzymes in anoles, and the current work highlights the importance of comparative studies in understanding the role of T-metabolising enzymes in development.
Table 1.
Primers used to clone anole-specific 5αR1, 5αR2 and aromatase.
| Primers (5′ to 3′) | Probe length (bp) | NCBI Accession number | |
|---|---|---|---|
|
| |||
| 5αR1 | Forward: TGATGCTGCCGCTGAGCAA | 658 | XM_003220033.1 |
| Reverse: TTCCTGTTGCGTGGATAGT | |||
|
| |||
| 5αR2 | Forward: CTTGGTTCCTGCAGGAGTT | 576 | XM_003215965.1 |
| Reverse: GGTAGCTGCTGAATGTCCT | |||
|
| |||
| Aromatase | Forward: GACATGCCGAAGCTGAA | 181 | XM_003225883 |
| Reverse: TTGGGAAGAACTCAAGCCGA | |||
Acknowledgments
We thank Camilla Peabody and Yu-Ping Tang for technical assistance, and Halie Kerver for help with validating our procedures. We also would like to thank Jennifer Yee and Kaitlyn Wilson for help with tissue processing, and members of the Wade lab for help with animal care. This work was supported by NSF grant IOS-0742833 and NIH grant T32 MH070343.
References
- 1.Phoenix CH, Goy RW, Gerall AA, Young WC. Organizing action of prenatally administered testosterone propionate on the tissues mediating mating behavior in the female guinea pig. Endocrinology. 1959;65:369–82. doi: 10.1210/endo-65-3-369. [DOI] [PubMed] [Google Scholar]
- 2.Arnold AP, Breedlove SM. Organizational and activational effects of sex steroids on brain and behavior: a reanalysis. Horm Behav. 1985;19(4):469–98. doi: 10.1016/0018-506x(85)90042-x. [DOI] [PubMed] [Google Scholar]
- 3.Lephart ED. A review of brain aromatase cytochrome P450. Brain Res Brain Res Rev. 1996;22(1):1–26. [PubMed] [Google Scholar]
- 4.Lephart ED, Lund TD, Horvath TL. Brain androgen and progesterone metabolizing enzymes: biosynthesis, distribution and function. Brain Res Brain Res Rev. 2001;37(1-3):25–37. doi: 10.1016/s0165-0173(01)00111-4. [DOI] [PubMed] [Google Scholar]
- 5.Aste N, Watanabe Y, Shimada K, Saito N. Sex- and age-related variation in neurosteroidogenic enzyme mRNA levels during quail embryonic development. Brain Res. 2008;1201:15–22. doi: 10.1016/j.brainres.2008.01.075. [DOI] [PubMed] [Google Scholar]
- 6.Schumacher M, Hutchison JB. Testosterone induces hypothalamic aromatase during early development in quail. Brain Res. 1986;377(1):63–72. doi: 10.1016/0006-8993(86)91191-1. [DOI] [PubMed] [Google Scholar]
- 7.Hutchison JB, Wozniak A, Beyer C, Karolczak M, Hutchison RE. Steroid metabolising enzymes in the determination of brain gender. J Steroid Biochem Mol Biol. 1999;69(1-6):85–96. doi: 10.1016/s0960-0760(99)00057-6. [DOI] [PubMed] [Google Scholar]
- 8.Hutchison JB, Beyer C, Hutchison RE, Wozniak A. Sex differences in the regulation of embryonic brain aromatase. J Steroid Biochem Mol Biol. 1997;61(3-6):315–22. doi: 10.1016/s0960-0760(97)80029-5. [DOI] [PubMed] [Google Scholar]
- 9.Hutchison JB, Beyer C, Green S, Wozniak A. Brain formation of oestrogen in the mouse: sex dimorphism in aromatase development. J Steroid Biochem Mol Biol. 1994;49(4-6):407–15. doi: 10.1016/0960-0760(94)90287-9. [DOI] [PubMed] [Google Scholar]
- 10.Romeo RD, Wade J, Venier JE, Sisk CL. Androgenic regulation of hypothalamic aromatase activity in prepubertal and postpubertal male golden hamsters. Endocrinology. 1999;140(1):112–7. doi: 10.1210/endo.140.1.6420. [DOI] [PubMed] [Google Scholar]
- 11.Hutchison JB, Wozniak A, Beyer C, Hutchison RE. Regulation of sex-specific formation of oestrogen in brain development: Endogenous inhibitors of aromatase. J Steroid Biochem Mol Biol. 1996;56(1-6):201–7. doi: 10.1016/0960-0760(95)00237-5. [DOI] [PubMed] [Google Scholar]
- 12.Bardet SM, Cornil CA, Balthazart J. Testosterone recruits new aromatase-imunoreactive cells in neonatal quail brain. Neuroreport. 2010;21(5):376–80. doi: 10.1097/WNR.0b013e3283378edf. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Houtsmuller EJ, Brand T, Dejonge FH, Joosten RNJMA, Vandepoll NE, Slob AK. Sdn-Poa Volume, Sexual-Behavior, and Partner Preference of Male-Rats Affected by Perinatal Treatment with Atd. Physiol Behav. 1994;56(3):535–41. doi: 10.1016/0031-9384(94)90298-4. [DOI] [PubMed] [Google Scholar]
