Abstract
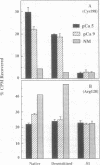
A change in the conformation of the active site of scallop myosin under the influence of regulatory amounts of Ca2+ has been identified by use of the ADP photoaffinity analog 2-[(4-azido-2-nitrophenyl)amino]ethyl diphosphate (NANDP). NANDP, trapped at the active site with Mn2+ and vanadate, photolabeled preferentially Arg-128 of the heavy chain in the absence of added Mg2+ and Ca2+ [Kerwin, B. & Yount, R. (1992) Bioconjugate Chem. 3, 328-336]. However, addition of 2 mM Mg2+ and regulatory amounts of Ca2+ (0.01-1 microM) shifted the predominant labeling to Cys-198 of the heavy chain in a Ca(2+)-dependent manner. This Ca(2+)-dependent change in the photolabeling pattern was absent when the regulatory light chains were removed or when the unregulated head (subfragment 1) was examined under similar conditions. These results demonstrate that both Arg-128 and Cys-198 are part of the purine binding site which undergoes a conformational change in response to Ca2+ binding to the regulatory domain.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adelstein R. S., Eisenberg E. Regulation and kinetics of the actin-myosin-ATP interaction. Annu Rev Biochem. 1980;49:921–956. doi: 10.1146/annurev.bi.49.070180.004421. [DOI] [PubMed] [Google Scholar]
- Ashiba G., Szent-Györgyi A. G. Essential light chain exchange in scallop myosin. Biochemistry. 1985 Nov 5;24(23):6618–6623. doi: 10.1021/bi00344a048. [DOI] [PubMed] [Google Scholar]
- Borejdo J., Werber M. M. Binding of calcium and magnesium to myosin in skeletal muscle myofibrils. Biochemistry. 1982 Feb 2;21(3):549–555. doi: 10.1021/bi00532a021. [DOI] [PubMed] [Google Scholar]
- Chantler P. D., Szent-Györgyi A. G. Regulatory light-chains and scallop myosin. Full dissociation, reversibility and co-operative effects. J Mol Biol. 1980 Apr 15;138(3):473–492. doi: 10.1016/s0022-2836(80)80013-1. [DOI] [PubMed] [Google Scholar]
- Chantler P. D., Szent-Györgyi A. G. Spectroscopic studies on invertebrate myosins and light chains. Biochemistry. 1978 Dec 12;17(25):5440–5448. doi: 10.1021/bi00618a018. [DOI] [PubMed] [Google Scholar]
- Cremo C. R., Grammer J. C., Yount R. G. Vanadate-mediated photocleavage of myosin. Methods Enzymol. 1991;196:442–449. doi: 10.1016/0076-6879(91)96038-s. [DOI] [PubMed] [Google Scholar]
- EBASHI S., EBASHI F. A NEW PROTEIN COMPONENT PARTICIPATING IN THE SUPERPRECIPITATION OF MYOSIN B. J Biochem. 1964 Jun;55:604–613. doi: 10.1093/oxfordjournals.jbchem.a127933. [DOI] [PubMed] [Google Scholar]
- Facemyer K. C., Cremo C. R. A new method to specifically label thiophosphorylatable proteins with extrinsic probes. Labeling of serine-19 of the regulatory light chain of smooth muscle myosin. Bioconjug Chem. 1992 Sep-Oct;3(5):408–413. doi: 10.1021/bc00017a009. [DOI] [PubMed] [Google Scholar]
- Goodwin E. B., Leinwand L. A., Szent-Györgyi A. G. Regulation of scallop myosin by mutant regulatory light chains. J Mol Biol. 1990 Nov 5;216(1):85–93. doi: 10.1016/S0022-2836(05)80062-2. [DOI] [PubMed] [Google Scholar]
- Hardwicke P. M., Szent-Györgyi A. G. Proximity of regulatory light chains in scallop myosin. J Mol Biol. 1985 May 25;183(2):203–211. doi: 10.1016/0022-2836(85)90213-x. [DOI] [PubMed] [Google Scholar]
- Hardwicke P. M., Wallimann T., Szent-Györgyi A. G. Light-chain movement and regulation in scallop myosin. Nature. 1983 Feb 10;301(5900):478–482. doi: 10.1038/301478a0. [DOI] [PubMed] [Google Scholar]
- Jackson A. P., Bagshaw C. R. Kinetic trapping of intermediates of the scallop heavy meromyosin adenosine triphosphatase reaction revealed by formycin nucleotides. Biochem J. 1988 Apr 15;251(2):527–540. doi: 10.1042/bj2510527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson A. P., Bagshaw C. R. Transient-kinetic studies of the adenosine triphosphatase activity of scallop heavy meromyosin. Biochem J. 1988 Apr 15;251(2):515–526. doi: 10.1042/bj2510515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kauer J. C., Erickson-Viitanen S., Wolfe H. R., Jr, DeGrado W. F. p-Benzoyl-L-phenylalanine, a new photoreactive amino acid. Photolabeling of calmodulin with a synthetic calmodulin-binding peptide. J Biol Chem. 1986 Aug 15;261(23):10695–10700. [PubMed] [Google Scholar]
