Abstract
Although visual processing impairments are common in schizophrenia, it is not clear to what extent these originate in the eye vs. the brain. This review highlights potential contributions, from the retina and other structures of the eye, to visual processing impairments in schizophrenia and high-risk states. A second goal is to evaluate the status of retinal abnormalities as biomarkers for schizophrenia. The review was motivated by known retinal changes in other disorders (e.g., Parkinson’s disease, multiple sclerosis), and their relationships to perceptual and cognitive impairments, and disease progression therein. The evidence reviewed suggests two major conclusions. One is that there are multiple structural and functional disturbances of the eye in schizophrenia, all of which could be factors in the visual disturbances of patients. These include retinal venule widening, retinal nerve fiber layer thinning, dopaminergic abnormalities, abnormal ouput of retinal cells as measured by electroretinography (ERG), maculopathies and retinopathies, cataracts, poor acuity, and strabismus. Some of these are likely to be illness-related, whereas others may be due to medication or comorbid conditions. The second conclusion is that certain retinal findings can serve as biomarkers of neural pathology, and disease progression, in schizophrenia. The strongest evidence for this to date involves findings of widened retinal venules, thinning of the retinal nerve fiber layer, and abnormal ERG amplitudes. These data suggest that a greater understanding of the contribution of retinal and other ocular pathology to the visual and cognitive disturbances of schizophrenia is warranted, and that retinal changes have untapped clinical utility.
Keywords: Schizophrenia; Vision; Perception; Retina; Macula; OCT, ERG
Visual processing impairments are well established in schizophrenia, including abnormalities in contrast sensitivity (Kelemen et al., 2013, Kiss et al., 2010); various excitatory and inhibitory functions (Kaplan and Lubow, 2011, Keri et al., 2005a, Robol et al., 2013) including those involved in masking (Green et al., 2011) and surround suppression (Tibber et al., 2013); and form and motion processing (Chen, 2011, Green et al., 2011, Javitt, 2009, Silverstein and Keane, 2011). There has been little work on color processing to date (Shuwairi et al., 2002), but clinical reports indicate frequent descriptions of increased intensity, or alterations in color perception (Chapman, 1966, Vollmer-Larsen et al., 2007). One study reported a 62% incidence of visual distortions in schizophrenia, with brightness, contrast, and motion increases being the most commonly reported (Phillipson and Harris, 1985). Visual distortions also had the highest predictive validity, among all basic symptoms, for conversion to a psychotic disorder (Klosterkotter et al., 2001), and visual distortions in help-seeking adolescents are associated with suicidal ideation, even after controlling for age, gender, depression, thought disorder, paranoia, and auditory distortions (Grano et al., 2015). Finally, visual impairments contribute substantially to poorer real-world functioning in people with schizophrenia (Green et al., 2012, Rassovsky et al., 2011).
In spite of this growing body of evidence, an unanswered question is the extent to which the problems observed in recent laboratory or clinical reports are due to changes in the eye vs. in the brain. It has already been shown that abnormalities (i.e., hypo- or hyper-activation) exist in occipital (Butler et al., 2013, Silverstein et al., 2009), temporal (Silverstein et al., 2010a), parietal (Dima et al., 2009), and prefrontal (Dima et al., 2009, Silverstein et al., 2009, Silverstein et al., 2010b) regions during visual processing tasks in schizophrenia, depending on the nature of the task requirements. These findings are thought to reflect both inefficient stimulus-driven (i.e., bottom-up) processing, as well as deficient top-down guidance or modulation of the feedforward sweep of information based on prior knowledge, expectations, and task strategies (Dima et al., 2009, Dima et al., 2010, Dima et al., 2011, Keane et al., 2013, Silverstein and Keane, 2009). However, degraded input to the visual system could contribute to both sets of findings, by adding noise to (or lowering the resolution of) sensory information, which would disrupt stimulus-driven processing, and by making it less likely that appropriate stimulus templates or memory representations would be activated to modulate processing. It is the purpose of this review to: 1) highlight potential mechanisms by which schizophrenia, or its treatment, can disrupt retinal function and lead to some of the common laboratory findings or visual distortions reported by patients; and 2) describe other types of ocular dysfunction that may contribute to these visual impairments. The extent to which these retinal and other ocular changes can serve as biomarkers for schizophrenia or psychosis will also be highlighted.
1. Retinal cellular and vascular changes in schizophrenia and high-risk states
The retina is an active information processor, in which, among other things, early forms of transformations traditionally thought to occur only in visual cortex, take place. For example, in response to moving stimuli, cone photoreceptors produce motion blur, which has been shown to enhance information about orientation and direction of movement, shape contour orientation and completion, changes in texture between stimulus regions, spatial frequency, and depth (van Tonder, 2010). Motion blur can also affect perception of complex stimuli such as facial emotions. Importantly, much input to cone photoreceptors (the photoreceptor type involved in color vision) is dopaminergic, and, as reviewed below, retinal dopamine (DA) abnormalities could contribute to several forms of visual impairments in schizophrenia. An implication of this is that anything that affects retinal function may result in intensified, degraded or noisy input to higher levels of processing in LGN and visual cortex, depending on the nature of the abnormality.
The retina develops from the same tissue (neuroectoderm) as the brain, and is the only part of the central nervous system that can be seen with the naked eye in its natural state in living organisms. Given that retinal changes may parallel, or mirror the integrity of brain structure and function (Chu et al., 2012, Kemenyova et al., 2014, Lee et al., 2013, Tian et al., 2011), it has been suggested that retinal changes can serve as a marker of progressive brain tissue loss. Given this, the well-documented structural and functional brain changes in schizophrenia, and the obvious implications of altered retinal function for changes in visual processing, what is the evidence for retinal abnormalities in schizophrenia?
Data from the Dunedin longitudinal study indicated that, at age 38, individuals with schizophrenia had wider retinal venules than cohort members who did not have schizophrenia, suggesting a history of chronic insufficient brain oxygen supply (Meier et al., 2013). This finding was not secondary to other health issues. Moreover, wider retinal venules were associated with extent of psychotic symptoms in adulthood, and in childhood. The association between wider retinal venule width and psychosis (assessed 6 years after the eye exam) was recently replicated in two Australian twin studies (Meier et al., 2015). Moreover, retinal venule width in unaffected twins of a psychotic sibling was characterized by values intermediate between controls and psychotic siblings. Arteriole width was unaffected by presence of psychosis in twins with psychosis or their unaffected siblings. These data suggested that retinal venule widening may be a proxy marker of familial risk for psychosis. Other evidence indicates that retinal venule width is significantly correlated with childhood IQ and with neuropsychological functioning at midlife, in the general population (Shalev et al., 2013). A similar recent finding is that, in the general population, color vision impairments [implying DA dysregulation in cone photoreceptors (see below)] predict difficulties with cognitive control (Colzato et al., 2014) — a function thought to involve dopaminergic projections to the prefrontal cortex (van Schouwenburg et al., 2010), and that is characteristically impaired in schizophrenia (Lesh et al., 2011). These data suggest that basic aspects of retinal structure and function may indicate increased risk for schizophrenia, and be impaired in the disorder, via reflecting reduced vascular and brain health.
Other evidence for structural retinal changes in schizophrenia comes from studies of optical coherence tomography (OCT). OCT is a recently developed, rapid, non-invasive imaging technique that can reveal retinal structure in vivo with axial resolutions of 5 microns or less. It has recently proven useful for identifying thinning of certain retinal layers in several neurological conditions such as multiple sclerosis (MS) (Khanifar et al., 2010, Martinez-Lapiscina et al., 2014), Parkinson’s disease (Satue et al., 2013, Satue et al., 2014, Tian et al., 2011), and Alzheimer’s disease (Moschos et al., 2012), in addition to focal eye diseases (Leung et al., 2010). Retinal nerve fiber layer (RNFL) thinning – reflecting loss of ganglion cell axons that leave the retina as the optic nerve and synapse onto the lateral geniculate nucleus – as revealed by OCT, is thought to be a good model of brain neurodegeneration, since retinal cells are unmyelinated, and so any thinning directly reflects cell loss (Lee et al., 2013). Consistent with this, progressive RNFL thinning parallels disease progression in Parkinson’s disease (Tian et al., 2011), where level of thinning correlates significantly with extent of visual hallucinations (Lee et al., 2014) and functional disability (Satue et al., 2014). In MS, RNFL thinning is associated with duration of illness, gray matter loss, poorer executive functioning, poorer overall cognitive functioning, illness progression, and relapse (Martinez-Lapiscina et al., 2014, Ratchford et al., 2013, Saidha et al., 2013, Sedighi et al., 2014, Toledo et al., 2008). Interestingly, even in healthy subjects, OCT indices correlate with intracranial volume (Saidha et al., 2013). OCT studies of schizophrenia have focused on RNFL thinning and reductions in macular volume (MV) (reflecting integrity of the fovea and surrounding tissue). While an initial small study (n=10 patients) reported RNFL thinning (Ascaso et al., 2010), this was not replicated in a subsequent study (Chu et al., 2012), although in the latter, reduced MV was related to increased positive symptoms. However, this study used an earlier generation (time domain) OCT device characterized by an ~ 10 micron axial resolution generating 400 scans per second. More recent studies, using spectral-domain OCT with resolution of ~ 5 microns and over 25,000 scans per second, have observed both RNFL and MV thinning (Lee et al., 2013), or RNFL thinning only (Cabezon et al., 2012), in schizophrenia (see Fig. 1, Fig. 2). As with retinal venule widening, the functional significance of these findings is not yet clear, although one study found that RNFL thinning was related to illness chronicity in schizophrenia (Lee et al., 2013). Studies investigating the relationship between retinal degeneration and visual processing are just beginning to be carried out with schizophrenia patients. However, it is thought that problems in visual processing (including in contrast sensitivity, color perception, and reading) in Parkinson’s disease may be due to retinal cell loss (Djamgoz et al., 1997, Harnois and Di Paolo, 1990, Hutton and Morris, 2001, Rodnitzky, 1998), and similar problems have been observed in schizophrenia (Butler et al., 2005, Cadenhead et al., 2013, Kelemen et al., 2013, Revheim et al., 2006b, Revheim et al., 2014) (see below). In addition, as described in the next section, ganglion cell loss (which may be the primary cause of RNFL thinning) may be a marker of other cellular processes that have been linked to specific visual impairments. An additional consideration for studies of schizophrenia is that diabetic retinal changes (maculopathy) detectable by OCT (Sikorski et al., 2013) could contribute to visual processing disturbances, and so must be ruled out in both eyes of patients.
