Abstract
Mindfulness-based stress reduction (MBSR) reduces symptoms of depression, anxiety, and fear of recurrence among breast cancer (BC) survivors. However, the effects of MBSR (BC) on telomere length (TL) and telomerase activity (TA), known markers of cellular aging, psychological stress, and disease risk, are not known. This randomized, wait-listed, controlled study, nested within a larger trial, investigated the effects of MBSR (BC) on TL and TA. BC patients (142) with Stages 0–III cancer who had completed adjuvant treatment with radiation and/or chemotherapy at least 2 weeks prior to enrollment and within 2 years of completion of treatment with lumpectomy and/or mastectomy were randomly assigned to either a 6-week MBSR for BC program or a usual care. Assessments of TA and TL were obtained along with psychological measurements at baseline, 6 weeks, and 12 weeks after completing the MBSR(BC) program. The mean age of 142 participants was 55.3 years; 72% were non-Hispanic White; 78% had Stage I or II cancer; and 36% received both chemotherapy and radiation. In analyses adjusted for baseline TA and psychological status, TA increased steadily over 12 weeks in the MBSR(BC) group (approximately 17%) compared to essentially no increase in the control group (approximately 3%, p < .01). In contrast, no between-group difference was observed for TL (p = .92). These results provide preliminary evidence that MBSR(BC) increases TA in peripheral blood mononuclear cells from BC patients and have implications for understanding how MBSR(BC) may extend cell longevity at the cellular level.
Keywords: telomere length, telomerase activity, stress, breast cancer, mindfulness based stress reduction, MBSR(BC)
A diagnosis of cancer can initiate a trajectory of distress starting with the diagnosis and continuing throughout treatment and survivorship when patients face fears of recurrence and death (French-Rosas, Moye, & Naik, 2011). Previous researchers have documented a relationship between physical health and psychological functioning in cancer patients (Miller, Chen, & Cole, 2009), yet the biological mechanism of action connecting the two is not clearly understood.
Recently, authors have proposed telomere length (TL) as an important “psychobiomarker” linking stress and disease (Epel, 2009) and have suggested that TL is regulated, in part, by psychological stress. Telomeres are short DNA–protein complexes capping the end of chromosomes. Their role is to preserve the integrity of the genome during cellular replication (Peres, 2011). With repetitive cellular replication, TL is gradually shortened due to the inability of deoxyribonucleic acid (DNA) polymerase to fully copy the entire length of the full DNA chain. Telomere shortening leads to chromosomal instability, mutagenesis (Desmaze, Soria, Freulet-Marriere, Mathieu, & Sabatier, 2003), and tumorigenesis (Fordyce et al., 2005; Gertler et al., 2004; Londono-Vallejo, 2008). Researchers consider TL to be a biological regulator and a predictive indicator of risk of disease, disease progression, and premature mortality (Ornish et al., 2008). The activity level of telomerase, a ribonucleoprotein DNA polymerase enzyme responsible for telomere maintenance through adding telomeric DNA (Epel, Daubenmier, Moskowitz, Folkman, & Blackburn, 2009), also serves as an indicator of disease risk and progression and is associated with psychological stress.
Current research has focused on more clearly defining the relationship between TL/telomerase activity (TA) and psychological symptoms as mediated by stress, depression, anxiety, and disease. Decreased TL and reduced TA are both related to disease and health risks (Blackburn, 2000; Lin et al., 2010; Serrano & Andres, 2004). Chronic psychological stress has been linked to shortened telomeres (Epel et al., 2009), reduced T-cell function, and increased proinflammatory cytokine responses, but the mechanisms for these associations remain unclear (Damjanovic et al., 2007; Goronzy, Fujii, & Weyand, 2006). Similarly, oxidative stress is associated with shorter telomeres and reduced TA, with greater levels and longer duration of perceived stress being associated with lower levels of TA. Research has also revealed associations between shortened telomeres and depression (Wikgren et al., 2011), lifetime depression exposure (Wolkowitz et al., 2011), and anxiety disorders in adults (Kananen et al., 2010).
Evidence is beginning to emerge that stress-reducing interventions may improve TL and TA. In a study among healthy volunteers randomly assigned to a 3-month meditation retreat or a control group, the 30 participants in the meditation group had higher TA relative to controls (Jacobs et al., 2011). In a nonrandomized study among prostate cancer patients, TA increased and psychological stress decreased following a stress-reducing, lifestyle-modification program (Ornish et al., 2008). Results of an intervention study among overweight women showed improvement in distress, eating behavior, and metabolic health in women participating in a mindfulness-based stress reduction (MBSR) program, all of which correlated with increases in TA (Daubenmier et al., 2011). Most recently, researchers explored the effects of a Kirtan Kriya yogic meditation intervention compared to exposure to relaxing music on telomerase activity (TA) among 39 dementia family caregivers. The yogic-meditation intervention group had a 43% increase in TA after the 8-week intervention period compared to the music group (p < .05; Lavretsky et al., 2013). Finally, among 22 patients with cervical cancer who were randomized to a psychosocial telephone counseling intervention (Biegler, Anderson, Wenzel, Osann, & Nelson, 2012), investigators found a significant association between increased TL and changes in psychological distress.
Although previous research has provided evidence both (1) that telomeres are dynamic structures that can be shortened or lengthened (Blackburn et al., 1989) and (2) that stress-reducing interventions may lead to improvements in TL and TA, the mechanisms of action have not yet been clearly identified. Epel, Daubenmier, Moskowitz, Folkman, and Blackburn (2009) proposed that certain types of stress cognitions lead to increased rumination and stress arousal, thus decreasing cell longevity. Authors have posited that the psychological process of mindfulness, a component of a number of stress-reduction interventions, disrupts the stress pathway and the physiological arousal system, thus changing the psychological context of the experience (Bishop et al., 2004). This change of context results in improved self-regulation of emotional bias and decreased rumination (Chambers, Lo, & Allen, 2008), thus decreasing the physiological stress response and promoting telomere maintenance (Epel et al., 2009).
MBSR is a standardized intervention that reduces psychological and physical stress through the use of four meditative practices (Lengacher et al., 2009). Investigators have demonstrated MBSR’s efficacy in reducing anxiety among patients with anxiety disorders (Kabat-Zinn et al., 1992; Miller, Fletcher, & Kabat-Zinn, 1995), avoiding recurrence of depression (Teasdale et al., 2000), and reducing stress among patients with chronic pain (Kabat-Zinn, Lipworth, & Burney, 1985). Our team has also empirically established the benefits of MBSR(BC), a form of MBSR specifically adapted for breast cancer (BC) survivors, in reducing psychological distress, depression, anxiety, and fear of recurrence, and improving quality of life and physical symptoms of fatigue and sleep (Lengacher, Johnson-Mallard, et al., 2011; Lengacher et al., 2009; Lengacher, Reich, et al., 2011).
