SUMMARY
Lung disease is a major cause of death in the USA, with current therapeutic approaches only serving to manage symptoms. The most common chronic and life-threatening genetic disease of the lung is Cystic fibrosis (CF) caused by mutations in the cystic fibrosis transmembrane regulator (CFTR). We have generated induced pluripotent stem cells (iPSC) from CF patients carrying a homozygous deletion of F508 in the CFTR gene, which results in defective processing of CFTR to the cell membrane. This mutation was precisely corrected using CRISPR to target corrective sequences to the endogenous CFTR genomic locus, in combination with a completely excisable selection system which significantly improved the efficiency of this correction. The corrected iPSC were subsequently differentiated to mature airway epithelial cells where recovery of normal CFTR expression and function was demonstrated. This isogenic iPSC-based model system for CF could be adapted for the development of new therapeutic approaches.
INTRODUCTION
In CF a thick sticky mucus forms in the lungs impairing breathing and providing a rich environment for pathogens to flourish leading to premature respiratory failure. It affects multiple organ systems leading to problems in the liver, pancreas and small bowel. There has been a substantial increase in the knowledge of molecular and cellular mechanisms over the past two decades, which has translated to improvement in care and an increase in the average life expectancy from 14 years in 1980 to 37 years in 2012. The disease still severely impacts the quality of life with a significant shortening of life expectancy. Therefore, development of new therapeutic approaches is critical in the absence of a potential cure. As mentioned above the underlying cause, mutations in the CFTR gene, prevents the expression or function of this chloride transporter at the cell membrane (Rogan et al., 2011). Studying the disease has proven difficult due to the shortcomings of animal models. In the mouse CFTR knockout model, for example, an upregulation of calcium activated chloride channels is sufficient to overcome the lack of functional CFTR (Boyd and Porteous, 2004). More recently, ferret and pig models of CF have been generated and thought to more closely replicate the human form of the disease (Rogers et al., 2008; Sun et al., 2010; Welsh et al., 2009). Studying the human disease still remains a challenge. There is a lack of availability of primary lung tissues and tissues from deceased CF patient lungs are inherently variable due to the differences in chronic infection and treatment regimens.
The ability to generate pluripotent stem cells from accessible tissues, such as skin, has opened the door for modeling human disease in a dish, increasing the potential for understanding the mechanisms of disease, testing novel therapeutic approaches and developing cell therapies in a human system (Takahashi et al., 2007a; Takahashi et al., 2007b). iPSC, together with the recent explosion in genome editing technologies, enables unprecedented capacity for patient-specific disease modeling, correction and therapy. The latest tool in genome editing is called clustered, regularly interspaced, short palindromic repeats (CRISPR)-associated (Cas) systems, which is found naturally in the adaptive immune system of bacteria and archaea (Hale et al., 2012; Millen et al., 2012; Wiedenheft et al., 2012). The CRISPR system is essentially comprised of a ribonucleoprotein endonuclease, Cas9, that can catalyze double strand cleavage of DNA in a sequence-specific manner defined by a guide RNA (gRNA) element complementary to the target DNA sequence. This simple two-component gene targeting system has been co-opted for broader use by engineering synthetic gRNA hairpins to replace the bipartite bacterial RNA element (Jinek et al., 2012) and codon-optimizing the bacterial Cas9 protein for optimized expression in higher eukaryotes (Cong et al., 2013; Mali et al., 2013). Recent work has shown that the CRISPR/Cas9 system can be used for efficient and multiplexed genome editing in a broad range of organisms including bacteria (Jiang et al., 2013), mice(Wang et al., 2013), zebrafish (Hwang et al., 2013), yeast (DiCarlo et al., 2013), Xenopus tropicalis (Nakayama et al., 2013), and human cells (Cho et al., 2013; Cong et al., 2013; Mali et al., 2013). The capability of Cas9 to mediate precise double stranded breaks (DSB) in the DNA genome opens the door to an infinite range of genome engineering possibilities.