- Kendrick-Jones J., Lehman W., Szent-Györgyi A. G. Regulation in molluscan muscles. J Mol Biol. 1970 Dec 14;54(2):313–326. doi: 10.1016/0022-2836(70)90432-8. [DOI] [PubMed] [Google Scholar]
- Kerwin B. A., Yount R. G. Photoaffinity labeling of scallop myosin with 2-[(4-azido-2-nitrophenyl)amino]ethyl diphosphate: identification of an active site arginine analogous to tryptophan-130 in skeletal muscle myosin. Bioconjug Chem. 1992 Jul-Aug;3(4):328–336. doi: 10.1021/bc00016a012. [DOI] [PubMed] [Google Scholar]
- Krebs J., Buerkler J., Guerini D., Brunner J., Carafoli E. 3-(Trifluoromethyl)-3-(m-[125I]iodophenyl)diazirine, a hydrophobic, photoreactive probe, labels calmodulin and calmodulin fragments in a Ca2+-dependent way. Biochemistry. 1984 Jan 31;23(3):400–403. doi: 10.1021/bi00298a002. [DOI] [PubMed] [Google Scholar]
- Kwon H., Goodwin E. B., Nyitray L., Berliner E., O'Neall-Hennessey E., Melandri F. D., Szent-Györgyi A. G. Isolation of the regulatory domain of scallop myosin: role of the essential light chain in calcium binding. Proc Natl Acad Sci U S A. 1990 Jun;87(12):4771–4775. doi: 10.1073/pnas.87.12.4771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamaye K. L., Wells J. A., Bridenbaugh R. L., Okamoto Y., Yount R. G. 2-[(4-Azido-2-nitrophenyl)amino]ethyl triphosphate, a novel chromophoric and photoaffinity analogue of ATP. Synthesis, characterization, and interaction with myosin subfragment 1. Biochemistry. 1985 Sep 10;24(19):5226–5235. doi: 10.1021/bi00340a041. [DOI] [PubMed] [Google Scholar]
- Nyitray L., Goodwin E. B., Szent-Györgyi A. G. Complete primary structure of a scallop striated muscle myosin heavy chain. Sequence comparison with other heavy chains reveals regions that might be critical for regulation. J Biol Chem. 1991 Oct 5;266(28):18469–18476. [PubMed] [Google Scholar]
- O'Neil K. T., Erickson-Viitanen S., DeGrado W. F. Photolabeling of calmodulin with basic, amphiphilic alpha-helical peptides containing p-benzoylphenylalanine. J Biol Chem. 1989 Aug 25;264(24):14571–14578. [PubMed] [Google Scholar]
- Okamoto Y., Yount R. G. Identification of an active site peptide of skeletal myosin after photoaffinity labeling with N-(4-azido-2-nitrophenyl)-2-aminoethyl diphosphate. Proc Natl Acad Sci U S A. 1985 Mar;82(6):1575–1579. doi: 10.1073/pnas.82.6.1575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Otsuki I., Masaki T., Nonomura Y., Ebashi S. Periodic distribution of troponin along the thin filament. J Biochem. 1967 Jun;61(6):817–819. doi: 10.1093/oxfordjournals.jbchem.a128619. [DOI] [PubMed] [Google Scholar]
- Pate E., Nakamaye K. L., Franks-Skiba K., Yount R. G., Cooke R. Mechanics of glycerinated muscle fibers using nonnucleoside triphosphate substrates. Biophys J. 1991 Mar;59(3):598–605. doi: 10.1016/S0006-3495(91)82275-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Penefsky H. S. Reversible binding of Pi by beef heart mitochondrial adenosine triphosphatase. J Biol Chem. 1977 May 10;252(9):2891–2899. [PubMed] [Google Scholar]
- Reisler E., Liu J., Cheung P. Role of magnesium binding to myosin in controlling the state of cross-bridges in skeletal rabbit muscle. Biochemistry. 1983 Oct 11;22(21):4954–4960. doi: 10.1021/bi00290a012. [DOI] [PubMed] [Google Scholar]
- Samuel M., Chowrashi P. K., Pepe F. A., Chacko S. Effects of phosphorylation, magnesium, and filament assembly on actin-activated ATPase of pig urinary bladder myosin. Biochemistry. 1990 Jul 31;29(30):7124–7132. doi: 10.1021/bi00482a026. [DOI] [PubMed] [Google Scholar]