Yet despite these promising findings, no previous study has explored the effects of MBSR on TL and TA among BC survivors. Thus, in the current study, we investigated whether enrollment in a 6-week MBSR(BC) program would be associated with favorable changes in TA and TL and whether these positive effects would be independent of psychological status. We also examined whether the amount of practice would be associated with increases in TL and TA. Consistent with Epel et al. (2009), we developed the following study hypotheses: (a) compared to usual care (UC), the MBSR(BC) program would be associated with more favorable changes in TL and TA over time; (b) changes in TL and TA would be related to changes in mindfulness and other psychological variables; and (c) among MBSR(BC) participants, the amount of practice (e.g., sitting meditation, yoga, etc.) would be positively associated with the magnitude of change in TL and TA (Epel et al., 2009).
Materials and Methods
Participants
We recruited 142 participants from the Moffitt Cancer Center and the Carol and Frank Morsani Center for Advanced Healthcare, both located at the University of South Florida in Tampa, Florida. Eligibility criteria for women to participate in this study included (a) age of 21 years or older; (b) a diagnosis of Stage 0, I, II, or III BC; (c) treatment with a lumpectomy and/or mastectomy; (d) completion of adjuvant radiation and/or chemotherapy at least 2 weeks prior to enrollment and within 2 years from end of primary treatment; and (e) the ability to read and speak English at an eighth-grade level. Patients were excluded if they had Stage IV cancer, a severe current psychiatric diagnosis, or a cancer recurrence. Clinical nurses assisted in identifying eligible patients. Those who met inclusion criteria were invited to an orientation session where we provided an overview of the MBSR(BC) program.
We postulated that the MBSR (BC) intervention would most likely yield a “small” effect size of approximately 0.25 in relation to TA and TL. With this in mind, the sample size of 142 participants would provide 90% power (i.e., desired level) to detect an effect size of 0.25 assuming a two-sided Type I error rate of 0.05 and inclusion of the baseline value of TA or TL (in an analysis of covariance model) with a modest R2 value of .15.
Procedures
Study design and randomization
We used a two-armed randomized controlled design among 142 BC survivors from a larger clinical trial. Participants were randomly assigned in a 1:1 ratio to one of two conditions: (a) the formal (in-class) 6-week MBSR(BC) program to commence within 1 week of the orientation session or (b) UC. Participant randomization was stratified by type of surgery (lumpectomy vs. mastectomy), adjuvant BC treatment (chemotherapy with or without radiation vs. radiation alone), and stage of BC (Stage 0/I vs. II/III). We obtained written informed consent prior to randomization. The Institutional Review Boards at the University of South Florida and the Moffitt Cancer Center approved the study protocol.
Data collection
At baseline, 6 weeks, and 12 weeks, we collected blood samples and clinical history and administered measures of psychological symptom status in both groups. We collected demographic data at baseline. MBSR(BC) participants were also instructed to record their formal and informal practice time in a daily diary during the intervention period as well as during the 6-week period following the intervention.
MBSR(BC) intervention
The MBSR(BC) program was modeled on the MBSR program developed by Jon Kabat-Zinn and colleagues at the Stress Reduction and Relaxation Clinic, Massachusetts Medical Center (Kabat-Zinn et al., 1985; Kabat-Zinn et al., 1992). MBSR(BC) was developed to assist BC patients in taking an active role in stress reduction and symptom management through the self-regulatory process of meditation. The MBSR(BC) intervention consists of three components: (a) educational material related to relaxation, meditation, the mind–body connection, and a healthy lifestyle for survivors; (b) practice of meditation in group meetings and homework assignments; and (c) group processes related to barriers to the practice of meditation, application of mindfulness in daily situations, and supportive group interaction (Speca, Carlson, Goodey, & Angen, 2000). Participants attended 6 weekly 2-hr sessions in which a trained psychologist led them in (a) sitting meditation (an awareness of bodily sensations, thoughts, and emotions while focusing on attention to breathing); (b) body scan (observing any sensations in the body from the head to the toes while focusing on attention to breathing); (c) gentle Hatha yoga (various postures and stretching thought to increase awareness and balance); and (d) walking meditation (increase awareness during walking). Participants were requested to formally meditate (mindfulness practice including sitting or walking meditation, yoga, or body scan exercises) for a minimum of 15–45 min per day and allocate 15–45 min per day for informal practice (deliberate awareness of and attention to being mindful of routine activities and events such as eating, weather, driving, walking, and interpersonal communications). We provided participants with CDs containing the guided mindfulness exercises they performed in the group as well as a daily diary in which we asked them to record the amount and type of meditative practice they performed.
UC
UC consisted of standard post-treatment clinic visits. We asked UC participants to refrain from practicing meditation or yoga techniques or participating in MBSR during the study. The UC group was wait-listed to receive the MBSR(BC) intervention within 6 months after study enrollment.
Measures
Demographic data and clinical history
We collected standard socioeconomic demographic data at baseline including age, ethnicity, highest level of education completed and marital, income, and employment status. At that time, we also collected data related to cancer diagnosis and treatment, including number of weeks on radiation and/or chemotherapy and date of treatment completion. We collected clinical medical history data at baseline, 6 weeks, and 12 weeks to determine whether there were any new problems of clinical significance.
TA analysis
We collected 5 ml of blood from each participant at baseline, 6 weeks, and 12 weeks. Peripheral blood mononuclear cells (PBMCs) were isolated by Histopaque-1077 (Sigma-Aldrich, St. Louis, MO) gradient centrifugation and counted using a hemocytometer. Then, a solution of 1 × 106 cells per ml was made with RPMI 1640 medium, washed with phosphate buffered saline, and pelleted by centrifugation at 15,000 rotations per min (rpm) at 4°C for 5 min in an Eppendorf-refrigerated microcentrifuge (Eppendorf, Westbury, NY). The cell pellets were stored at −80°C until samples were ready for TA assay.
TA was quantified from PBMC pellets using the Telomeric Repeat Amplification Protocol (TRAP)eze®RT: Telomerase Detection Kit (Chemicon International, Temecula, CA). First, cells were lysed with 1× CHAPS, 3-[(3-cholamidopropyl)-dimethyl-ammonio]-1-propanesulfonate buffer (10 mM tris-hydrochloride, pH 7.5, 1 mM MgCl2, 1 mM ethylene-glycotetraacetic acid [EGTA], 0.1 mM benzamidine, 5 mM β-mercaptoethanol, 0.5% CHAPS, 3-[(3-cholamidopro-pyl)dimethyl-ammonio]-1-propanesulfonate, and 10% glycerol). We used 10,000 PBMCs per the manufacturer’s suggested TRAPeze®RT protocol. Relative TA was determined by normalization with the TSK (internal positive control) value after extrapolation based upon TSR8 (reference template) standard curves with two controls on each samples: heat-treated telomerase negative control and polymerase chain reaction (PCR) positive control. Relative TA was defined as follows: 1 unit equals the ratio between the amount of PCR product from 10,000 PBMC to TSR8 after PCR inhibitor normalization. Measurement of five repeated samples on different assays provided an interassay coefficient of variation (CV) of 10.7%.