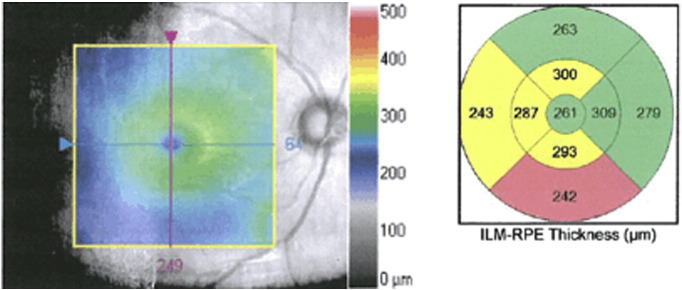
Fig. 1.
Macular thinning, as shown in a representative patient with schizophrenia in an ongoing study of the authors. Left panel: gray-scale image of the macula from right eye of patient. Central colorized panel is a topographic map overlay (50% transparency) showing thickness (in μm) from the inner limiting membrane (ILM, which covers the retinal nerve fiber layer; see Fig. 2) to the retinal pigment epithelium (RPE, which is beneath the photoreceptor layer). Thickness scale is depicted by the legend on right side of figure. Right panel: average thickness values of the segments are colorized relative to age-matched control subjects. Regions in yellow denote values observed in less than 5% of age-matched subjects; regions in pink–red denote values observed in less than 1% of age-matched subjects. This patient demonstrates borderline significant or significant thinning in more than half of the macular subregions in this eye.
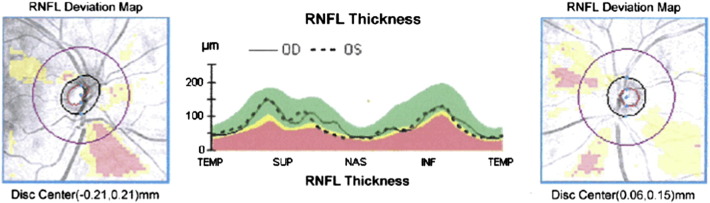
Fig. 2.
Retinal nerve fiber layer (RNFL) thickness map represents the thickness of the layer of ganglion cell axons that leave the retina as the optic nerve. There is thinning of focal sectors from a representative patient with schizophrenia in an ongoing study of the authors. Left and right panels depict right and left eyes, respectively. Areas shaded yellow represent age-corrected thickness values observed in less than 5% of the general population. Areas in pink–red represent values observed in less than 1% of the general population. Center panel depicts actual thickness values in temporal (TEMP), superior (SUP), nasal (NAS), and inferior (INF) retinal quadrants, for the left (dashed line) and right (continuous line) eyes. Yellow and pink–red shaded areas represent the same age-normed values as in the left and right panels. This patient demonstrates significant (< 1% of the population) thinning in both left and right inferior RNFL quadrants, and borderline significant (< 5% of the population) thinning in the left nasal quadrant.
2. Dopamine and retinal function
DA is a major neurotransmitter in the vertebrate retina, and its cellular localization and functions are similar in organisms as diverse as fish and primates (Djamgoz et al., 1997, Masson et al., 1993). In the retina, DA originates in one class of amacrine cells (ACs) and in interplexiform cells. It is transmitted via standard synaptic transmission, as well as by volume transmission, where it can diffuse up to 3 mm through retinal tissue to potentially influence every type of retinal neuron, as all have receptors for DA (Yazulla and Studholme, 1995). One important function of DA in the retina is to weaken the gap junctions that couple horizontal cells (Piccolino et al., 1984, Teranishi et al., 1983). Because horizontal cells (HCs) pool the activity of photoreceptor cells across space, this DA-related uncoupling leads to significant reduction of the normally large HC receptive fields (Xin and Bloomfield, 2000), and to increased sensitivity to local (relative to contextual) stimulation (Brandies and Yehuda, 2008). A second effect of the uncoupling of HCs is reduced interaction between neurons signaling light and dark portions of space, leading to enhanced center responses (and reduced effects of surround responses) (Hedden and Dowling, 1978).
The DA receptor types found in the retina are the same as those found in the brain (e.g., D1–D5), and can be roughly divided into D1 and D2 types (Brandies and Yehuda, 2008). Endogenous DA inhibits suprathreshold rod-mediated ON and OFF responses. However, with cones, DA increases excitation for ON responses, and increases inhibition for OFF responses in most cases (Popova and Kupenova, 2013). It is thought that D2 receptors are primarily involved in generating ON responses, whereas D1 receptors are mainly responsible for OFF responses (Popova and Kupenova, 2013). As a result of these asymmetries, excess DA at D2 receptors can lead to hyper-intense color perception (as seen, for example, in early schizophrenia, see below), whereas reduced DA can lead to reductions in color perception (as seen, for example, in Parkinson’s disease, see below).
Taken together, these data suggest that the excesses and reductions in DA activity repeatedly demonstrated in the brain in schizophrenia are likely to also occur in the retina, where they may play a significant role in visual processing disturbances. In the following two subsections, each of these scenarios, and their implications for understanding results of visual experiments in schizophrenia, is discussed.
2.1. Effects of reduced retinal DA on vision
Through studies of Parkinson’s disease patients (including post-mortem studies), along with animal and human studies of drug-induced Parkinsonian symptoms (Bodis-Wollner and Tagliati, 1993, Bulens et al., 1989), it is known that retinal DA is reduced in this condition (Tian et al., 2011). Reduced retinal DA leads to excessively strong coupling of HCs and ACs, leading to increased inhibition, and subsequently to reduced contrast sensitivity and poorer color vision and visual acuity (Djamgoz et al., 1997). Monkeys administrated MPTP or intraocular 6-OHDA (both of which destroy retinal DA neurons and reduce levels of retinal DA) show reduced processing of mid-range spatial frequencies (e.g., 2.5–3.5 cycles/degree) (Bodis-Wollner and Tagliati, 1993), and these changes were temporarily reversed by L-DOPA administration (Bodis-Wollner, 1990). Humans with drug-induced Parkinsonism have demonstrated a general reduction in contrast sensitivity across all spatial frequencies (Bulens et al., 1989), and the contrast sensitivity impairments in patients with Parkinson’s Disease can be reversed (with less strong effects at high spatial frequencies) after L-DOPA administration (Bulens et al., 1987). In addition, elderly subjects from the general population, who typically show poor low spatial frequency (LSF) processing, demonstrated improved processing across the full range of spatial frequencies after 3 months of treatment with the D2 agonist piribedil (Corbe et al., 1992). Moreover, in children and/or adults with amblyopia, a single dose of L-DOPA (but not placebo) significantly increased contrast sensitivity (Gottlob and Stangler-Zuschrott, 1990) and visual acuity (Leguire et al., 1992), and effects on acuity were 3 times stronger after a week of L-DOPA (Gottlob et al., 1992). Of note, these effects were obtained regardless of whether contrast was measured at, or above, detection threshold (Brandies and Yehuda, 2008). Finally, in healthy rats and humans, haloperidol (Haldol), including a single dose, delayed visual evoked potentials (VEPs) (reviewed in (Brandies and Yehuda, 2008)). These data are relevant to schizophrenia, because people with this condition typically take DA receptor antagonists, and have also demonstrated longer latency VEPs (Malyszczak et al., 2003), and problems in visual acuity (Viertio et al., 2007), contrast sensitivity (Kelemen et al., 2013, Kiss et al., 2010, Slaghuis, 1998), and LSF processing (Martinez et al., 2008, Martinez et al., 2012), although the latter impairment is not always found (Keane et al., 2014a, Laprevote et al., 2010, Laprevote et al., 2013), and it may not be specific to low spatial frequencies (Skottun and Skoyles, 2007).
The similarities between schizophrenia and Parkinson’s disease discussed above raise the question: are the findings in schizophrenia due to disease-related retinal DA reductions, or to receptor blockade secondary to antipsychotic medication? Data on this issue suggest that antipsychotic medication can have negative effects on visual function. For example, while antipsychotic medication in general did not impair visual acuity (which was reduced in subjects with schizophrenia), phenothiazines in particular were shown to impair near (but not far) acuity through their effect on ciliary muscle accommodation responses (Viertio et al., 2007), which is most relevant to reading and performing laboratory visual processing tasks. A recent study found impaired contrast sensitivity for both chromatic and achromatic stimuli in schizophrenia, with some evidence for more normal chromatic contrast sensitivity in unmedicated patients (Cadenhead et al., 2013). Similarly, in an earlier study, in a mixed group of psychiatric patients that included many with schizophrenia, administration of antipsychotic mediation led to a global decrease in contrast sensitivity compared to controls (Bulens et al., 1987). This was later replicated in a study demonstrating that schizophrenia patients on typical antipsychotic medications demonstrated poorer contrast sensitivity compared to both healthy controls and patients on atypical medications (Chen et al., 2003). In some cases, these decreases in contrast sensitivity are dose-dependent (Keri et al., 2002). Some studies of schizophrenia patients, however, have found that administration of antipsychotic medications decreases contrast sensitivity for medium and high spatial frequencies (Harris et al., 1990, Keri et al., 2002), and increases it at low spatial frequencies (Harris et al., 1990). Further evidence suggests that the combination of antipsychotic and antidepressant medication [a combination frequently prescribed due to high rates of depression in schizophrenia (Siris, 2000)] is especially detrimental to contrast sensitivity (Sheremata and Chen, 2004). It should be noted here that antipsychotic medications can interfere with vision in ways other than by reducing retinal DA, as instances of toxic maculopathy (Lee and Fern, 2004) and increased risk for cataract (McCarty et al., 1999) have been reported. However, these are far less likely to occur than the DA-depleting effects. Finally, in over half of a group of previously untreated schizophrenia patients, VEP latency increased after initiation of antipsychotic medication (Bodis-Wollner et al., 1982).