- 14.Bakker J, Honda S, Harada N, Balthazart J. The aromatase knockout (ArKO) mouse provides new evidence that estrogens are required for the development of the female brain. Ann N Y Acad Sci. 2003;1007:251–62. doi: 10.1196/annals.1286.024. [DOI] [PubMed] [Google Scholar]
- 15.Brock O, Baum MJ, Bakker J. The Development of Female Sexual Behavior Requires Prepubertal Estradiol. J Neuro. 2011;31(15):5574–8. doi: 10.1523/JNEUROSCI.0209-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hosokawa N, Chiba A. Embryonic and posthatching treatments with sex steroids demasculinize the motivational aspects of crowing behavior in male Japanese quail. Horm Behav. 2009;55(1):139–48. doi: 10.1016/j.yhbeh.2008.09.005. [DOI] [PubMed] [Google Scholar]
- 17.Wibbels T, Crews D. Putative aromatase inhibitor induces male sex determination in a female unisexual lizard and in a turtle with temperature-dependent sex determination. J Endocrinol. 1994;141(2):295–9. doi: 10.1677/joe.0.1410295. [DOI] [PubMed] [Google Scholar]
- 18.Pieau C, Dorizzi M, Richard-Mercier N. Temperature-dependent sex determination and gonadal differentiation in reptiles. Cell Mol Life Sci. 1999;55(6-7):887–900. doi: 10.1007/s000180050342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lance VA. Is regulation of aromatase expression in reptiles the key to understanding temperature-dependent sex determination? J Exp Zool Part A Ecol Genet Physiol. 2009;311(5):314–22. doi: 10.1002/jez.465. [DOI] [PubMed] [Google Scholar]
- 20.Belaid B, Richard-Mercier N, Pieau C, Dorizzi M. Sex reversal and aromatase in the European pond turtle: Treatment with letrozole after the thermosensitive period for sex determination. J Exp Zool. 2001;290(5):490–7. doi: 10.1002/jez.1092. [DOI] [PubMed] [Google Scholar]
- 21.Wennstrom KL, Crews D. Making males from females: the effects of aromatase inhibitors on a parthenogenetic species of whiptail lizard. Gen Comp Endocrinol. 1995;99(3):316–22. doi: 10.1006/gcen.1995.1115. [DOI] [PubMed] [Google Scholar]
- 22.Ganesh S, Choudhary B, Raman R. Temporal difference between testis and ovary determinations with possible involvement of testosterone and aromatase in gonadal differentiation in TSD lacking lizard, Calotes versicolor. J Exp Zool. 1999;283(6):600–7. doi: 10.1002/(sici)1097-010x(19990501)283:6<600::aid-jez12>3.0.co;2-7. [DOI] [PubMed] [Google Scholar]
- 23.Endo D, Kanaho YI, Park MK. Expression of sex steroid hormone-related genes in the embryo of the leopard gecko. Gen Comp Endocrinol. 2008;155(1):70–8. doi: 10.1016/j.ygcen.2007.04.010. [DOI] [PubMed] [Google Scholar]
- 24.Milnes MR, Roberts RN, Guillette LJ. Effects of incubation temperature and estrogen exposure on aromatase activity in the brain and gonads of embryonic alligators. Environ Health Persp. 2002;110:393–6. doi: 10.1289/ehp.02110s3393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Crews D, Fleming A, Willingham E, Baldwin R, Skipper JK. Role of steroidogenic factor I and aromatase in temperature-dependent sex determination in the red-eared slider turtle. J Exp Zool. 2001;290(6):597–606. doi: 10.1002/jez.1110. [DOI] [PubMed] [Google Scholar]
- 26.Poletti A, Negri-Cesi P, Rabuffetti M, Colciago A, Celotti F, Martini L. Transient expression of the 5 alpha-reductase type 2 isozyme in the rat brain in late fetal and early postnatal life. Endocrinology. 1998;139(4):2171–8. doi: 10.1210/endo.139.4.5866. [DOI] [PubMed] [Google Scholar]
- 27.Urbatzka R, Lutz I, Kloas W. Aromatase, steroid-5-alpha-reductase type 1 and type 2 mRNA expression in gonads and in brain of Xenopus laevis during ontogeny. Gen Comp Endocrinol. 2007;153(1-3):280–8. doi: 10.1016/j.ygcen.2007.01.041. [DOI] [PubMed] [Google Scholar]