TL analysis
TL was measured with a quantitative real-time PCR assay that determines the relative ratio of telomere repeat copy number to single-copy gene (human β globin) number (T/S ratio) in experimental samples as compared to a reference DNA sample (Cawthon, 2002; Liu et al., 2007). Genomic DNA was freshly extracted from the PBMC pellet on the day the sample arrived in the lab using the FlexiGene DNA kit (Qiagen Inc., Valencia, CA) with modifications then quantified using a nanospectrophotometer and stored at −80° C until TL assay.
Amplification of DNA from experimental samples and serial dilutions of reference DNA were conducted in identical 386-well plates using mastermixes of PCR reagents prepared at 2.4 nmol/L: one with the T primer pair (tel1,5′-GGTTTTTGAGGGTG-AGGGTGAGGGTGAGGGTGAGGGT-3′; tel2, 5′-TCCCGACTA-TCCCTATCCCTATCCCTATCCCTATCCCTA-3′) and one with the S primer pair (36B4u, 5′-CAGCAAGTGG-GAAGGTGTAATCC-3′; 36B 4d, 5′-CCCATTCTATCATCAACGGGTACAA-3′). Each reaction contained 25 ng template DNA, 12.5 μl SYBR Green PCR Mastermix, and 7.5 μl primers mixture. Genomic DNA derived from the T47D cell line was serially diluted to produce a standard curve with five different final concentrations (0.1, 0.5, 1.0, 2.0 and 5.0 ng). The standard curve and negative control (water) were included in each run. Real-time PCR was performed using the following cycling profiles: for telomere amplification, 95°C for 10 min followed by 30 cycles of 95°C for 15 s and 54°C for 2 min; for 36B4, 95°C for 10 min followed by 40 cycles of 95°C for 15 s and 60°C for 1 min.
The ratio of telomere (T) repeat copy number to a single-copy gene (S) copy number (T/S) for each sample was determined by subtracting the average threshold cycle value (Ct) for 36B4 from the average telomere Ct. The single-copy gene used as a reference was 36B4, which encodes acidic ribosomal phosphoprotein. The relative T/S ratio was determined by subtracting the T/S ratio of the standard curve point from the T/S ratio of each unknown sample. Laboratory personnel were masked to the case–control status of all samples. Both reactions for TL and 36B4 were performed in triplicate. To control for interassay variability, DNA samples from the same individual were included in each run. Measurement of nine same-DNA samples on different TL assays produced an interassay CV of 8.7%.
Psychological measures
We measured fear of recurrence (cancer) with the Concerns About Recurrence Scale, a 30-item Likert-type instrument that measures the extent and nature of women’s fears about the possibility of BC recurrence (Vickberg, 2003). The scale is composed of two sections. The first has 4 items that assess the extent of worry related to recurrence, scored on a scale of 1 (I don’t think about it at all) to 6 (I think about it all the time). The second includes 26 items that assess the nature of the concerns related to recurrence, and this section is scored on a 5-point scale ranging from 0 (Not at all) to 4 (Extremely). Overall, internal consistency reliability was .87 for BC subjects.
We measured depression using the Center for Epidemiological Studies Depression Scale, a 20-item measure of depressive symptomatology (Radloff, 1977). Respondents rate how frequently they have experienced each depressive symptom during the previous week on a 4-point scale from 0 (Rarely or none of the time) to 3 (Most or all of the time). A total depression score was computed, with higher scores indicative of more depressive symptoms. This instrument has been used in several cancer studies and, despite having a mix of psychological- and physical-symptom items, it principally measures emotional/psychological status. The reported coefficient α reliability was .92 for BC subjects.
We measured anxiety with the State-Trait Anxiety Inventory (Spielberger, Gorsuch, & Luschene, 1983). The instrument contains two subscales: the one that we used in this study, which measures state anxiety, evaluating how the respondent feels “right now, at this moment,” and another that measures trait anxiety, or how the respondent feels “generally.” The state anxiety subscale contains 20 items that measure the present experience of anxiety on a Likert-type scale ranging from 1 (Not at all) to 4 (Very much so). Higher scores indicate greater anxiety. Internal consistency reliability is reported to be .95.
We measured perceived stress using the Perceived Stress Scale (Cohen, Kamarck, & Mermelstein, 1983), a 14-item, Likert-type instrument that assesses how often in the past month one appraised life situations as “stressful.” Items are scored from 0 (Never) to 4 (Very often). Higher scores indicate a greater level of perceived stress. Internal consistency reliability has been reported ranging from .84 to .86.
We measured mindfulness using the Cognitive and Affective Mindfulness Scale–Revised, a self-report, 12-item, Likert-type scale (Feldman, Hayes, Kumar, Greeson, & Laurenceau, 2007). Items query mindfulness approaches to thoughts and feelings and respondents rate how each often item applies to them, from 1 (Rarely/not at all) to 4 (Almost always). The subscales measure the four domains of mindfulness (attention, present focus, awareness, and acceptance/nonjudgment). The 12-item total mindfulness score had an acceptable level of internal consistency (α = .74–.77).
Statistical Methods
Statistical package for the Social Sciences (SPSS) v. 19.0 was used for all data analyses. Baseline characteristics, including TL and TA, were compared by random assignment using Wilcoxon rank-sum tests for continuous variables and χ2 tests for categorical variables. To examine whether TL and TA increased based on study condition, linear mixed models were used. The models included main effects terms for condition (MBSR[BC] vs. UC), time, and the interaction between condition and time to assess for differential slope. The intention-to-treat principle was used for the mixed model, meaning participants randomly assigned to MBSR(BC) were included in the MBSR(BC) group whether they attended all classes or not. However, missing follow-up measurements among eight participants were not imputed (i.e., no change ssumed), as they would be in a pure intention-to-treat analysis. Spearman correlations were used to determine whether change in TL or TA was related to change in mindfulness or other psychological variables. Finally, partial correlations (rp) were used to estimate the effect of individual types of MBSR(BC) practice on TL and TA, while controlling for baseline TL and TA. This analysis was restricted to MBSR(BC) participants because UC participants did not have practice scores. When required for parametric analyses, skewed distributions, including TA, were modeled using an appropriate transformation (e.g., natural logarithm or square root). A nominal α level of .05 was used to define statistical significance for the analysis of Hypothesis a (MBSR’s effect on TL and TA). Due to multiple tests, a nominal α level of .01 was used for Hypotheses b and c.