RESULTS
Derivation of CF iPSC and strategy for gene correction of CFTR ΔF508
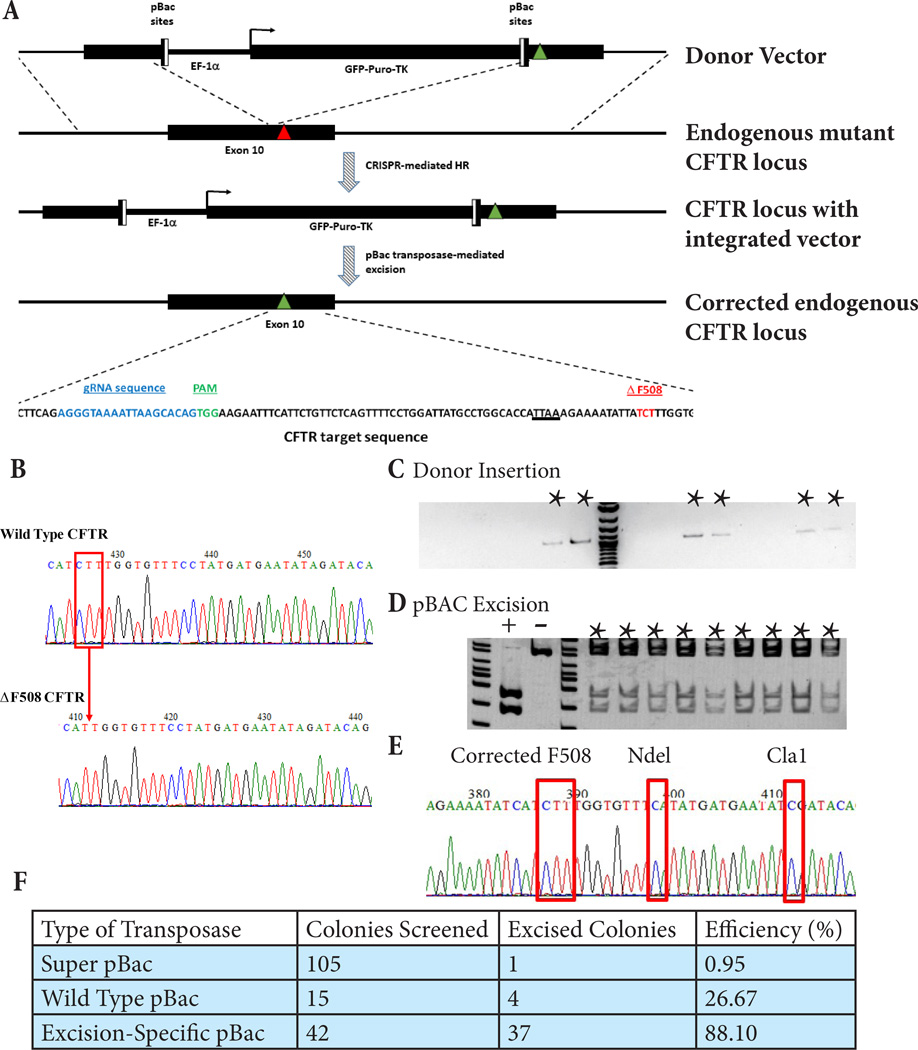
We obtained skin biopsy from a CF patient organ donor, isolated and expanded fibroblasts for reprogramming. iPSC were generated either by using a 6-factor polycistronic lentivirus followed by successful excision using Cre-RNA (Firth et al., 2014) or by using 4 individual factor Sendai virus (Fusaki et al., 2009). All iPSC lines generated retained all the hallmarks of pluripotent stem cells (Figure S1). To correct the causative ΔF508 mutation in the patient-derived CF iPSC, we developed a customized CRISPR system, consisting of two components: a plasmid encoding the full-length Cas9 protein, codon optimized for optimal expression in human cells and driven by the stem cell-compatible EF-1α promoter, and a separate plasmid containing a U6 promoter-driven gRNA hairpin cassette. The gRNA cassette was designed so that we could clone unique guide sequences into the RNA hairpin by using specifically designed primers containing the desired target guide sequences and PCR cloning into the vector backbone. gRNAs were designed to target sequences in the vicinity of the ΔF508 mutation in the endogenous CFTR gene (Fig. 1A). The patient derived fibroblasts and iPSC were confirmed to be homozygous for the phenylalanine deletion at position 508 (ΔF508) (Fig. 1B). The functional activity of these gRNAs in combination with our customized CRISPR system was validated in HEK293T cells and in CF iPSC by Surveyor assay (Figure S2A).