- 28.Karolczak M, Kuppers E, Beyer C. Developmental expression and regulation of aromatase- and 5 alpha-reductase type I mRNA in the male and female mouse hypothalamus. J Neuroendocrinol. 1998;10(4):267–74. doi: 10.1046/j.1365-2826.1998.00200.x. [DOI] [PubMed] [Google Scholar]
- 29.Grisham W, Tam A, Greco CM, Schlinger BA, Arnold AP. A putative 5 alpha-reductase inhibitor demasculinizes portions of the zebra finch song system. Brain Res. 1997;750(1-2):122–8. doi: 10.1016/s0006-8993(96)01336-4. [DOI] [PubMed] [Google Scholar]
- 30.Ribeiro CM, Pereira OCM. 5alpha-reductase 2 inhibition impairs brain defeminization of male rats: Reproductive aspects. Pharm Biochem Behav. 2005;82(1):228–35. doi: 10.1016/j.pbb.2005.08.015. [DOI] [PubMed] [Google Scholar]
- 31.Schlinger BA, Arnold AP. Androgen effects on the development of the zebra finch song system. Brain Res. 1991;561(1):99–105. doi: 10.1016/0006-8993(91)90754-j. [DOI] [PubMed] [Google Scholar]
- 32.Cohen RE, Wade J. Distribution of two isozymes of 5alpha-reductase in the brains of adult male and female green anole lizards. Brain Behav Evol. 2010;76(3-4):279–88. doi: 10.1159/000322096. [DOI] [PubMed] [Google Scholar]
- 33.Cohen RE, Wade J. Aromatase mRNA in the brain of adult green anole lizards: effects of sex and season. J Neuroendocrinol. 2011;23(3):254–60. doi: 10.1111/j.1365-2826.2010.02102.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wade J. Relationships among hormones, brain and motivated behaviors in lizards. Horm Behav. 2011;59(5):637–44. doi: 10.1016/j.yhbeh.2010.08.014. [DOI] [PubMed] [Google Scholar]
- 35.Latham S, Wade J. Estradiol facilitates mounting behavior in male green anole lizards. Physiol Behav. 2010;99(1):78–81. doi: 10.1016/j.physbeh.2009.10.013. [DOI] [PubMed] [Google Scholar]
- 36.Winkler SM, Wade J. Aromatase activity and regulation of sexual behaviors in the green anole lizard. Physiol Behav. 1998;64(5):723–31. doi: 10.1016/s0031-9384(98)00138-3. [DOI] [PubMed] [Google Scholar]
- 37.Rosen GJ, Wade J. The role of 5alpha-reductase activity in sexual behaviors of the green anole lizard. Physiol Behav. 2000;69(4-5):487–98. doi: 10.1016/s0031-9384(00)00207-9. [DOI] [PubMed] [Google Scholar]
- 38.Cohen RE, Wade J. Testosterone selectively affects aromatase and 5alpha-reductase activities in the green anole lizard brain. Gen Comp Endocrinol. 2010;166(1):128–33. doi: 10.1016/j.ygcen.2009.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Cohen RE, Wade J. Aromatase and 5α-reductase type 2 mRNA in the green anole forebrain: An investigation of the effects of sex, season and testosterone manipulation. Gen Comp Endocrinol. doi: 10.1016/j.ygcen.2012.01.018. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lovern MB, McNabb FM, Jenssen TA. Developmental effects of testosterone on behavior in male and female green anoles (Anolis carolinensis) Horm Behav. 2001;39(2):131–43. doi: 10.1006/hbeh.2000.1637. [DOI] [PubMed] [Google Scholar]
- 41.Beck LA, Wade J. Morphology and estrogen receptor alpha mRNA expression in the developing green anole forebrain. J Exp Zool Part A Ecol Genet Physiol. 2009;311(3):162–71. doi: 10.1002/jez.514. [DOI] [PubMed] [Google Scholar]
- 42.Jacobson NA, Ladle DR, Lephart ED. Aromatase cytochrome P450 and 5 alpha-reductase in the amygdala and cortex of perinatal rats. Neuroreport. 1997;8(11):2529–33. doi: 10.1097/00001756-199707280-00022. [DOI] [PubMed] [Google Scholar]
- 43.Hutchison JB, Schumacher M. Development of testosterone-metabolizing pathways in the avian brain: enzyme localization and characteristics. Brain Res. 1986;390(1):33–42. doi: 10.1016/0165-3806(86)90149-5. [DOI] [PubMed] [Google Scholar]
- 44.Melcangi RC, Poletti A, Cavarretta I, Celotti F, Colciago A, Magnaghi V, Motta M, Negri-Cesi P, Martini L. The 5alpha-reductase in the central nervous system: expression and modes of control. J Steroid Biochem Mol Biol. 1998;65(1-6):295–9. doi: 10.1016/s0960-0760(98)00030-2. [DOI] [PubMed] [Google Scholar]