Results
Participant Characteristics
Table 1 provides demographic and medical history data for the participants. The mean age of the 142 study participants was 55.3 years. Most were White non-Hispanic. The majority had either Stage I or II cancer, and more than one-third had received both chemotherapy and radiation therapy. Slightly more women had undergone a mastectomy versus a lumpectomy. Importantly, the two groups were well balanced on baseline characteristics, with no significant differences between them. Of the 142 participants enrolled and randomized into groups, 74 were assigned to the intervention group and 68 were assigned to the control group (Figure 1). Although all 142 were assessed at baseline, 4 in each group failed to return for follow-up, for a total of 134 participants included in post-treatment analyses (Figure 1).
Table 1.
Demographic and Medical History Characteristics of Participants by Group.
| Characteristic | Total (N = 142) | Usual Care (N = 68) | MBSR(BC) (N = 74) | p value |
|---|---|---|---|---|
| Mean age (SD), years | 55.31 (9.84) | 55.19 (9.15) | 55.42 (10.49) | .89 |
| Mean days since the end of adjuvant treatment (SD) | 238 (224) | 212 (258) | 261 (187) | .20 |
| Race/ethnicity, n (%) | .71 | |||
| White, non-Hispanic | 101 (71.1) | 49 (72.1) | 52 (70.3) | .55 |
| White, Hispanic | 15 (10.6) | 7 (10.3) | 8 (10.8) | .47 |
| Black, non-Hispanic | 17 (12.0) | 10 (14.7) | 7 (9.5) | .24 |
| Other | 9 (5.6) | 2 (3.0) | 7 (9.5) | .13 |
| Educational status, n (%)a | .87 | |||
| High school or less | 24 (17.0) | 12 (17.9) | 12 (16.2) | |
| Some college | 57 (40.4) | 28 (41.8) | 29 (39.2) | |
| College or professional degrees | 60 (42.6) | 27 (40.3) | 33 (44.6) | |
| Employment status, n (%)a | .07 | |||
| Employed | 60 (42.6) | 34 (50.7) | 26 (35.1) | |
| Retired | 31 (22.0) | 11 (16.4) | 20 (27.0) | |
| Disabled/medical leave | 13 (9.2) | 4 (6.0) | 9 (12.2) | |
| Unemployed | 17 (12.1) | 11 (16.4) | 6 (8.1) | |
| Other | 20 (14.2) | 7 (10.4) | 13 (17.6) | |
| Breast cancer stage, n (%) | .41 | |||
| Stage 0 | 14 (9.9) | 8 (11.8) | 6 (8.1) | |
| Stage I | 57 (40.1) | 31 (45.6) | 26 (35.1) | |
| Stage II | 54 (38.0) | 22 (32.4) | 32 (43.2) | |
| Stage III | 17 (12.0) | 7 (10.3) | 10 (13.5) | |
| Adjuvant treatment type, n (%) | .57 | |||
| Chemotherapy | 24 (16.9) | 14 (20.6) | 10 (13.5) | |
| Radiation | 35 (24.6) | 17 (25.0) | 18 (24.3) | |
| Chemotherapy and radiation | 51 (35.9) | 21 (30.9) | 30 (40.5) | |
| None | 32 (22.5) | 16 (23.5) | 16 (21.6) | |
| Surgery type, n (%) | .87 | |||
| Lumpectomy | 60 (42.3) | 28 (41.2) | 32 (43.2) | |
| Mastectomy | 82 (57.7) | 40 (58.8) | 42 (56.8) | |
| Comorbidities, n (%) | .37 | |||
| Diabetes | 15 (14.4) | 8 (15.7) | 7 (13.2) | |
| Cardiovascular problems | 9 (6.3) | 3 (4.4) | 6 (8.1) |
Note. MBSR(BC) = mindfulness-based stress reduction intervention for breast cancer survivors.
One usual care participant did not respond to the questions on education or employment.
Figure 1.
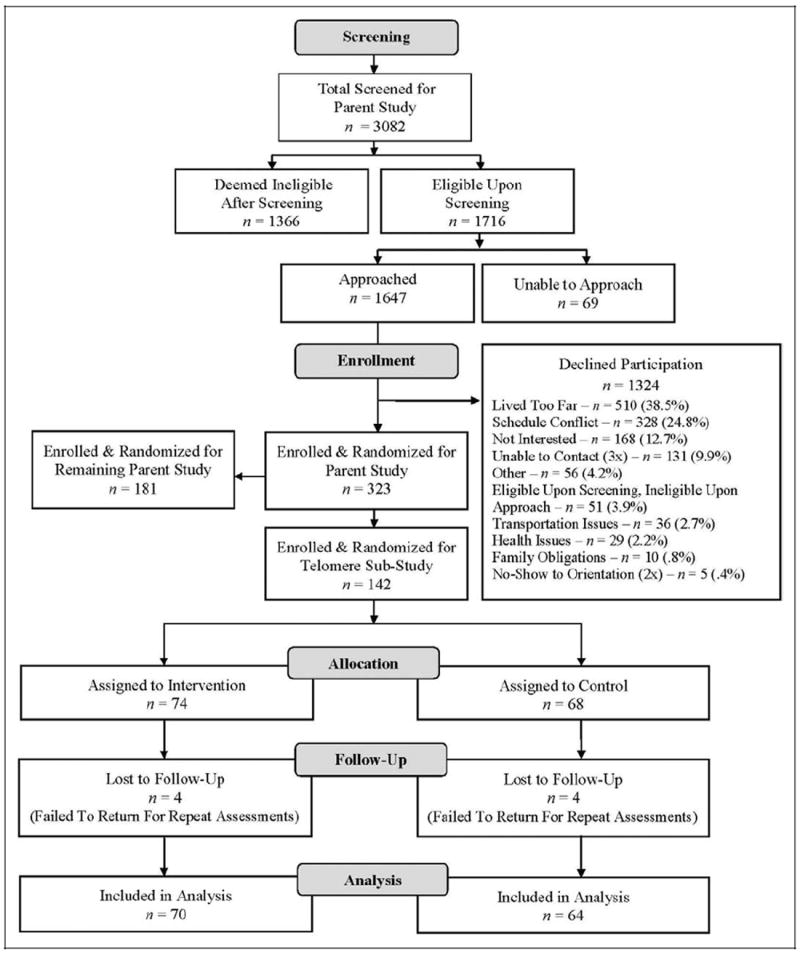
CONSORT (Consolidated Standards of Reporting Trials) flow diagram of participant recruitment, screening, and enrollment from parent R01 trial to study subset.
Change in TL and TA Following MBSR(BC)
Mean (x̄) TL values were similar at baseline, 6-week follow-up, and 12-week follow-up between the MBSR(BC) and UC groups (Figure 2, p > .05 for all comparisons). Thus, there was no indication that participation in the MBSR(BC) program differentially influenced change in TL (Hypothesis a). In contrast, TA increased steadily over time in the MBSR(BC) group while remaining relatively constant at baseline x̄ in the UC group (Figure 3). At baseline, participants who were randomly assigned to MBSR(BC) had lower baseline (intercept) TA values than those assigned to UC (p = .04). Accounting for this baseline difference, the linear mixed model confirmed a steeper slope (greater improvement) in TA at 12 weeks in the MBSR(BC) group compared to the UC group, F(2, 392) = 5.48, p = .004. This slope remained significant (p < .01) even while controlling for baseline TA and the number of days since the end of adjuvant cancer treatment.