Figure 1. Footprint-free CRISPR-mediated correction of CF iPSC.
A) Schematic of strategy used for CRISPR-mediated correction of the CFTR ΔF508 mutation. Red triangle denotes the ΔF508 deletion (shown in red in the sequence at the bottom) and green triangles the correction of the deleted triplet of bases. The TTAA pBac recognition site is underlined. The actual CFTR target sequence is shown below with the gRNA target sequence in blue and the PAM in green. B) Sequencing analysis of the endogenous CFTR gene at the genomic locus of the ΔF508 mutation from wild type and CF patient-derived iPSC. The position of the CTT deletion in the mutant iPSC as compared to the wild type is indicated by the red box and arrow. C) Integration-specific PCR of puromycin resistant single-cell clones of CF iPSC after CRISPR treatment. Indicated clones contain the integrated selection cassette and corrective sequence in the correct position at the CFTR genomic locus. D) Screening by ClaI digest of a single-cell corrected clone after excision and negative selection of unexcised clones by ganciclovir. Indicated clones show precise and complete excision of the selection cassette from the endogenous CFTR genomic locus leaving behind only the corrected F508 sequence and the intended silent mutations encoding the restrictions sites used for screening here. E) Sequence analysis of corrected, excised clone showing the corrected ΔF508 mutation and adjacent silent mutations introduced in the endogenous CFTR gene. F) Table of excision screening done with different variations of the pBac transposase.
For targeting the ΔF508 mutation at its endogenous genomic locus, a donor vector was developed in order to achieve efficient, footprint-free correction of the CFTR gene (Fig. 1A). The vector includes an EF-1α promoter-driven GFP-Puro-TK cassette flanked with recognition sites for the piggyBac transposase along with the homology arms with the corrected CFTR sequence for integration in to the endogenous genomic locus. Upon successful CRISPR-mediated integration into the genomic target site, we can select for integration using puromycin and then seamlessly excise the selection cassette by expressing the pBac transposase (System Biosciences). This excision should proceed without leaving behind any genomic footprint from the donor vector other than the desired correction of the target ΔF508 mutation, as it uses a suitably placed TTAA pBac recognition site which occurs naturally within the target genomic sequence, as outlined in Fig. 1A. We can then select against unexcised colonies in a subsequent round of clonal selection using ganciclovir sensitivity of clones expressing the unexcised thymidine kinase (TK) gene.
CRISPR-mediated gene targeting of endogenous CFTR in CF iPSC
The Cas9 vector, CFTR gRNA vector, donor vector and/or GFP were co-nucleofected in various combinations into CF iPSC. Forty-eight hours after plating at a single-cell density the colonies were treated with puromycin and the surviving colonies were selected and analyzed. The CFTR locus-specific segment of DNA was amplified using an integration-specific primer pair, with one primer contained within the selection cassette, and the other outside the homology arm of the donor vector (Fig. 1C). PCR products arising from clones screened with this combination of primers, spanning the junction between the target and insert, indicate that these puromycin-resistant clones contain the integrated selection cassette and corrective sequences in the correct location and orientation. Of the 36 clones picked and analyzed, 6 were positive for correct integration, at an overall efficiency of 16.7%. The sequence of the integration-specific product at the correct target locus in at least one allele of the endogenous CFTR gene in each the positive clones was confirmed by sequencing.
Complete excision of selection markers using piggyBac transposase
The selection cassette was subsequently excised using the piggyBac transposase system, which allows removal of the pBac site-flanked sequence without leaving behind any genomic footprint at the target site. Three variations of the piggyBac transposase (wild type, integration-specific Super PiggyBac or an excision-specific version from System Biosciences) were nucleofected into one of the integration-positive iPSC clones, to compare their relative efficiencies at excising the integrated cassette. The genomic DNA for each of the Ganciclovir resistant iPSC colonies was analyzed by PCR amplification of the CFTR locus and ClaI restriction enzyme digest of the amplicon (Fig. 1D). The donor plasmid contained a silent mutation to introduce a ClaI site in close vicinity of the correction site. A number of corrected, excised clones were isolated, with nearly 90% efficiency when we used the excision-specific pBac transposase. A summary of excision experiments is provided in Figure 1F.