One consequence of retinal DA receptor blockade is less input to retinal ganglion cells, which has been proposed as a mechanism of ganglion cell atrophy in Parkinson’s Disease (Tian et al., 2011). This raises the intriguing possibility that the RNFL abnormalities reviewed in the section above may be secondary to antipsychotic (and perhaps other) medication use.
2.2. Effects of excess retinal dopamine on vision
Evidence from single-photon emission computed tomography (SPECT) and positron emission tomography (PET) studies supports the traditionally held view that the acute psychotic phase of schizophrenia is characterized by hyperdopaminergia (e.g., an exaggerated response of DA neurons), whereas functioning of the DA system approaches normal levels in periods of symptom remission (Kegeles et al., 2010, Laruelle et al., 1999). Therefore, changes in vision during acute psychotic episodes should be expected to be the opposite of those found with antipsychotic medication administration. Some evidence suggests that this is so. For example, in studies with healthy adult volunteers, L-DOPA administration improved contrast sensitivity at medium to high spatial frequencies, and decreased it at lower frequencies (Domenici et al., 1985), and apomorphine also reduced contrast sensitivity at low frequencies in a dose-dependent fashion (Blin et al., 1991). These effects were shown not to be a function of attention span or pupil diameter, suggesting a dopaminergic effect (Blin et al., 1991). Similar findings were demonstrated in unmedicated schizophrenia patients, who demonstrated higher contrast sensitivity compared to healthy controls (Chen et al., 2003). A more complex pattern of results was reported in a recent study of previously untreated first episode schizophrenia patients (Kelemen et al., 2013). This group demonstrated greater sensitivity than controls to low, but not medium, spatial frequencies, prior to antipsychotic treatment. After initiation of treatment, this superiority disappeared, due to a reduction in sensitivity to low spatial frequencies in the schizophrenia group (i.e., not due to changes in the control group) during the treatment period. Moreover, the normalization (reduction) in LSF processing after treatment occurred in conjunction with a reduction in self-reported anomalous visual experiences. Importantly, these visual effects were independent of GABA levels in the visual cortex, which affect visual processing (Rokem et al., 2011, Yoon et al., 2010) but were unaffected by this treatment, suggesting their possible retinal origin.
Can the findings of increased LSF processing in untreated first episode patients, with a decrease after treatment (Kelemen et al., 2013), be reconciled with the findings of an increase in LSF processing (and decreases in medium and high spatial frequency processing) after treatment in chronic schizophrenia patients (Harris et al., 1990)? This apparent paradox may reflect important differences between the groups: while the first episode patients were characterized by a hyperdopaminergic state prior to treatment, the chronic patients had many years of treatment, were clinically stable and living in the community, and were therefore presumably not in a hyperdopaminergic state when they were switched to an injectable medication (after which, the second testing was done at the point where blood levels of the medication peaked). It is therefore possible that the results with first episode cases reflect movement from hyperdopaminergic to normal levels, whereas the results with chronic cases reflect movement from normal levels towards hypodopaminergia, similar to what is observed in studies of Parkinson’s Disease (Bodis-Wollner and Tagliati, 1993). Even so, the increase in LSF processing after injectable medication in chronic patients is curious, and may be a spurious result, given the small sample size (n = 8), the unclear diagnostic criteria used, and the fact that an increase in LSF processing after treatment was not reported in other studies. Still, the decrease in medium–high spatial frequency processing after treatment in these patients is consistent with effects observed in Parkinson’s disease.
As noted above, excessive retinal DA can be expected to cause effects such as increased brightness and heightened intensity of color. Each of these has been reported (in clinical reports) in first episode schizophrenia (Chapman, 1966), along with a high level of visual distortions (Bunney et al., 1999, Klosterkotter et al., 2001), and, as predicted, all of these phenomena decrease after initiation of antipsychotic medications (Kelemen et al., 2013). In short, available, albeit limited, evidence suggests that hyperdopaminergia as found in schizophrenia is associated with excessive LSF processing, and this is consistent with other evidence of exaggerated magnocellular responsivity, and subjective changes in vision early in the illness (Keri and Benedek, 2007) and prior to long-term antipsychotic treatment.
3. Other neurotransmitters and retinal function
Glutamate is the major neurotransmitter in the vertebrate retina (de Souza et al., 2012), is the only output neurotransmitter of the photoreceptors (Copenhagen and Jahr, 1989), and is also released by bipolar and ganglion cells (Massey and Miller, 1990) (i.e., cells providing the feedforward sweep of information within and out of the retina). Moreover, glutamate output from photoreceptors is gated by the NMDA-type of glutamate receptor (Copenhagen and Jahr, 1989). This is potentially relevant to vision in schizophrenia since: 1) various lines of evidence converge in indicating that schizophrenia is characterized by NMDA dysregulation and NMDA receptor hypoactivity, leading to increased glutamate release, and this evidence forms the basis of a leading theory of the etiology of the condition (Kantrowitz and Javitt, 2010a, Kantrowitz and Javitt, 2010b, Moghaddam and Javitt, 2012, Olney and Farber, 1995); 2) NMDA dysregulation is known to cause increased DA release (Javitt, 2007), and to produce visual distortions, hallucinations, and performance on psychophysical tests of vision that resemble those found in schizophrenia (Phillips and Silverstein, 2003, Uhlhaas et al., 2007); 3) NMDA antagonists such as ketamine can cause pathological changes in the retina, including retinal hypoxia and cell death (Antal, 1979), and can cause reductions in the early (sensory) visual P1 evoked potential (Lalonde et al., 2006), which is also found in schizophrenia where it is related to level of positive symptoms (Gonzalez-Hernandez et al., 2014); and 4) many schizophrenia patients have diabetes, in many cases due to antipsychotic medication-related metabolic syndrome and weight gain, and diabetes is associated with increased retinal glutamate and retinopathy (Kowluru et al., 2001). To date, it has not been established that changes in glutamate function exist in the retina of people with schizophrenia (and therefore that any such changes contribute to visual functioning in people with schizophrenia). However, given the evidence noted here, we believe this is as fruitful an area to explore as that of retinal DA changes and vision in schizophrenia. One question that is particularly relevant is whether the glutamatergic input to, or from, two types of retinal cells in particular, midget and parasol cells, is altered. While the functional properties of these cell types continue to be topics of study, it is generally agreed that midget bipolar and ganglion cells have small receptive fields, are involved in color processing, and project into the LGN parvocellular pathway (Kolb, 1995, Kolb and Marshak, 2003). In contrast, parasol ganglion cells, which receive input from DB2 and DB3 type bipolar cells (Jacoby et al., 2000), have large receptive fields, project to the LGN magnocellular pathway, and play a role in contrast sensitivity (Crook et al., 2014). The latter has been found to be variously underactive or overactive in schizophrenia, depending on the task, and phase of illness (Butler et al., 2001, Javitt, 2009, Kelemen et al., 2013, Mcclure, 2001, Schechter et al., 2003), but retinal contributions to these effects have yet to be described.
Two other prevalent neurotransmitters in the mammalian retina are glycine and GABA (Brandstatter et al., 1998). These neurotransmitters are involved in the inhibitory connections at horizontal and amacrine cells, which modulate the output of photoreceptor and bipolar cells. Studies of retinal connectomics have revealed multiple modes of inhibitory input for both GABA and glycine (Robert et al., 2014). Studies of schizophrenia have also noted abnormalities in these neurotransmitter systems (Ohnuma et al., 2008, Stan and Lewis, 2012, Yamamori et al., 2014). To date, however, the issue of contributions of retinal levels of these neurotransmitters to visual processing disturbances in schizophrenia has not been explored.
4. Electroretinographic changes in schizophrenia
Several studies indicate abnormal a- or b-wave activity in the ERG in schizophrenia (Lavoie et al., 2014b). The a-wave (late receptor potential) reflects activity in the photoreceptors, whereas the b-wave primarily reflects activity in bipolar and Müller cells (other waveforms exist as well, but, with one exception, see below, these have not been studied in schizophrenia). Depending on the type of stimulation and background illumination used, these waveforms can reflect varying degrees of rod vs. cone function. All published studies to date have used the flash ERG method (pattern ERG and multifocal ERG are also commonly used in ophthalmology). The initial study of this issue (Marmor et al., 1988) focused on oscillatory potentials (fluctuations in the rise of the b-wave) which are thought to reflect output of DA-containing amacrine cells. This study found normal waveforms, but it may have been underpowered (only 12 patients and 9 controls were studied), no statistical tests were reported, and all patients were medication withdrawn, suggesting a possible self-section bias towards less severely ill patients. A later, also small, study of schizophrenia patients with healthy eyes demonstrated cone a-wave and rod a- and b-wave amplitude reductions, with these findings being unrelated to medication dose (Warner et al., 1999). Reduced a-wave amplitude was confirmed in a later study, but the effect was only found after hospitalization for an acute psychotic episode; after 8 weeks of treatment, ERG indices were normal, suggesting a state effect. ERG data were normal at both time points in a group of bipolar disorder patients (Balogh et al., 2008). In the largest study to date, comparing 105 schizophrenia patients to 150 controls, patients differed significantly from controls on cone a-wave amplitude, mixed rod-cone b-wave amplitude and pure rod b-wave amplitude, with trends noted for other amplitude and latency variables (Hebert et al., 2015). As with earlier studies, the findings were not related to level of medication, or tobacco, use. Finally, reduced rod b-wave amplitude and longer latencies were found in a group of unaffected offspring of parents with schizophrenia or bipolar disorder (there was no effect of parental diagnosis; Hébert et al., 2010). Cone activity was normal, as was rod a-wave activity in this sample. Although much more data are need on this issue, existing data suggest abnormal retinal function at the level of photoreceptors during acute psychosis, and at the level of the bipolar-Müller cells in patients and high-risk subjects regardless of clinical state. As with OCT data, it is not clear whether these findings are causes of later visual processing impairments, or whether they reflect parallel disturbances in brain function, or both. One proposed mechanism of ERG abnormalities in schizophrenia is reduced omega-3 fatty acid concentration in retinal tissue, where it is normally very dense. This hypothesis is consistent with data on reduced omega-3 fatty acid levels in the brain in schizophrenia (Ohara, 2007), with the therapeutic effects of omega-3 supplementation in schizophrenia (McNamara and Strawn, 2013), and with known effects of fatty acid depletion on retinal function and ERG indices (Neuringer et al., 1986). However, it is also hypothesized that ERG abnormalities reflect DA and serotonin changes, paralleling similar changes in the brain (Lavoie et al., 2014a).