Figure 2.
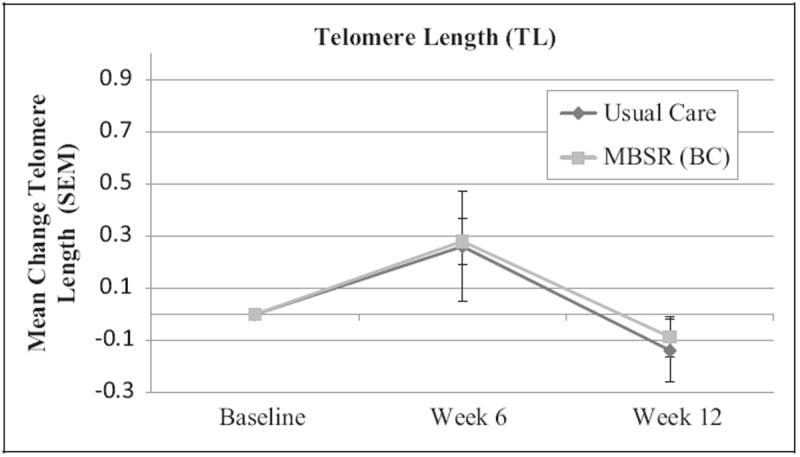
Mean change from baseline in log-transformed telomere length (with standard error of the mean) among breast cancer survivors randomized to the mindfulness-based stress reduction [MBSR(BC)] or the UC group. Baseline measures were taken before the start of the intervention, week 6 measures at the end of the intervention, and week 12 follow-up measures 6 weeks after the end of the intervention.
Figure 3.
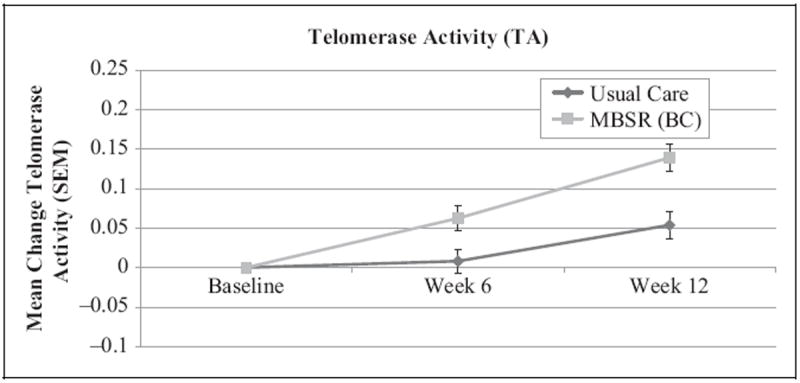
Mean change from baseline in log-transformed telomerase activity (with standard error of the mean) among breast cancer survivors randomized to the mindfulness-based stress reduction for breast cancer [MBSR(BC)] or UC groups. A linear mixed model indicated a greater slope (increase) in TA for the MBSR(BC) group compared to UC (p < .01). Baseline measures were taken before the start of the intervention, week 6 measures at the end of the intervention, and week 12 follow-up measures 6 weeks after the end of the intervention.
Associations Between Change in TA and Changes in Mindfulness and Other Psychological Variables (Hypothesis b)
Given that MBSR(BC) was associated with change in TA but not TL, we restricted our remaining analyses to TA. Change in TA was not significantly related to change in mindfulness (r = .08), depression (r = −.08), anxiety (r = −.01), stress (r = −.11), or fear of recurrence (r = −.09). All p values were greater than .19. Nonetheless, the nominal correlation coefficients were all in the direction of higher mindfulness and more favorable psychological status was associated with higher TA.
Relationship of Practice With TL and TA (Hypothesis c)
We conducted analyses to assess whether the amount of practice time for sitting meditation, yoga, body scan, and walking meditation was associated with changes in TL or TA. All types of practice (i.e., minutes per week), with the exception of walking meditation, trended toward a positive association with higher TA at 12 weeks (Table 2). However, only the relationship between practice of body scan and TA approached significance, rp(67) = .24, p = .049. Given the nominal α of .01, this value did not reach statistical significance. The two practice subtypes that had the weakest relationships with TA, walking meditation and yoga, were the practice types that participants were least likely to perform. Based on these results, we see more definitive evidence for the MBSR(BC) program being independently associated with TA as compared to individual subtypes of practice that comprise the MBSR(BC) program. In addition, individual types of practice were not associated with TA at Week 6 or TL at Weeks 6 or 12 (data not shown).
Table 2.
Mindfulness-Based Stress Reduction for Breast Cancer (MBSR[BC]) Practice Subtypes: Practice Hours and Correlation With Telomerase Activity (TA) While Controlling for Baseline TA.
| Practice type | Practice hours median | Practice hours 25th percentile | Practice hours 75th percentile | % of Participants not practicing | Partial correlation with TA (12 weeks) | p |
|---|---|---|---|---|---|---|
| Sitting meditation | 12.75 | 5.22 | 23.83 | 2.7 | 0.18 | .138 |
| Walking meditation | 3.25 | .81 | 12.44 | 12.2 | −0.11 | .364 |
| Body scan | 5.48 | 2.40 | 11.17 | 8.1 | 0.24 | .049 |
| Yoga | 3.42 | .24 | 10.25 | 23.3 | 0.09 | .481 |
| Formal practice | 33.11 | 14.03 | 55.65 | 2.7 | 0.18 | .154 |
| Informal practice | 30.55 | 17.03 | 55.98 | 4.1 | 0.20 | .106 |
| All practice | 44.25 | 22.92 | 75.57 | 2.7 | 0.21 | .097 |
Note. N = 74.
Discussion
To our knowledge, the present study is the first randomized controlled trial to have examined the effects of MBSR(BC) on TL and TA in PBMCs from BC survivors. Although our findings did not support two of our hypotheses, the primary hypothesis pertaining to MBSR(BC)’s effect on TA was supported. Specifically, the MBSR(BC) intervention significantly increased TA among BC survivors. TA steadily increased over time from baseline to 12 weeks in the MBSR(BC) condition. These data suggest a continuing post-treatment effect of the MBSR(BC) program on TA, at least in the short term (from the end of MBSR(BC) at week 6 through week 12). The changes in TA were not significantly correlated with changes in mindfulness, other psychological measures, or the amount of meditation practice. However, our findings were in the direction of more favorable psychological status being associated with higher TA. We postulate that the lack of formal statistical association may be due, in part, to the study population experiencing “competing” concomitant biological processes associated with recovery from radiation and/or chemotherapy. We found no evidence that the MBSR(BC) program influenced a change in TL.