Analysis of off-target effects of genome editing approach
Corrected, excised clones were verified by sequencing (Fig. 1E) and one particular clone was then analyzed in detail for off-target effects of CRISPR by whole exome sequencing. We compared observed mutations to predicted off target sites using the CRISPR design tool (http://crispr.mit.edu/) (Cong et al., 2013). No mutations (SNVs, indels) occurred within 300 base pairs of a predicted off target site. After filtering, the predominant type of mutation was single nucleotide variants (80%) with the remainder being insertions and deletions at roughly the same proportion (Figure S2B). With regards to the length of these observed insertions and deletions, a heavy skew was observed to lengths which were less than 3 nucleotides (Figure S2C). Given the type of mutations, short length of indels, and lack of predicted off target events, the acquired mutations are not due to the correction technology itself. PCR analysis of genomic DNA of corrected clones, both before and after excision, using primers specific to the selection cassette was negative indicating there was no random integration of the selection cassette elsewhere in the genome (data not shown).
Recovery of CFTR function in airway epithelial cells derived from iPSC
Differentiation to proximal airway epithelial cells was carried out as previously described (Firth et al., 2014). Briefly, our mutant and gene corrected CF iPSC lines were differentiated through definitive endoderm, anterior foregut endoderm, NKx2.1 lung progenitors and matured in an air-liquid interface. All iPSC lines were capable of generating definitive endoderm, as shown in Figure 2A by FOXA2 staining and maturing to lung epithelial cells with tight junctions after 48 days of differentiation, as indicated by ZO-1 staining occludin (red) in the lower images of Figure 2A. Quantitative PCR analysis of key genes representing different stages of lung differentiation is shown in Figure 2B for wild type, CF mutant and CF corrected iPSC. Definitive endoderm markers FOXA2, GATA6 and SOX17 all increased by Day 5 of differentiation. NKx2.1, one of the earliest transcription factors specifying lung, increased over the differentiation time course as did club cell 10kDa protein (CC10, club cells), keratin 5 (KRT5) and TP63 (basal cells), Mucin 5AC (MUC5AC, goblet cells) and CFTR (functional epithelial cells).
Figure 2. Generation of Functional Respiratory Epithelial Cells from CF mutant and gene corrected iPSC.
A) Representative images of CF corrected and CF mutant iPSC differentiation to DE (Day 5); DAPI (Blue) and FOXA2 (Cyan), Scale bar white =100µm, yellow =20µm. B) mRNA expression of DE, AFE and lung epithelial cells markers over the time course of differentiation for wildtype, CF mutant and CF corrected iPSC; data are corrected for internal controls and normalized to wildtype iPSC cDNA (mean ±SEM, n=6–15 from a minimum of 3 experimental replicates). C) Averaged current/voltage plots for baseline (blue), in presence of Forskolin, genistein and isobutylmethyl xanthine (IBMX) (pink) and with the addition of CFTRinh-172 (green).Data represented are mean ±SEM for 0 of 4 patched mutant cells and 3 of 8 patched gene corrected cells. Lower panels are representative current traces from one gene corrected differentiated epithelial cell at baseline, and in the presence of FSK cocktail and CFTRinh-172 (L to R), traces represent 20mV increments from −80 to +80mV. D) Western blot showing the presence of the membrane translocated and glycosylated protein in the wildtype and gene corrected cells (Band B) and the unglycoslylated CFTR band in all 3 cell lines (Band A). WT= wildtype, M = mutant, C= gene corrected.