5. Visual acuity changes and their implications in schizophrenia
Poor performance on tests of visual perception (and other cognitive tasks involving visual stimuli) is assumed to reflect the process under investigation, and little attention has been paid to the possibility that reduced visual acuity could play a role. We believe this needs to change, given past findings of poorer visual acuity in schizophrenia, but not other psychotic disorder, patients (including that caused by medication) (Viertio et al., 2007), with one study reporting that close to 70% of a schizophrenia patient sample had untreated visual acuity problems (Smith et al., 1997) [although some studies reported lower rates [e.g., 26% (Souza et al., 2008)] this is still excessive]. While the role of acuity changes in performance on perceptual (and other) tasks in driving between group differences in studies of schizophrenia is generally not known, recent evidence suggests a role. For example, in a study that matched patients and controls on visual acuity, no differences were found in low and high spatial frequency processing conditions (in contrast to many past studies), and, in the sample as a whole, reduced high spatial frequency processing was related to lower acuity (Silverstein et al., 2014). This suggests that at least some prior findings of group differences in spatial frequency processing, or where detail processing is critical to task performance, or where visual stimuli are degraded (Knott et al., 1999, Nuechterlein et al., 1986), or even in reading (Revheim et al., 2006a, Revheim et al., 2014), could be due, at least in part, to between-group acuity differences (even when these are within the normal range, see below). It also raises the possibility that the observed bias towards LSF processing in some studies (Laprevote et al., 2010, Laprevote et al., 2013) may be due to an illness-related bias that has developed to preferentially process LSFs, even when HSF processing is adequate for the task. But, even within the range of normal vision (i.e., 20/32 or better), acuity makes a difference. Another recent study demonstrated that, among healthy controls, people with 20/16 vision performed significantly better than subjects with 20/20 vision on tests of contour integration and collinear facilitation (Keane et al., 2014b), two measures on which schizophrenia patients have performed abnormally in multiple studies (Keri et al., 2005a, Keri et al., 2005b, Keri et al., 2009, Silverstein et al., 2000, Silverstein et al., 2009, Silverstein et al., 2012a). This was recently replicated in a separate sample of schizophrenia patients and controls (Keane et al., 2014c). The implication of this is that demonstrating that all subjects (patients and controls) have “normal or corrected to normal vision” is not enough, especially since schizophrenia patients, with their poorer health care (Marder et al., 2004) including less frequent eye examinations (Viertio et al., 2007) [including in patients with diabetes (De Hert et al., 2011)], can be assumed to have poorer acuity than controls even when it is roughly within the normal range.
Importantly, acuity differences may precede diagnosis. For example, poorer visual acuity, but not other sensory impairments, at 4 years old predicted a diagnosis of schizophrenia, but not other psychiatric diagnoses, in adulthood (Schubert et al., 2005).
Finally, since many antipsychotic medications have anticholinergic effects, and these are known to include dilated pupils and blurred vision (Lieberman, 2004), and similar effects are caused by medications used to treat movement side effects in schizophrenia (e.g., benztropine), medication use may compound visual acuity impairments in people with schizophrenia.
In short, future studies of perceptual impairment in schizophrenia, or of other functions that involve processing visual stimuli should describe the extent to which reduced visual acuity contributes to abnormal performance.
6. Ocular abnormalities and their implications for schizophrenia
In addition to acuity differences, other ocular changes related to schizophrenia, lack of good health care in people with this condition, and/or to its treatment can affect performance on visual processing tasks. For example, Smith et al. (1997) reported that 82.6% of a sample of schizophrenia patients had lens opacities, cataracts, or corneal pigmentation. This study also found that cataracts were more common in patients on first-generation (40%), compared to second-generation antipsychotic medications (18%).
Another condition more common in schizophrenia than the general population is strabismus (Yoshitsugu et al., 2006), or differential alignment of the eyes. Elevated rates of strabismus have also been found in children who later develop schizophrenia (Schiffman et al., 2006). This can result in amblyopia, and depth perception difficulties, and people with schizophrenia have shown poor performance on visual tasks such as contour integration that resembles that of people with amblyopia (Hess et al., 1997, Kiorpes, 2006, Kovacs et al., 2000, Silverstein et al., 2000, Silverstein et al., 2009, Silverstein et al., 2012a), as well as problems with depth perception (Schechter et al., 2006).
Whether resulting from illness-related processes, general neurological vulnerabilities, antipsychotic medication, or a lack of adequate health care, these conditions can affect visual processing. However, at least some of these problems are likely to be primary, because children who later developed schizophrenia in adulthood have been shown to have poorer ocular alignment (i.e., higher strabismus scale scores) than children who did not develop schizophrenia (Schiffman et al., 2006). Whether primary or secondary, however, studies of perception in schizophrenia typically do not screen for ocular problems, and thus their contribution to past findings is not known.
7. Does congenital blindness exert a protective effect against schizophrenia?
Since 1950 (Chevigny and Braverman, 1950), it has been proposed multiple times that, since there have been no reports of people with schizophrenia who were born blind, congenital blindness may confer a complete or partially protective effect against schizophrenia. Two recent reviews have summarized this literature, and explored mechanisms whereby this effect may operate (Landgraf and Osterheider, 2013, Silverstein et al., 2012b). These include: 1) abnormal visual input that precedes, and characterizes, schizophrenia is eliminated; 2) many of the cognitive functions that are impaired in schizophrenia are enhanced among the congenitally blind (see Table 1), suggesting that the cortical reorganization following birth results in a brain that is essentially protected against the development of this condition; and 3) congenital blindness involves reduced flexibility in language and in dynamic representation of the body, and these reductions may protect against thought disorder and alterations in experience of the self, two additional aspects of schizophrenia. Importantly: 1) these effects are much reduced if onset of blindness is after the first few months of life; 2) other forms of congenital sensory loss do not protect against schizophrenia (and some are associated with increased risk); 3) congenital blindness does not protect against other psychiatric disorders suggesting that there is a special link between schizophrenia and visual processing; and 4) past findings of an inverse relationship between the conditions are not due to their relatively low base rates (Silverstein et al., 2013). However, much remains to be learned about this inverse relationship, which parallels, but appears to be stronger than, the one between schizophrenia and rheumatoid arthritis (Gorwood et al., 2004, Mors et al., 1999). For example, there are multiple ways that blindness can occur, with varying contributions of the eyes, subcortical structures (e.g., LGN), and visual cortex, and the extent to which these contributions may vary in their association with schizophrenia is unclear.
Table 1.
Summary of cognitive and brain function enhancements (+) and impairments (−), compared to healthy sighted individuals, in congenital blindness and schizophrenia. Data reviewed in Silverstein et al. (2012b).
| Function | Congenital Blindness | Schizophrenia |
|---|---|---|
| Auditory Perception | ||
| ▪ Localization | + | − |
| ▪ Acuity | + | − |
| ▪ Discrimination | + | − |
| ▪ Comprehension | + | − |
| ▪ Categorization | + | − |
| ▪ Temporal resolution | + | − |
| ▪ Latency of auditory ERPs | +a | −b |
| Auditory Attention | ||
| ▪ Preattentive processing (e.g., MMN) | + | − |
| ▪ Selective attention | + | − |
| ▪ Divided attention | + | − |
| Memory | ||
| ▪ Working memory | + | − |
| ▪ Short-term memory | + | − |
| ▪ Long-term memory | + | − |
| Language | ||
| ▪ Lexical decision making | +a | −b |
| ▪ Abstraction | − | + |
| ▪ Conceptual inclusiveness | − | + |
| ▪ Word inventions | − | + |
| Construction of subjective experience | ||
| ▪ Integration via serial processing | + | − |
| ▪ Holistic processing | + | − |
| Olfaction | + | − |
| Motor control | + | − |
| Body perception | + | − |
| Plasticity | + | − |
Faster than normal.
Slower than normal.