Overall, findings were consistent with previous studies that demonstrated increases in TA following stress-reducing interventions (Daubenmier et al., 2011; Jacobs et al., 2011; Ornish et al., 2008). In contrast to previous research, however, our study featured a larger sample size and statistical adjustment for baseline levels of TA. Thus, to our knowledge, our study provides the strongest empirical support for a positive relationship between a stress-reducing intervention and increased TA.
As depicted in Figure 3, TA increased approximately 17% over time in the MBSR(BC) group compared to approximately 3% in the UC group. The magnitude of the difference between the groups is approximately 1 standard deviation (SD), which corresponds to a large effect size (Cohen, 1988). TA has the capacity to add DNA sequences, thereby actively increasing TL and preserving healthy cell function (Kim, Han, You, Chen, & Campisi, 2003). Although we did not observe significant increases in TL in the MBSR(BC) group, such increases may have been evident had we collected data over a longer follow-up period. Ornish et al. (2008) suggested that changes in TL due to behavioral interventions make take at least 1 year. In addition, Lin and colleagues (2010) have reported significant variations of TA among cell types, with B cells showing higher TA than T cells. Thus, measurement of TA may be more sensitive to subtle changes over relatively short times as compared to TL. Future longer term studies are required to quantify the net clinical benefits of increased TA over time in both cancer patients and noncancer patient populations. At this time, we can simply state that the increased TA associated with the MBSR(BC) program shows promise in reducing cellular aging.
Regarding the specific mechanism of how MBSR may impact TA and TL, Epel et al. (2009) proposed that two types of stress cognition may negatively impact TL (and thus, cell life span): (1) threat appraisal and (2) rumination. Meditation may lead to an increase in positive cognitions and reductions of stress, exaggerated threat appraisals, and rumination, which may ultimately increase cellular longevity. Prior research has shown, in fact, that patients receiving MBSR have higher levels of mindful attentiveness (Matchim, Armer, & Stewart, 2010) and lower levels of ruminative thinking (Campbell, Labelle, Bacon, Faris, & Carlson, 2011). Decreased rumination following MBSR may lead to lower distress (Jain et al., 2007; Labelle, Campbell, & Carlson, 2010). As a mediator, increased mindfulness has been found to lead to (1) lower perceived stress, less rumination, and improved quality of life in nonclinical populations (Nyklicek & Kuijpers, 2008; Shapiro, Oman, Thoresen, Plante, & Flinders, 2008); (2) lower perceived stress and posttraumatic avoidance symptoms and improved positive states of mind in cancer patients not on treatment (Branstrom, Kvillemo, Brandberg, & Moskowitz, 2010); (3) less worry and trait anxiety among patients with anxiety disorders (Vollestad, Sivertsen, & Nielsen, 2011); and (4) greater perceived control and less neuroticism overall (two major contributors of stress), both of which have been shown to be associated with TA (Jacobs et al., 2011).
Additional mechanisms by which meditation may improve cellular longevity include decreasing the levels of stress hormones and oxidative stress and increasing the levels of hormones beneficial to telomeres (Epel et al., 2009) and TA (Daubenmier et al., 2011). Research has shown that low TA is associated with exaggerated autonomic reactivity to acute mental stress and elevated nocturnal epinephrine, while shorter TL is associated with increases in catecholamine and cortisol levels (Epel et al., 2006). Therefore, it appears that TA level is somewhat responsive at the cellular level to changes in psychological well-being (Epel, 2012).
Limitations
While restricted to BC patients, our sample of women was heterogeneous in terms of treatment received (i.e., radiation and/or chemotherapy) and time since treatment completion. Whether or not the apparent beneficial effects of MBSR(BC) on TA are more profound in specific patient subsets is unknown. Similarly, the 12-week length of participant follow-up, which included 6 weeks of participation in the MBSR(BC) program, was quite short and permitted assessment of acute effects only. Additionally, the study was designed to compare the MBSR(BC) to the usual treatment given to BC survivors and did not control for the effect of group dynamics on study outcomes. Future studies should examine the effect of MBSR(BC) on TL and TA over a longer time frame, including the extent of meditative practice after cessation of the formal MBSR(BC) program. Finally, we observed an apparent variation in absolute values of TL at the three different measurement intervals (Figure 2). This fluctuation in absolute values of TL may reflect interassay variation; however, it has no meaningful impact on the validity of the between-group (randomized) comparisons of TL.
Conclusion
In addition to the established effects of MBSR(BC) in reducing psychological and physical symptoms in BC survivors, the present randomized controlled study provides evidence that MBSR(BC) also increases TA in this population. Although the net clinical benefits from this increased activity are unknown, these data provide a rationale for long-term follow-up of cancer patients who participate in MBSR programs as well as consideration of TL and TA as outcome variables in future intervention studies.
Acknowledgments
Funding
The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported by the USF Established Researcher Grant Award and, in part, by the National Cancer Institute (R01-CA131080).