We isolated epithelial calmodulin (EpCAM) positive cells at Day 48 of differentiation for analysis of CFTR currents by whole cell patch clamp methods. In the mutant differentiated cells no increase in CFTR chloride current was observed in response to a cocktail of forskolin, genistein and isobutylmethyl xanthine (IBMX). In the CRISPR gene corrected epithelial cells, half of the cells stably patched responded to stimulation, similar to our observations in wild type iPSC derived lung epithelial cells (Firth et al., 2014). The increase in current was blocked by specific CFTR inhibitor CFTRinh-172 (Fig. 2C). Additionally, CFTR undergoes N-Glycosylation enabling conformational changes essential to its translocation to the cell membrane. CFTR contains two glycosylation sites which can be detected by gel electrophoresis of the protein. Core glycosylation produced a band of ~150kDa (Band A) and complex glycosylation a band of ~170kDa (Band B); ΔF508 mutant CFTR is unable to be glycosylated and only band A can be detected (Patrick et al., 2011). The western blot in Figure 2D shows the presence of the un-glycosylated and glycosylated bands in differentiated wild type and gene corrected iPSC but only the un-glycosylated band in the differentiated CF mutant iPSC.
DISCUSSION
While genomic correction of CF patient iPSC has recently been demonstrated using zinc finger nucleases (ZFN) (Crane et al., 2015), we believe this report is the first to describe correction of iPSC derived from a CF patient carrying the most commonly found homozygous ΔF508 mutation using CRISPR. CRISPR has also recently been used to correct CFTR in adult intestinal stem cells however these cells cannot be utilized to study the pathophysiology of CF in the lung (Schwank et al., 2013). Our footprint-free CRISPR-based approach is also able to address some of the technical limitations regarding the persistence of a single LoxP site after CRE excision of the donor sequence and the use of integrating vector used to generate the iPSC (Crane et al., 2015), thus providing a more optimal approach when considering clinical applications. In addition, we observe a much-improved efficiency of genomic correction using our selection-based methodology using CRISPR, while completely eliminating any local genomic footprint in the endogenous CFTR gene.
Having such a “footprint free” approach to gene editing has enabled us to precisely and efficiently edit a point mutation in the genome leaving no genomic scar in patient cells. It is hoped that eventually this approach could be utilized as a cell therapy approach for patients with lung disease. A more immediate use for such cells would be for their utilization in research and drug development. Further refinement of the differentiation protocol will lead to effective in vitro isogenic models of human lung diseases like CF which can be used to study mechanisms of disease, screen for novel therapeutic approaches and even clinically to identify responders to currently available therapeutic options.
EXPERIMENTAL PROCEDURES
Nucleofection of iPSC
Adherent cells were dissociated using Tryple (Gibco), centrifuged and resuspended in TeSR containing 10µM ROCK inhibitor, Y27632. 1×106 cells were resuspended in 100µl of Lonza® Nucleofection solution (according to manufacturer’s protocol). The following amounts of DNA were added; Cas9 2µg, gRNA 2 µg, PBHR 5µg, pmaxGFP 2µg, and/or PiggyBac Transposase 2.5µg in the required experimental combination. The samples were then nucleofected using the B-16 protocol on the device. After nucleofection, TeSR was added and cells transferred to a 6-well Matrigel-coated plate. After 24–48 hours of incubation, the nucleofected iPSC were split onto 10cm MEF plates at single-cell density for colony screening or harvested for genomic DNA analysis.
Colony Isolation and Screening
After puromycin (1µg/ml) or ganciclovir (3µg/ml) treatment, colonies were mechanically passaged into a matrigel-coated 12-well plate in TeSR with ROCK inhibitor. Once the isolated iPSC clones were grown to the appropriate size, each well was split into duplicate 12-well matrigel plates. The cells were dissociated using trypsin, centrifuged at 1000rpm for 5min and resuspended in 1ml TeSR with ROCK inhibitor. 2/3 of each cell suspension is added to a matrigel plate for genomic DNA isolation, while the other 1/3 is carried to another matrigel plate designated for clonal maintenance and expansion. Genomic DNA was isolated as described in Supplemental Experimental Procedures. To screen genomic DNA samples for integration of the corrective/selective sequences, the CFTR target locus was amplified by PCR, to yield an integration-specific product using one primer within the selection cassette and one outside the homology arm in the genomic sequence upstream of the integration site (F: CTTCCTCTGCTACCTCCTTTCC, R: CCGATAAAACACATGCGTCA). For screening excised colonies, PCR products were digested with ClaI, a silent restriction site for which was introduced via the targeting vector, at 37°C incubator for at least 1 hour. Digests were run on a 1% agarose gel and excised clones were identified by the presence of cleavage products of the expected sizes.