8. Conclusions
The evidence reviewed above suggests two major conclusions. One is that there are multiple structural and physiological disturbances of the eye associated with schizophrenia, all of which could contribute to the visual processing impairments, and altered visual experiences found in the disorder. These include retinal venule widening (possibly secondary to tissue loss), ganglion cell axon (and possibly cell body) loss, dopaminergic abnormalities in photoreceptors, reduced GABA- and glycine-related lateral inhibition, various maculopathies and retinopathies, cataracts, poor visual acuity, and strabismus. Some of these are likely to be related to the illness itself, whereas others may be due to medication use or comorbid conditions such as diabetes (i.e., diabetic retinopathy). In some cases, it has already been shown that variability in these phenomena (e.g., visual acuity) affects performance on visual processing tasks on which schizophrenia patients have demonstrated impairment. A second conclusion suggested by the evidence is that aspects of retinal structure and function can serve as biomarkers of brain pathology, and disease progression, in schizophrenia. The strongest evidence for this to date involves the markers of widened retinal venules, thinning of the RNFL, and abnormal ERG (a and b) waveforms. This and other evidence suggests that the fast and accurate indices provided by ERG, digital retinal imaging, OCT, and even color vision assessment may be useful surrogate measures of global cognitive functioning and specific cognitive impairments (e.g., cognitive control), as well as of disease progression over time, as is the case in other CNS disorders such as Parkinson’s disease and MS. Regarding color perception in particular, more definitive studies in medicated patients are needed, as are studies investigating the possibility of heightened color sensitivity in unmedicated first-episode patients (especially in those reporting color hue and intensity distortions), to determine whether there is excessive activity at cone D2 receptors. To our knowledge, such studies have not yet been conducted.
The goal of this review was to highlight potential contributors to the visual processing abnormalities of schizophrenia and high-risk states that are local to the retina or other structures of the eye. The main weakness of the argument is the relatively small amount of direct evidence of these effects to date. However, we believe that findings from other disorders such as Parkinson’s disease and MS, along with the known problems in acuity and eye disease in schizophrenia, and the relative consistency in the albeit small number of OCT and ERG studies of schizophrenia, provide sufficiently compelling evidence to suggest the need for further investigation.
Finally, because post-ocular structures, such as the LGN and visual cortex, also rely on DA, glutamate, GABA and other neurotransmitters, it will be a challenge to distinguish which effects are local to the retina, as opposed to subcortical or cortical. Some aspects of visual impairment in schizophrenia (e.g., involving orientation discrimination) clearly involve cortical contributions due to known properties of visual cortex (e.g., orientation specific columns). Others, such as reduced susceptibility to certain visual illusions, appear to involve reduced influences of stored knowledge on perception, reflecting altered connectivity between frontal and parietal regions and higher level visual regions (Dima et al., 2009, Keane et al., 2013). In addition, since, for example, there are ~ 20 types of retinal ganglion cells, which cover the retina in a mosaic-type pattern, and whose output is integrated in the LGN, with each contributing a different aspect of information about the visual stimulus (Roska and Meister, 2014), some visual processing impairments may reflect abnormal coordination of ganglion cell outputs in the LGN. This is particularly relevant given that there are abnormalities in coordinating neural activity that are found throughout the cortex, and that characterize intermediate and higher-level visual processing and cognitive impairments (Phillips and Silverstein, 2003). However, even with functions such as coordination of ganglion cell output, orientation discrimination, and perceptual organization, as well as higher-level processes such as illusion perception, visual memory, and visual learning, degraded retinal input could serve as a rate-limiting factor. In contrast, with lower level visual processes such as brightness and motion representation, contrast sensitivity, spatial frequency processing, and color processing, retinal contributions have long been acknowledged. Therefore, we believe that a more comprehensive understanding of the nature of the full range of visual processing and visual cognition impairments in schizophrenia requires that retinal contributions be more precisely determined.
Role of Funding Source
This work was supported by NIMH grant R01MH093439 to the first author.
Contributors
Steven Silverstein and Richard Rosen both contributed to the literature review that formed the basis of this paper, and both contributed to the writing of the manuscript.
Conflict of Interest
Steven Silverstein has received funding from the National Institute of Mental Health, NARSAD, the Jacob and Valeria Langaloth Foundation, the van Ameringen Foundation, the Scottish Rite Schizophrenia Research Program, the New York State Office of Mental Health, the New Jersey Division of Mental Health and Addiction Services, the New England Research Institutes, the Brain Resource Company, the Committee to Aid Research to End Schizophrenia, Janssen Pharmaceutica, Pfizer, Novartis, and Astra-Zeneca. Richard Rosen has received funding from the Gladys Brooks Foundation, the Lane Foundation, the Lowenstein Foundation, the Achelis-Bodman Foundation, the Cancer Care Fund, the Lavoe Trust Foundation, the Audrey Monel Foundation, the JP Morgan Chase Foundation, Allergan Pharmaceuticals, the Aborn Family Foundation, the Wise Foundation, the J. Milbank Foundation, the Marty Richards Foundation, the Marcelle Myers Estate Foundation, and the Marrus Family Foundation. Both authors declare that they have no conflicts of interest regarding material presented in this paper.
Acknowledgements
The authors thank Drs. Stuart Green and Brian Keane for helpful discussions of some of the issues covered in this paper.
References
- Antal M. Ketamine-induced ultrastructural changes in the retina. Albrecht Von Graefes Arch. Klin. Exp. Ophthalmol. 1979;210:43–53. doi: 10.1007/BF00414788. [DOI] [PubMed] [Google Scholar]
- Ascaso F.J., Cabezón L., Quintanilla M.A., Galve L.G., Antón R.L., Cristóbal J.A., Lobo A. Retinal nerve fiber layer thickness measured by optical coherence tomography in patients with schizophrenia: A short report. Eur J Psychiatry. 2010;24:227–235. [Google Scholar]
- Balogh Z., Benedek G., Keri S. Retinal dysfunctions in schizophrenia. Prog. Neuropsychopharmacol. Biol. Psychiatry. 2008;32:297–300. doi: 10.1016/j.pnpbp.2007.08.024. [DOI] [PubMed] [Google Scholar]
- Blin O., Mestre D., Masson G., Serratrice G. Selective effects of low doses of apomorphine on spatiotemporal contrast sensitivity in healthy volunteers: a double-blind placebo-controlled study. Br. J. Clin. Pharmacol. 1991;32:551–556. doi: 10.1111/j.1365-2125.1991.tb03950.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bodis-Wollner I. Visual deficits related to dopamine deficiency in experimental animals and Parkinson's disease patients. Trends Neurosci. 1990;13:296–302. doi: 10.1016/0166-2236(90)90113-o. [DOI] [PubMed] [Google Scholar]
- Bodis-Wollner I., Tagliati M. The visual system in Parkinson's disease. Adv. Neurol. 1993;60:390–394. [PubMed] [Google Scholar]
- Bodis-Wollner I., Yahr M.D., Mylin L., Thornton J. Dopaminergic deficiency and delayed visual evoked potentials in humans. Ann. Neurol. 1982;11:478–483. doi: 10.1002/ana.410110507. [DOI] [PubMed] [Google Scholar]
- Brandies R., Yehuda S. The possible role of retinal dopaminergic system in visual performance. Neurosci. Biobehav. Rev. 2008;32:611–656. doi: 10.1016/j.neubiorev.2007.09.004. [DOI] [PubMed] [Google Scholar]
- Brandstatter J.H., Koulen P., Wassle H. Diversity of glutamate receptors in the mammalian retina. Vis. Res. 1998;38:1385–1397. doi: 10.1016/s0042-6989(97)00176-4. [DOI] [PubMed] [Google Scholar]
- Bulens C., Meerwaldt J.D., Van Der Wildt G.J., Van Deursen J.B. Effect of levodopa treatment on contrast sensitivity in Parkinson's disease. Ann. Neurol. 1987;22:365–369. doi: 10.1002/ana.410220313. [DOI] [PubMed] [Google Scholar]
- Bulens C., Meerwaldt J.D., Van Der Wildt G.J., Keemink C.J. Visual contrast sensitivity in drug-induced Parkinsonism. J. Neurol. Neurosurg. Psychiatry. 1989;52:341–345. doi: 10.1136/jnnp.52.3.341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bunney W.E., Jr., Hetrick W.P., Bunney B.G., Patterson J.V., Jin Y., Potkin S.G. Structured Interview for Assessing Perceptual Anomalies (SIAPA) Schizophr. Bull. 1999;25:577–592. doi: 10.1093/oxfordjournals.schbul.a033402. [DOI] [PubMed] [Google Scholar]