Footnotes
Declaration of Conflicting Interests
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
References
- Biegler K, Anderson A, Wenzel L, Osann K, Nelson E. Longitudinal change in telomere length and the chronic stress response in a randomized pilot biobehavioral clinical study: Implications for cancer prevention. Cancer Prevention Research. 2012;5:1173–1182. doi: 10.1158/1940-6207.CAPR-12-0008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bishop SR, Lau M, Shapiro S, Carlson L, Anderson ND, Carmody J, Devins G, et al. Mindfulness: A proposed operational definition. Clinical Psychology—Science and Practice. 2004;11:230–241. doi: 10.1093/clipsy/bph077. [DOI] [Google Scholar]
- Blackburn EH. Telomere states and cell fates. Nature. 2000;408:53–56. doi: 10.1038/35040500. [DOI] [PubMed] [Google Scholar]
- Blackburn EH, Greider CW, Henderson E, Lee MS, Shampay J, Shippen-Lentz D. Recognition and elongation of telomeres by telomerase. Genome. 1989;31:553–560. doi: 10.1139/g89-104. [DOI] [PubMed] [Google Scholar]
- Branstrom R, Kvillemo P, Brandberg Y, Moskowitz JT. Self-report mindfulness as a mediator of psychological well-being in a stress reduction intervention for cancer patients—A randomized study. Annuals of Behavioral Medicine. 2010;39:151–161. doi: 10.1007/s12160-010-9168-6. [DOI] [PubMed] [Google Scholar]
- Campbell TS, Labelle LE, Bacon SL, Faris P, Carlson LE. Impact of mindfulness-based stress reduction (MBSR) on attention, rumination and resting blood pressure in women with cancer: A waitlist-controlled study. Journal of Behavioral Medicine. 2011;35:262–271. doi: 10.1007/s10865-011-9357-1. [DOI] [PubMed] [Google Scholar]
- Cawthon R. Telomere measurement by quantitative PCR. Nucleic Acids Research. 2002;30:e47. doi: 10.1093/nar/30.10.e47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chambers R, Lo BCY, Allen NB. The impact of intensive mindfulness training on attentional control, cognitive style, and affect. Cognitive Therapy and Research. 2008;32:303–322. doi: 10.1007/s10608-007-9119-0. [DOI] [Google Scholar]
- Cohen J. Statistical power analysis for the behavioral sciences. 2. Hillsdale, NJ: Lawrence Erlbaum; 1988. [Google Scholar]
- Cohen S, Kamarck T, Mermelstein R. A global measure of perceived stress. Journal of Health and Social Behavior. 1983;24:385–396. [PubMed] [Google Scholar]
- Damjanovic A, Yang Y, Glaser R, Kiecolt-Glaser J, Nguyen H, Laskowski B, Weng N, et al. Accelerated telomere erosion is associated with a declining immune function of caregivers of Alzheimer’s disease patients. Journal of Immunology. 2007;179:4249–4254. doi: 10.4049/jimmunol.179.6.4249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daubenmier J, Lin J, Blackburn E, Hecht FM, Kristeller J, Maninger N, Epel E, et al. Changes in stress, eating, and metabolic factors are related to changes in telomerase activity in a randomized mindfulness intervention pilot study. Psychoneuroendocrinology. 2011;37:917–928. doi: 10.1016/j.psyneuen.2011.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desmaze C, Soria JC, Freulet-Marriere MA, Mathieu N, Sabatier L. Telomere-driven genomic instability in cancer cells. Cancer Letters. 2003;194:173–182. doi: 10.1016/s0304-3835(02)00704-8. [DOI] [PubMed] [Google Scholar]
- Epel E. Telomeres in a life-span perspective: A new “psychobiomarker”? Current Directions in Psychological Science. 2009;18:6–10. doi: 10.1111/j.1467-8721.2009.01596.x. [DOI] [Google Scholar]
- Epel E. How “reversible” is telomeric aging? Cancer Prevention Research. 2012;5:1163–1168. doi: 10.1158/1940-6207.CAPR-12-0370. [DOI] [PubMed] [Google Scholar]
- Epel E, Daubenmier J, Moskowitz J, Folkman S, Blackburn E. Can meditation slow rate of cellular aging? Cognitive stress, mindfulness, and telomeres. Annals of the New York Academy of Sciences. 2009;1172:34–53. doi: 10.1111/j.1749-6632.2009.04414.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Epel ES, Lin J, Wilhelm FH, Wolkowitz OM, Cawthon R, Adler NE, Blackburn EH, et al. Cell aging in relation to stress arousal and cardiovascular disease risk factors. Psychoneuroendocrinology. 2006;31:277–287. doi: 10.1016/j.psyneuen.2005.08.011. [DOI] [PubMed] [Google Scholar]
- Feldman G, Hayes A, Kumar S, Greeson J, Laurenceau J. Mindfulness and emotion regulation: The development and initial validation of the Cognitive and Affective Mindfulness Scale-Revised (CAMS-R) Journal of Psychopathology and Behavioral Assessment. 2007;29:177–190. doi: 10.1007/s10862-006-9035-8. [DOI] [Google Scholar]
- Fordyce CA, Heaphy CM, Joste NE, Smith AY, Hunt WC, Griffith JK. Association between cancer-free survival and telomere DNA content in prostate tumors. Journal of Urology. 2005;173:610–614. doi: 10.1097/01.ju.0000143195.49685.ce. [DOI] [PubMed] [Google Scholar]
- French-Rosas LN, Moye J, Naik AD. Improving the recognition and treatment of cancer-related posttraumatic stress disorder. Journal of Psychiatric Practice. 2011;17:270–276. doi: 10.1097/01.pra.0000400264.30043.ae. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gertler R, Rosenberg R, Stricker D, Friederichs J, Hoos A, Werner M, Siewert JR, et al. Telomere length and human telomerase reverse transcriptase expression as markers for progression and prognosis of colorectal carcinoma. Journal of Clinical Oncology. 2004;22:1807–1814. doi: 10.1200/JCO.2004.09.160. [DOI] [PubMed] [Google Scholar]
- Goronzy JJ, Fujii H, Weyand CM. Telomeres, immune aging and autoimmunity. Experimental Gerontology. 2006;41:246–251. doi: 10.1016/j.exger.2005.12.002. [DOI] [PubMed] [Google Scholar]
- Jacobs TL, Epel ES, Lin J, Blackburn EH, Wolkowitz OM, Bridwell DA, Saron CD. Intensive meditation training, immune cell telomerase activity, and psychological mediators. Psychoneuroendocrinology. 2011;36:664–681. doi: 10.1016/j.psyneuen.2010.09.010. [DOI] [PubMed] [Google Scholar]
- Jain S, Shapiro SL, Swanick S, Roesch SC, Mills PJ, Bell I, Schwartz GE. A randomized controlled trial of mindfulness meditation versus relaxation training: Effects on distress, positive states of mind, rumination, and distraction. Annals of Behavioral Medicine. 2007;33:11–21. doi: 10.1207/s15324796abm3301_2. [DOI] [PubMed] [Google Scholar]
- Kabat-Zinn J, Lipworth L, Burney R. The clinical use of mindfulness meditation for the self-regulation of chronic pain. Journal of Behavioral Medicine. 1985;8:163–190. doi: 10.1007/BF00845519. [DOI] [PubMed] [Google Scholar]
- Kabat-Zinn J, Massion AO, Kristeller J, Peterson LG, Fletcher KE, Pbert L, Santorelli SF, et al. Effectiveness of a meditation-based stress reduction program in the treatment of anxiety disorders. American Journal of Psychiatry. 1992;149:936–943. doi: 10.1176/ajp.149.7.936. [DOI] [PubMed] [Google Scholar]