PiggyBac Transposase Nucleofection (excision)
Excision of the integrated selection cassette from corrected clones was done by overexpressing piggyBac transposase in clonal iPSC lines expressing puromycin resistance and sensitivity to ganciclovir. 4µg of various piggyBac transposase vectors (System Biosciences) were nucleofected into 1×106 iPSC using the Amaxa Nucleofector Device as described above, followed by selection with 3µg/ml ganciclovir to eliminate unexcised cells.
Differentiation to Respiratory Epithelial Cells
Differentiation was carried out as previously described (Firth et al., 2014). Briefly, transwell inserts were coated with a combination of Fibronectin (50µg/ml), Laminin (5µg/ml) and Collagen IV (60mg/ml, Sigma-Aldrich). A single cell suspension of iPSC was generated using Accutase. 300,000 iPSC were plated per 30mm insert in TeSR with ROCK inhibitor for 24 hours. Differentiation to airway epithelial cells proceeded with media changes as detailed in Firth et al, 2014 (Firth et al., 2014). Data was analyzed at Day 42 of differentiation (Day 28 of ALI). Methodology for immunofluorescence imaging is included in the Supplemental Methods and antibodies in Table S2.
Analysis of the Epithelial Differentiation
Immunocytochemistry was carried out on paraffin embossed sections as previously detailed (Firth et al., 2014). RNA was isolated using the Qiagen RNeasy kit as per the manufacturer’s instructions and 500ng of RNA reverse transcribed using High capacity cDNA kit (Applied Biosciences) as previously described (Firth et al., 2014). Real time quantitative PCR was carried out on the 7900HT Fast Real-Time PCR System in the Salk Functional Genomics Core facility and data analyzed using SDS2.3 and Microsoft Excel. Data is expressed as relative expression (ct) corrected for internal control GAPDH and normalized to iPSC ± SEM.
Western Blot
Protein was isolated from the samples using RIPA Buffer with protease inhibitors for 30 minutes on ice. 40µg protein lysate was loaded onto a 4–12% Bis-Tris gel (Life Technologies, Carlsbad, CA) and run in MOPS buffer(Life Technologies, Carlsbad, CA). Protein was transferred to a nitrocellulose membrane The membrane was probed with mouse anti-human CFTR-570 (1:1000) and anti-human beta actin (1:5000).
Patch Clamp Electrophysiology
Patch Clamp Experiments were carried out as previously described (Firth et al., 2014). Briefly, the pipette (intracellular) solution contained (in mM): NaCl 5, MgCl2 2, CsCl 145, EGTA 10, HEPES 10, MgATP 5, CsOH to pH7.2. The bath (extracellular) solution contained (in mM): CaCl2 2, NaCl 150, MgCl2 2, HEPES 10, Tris HCl to pH7.4. Cells were held at a holding potential of −40mV and 200ms depolarizing steps in 20mV increments from −80 to +80mV were applied. The data was filtered at 3.3kHz and digitized at 4kHz. Cm and Rs were routinely compensated for. Pipette resistance 2–4MOhm. For CFTR activation Forskolin (10µM), isobutylmethyl xanthine (IBMX,100µM) and Genistein (50µM) was added to the extracellular solution and the cells were perfused for 5 minutes prior to recording. After CFTR activation cells were perfused with CFTR-inh172 (3-[(3-trifluoromethyl) phenyl]-5-[(4-carboxyphenyl)methylene]-2-thioxo-4-thiazolidinone,10µM) for 5 min prior to recording.