- 45.Sanchez P, Torres JM, Del Moral RG, Ortega E. Effects of testosterone on brain mRNA levels of steroid 5alpha-reductase isozymes in early postnatal life of rat. Neurochem Int. 2006;49(6):626–30. doi: 10.1016/j.neuint.2006.05.003. [DOI] [PubMed] [Google Scholar]
- 46.Torres JM, Ortega E. Steroid 5alpha-reductase isozymes in the adult female rat brain: central role of dihydrotestosterone. J Mol Endocrinol. 2006;36(2):239–45. doi: 10.1677/jme.1.01907. [DOI] [PubMed] [Google Scholar]
- 47.Young LJ, Greenberg N, Crews D. The Effects of Progesterone on Sexual-Behavior in Male Green Anole Lizards (Anolis-Carolinensis) Horm Behav. 1991;25(4):477–88. doi: 10.1016/0018-506x(91)90015-a. [DOI] [PubMed] [Google Scholar]
- 48.Wu J, Whittier JM, Crews D. Role of Progesterone in the Control of Female Sexual Receptivity in Anolis-Carolinensis. Gen Comp Endocrinol. 1985;58(3):402–6. doi: 10.1016/0016-6480(85)90112-1. [DOI] [PubMed] [Google Scholar]
- 49.Rissman EF. Roles of oestrogen receptors alpha and beta in behavioural neuroendocrinology: beyond Yin/Yang. J Neuroendocrinol. 2008;20(6):873–9. doi: 10.1111/j.1365-2826.2008.01738.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Cohen RE, Roach J, Wade J. The distribution of estrogen receptor beta mRNA in male and female green anole lizards. Brain Res. 2012;1430:43–51. doi: 10.1016/j.brainres.2011.10.047. [DOI] [PubMed] [Google Scholar]
- 51.Hutchison JB, Beyer C, Hutchison RE, Wozniak A. Sexual dimorphism in the developmental regulation of brain aromatase. J Steroid Biochem Mol Biol. 1995;53(1-6):307–13. doi: 10.1016/0960-0760(95)00068-b. [DOI] [PubMed] [Google Scholar]
- 52.Konkle ATM, McCarthy MM. Developmental Time Course of Estradiol, Testosterone, and Dihydrotestosterone Levels in Discrete Regions of Male and Female Rat Brain. Endocrinology. 2011;152(1):223–35. doi: 10.1210/en.2010-0607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Blaquez M, Gonzalez A, Papadaki M, Mylonas C, Piferrer F. Sex-related changes in estrogen receptors and aromatase gene expression and enzymatic activity during early development and sex differentiation in the European sea bass (Dicentrarchus labrax) Gen Comp Endocrinol. 2008;158(1):95–101. doi: 10.1016/j.ygcen.2008.06.001. [DOI] [PubMed] [Google Scholar]
- 54.Bakker J, De Mees C, Douhard Q, Balthazart J, Gabant P, Szpirer J, Szpirer C. Alpha-fetoprotein protects the developing female mouse brain from masculinization and defeminization by estrogens. Nat Neurosci. 2006;9(2):220–6. doi: 10.1038/nn1624. [DOI] [PubMed] [Google Scholar]
- 55.Bakker J, Honda S, Harada N, Balthazart J. Restoration of male sexual behavior by adult exogenous estrogens in male aromatase knockout mice. Horm Behav. 2004;46(1):1–10. doi: 10.1016/j.yhbeh.2004.02.003. [DOI] [PubMed] [Google Scholar]
- 56.ten Donkelaar HJNR. The brainstem. In: Gans CNR, Ulinski PS, editors. Biology of the Reptilia. San Francisco: Academic Press; 1979. pp. 133–200. [Google Scholar]
- 57.Barbas-Henry HA, Lohman AH. The motor nuclei and primary projections of the IXth, Xth, XIth and XIIth cranial nerves in the monitor lizard, Varanus exanthematicus. J Comp Neurol. 1984;226(4):565–79. doi: 10.1002/cne.902260409. [DOI] [PubMed] [Google Scholar]
- 58.Greenberg N. A forebrain atlas and stereotaxic technique for the lizard, Anolis carolinensis. J Morphol. 1982;174:217–36. doi: 10.1002/jmor.1051740210. [DOI] [PubMed] [Google Scholar]
- 59.Marsh KE, Creutz LM, Hawkins MB, Godwin J. Aromatase immunoreactivity in the bluehead wrasse brain, Thalassoma bifasciatum: immunolocalization and co-regionalization with arginine vasotocin and tyrosine hydroxylase. Brain Res. 2006;1126(1):91–101. doi: 10.1016/j.brainres.2006.09.017. [DOI] [PMC free article] [PubMed] [Google Scholar]