- Butler P.D., Schechter I., Zemon V., Schwartz S.G., Greenstein V.C., Gordon J. Dysfunction of early-stage visual processing in schizophrenia. Am. J. Psychiatry. 2001;158:1126–1133. doi: 10.1176/appi.ajp.158.7.1126. [DOI] [PubMed] [Google Scholar]
- Butler P.D., Zemon V., Schechter I., Saperstein A.M., Hoptman M.J., Lim K.O. Early-stage visual processing and cortical amplification deficits in schizophrenia. Arch. Gen. Psychiatry. 2005;62:495–504. doi: 10.1001/archpsyc.62.5.495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butler P.D., Abeles I.Y., Silverstein S.M., Dias E.C., Weiskopf N.G., Calderone D.J. An event-related potential examination of contour integration deficits in schizophrenia. Front. Psychol. 2013;4:132. doi: 10.3389/fpsyg.2013.00132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cabezon L., Ascaso F., Ramiro P., Quintanilla M., Gutierrez L., Lobo A. Optical coherence tomography: a window into the brain of schizophrenic patients. Acta Ophthalmol. (Copenh) 2012;90 [Google Scholar]
- Cadenhead K.S., Dobkins K., Mcgovern J., Shafer K. Schizophrenia spectrum participants have reduced visual contrast sensitivity to chromatic (red/green) and luminance (light/dark) stimuli: new insights into information processing, visual channel function, and antipsychotic effects. Front. Psychol. 2013;4:535. doi: 10.3389/fpsyg.2013.00535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chapman J. The early symptoms of schizophrenia. Br. J. Psychiatry. 1966;112:225–251. doi: 10.1192/bjp.112.484.225. [DOI] [PubMed] [Google Scholar]
- Chen Y. Abnormal visual motion processing in schizophrenia: a review of research progress. Schizophr. Bull. 2011;37:709–715. doi: 10.1093/schbul/sbr020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y., Levy D.L., Sheremata S., Nakayama K., Matthysse S., Holzman P.S. Effects of typical, atypical, and no antipsychotic drugs on visual contrast detection in schizophrenia. Am. J. Psychiatry. 2003;160:1795–1801. doi: 10.1176/appi.ajp.160.10.1795. [DOI] [PubMed] [Google Scholar]
- Chevigny H., Braverman S. Yale University Press; New Haven: 1950. The Adjustment of the Blind. [Google Scholar]
- Chu E.M., Kolappan M., Barnes T.R., Joyce E.M., Ron M.A. A window into the brain: an in vivo study of the retina in schizophrenia using optical coherence tomography. Psychiatry Res. 2012;203:89–94. doi: 10.1016/j.pscychresns.2011.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colzato L.S., Sellaro R., Hulka L.M., Quednow B.B., Hommel B. Cognitive control predicted by color vision, and vice versa. Neuropsychologia. 2014;62:55–59. doi: 10.1016/j.neuropsychologia.2014.07.010. [DOI] [PubMed] [Google Scholar]
- Copenhagen D.R., Jahr C.E. Release of endogenous excitatory amino acids from turtle photoreceptors. Nature. 1989;341:536–539. doi: 10.1038/341536a0. [DOI] [PubMed] [Google Scholar]
- Corbe C., Arnaud F., Brault Y., Janiak-Bolzinger C. Effect of a dopaminergic agonist, piribedil (Trivastal 50 mg LP), on visual and spatial integration in elderly subjects. J. Neurol. 1992;239(Suppl. 1):S22–S27. doi: 10.1007/BF00819563. [DOI] [PubMed] [Google Scholar]
- Crook J.D., Packer O.S., Dacey D.M. A synaptic signature for ON- and OFF-center parasol ganglion cells of the primate retina. Vis. Neurosci. 2014;31:57–84. doi: 10.1017/S0952523813000461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Hert M., Correll C.U., Bobes J., Cetkovich-Bakmas M., Cohen D., Asai I. Physical illness in patients with severe mental disorders. I. Prevalence, impact of medications and disparities in health care. World Psychiatry. 2011;10:52–77. doi: 10.1002/j.2051-5545.2011.tb00014.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Souza C.F., Kalloniatis M., Polkinghorne P.J., Mcghee C.N., Acosta M.L. Functional activation of glutamate ionotropic receptors in the human peripheral retina. Exp. Eye Res. 2012;94:71–84. doi: 10.1016/j.exer.2011.11.008. [DOI] [PubMed] [Google Scholar]
- Dima D., Roiser J.P., Dietrich D.E., Bonnemann C., Lanfermann H., Emrich H.M. Understanding why patients with schizophrenia do not perceive the hollow-mask illusion using dynamic causal modelling. Neuroimage. 2009;46:1180–1186. doi: 10.1016/j.neuroimage.2009.03.033. [DOI] [PubMed] [Google Scholar]
- Dima D., Dietrich D.E., Dillo W., Emrich H.M. Impaired top-down processes in schizophrenia: a DCM study of ERPs. Neuroimage. 2010;52:824–832. doi: 10.1016/j.neuroimage.2009.12.086. [DOI] [PubMed] [Google Scholar]
- Dima D., Dillo W., Bonnemann C., Emrich H.M., Dietrich D.E. Reduced P300 and P600 amplitude in the hollow-mask illusion in patients with schizophrenia. Psychiatry Res. 2011;191:145–151. doi: 10.1016/j.pscychresns.2010.09.015. [DOI] [PubMed] [Google Scholar]
- Djamgoz M.B., Hankins M.W., Hirano J., Archer S.N. Neurobiology of retinal dopamine in relation to degenerative states of the tissue. Vis. Res. 1997;37:3509–3529. doi: 10.1016/S0042-6989(97)00129-6. [DOI] [PubMed] [Google Scholar]
- Domenici L., Trimarchi C., Piccolino M., Fiorentini A., Maffei L. Dopaminergic drugs improve human visual contrast sensitivity. Hum. Neurobiol. 1985;4:195–197. [PubMed] [Google Scholar]
- Gonzalez-Hernandez J.A., Pita-Alcorta C., Padron A., Finale A., Galan L., Martinez E. Basic visual dysfunction allows classification of patients with schizophrenia with exceptional accuracy. Schizophr. Res. 2014;159:226–233. doi: 10.1016/j.schres.2014.07.052. [DOI] [PubMed] [Google Scholar]
- Gorwood P., Pouchot J., Vinceneux P., Puechal X., Flipo R.M., De Bandt M. Rheumatoid arthritis and schizophrenia: a negative association at a dimensional level. Schizophr. Res. 2004;66:21–29. doi: 10.1016/s0920-9964(03)00017-3. [DOI] [PubMed] [Google Scholar]
- Gottlob I., Stangler-Zuschrott E. Effect of levodopa on contrast sensitivity and scotomas in human amblyopia. Invest. Ophthalmol. Vis. Sci. 1990;31:776–780. [PubMed] [Google Scholar]
- Gottlob I., Charlier J., Reinecke R.D. Visual acuities and scotomas after one week levodopa administration in human amblyopia. Invest. Ophthalmol. Vis. Sci. 1992;33:2722–2728. [PubMed] [Google Scholar]
- Grano N., Salmijarvi L., Karjalainen M., Kallionpaa S., Roine M., Taylor P. Early signs of worry: psychosis risk symptom visual distortions are independently associated with suicidal ideation. Psychiatry Res. 2015;225:263–267. doi: 10.1016/j.psychres.2014.12.031. [DOI] [PubMed] [Google Scholar]
- Green M.F., Lee J., Wynn J.K., Mathis K.I. Visual masking in schizophrenia: overview and theoretical implications. Schizophr. Bull. 2011;37:700–708. doi: 10.1093/schbul/sbr051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green M.F., Hellemann G., Horan W.P., Lee J., Wynn J.K. From perception to functional outcome in schizophrenia: modeling the role of ability and motivation. Arch. Gen. Psychiatry. 2012;69:1216–1224. doi: 10.1001/archgenpsychiatry.2012.652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harnois C., Di Paolo T. Decreased dopamine in the retinas of patients with Parkinson's disease. Invest. Ophthalmol. Vis. Sci. 1990;31:2473–2475. [PubMed] [Google Scholar]
- Harris J.P., Calvert J.E., Leendertz J.A., Phillipson O.T. The influence of dopamine on spatial vision. Eye (Lond.) 1990;4(Pt 6):806–812. doi: 10.1038/eye.1990.127. [DOI] [PubMed] [Google Scholar]
- Hébert M., Gagné A.M., Paradis M.E., Jomphe V., Roy M.A., Mérette C., Maziade M. Retinal response to light in young nonaffected offspring at high genetic risk of neuropsychiatric brain disorders. Biol Psychiatry. 2010;67:270–274. doi: 10.1016/j.biopsych.2009.08.016. [DOI] [PubMed] [Google Scholar]
- Hebert M., Merette C., Paccalet T., Emond C., Gagne A.M., Sasseville A. Light evoked potentials measured by electroretinogram may tap into the neurodevelopmental roots of schizophrenia. Schizophr. Res. 2015;162:294–295. doi: 10.1016/j.schres.2014.12.030. [DOI] [PubMed] [Google Scholar]
- Hedden W.L., Jr., Dowling J.E. The interplexiform cell system. II. Effects of dopamine on goldfish retinal neurones. Proc. R. Soc. Lond. B Biol. Sci. 1978;201:27–55. doi: 10.1098/rspb.1978.0031. [DOI] [PubMed] [Google Scholar]
- Hess R.F., Mcilhagga W., Field D.J. Contour integration in strabismic amblyopia: the sufficiency of an explanation based on positional uncertainty. Vis. Res. 1997;37:3145–3161. doi: 10.1016/s0042-6989(96)00281-7. [DOI] [PubMed] [Google Scholar]
- Hutton J.T., Morris J.L. Vision in Parkinson's disease. Adv. Neurol. 2001;86:279–288. [PubMed] [Google Scholar]
- Jacoby R.A., Wiechmann A.F., Amara S.G., Leighton B.H., Marshak D.W. Diffuse bipolar cells provide input to OFF parasol ganglion cells in the macaque retina. J. Comp. Neurol. 2000;416:6–18. doi: 10.1002/(sici)1096-9861(20000103)416:1<6::aid-cne2>3.0.co;2-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Javitt D.C. Glutamate and schizophrenia: phencyclidine, N-methyl-d-aspartate receptors, and dopamine-glutamate interactions. Int. Rev. Neurobiol. 2007;78:69–108. doi: 10.1016/S0074-7742(06)78003-5. [DOI] [PubMed] [Google Scholar]