Supplementary Material
ACKNOWLEDGEMENTS
We would like to acknowledge Gerald M. Pao, Ph.D. for valuable technical expertise and scientific discussions. Research reported in this article made use of the Waitt Advanced Biophotonics Center Core Facility (supported by the Waitt Foundation, NCI P30 CA014195-40 and NINDS P30 NS072031-03), the Flow Cytometry and Functional Genomics Core Facilities (supported by NCI P30 CA014195-40) and the Stem Cell Core Facility TM was supported by a Pioneer Fund Postdoctoral Scholar Award. ALF was supported by a CIRM Postdoctoral Training Fellowship #TG2-01158. CTD (2012), GSP (2013) and BML (2014) were supported by CIRM-Bridges Internship #TB1-01175. IMV is an American Cancer Society Professor of Molecular Biology, and holds the Irwin and Joan Jacobs Chair in Exemplary Life Science. This work was supported in part by grants from the NIH (P30 CA014195-38, AI048034, HL053670), CIRM (CL1-00500-1.2), Ipsen, Sanofi Aventis, and the H.N. and Frances C. Berger Foundation. The Leona M. and Harry B. Helmsley Charitable Trust grant #2012-PG-MED002, LifesharingR of San Diego for Tissue recovery.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
AUTHOR CONTRIBUTIONS
ALF & TM generated the idea, designed experiments, performed the experiments, analyzed data and wrote the manuscript, GSP assisted with stem cell and gene correction experiments, SJQ & CTD assisted with all stem cell experiments, BML assisted with the gene correction experiments, EK analyzed and presented data from the exome sequencing, RW performed the electrophysiology experiments, AK provided patient tissues for the experiments, academic input and reviewed the manuscript, FHG provided support and coordinated the electrophysiology experiments, IMV designed and coordinated the research, provided support and wrote the manuscript.
REFERENCES
- Boyd AC, Porteous DJ. Revisiting the mouse lung model for CF. Gene Ther. 2004;11:737–738. doi: 10.1038/sj.gt.3302257. [DOI] [PubMed] [Google Scholar]
- Cho SW, Kim S, Kim JM, Kim JS. Targeted genome engineering in human cells with the Cas9 RNA-guided endonuclease. Nat Biotechnol. 2013;31:230–232. doi: 10.1038/nbt.2507. [DOI] [PubMed] [Google Scholar]
- Cong L, Ran FA, Cox D, Lin S, Barretto R, Habib N, Hsu PD, Wu X, Jiang W, Marraffini LA, et al. Multiplex genome engineering using CRISPR/Cas systems. Science. 2013;339:819–823. doi: 10.1126/science.1231143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crane AM, Kramer P, Bui JH, Chung WJ, Li XS, Gonzalez-Garay ML, Hawkins F, Liao W, Mora D, Choi S, et al. Targeted Correction and Restored Function of the CFTR Gene in Cystic Fibrosis Induced Pluripotent Stem Cells. Stem Cell Reports. 2015 doi: 10.1016/j.stemcr.2015.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DiCarlo JE, Norville JE, Mali P, Rios X, Aach J, Church GM. Genome engineering in Saccharomyces cerevisiae using CRISPR-Cas systems. Nucleic Acids Res. 2013;41:4336–4343. doi: 10.1093/nar/gkt135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Firth AL, Dargitz CT, Qualls SJ, Menon T, Wright R, Singer O, Gage FH, Khanna A, Verma IM. Generation of multiciliated cells in functional airway epithelia from human induced pluripotent stem cells. Proc Natl Acad Sci U S A. 2014;111:E1723–E1730. doi: 10.1073/pnas.1403470111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fusaki N, Ban H, Nishiyama A, Saeki K, Hasegawa M. Efficient induction of transgene-free human pluripotent stem cells using a vector based on Sendai virus, an RNA virus that does not integrate into the host genome. Proc Jpn Acad Ser B Phys Biol Sci. 2009;85:348–362. doi: 10.2183/pjab.85.348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hale CR, Majumdar S, Elmore J, Pfister N, Compton M, Olson S, Resch AM, Glover CV, 3rd, Graveley BR, Terns RM, et al. Essential features and rational design of CRISPR RNAs that function with the Cas RAMP module complex to cleave RNAs. Mol Cell. 2012;45:292–302. doi: 10.1016/j.molcel.2011.10.