- Sherry J. M., Górecka A., Aksoy M. O., Dabrowska R., Hartshorne D. J. Roles of calcium and phosphorylation in the regulation of the activity of gizzard myosin. Biochemistry. 1978 Oct 17;17(21):4411–4418. doi: 10.1021/bi00614a009. [DOI] [PubMed] [Google Scholar]
- Sobieszek A., Small J. V. Regulation of the actin-myosin interaction in vertebrate smooth muscle: activation via a myosin light-chain kinase and the effect of tropomyosin. J Mol Biol. 1977 Jun 5;112(4):559–576. doi: 10.1016/s0022-2836(77)80164-2. [DOI] [PubMed] [Google Scholar]
- Stafford W. F., 3rd, Szentkiralyi E. M., Szent-Györgyi A. G. Regulatory properties of single-headed fragments of scallop myosin. Biochemistry. 1979 Nov 27;18(24):5273–5280. doi: 10.1021/bi00591a002. [DOI] [PubMed] [Google Scholar]
- Suzuki H., Suzuki S., Watanabe S. High concentrations of magnesium for formation of thick-filaments of chicken gizzard myosin. J Biochem. 1981 Mar;89(3):871–878. doi: 10.1093/oxfordjournals.jbchem.a133270. [DOI] [PubMed] [Google Scholar]
- Szent-Györgyi A. G., Szentkiralyi E. M., Kendrick-Jonas J. The light chains of scallop myosin as regulatory subunits. J Mol Biol. 1973 Feb 25;74(2):179–203. doi: 10.1016/0022-2836(73)90106-x. [DOI] [PubMed] [Google Scholar]
- Szentkiralyi E. M. Tryptic digestion of scallop S1: evidence for a complex between the two light-chains and a heavy-chain peptide. J Muscle Res Cell Motil. 1984 Apr;5(2):147–164. doi: 10.1007/BF00712153. [DOI] [PubMed] [Google Scholar]
- Trotter J. A., Adelstein R. S. Macrophage myosin. Regulation of actin-activated ATPase, activity by phosphorylation of the 20,000-dalton light chain. J Biol Chem. 1979 Sep 25;254(18):8781–8785. [PubMed] [Google Scholar]
- Trybus K. M. Regulation of smooth muscle myosin. Cell Motil Cytoskeleton. 1991;18(2):81–85. doi: 10.1002/cm.970180202. [DOI] [PubMed] [Google Scholar]
- Vibert P., Cohen C. Domains, motions and regulation in the myosin head. J Muscle Res Cell Motil. 1988 Aug;9(4):296–305. doi: 10.1007/BF01773873. [DOI] [PubMed] [Google Scholar]
- Vibert P., Craig R. Structural changes that occur in scallop myosin filaments upon activation. J Cell Biol. 1985 Sep;101(3):830–837. doi: 10.1083/jcb.101.3.830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vibert P., Szentkiralyi E., Hardwicke P., Szent-Györgyi A. G., Cohen C. Structural models for the regulatory switch of Myosin. Biophys J. 1986 Jan;49(1):131–133. doi: 10.1016/S0006-3495(86)83622-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallimann T., Hardwicke P. M., Szent-Györgyi A. G. Regulatory and essential light-chain interactions in scallop myosin. II. Photochemical cross-linking of regulatory and essential light-chains by heterobifunctional reagents. J Mol Biol. 1982 Mar 25;156(1):153–173. doi: 10.1016/0022-2836(82)90464-8. [DOI] [PubMed] [Google Scholar]
- Wallimann T., Szent-Györgyi A. G. An immunological approach to myosin light-chain function in thick filament linked regulation. 2. Effects of anti-scallop myosin light-chain antibodies. Possible regulatory role for the essential light chain. Biochemistry. 1981 Mar 3;20(5):1188–1197. doi: 10.1021/bi00508a021. [DOI] [PubMed] [Google Scholar]
- Wells C., Bagshaw C. R. Calcium regulation of molluscan myosin ATPase in the absence of actin. Nature. 1985 Feb 21;313(6004):696–697. doi: 10.1038/313696a0. [DOI] [PubMed] [Google Scholar]
- Yanagisawa M., Hamada Y., Katsuragawa Y., Imamura M., Mikawa T., Masaki T. Complete primary structure of vertebrate smooth muscle myosin heavy chain deduced from its complementary DNA sequence. Implications on topography and function of myosin. J Mol Biol. 1987 Nov 20;198(2):143–157. doi: 10.1016/0022-2836(87)90302-0. [DOI] [PubMed] [Google Scholar]
- el-Saleh S. C., Warber K. D., Potter J. D. The role of tropomyosin-troponin in the regulation of skeletal muscle contraction. J Muscle Res Cell Motil. 1986 Oct;7(5):387–404. doi: 10.1007/BF01753582. [DOI] [PubMed] [Google Scholar]