- Javitt D.C. Sensory processing in schizophrenia: neither simple nor intact. Schizophr. Bull. 2009;35:1059–1064. doi: 10.1093/schbul/sbp110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kantrowitz J.T., Javitt D.C. N-methyl-d-aspartate (NMDA) receptor dysfunction or dysregulation: the final common pathway on the road to schizophrenia? Brain Res. Bull. 2010;83:108–121. doi: 10.1016/j.brainresbull.2010.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kantrowitz J.T., Javitt D.C. Thinking glutamatergically: changing concepts of schizophrenia based upon changing neurochemical models. Clin. Schizophr. Relat. Psychoses. 2010;4:189–200. doi: 10.3371/CSRP.4.3.6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaplan O., Lubow R.E. Ignoring irrelevant stimuli in latent inhibition and Stroop paradigms: the effects of schizotypy and gender. Psychiatry Res. 2011;186:40–45. doi: 10.1016/j.psychres.2010.07.025. [DOI] [PubMed] [Google Scholar]
- Keane B.P., Silverstein S.M., Wang Y., Papathomas T.V. Reduced depth inversion illusions in schizophrenia are state-specific and occur for multiple object types and viewing conditions. J. Abnorm. Psychol. 2013;122:506–512. doi: 10.1037/a0032110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keane B., Erlikhman G., Kastner S., Paterno D., Silverstein S. Multiple forms of contour grouping deficits in schizophrenia: what is the role of spatial frequency? Neuropsychologia. 2014;65:221–233. doi: 10.1016/j.neuropsychologia.2014.10.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keane B.P., Kastner S., Paterno D., Silverstein S.M. Is 20/20 vision good enough? Visual acuity differences within the normal range predict contour element detection and integration. Psychon. Bull. Rev. 2014;22:121–127. doi: 10.3758/s13423-014-0647-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keane B.P., Erlikhman G., Kastner S., Paterno D., Silverstein S.M. Multiple forms of contour grouping deficits in schizophrenia: what is the role of spatial frequency? Neuropsychologia. 2014:221–233. doi: 10.1016/j.neuropsychologia.2014.10.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kegeles L.S., Abi-Dargham A., Frankle W.G., Gil R., Cooper T.B., Slifstein M. Increased synaptic dopamine function in associative regions of the striatum in schizophrenia. Arch. Gen. Psychiatry. 2010;67:231–239. doi: 10.1001/archgenpsychiatry.2010.10. [DOI] [PubMed] [Google Scholar]
- Kelemen O., Kiss I., Benedek G., Keri S. Perceptual and cognitive effects of antipsychotics in first-episode schizophrenia: the potential impact of GABA concentration in the visual cortex. Prog. Neuropsychopharmacol. Biol. Psychiatry. 2013;47:13–19. doi: 10.1016/j.pnpbp.2013.07.024. [DOI] [PubMed] [Google Scholar]
- Kemenyova P., Turcani P., Sutovsky S., Waczulikova I. Optical coherence tomography and its use in optical neuritis and multiple sclerosis. Bratisl. Lek. Listy. 2014;115:723–729. doi: 10.4149/bll_2014_140. [DOI] [PubMed] [Google Scholar]
- Keri S., Benedek G. Visual contrast sensitivity alterations in inferred magnocellular pathways and anomalous perceptual experiences in people at high-risk for psychosis. Vis. Neurosci. 2007;24:183–189. doi: 10.1017/S0952523807070253. [DOI] [PubMed] [Google Scholar]
- Keri S., Antal A., Szekeres G., Benedek G., Janka Z. Spatiotemporal visual processing in schizophrenia. J. Neuropsychiatry Clin. Neurosci. 2002;14:190–196. doi: 10.1176/jnp.14.2.190. [DOI] [PubMed] [Google Scholar]
- Keri S., Kelemen O., Benedek G., Janka Z. Lateral interactions in the visual cortex of patients with schizophrenia and bipolar disorder. Psychol. Med. 2005;35:1043–1051. doi: 10.1017/s0033291705004381. [DOI] [PubMed] [Google Scholar]
- Keri S., Kiss I., Kelemen O., Benedek G., Janka Z. Anomalous visual experiences, negative symptoms, perceptual organization and the magnocellular pathway in schizophrenia: a shared construct? Psychol. Med. 2005;35:1445–1455. doi: 10.1017/S0033291705005398. [DOI] [PubMed] [Google Scholar]
- Keri S., Kelemen O., Benedek G. Attentional modulation of perceptual organisation in schizophrenia. Cogn. Neuropsychiatry. 2009;14:77–86. doi: 10.1080/13546800902757936. [DOI] [PubMed] [Google Scholar]
- Khanifar A.A., Parlitsis G.J., Ehrlich J.R., Aaker G.D., D'amico D.J., Gauthier S.A. Retinal nerve fiber layer evaluation in multiple sclerosis with spectral domain optical coherence tomography. Clin. Ophthalmol. 2010;4:1007–1013. doi: 10.2147/opth.s13278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiorpes L. Visual processing in amblyopia: animal studies. Strabismus. 2006;14:3–10. doi: 10.1080/09273970500536193. [DOI] [PubMed] [Google Scholar]
- Kiss I., Fabian A., Benedek G., Keri S. When doors of perception open: visual contrast sensitivity in never-medicated, first-episode schizophrenia. J. Abnorm. Psychol. 2010;119:586–593. doi: 10.1037/a0019610. [DOI] [PubMed] [Google Scholar]
- Klosterkotter J., Hellmich M., Steinmeyer E.M., Schultze-Lutter F. Diagnosing schizophrenia in the initial prodromal phase. Arch. Gen. Psychiatry. 2001;58:158–164. doi: 10.1001/archpsyc.58.2.158. [DOI] [PubMed] [Google Scholar]
- Knott V., Mahoney C., Labelle A., Ripley C., Cavazzoni P., Jones B. Event-related potentials in schizophrenic patients during a degraded stimulus version of the visual continuous performance task. Schizophr. Res. 1999;35:263–278. doi: 10.1016/s0920-9964(98)00122-4. [DOI] [PubMed] [Google Scholar]
- Kolb H. Midget pathways of the primate retina underlie resolution and red green color opponency. In: Kolb H., Fernandez E., Nelson R., editors. Webvision: The Organization of the Retina and Visual System. 1995. (Salt Lake City, UT) [PubMed] [Google Scholar]
- Kolb H., Marshak D. The midget pathways of the primate retina. Doc. Ophthalmol. 2003;106:67–81. doi: 10.1023/a:1022469002511. [DOI] [PubMed] [Google Scholar]
- Kovács I., Polat U., Pennefather Pm, Chandna A., Norcia A.M. A new test of contour integration deficits in patients with a history of disrupted binocular experience during visual development. Vis. Res. 2000;40:1775–1783. doi: 10.1016/s0042-6989(00)00008-0. [DOI] [PubMed] [Google Scholar]
- Kowluru R.A., Engerman R.L., Case G.L., Kern T.S. Retinal glutamate in diabetes and effect of antioxidants. Neurochem. Int. 2001;38:385–390. doi: 10.1016/s0197-0186(00)00112-1. [DOI] [PubMed] [Google Scholar]
- Lalonde M.R., Chauhan B.C., Tremblay F. Retinal ganglion cell activity from the multifocal electroretinogram in pig: optic nerve section, anaesthesia and intravitreal tetrodotoxin. J. Physiol. 2006;570:325–338. doi: 10.1113/jphysiol.2005.098046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Landgraf S., Osterheider M. "To see or not to see: that is the question." The "Protection-Against-Schizophrenia" (PaSZ) model: evidence from congenital blindness and visuo-cognitive aberrations. Front. Psychol. 2013;4:352. doi: 10.3389/fpsyg.2013.00352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laprevote V., Oliva A., Delerue C., Thomas P., Boucart M. Patients with schizophrenia are biased toward low spatial frequency to decode facial expression at a glance. Neuropsychologia. 2010;48:4164–4168. doi: 10.1016/j.neuropsychologia.2010.10.017. [DOI] [PubMed] [Google Scholar]
- Laprevote V., Oliva A., Ternois A.S., Schwan R., Thomas P., Boucart M. Low spatial frequency bias in schizophrenia is not face specific: when the integration of coarse and fine information fails. Front. Psychol. 2013;4:248. doi: 10.3389/fpsyg.2013.00248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laruelle M., Abi-Dargham A., Gil R., Kegeles L., Innis R. Increased dopamine transmission in schizophrenia: relationship to illness phases. Biol. Psychiatry. 1999;46:56–72. doi: 10.1016/s0006-3223(99)00067-0. [DOI] [PubMed] [Google Scholar]
- Lavoie J., Illiano P., Sotnikova T.D., Gainetdinov R.R., Beaulieu J.M., Hebert M. The electroretinogram as a biomarker of central dopamine and serotonin: potential relevance to psychiatric disorders. Biol. Psychiatry. 2014;75:479–486. doi: 10.1016/j.biopsych.2012.11.024. [DOI] [PubMed] [Google Scholar]
- Lavoie J., Maziade M., Hebert M. The brain through the retina: the flash electroretinogram as a tool to investigate psychiatric disorders. Prog. Neuropsychopharmacol. Biol. Psychiatry. 2014;48:129–134. doi: 10.1016/j.pnpbp.2013.09.020. [DOI] [PubMed] [Google Scholar]
- Lee M.S., Fern A.I. Fluphenazine and its toxic maculopathy. Ophthalmic Res. 2004;36:237–239. doi: 10.1159/000078784. [DOI] [PubMed] [Google Scholar]
- Lee W.W., Tajunisah I., Sharmilla K., Peyman M., Subrayan V. Retinal nerve fiber layer structure abnormalities in schizophrenia and its relationship to disease state: evidence from optical coherence tomography. Invest. Ophthalmol. Vis. Sci. 2013;54:7785–7792. doi: 10.1167/iovs.13-12534. [DOI] [PubMed] [Google Scholar]
- Lee J.Y., Kim J.M., Ahn J., Kim H.J., Jeon B.S., Kim T.W. Retinal nerve fiber layer thickness and visual hallucinations in Parkinson's Disease. Mov. Disord. 2014;29:61–67. doi: 10.1002/mds.25543. [DOI] [PubMed] [Google Scholar]
- Leguire L.E., Rogers G.L., Bremer D.L., Walson P., Hadjiconstantinou-Neff M. Levodopa and childhood amblyopia. J. Pediatr. Ophthalmol. Strabismus. 1992;29:290–298. doi: 10.3928/0191-3913-19920901-08. (discussion 299) [DOI] [PubMed] [Google Scholar]