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hwang WY, Fu Y, Reyon D, Maeder ML, Tsai SQ, Sander JD, Peterson RT, Yeh JR, Joung JK. Efficient genome editing in zebrafish using a CRISPR-Cas system. Nat Biotechnol. 2013;31:227–229. doi: 10.1038/nbt.2501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang W, Bikard D, Cox D, Zhang F, Marraffini LA. RNA-guided editing of bacterial genomes using CRISPR-Cas systems. Nat Biotechnol. 2013;31:233–239. doi: 10.1038/nbt.2508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jinek M, Chylinski K, Fonfara I, Hauer M, Doudna JA, Charpentier E. A programmable dual-RNA-guided DNA endonuclease in adaptive bacterial immunity. Science. 2012;337:816–821. doi: 10.1126/science.1225829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mali P, Yang L, Esvelt KM, Aach J, Guell M, DiCarlo JE, Norville JE, Church GM. RNA-guided human genome engineering via Cas9. Science. 2013;339:823–826. doi: 10.1126/science.1232033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Millen AM, Horvath P, Boyaval P, Romero DA. Mobile CRISPR/Cas-mediated bacteriophage resistance in Lactococcus lactis. PLoS One. 2012;7:e51663. doi: 10.1371/journal.pone.0051663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakayama T, Fish MB, Fisher M, Oomen-Hajagos J, Thomsen GH, Grainger RM. Simple and efficient CRISPR/Cas9-mediated targeted mutagenesis in Xenopus tropicalis. Genesis. 2013;51:835–843. doi: 10.1002/dvg.22720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patrick AE, Karamyshev AL, Millen L, Thomas PJ. Alteration of CFTR transmembrane span integration by disease-causing mutations. Mol Biol Cell. 2011;22:4461–4471. doi: 10.1091/mbc.E11-05-0396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogan MP, Stoltz DA, Hornick DB. Cystic fibrosis transmembrane conductance regulator intracellular processing, trafficking, and opportunities for mutation-specific treatment. Chest. 2011;139:1480–1490. doi: 10.1378/chest.10-2077. [DOI] [PubMed] [Google Scholar]
- Rogers CS, Stoltz DA, Meyerholz DK, Ostedgaard LS, Rokhlina T, Taft PJ, Rogan MP, Pezzulo AA, Karp PH, Itani OA, et al. Disruption of the CFTR gene produces a model of cystic fibrosis in newborn pigs. Science. 2008;321:1837–1841. doi: 10.1126/science.1163600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwank G, Koo BK, Sasselli V, Dekkers JF, Heo I, Demircan T, Sasaki N, Boymans S, Cuppen E, van der Ent CK, et al. Functional repair of CFTR by CRISPR/Cas9 in intestinal stem cell organoids of cystic fibrosis patients. Cell Stem Cell. 2013;13:653–658. doi: 10.1016/j.stem.2013.11.002. [DOI] [PubMed] [Google Scholar]
- Sun X, Sui H, Fisher JT, Yan Z, Liu X, Cho HJ, Joo NS, Zhang Y, Zhou W, Yi Y, et al. Disease phenotype of a ferret CFTR-knockout model of cystic fibrosis. J Clin Invest. 2010;120:3149–3160. doi: 10.1172/JCI43052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi K, Okita K, Nakagawa M, Yamanaka S. Induction of pluripotent stem cells from fibroblast cultures. Nat Protoc. 2007a;2:3081–3089. doi: 10.1038/nprot.2007.418. [DOI] [PubMed] [Google Scholar]
- Takahashi K, Tanabe K, Ohnuki M, Narita M, Ichisaka T, Tomoda K, Yamanaka S. Induction of pluripotent stem cells from adult human fibroblasts by defined factors. Cell. 2007b;131:861–872. doi: 10.1016/j.cell.2007.11.019. [DOI] [PubMed] [Google Scholar]
- Wang H, Yang H, Shivalila CS, Dawlaty MM, Cheng AW, Zhang F, Jaenisch R. One-step generation of mice carrying mutations in multiple genes by CRISPR/Cas-mediated genome engineering. Cell. 2013;153:910–918. doi: 10.1016/j.cell.2013.04.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Welsh MJ, Rogers CS, Stoltz DA, Meyerholz DK, Prather RS. Development of a porcine model of cystic fibrosis. Trans Am Clin Climatol Assoc. 2009;120:149–162. [PMC free article] [PubMed] [Google Scholar]
- Wiedenheft B, Sternberg SH, Doudna JA. RNA-guided genetic silencing systems in bacteria and archaea. Nature. 2012;482:331–338. doi: 10.1038/nature10886. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.