- Kananen L, Surakka I, Pirkola S, Suvisaari J, Lonnqvist J, Peltonen L, Hovatta I, et al. Childhood adversities are associated with shorter telomere length at adult age both in individuals with an anxiety disorder and controls. PLos One. 2010;5:e10826. doi: 10.1371/journal.pone.0010826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim SH, Han S, You YH, Chen DJ, Campisi J. The human telomere-associated protein TIN2 stimulates interactions between telomeric DNA tracts in vitro. EMBO Reports. 2003;4:685–691. doi: 10.1038/sj.embor.embor872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Labelle LE, Campbell TS, Carlson LE. Mindfulness-based stress reduction in oncology: Evaluating mindfulness and rumination as mediators of change in depressive symptoms. Mindfulness. 2010;1:28–40. doi: 10.1007/s12671-010-0005-6. [DOI] [Google Scholar]
- Lavretsky H, Epel ES, Siddarth P, Nazarian N, Cyr NS, Khalsa DS, Irwin MR, et al. A pilot study of yogic meditation for family dementia caregivers with depressive symptoms: Effects on mental health, cognition, and telomerase activity. International Journal of Geriatric Psychiatry. 2013;28:57–65. doi: 10.1002/gps.3790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lengacher C, Johnson-Mallard V, Barta M, Fitzgerald S, Moscoso M, Post-White J, Kip K, et al. Feasibility of a mindfulness-based stress reduction program for early-stage breast cancer survivors. Journal of Holistic Nursing. 2011;29:107–117. doi: 10.1177/0898010110385938. [DOI] [PubMed] [Google Scholar]
- Lengacher C, Reich R, Post-White J, Moscoso M, Shelton M, Barta M, Budhrani P, et al. Mindfulness based stress reduction in post-treatment breast cancer patients: An examination of symptoms and symptom clusters. Journal of Behavioral Medicine. 2011;35:86–94. doi: 10.1007/s10865-011-9346-4. [DOI] [PubMed] [Google Scholar]
- Lengacher CA, Johnson-Mallard V, Post-White J, Moscoso MS, Jacobsen PB, Klein TW, Kip KE, et al. Randomized controlled trial of mindfulness-based stress reduction (MBSR) for survivors of breast cancer. Psycho-Oncology. 2009;18:1261–1272. doi: 10.1002/pon.1529. [DOI] [PubMed] [Google Scholar]
- Lin J, Epel E, Cheon J, Kroenke C, Sinclair E, Bigos M, Blackburn E, et al. Analyses and comparisons of telomerase activity and telomere length in human T and B cells: Insights for epidemiology of telomere maintenance. Journal of Immunological Methods. 2010;352:71–80. doi: 10.1016/j.jim.2009.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu L, Bailey SM, Okuka M, Munoz P, Li C, Zhou L, Keefe DL, et al. Telomere lengthening early in development. Nature Cell Biology. 2007;9:1436–1441. doi: 10.1038/ncb1664. [DOI] [PubMed] [Google Scholar]
- Londono-Vallejo JA. Telomere instability and cancer. Biochimie. 2008;90:73–82. doi: 10.1016/j.biochi.2007.07.009. [DOI] [PubMed] [Google Scholar]
- Matchim Y, Armer JM, Stewart BR. Effects of mindfulness-based stress reduction (MBSR) on health among breast cancer survivors. Western Journal of Nursing Research. 2010;33:996–1016. doi: 10.1177/0193945910385363. [DOI] [PubMed] [Google Scholar]
- Miller G, Chen E, Cole SW. Health psychology: Developing biologically plausible models linking the social world and physical health. Annual Review of Psychology. 2009;60:501–524. doi: 10.1146/annurev.psych.60.110707.163551. [DOI] [PubMed] [Google Scholar]
- Miller J, Fletcher K, Kabat-Zinn J. Three-year follow-up and clinical implications of a mindfulness meditation-based stress reduction intervention in the treatment of anxiety disorders. General Hospital Psychiatry. 1995;17:192–200. doi: 10.1016/0163-8343(95)00025-m. [DOI] [PubMed] [Google Scholar]
- Nyklicek I, Kuijpers KF. Effects of mindfulness-based stress reduction intervention on psychological well-being and quality of life: Is increased mindfulness indeed a mechanism? Annals of Behavioral Medicine. 2008;35:331–340. doi: 10.1007/s12160-008-9030-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ornish D, Lin J, Daubenmier J, Weidner G, Epel E, Kemp C, Blackburn EH, et al. Increased telomerase activity and comprehensive lifestyle changes: A pilot study. Lancet Oncology. 2008;9:1048–1057. doi: 10.1016/S1470-2045(08)70234-1. [DOI] [PubMed] [Google Scholar]
- Peres J. Telomere research offers insight on stress-disease link. Journal of the National Cancer Institute. 2011;103:848–850. doi: 10.1093/jnci/djr214. [DOI] [PubMed] [Google Scholar]
- Radloff L. The CES-D scale: A self-report depression scale for researching the general population. Application of Psychological Measures. 1977;1:385–401. [Google Scholar]
- Serrano AL, Andres V. Telomeres and cardiovascular disease: Does size matter? Circulation Research. 2004;94:575–584. doi: 10.1161/01.RES.0000122141.18795.9C. [DOI] [PubMed] [Google Scholar]
- Shapiro SL, Oman D, Thoresen CE, Plante TG, Flinders T. Cultivating mindfulness: Effects on well-being. Journal of Clinical Psychology. 2008;64:840–862. doi: 10.1002/jclp.20491. [DOI] [PubMed] [Google Scholar]
- Speca M, Carlson LE, Goodey E, Angen M. A randomized, wait-list controlled clinical trial: The effect of a mindfulness meditation-based stress reduction program on mood and symptoms of stress in cancer outpatients. Journal of Psychosomatic Medicine. 2000;62:613–622. doi: 10.1097/00006842-200009000-00004. [DOI] [PubMed] [Google Scholar]
- Spielberger CD, Gorsuch RL, Luschene RE. Manual for the State-Trait Anxiety Inventory. Palo Alto, CA: Consulting Psychologists; 1983. [Google Scholar]
- Teasdale JD, Segal ZV, Williams JM, Ridgeway VA, Soulsby JM, Lau MA. Prevention of relapse/recurrence in major depression by mindfulness-based cognitive therapy. Journal of Consulting and Clinical Psychology. 2000;68:615–623. doi: 10.1037//0022-006x.68.4.615. [DOI] [PubMed] [Google Scholar]
- Vickberg SM. The Concerns About Recurrence Scale (CARS): A systematic measure of women’s fears about the possibility of breast cancer recurrence. Annals of Behavioral Medicine. 2003;25:16–24. doi: 10.1207/S15324796ABM2501_03. [DOI] [PubMed] [Google Scholar]
- Vollestad J, Sivertsen B, Nielsen GH. Mindfulness-based stress reduction for patients with anxiety disorders: Evaluation in a randomized controlled trial. Behaviour Research and Therapy. 2011;49:281–288. doi: 10.1016/j.brat.2011.01.007. [DOI] [PubMed] [Google Scholar]
- Wikgren M, Maripuu M, Karlsson T, Nordfjall K, Bergdahl J, Hultdin J, Norrback KF, et al. Short telomeres in depression and the general population are associated with a hypocortiso-lemic state. Biological Psychiatry. 2011;71:294–300. doi: 10.1016/j.biopsych.2011.09.015. [DOI] [PubMed] [Google Scholar]
- Wolkowitz OM, Mellon SH, Epel ES, Lin J, Dhabhar FS, Su Y, Blackburn EH, et al. Leukocyte telomere length in major depression: Correlations with chronicity, inflammation and oxidative stress—preliminary findings. PLos One. 2011;6:e17837. doi: 10.1371/journal.pone.0017837. [DOI] [PMC free article] [PubMed] [Google Scholar]