- Lesh T.A., Niendam T.A., Minzenberg M.J., Carter C.S. Cognitive control deficits in schizophrenia: mechanisms and meaning. Neuropsychopharmacology. 2011;36:316–338. doi: 10.1038/npp.2010.156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leung C.K., Cheung C.Y., Weinreb R.N., Qiu K., Liu S., Li H. Evaluation of retinal nerve fiber layer progression in glaucoma: a study on optical coherence tomography guided progression analysis. Invest. Ophthalmol. Vis. Sci. 2010;51:217–222. doi: 10.1167/iovs.09-3468. [DOI] [PubMed] [Google Scholar]
- Lieberman J.A. Managing anticholinergic side effects. Prim. Care Companion CNS Disord. 2004;6(Suppl 2):20–23. [PMC free article] [PubMed] [Google Scholar]
- Malyszczak K., Kubiszewski M., Pilecki W., Maciejowski A., Sobieszczanska M. Distribution of latencies of visual evoked potentials in a sample of schizophrenic patients. Psychiatr. Pol. 2003;37:989–997. [PubMed] [Google Scholar]
- Marder S.R., Essock S.M., Miller A.L., Buchanan R.W., Casey D.E., Davis J.M. Physical health monitoring of patients with schizophrenia. Am. J. Psychiatry. 2004;161:1334–1349. doi: 10.1176/appi.ajp.161.8.1334. [DOI] [PubMed] [Google Scholar]
- Marmor M.F., Hock P., Schechter G., Pfefferbaum A., Berger P.A., Maurice R. Oscillatory potentials as a marker for dopaminergic disease. Doc. Ophthalmol. 1988;69:255–261. doi: 10.1007/BF00154406. [DOI] [PubMed] [Google Scholar]
- Martinez A., Hillyard S.A., Dias E.C., Hagler D.J., Jr., Butler P.D., Guilfoyle D.N. Magnocellular pathway impairment in schizophrenia: evidence from functional magnetic resonance imaging. J. Neurosci. 2008;28:7492–7500. doi: 10.1523/JNEUROSCI.1852-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinez A., Hillyard S.A., Bickel S., Dias E.C., Butler P.D., Javitt D.C. Consequences of magnocellular dysfunction on processing attended information in schizophrenia. Cereb. Cortex. 2012;22:1282–1293. doi: 10.1093/cercor/bhr195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinez-Lapiscina E., Ortiz-Pérez S., Fraga-Pumar E., Martinez-Heras E., Gabliondo I., Llufriu S. Color vision impairment is associated with disease severity in multiple sclerosis. Mult. Scler. 2014;20:1207–1216. doi: 10.1177/1352458513517591. [DOI] [PubMed] [Google Scholar]
- Massey S.C., Miller R.F. N-methyl-d-aspartate receptors of ganglion cells in rabbit retina. J. Neurophysiol. 1990;63:16–30. doi: 10.1152/jn.1990.63.1.16. [DOI] [PubMed] [Google Scholar]
- Masson G., Mestre D., Blin O. Dopaminergic modulation of visual sensitivity in man. Fundam. Clin. Pharmacol. 1993;7:449–463. doi: 10.1111/j.1472-8206.1993.tb01041.x. [DOI] [PubMed] [Google Scholar]
- Mccarty C.A., Wood C.A., Fu C.L., Livingston P.M., Mackersey S., Stanislavsky Y. Schizophrenia, psychotropic medication, and cataract. Ophthalmology. 1999;106:683–687. doi: 10.1016/S0161-6420(99)90151-3. [DOI] [PubMed] [Google Scholar]
- Mcclure R.K. The visual backward masking deficit in schizophrenia. Prog. Neuropsychopharmacol. Biol. Psychiatry. 2001;25:301–311. doi: 10.1016/s0278-5846(00)00166-4. [DOI] [PubMed] [Google Scholar]
- Mcnamara R.K., Strawn J.R. Role of long-chain omega-3 fatty acids in psychiatric practice. PharmaNutrition. 2013;1:41–49. doi: 10.1016/j.phanu.2012.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meier M.H., Shalev I., Moffitt T.E., Kapur S., Keefe R.S., Wong T.Y. Microvascular abnormality in schizophrenia as shown by retinal imaging. Am. J. Psychiatry. 2013;170:1451–1459. doi: 10.1176/appi.ajp.2013.13020234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meier M.H., Gillespie N.A., Hansell N.K., Hewitt A.W., Hickie I.B., Lu Y. Retinal microvessels reflect familial vulnerability to psychotic symptoms: a comparison of twins discordant for psychotic symptoms and controls. Schizophr. Res. 2015 doi: 10.1016/j.schres.2015.01.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moghaddam B., Javitt D. From revolution to evolution: the glutamate hypothesis of schizophrenia and its implication for treatment. Neuropsychopharmacology. 2012;37:4–15. doi: 10.1038/npp.2011.181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mors O., Mortensen P.B., Ewald H. A population-based register study of the association between schizophrenia and rheumatoid arthritis. Schizophr. Res. 1999;40:67–74. doi: 10.1016/s0920-9964(99)00030-4. [DOI] [PubMed] [Google Scholar]
- Moschos M.M., Markopoulos I., Chatziralli I., Rouvas A., Papageorgiou S.G., Ladas I. Structural and functional impairment of the retina and optic nerve in Alzheimer's disease. Curr. Alzheimer Res. 2012;9:782–788. doi: 10.2174/156720512802455340. [DOI] [PubMed] [Google Scholar]
- Neuringer M., Connor W.E., Lin D.S., Barstad L., Luck S. Biochemical and functional effects of prenatal and postnatal omega 3 fatty acid deficiency on retina and brain in rhesus monkeys. Proc Natl Acad Sci U S A. 1986;83:4021–4025. doi: 10.1073/pnas.83.11.4021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nuechterlein K.H., Edell W.S., Norris M., Dawson M.E. Attentional vulnerability indicators, thought disorder, and negative symptoms. Schizophr. Bull. 1986;12:408–426. doi: 10.1093/schbul/12.3.408. [DOI] [PubMed] [Google Scholar]
- Ohara K. The n-3 polyunsaturated fatty acid/dopamine hypothesis of schizophrenia. Prog. Neuropsychopharmacol. Biol. Psychiatry. 2007;31:469–474. doi: 10.1016/j.pnpbp.2006.11.013. [DOI] [PubMed] [Google Scholar]
- Ohnuma T., Sakai Y., Maeshima H., Hatano T., Hanzawa R., Abe S. Changes in plasma glycine, L-serine, and D-serine levels in patients with schizophrenia as their clinical symptoms improve: results from the Juntendo University Schizophrenia Projects (JUSP) Prog. Neuropsychopharmacol. Biol. Psychiatry. 2008;32:1905–1912. doi: 10.1016/j.pnpbp.2008.07.022. [DOI] [PubMed] [Google Scholar]
- Olney J.W., Farber N.B. Glutamate receptor dysfunction and schizophrenia. Arch. Gen. Psychiatry. 1995;52:998–1007. doi: 10.1001/archpsyc.1995.03950240016004. [DOI] [PubMed] [Google Scholar]
- Phillips W.A., Silverstein S.M. Convergence of biological and psychological perspectives on cognitive coordination in schizophrenia. Behav. Brain Sci. 2003;26:65–82. doi: 10.1017/s0140525x03000025. (discussion 82-137) [DOI] [PubMed] [Google Scholar]
- Phillipson O.T., Harris J.P. Perceptual changes in schizophrenia: a questionnaire survey. Psychol. Med. 1985;15:859–866. doi: 10.1017/s0033291700005092. [DOI] [PubMed] [Google Scholar]
- Piccolino M., Neyton J., Gerschenfeld H.M. Decrease of gap junction permeability induced by dopamine and cyclic adenosine 3':5'-monophosphate in horizontal cells of turtle retina. J. Neurosci. 1984;4:2477–2488. doi: 10.1523/JNEUROSCI.04-10-02477.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Popova E., Kupenova P. Effects of dopamine receptor blockade on the intensity-response function of ERG b- and d-waves in dark adapted eyes. Vis. Res. 2013;88:22–29. doi: 10.1016/j.visres.2013.06.004. [DOI] [PubMed] [Google Scholar]
- Rassovsky Y., Horan W.P., Lee J., Sergi M.J., Green M.F. Pathways between early visual processing and functional outcome in schizophrenia. Psychol. Med. 2011;41:487–497. doi: 10.1017/S0033291710001054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ratchford J.N., Saidha S., Sotirchos E.S., Oh J.A., Seigo M.A., Eckstein C. Active MS is associated with accelerated retinal ganglion cell/inner plexiform layer thinning. Neurology. 2013;80:47–54. doi: 10.1212/WNL.0b013e31827b1a1c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Revheim N., Butler P.D., Schechter I., Jalbrzikowski M., Silipo G., Javitt D.C. Reading impairment and visual processing deficits in schizophrenia. Schizophr. Res. 2006;87:238–245. doi: 10.1016/j.schres.2006.06.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Revheim N., Schechter I., Kim D., Silipo G., Allingham B., Butler P. Neurocognitive and symptom correlates of daily problem-solving skills in schizophrenia. Schizophr. Res. 2006;83:237–245. doi: 10.1016/j.schres.2005.12.849. [DOI] [PubMed] [Google Scholar]
- Revheim N., Corcoran C.M., Dias E., Hellmann E., Martinez A., Butler P.D. Reading deficits in schizophrenia and individuals at high clinical risk: relationship to sensory function, course of illness, and psychosocial outcome. Am. J. Psychiatry. 2014;171:949–959. doi: 10.1176/appi.ajp.2014.13091196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robert E., Jones B., Lauritzen J., Watt C., Anderson J. Retinal conectomics: a new era for connectivity analysis. In: Werner J., Chalupa L., editors. The New Visual Neurosciences. MIT Press; Cambridge, MA: 2014. [Google Scholar]
- Robol V., Tibber M.S., Anderson E.J., Bobin T., Carlin P., Shergill S.S. Reduced crowding and poor contour detection in schizophrenia are consistent with weak surround inhibition. PLoS One. 2013;8:e60951. doi: 10.1371/journal.pone.0060951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodnitzky R.L. Visual dysfunction in Parkinson's disease. Clin. Neurosci. 1998;5:102–106. [PubMed] [Google Scholar]
- Rokem A., Yoon J.H., Ooms R.E., Maddock R.J., Minzenberg M.J., Silver M.A. Broader visual orientation tuning in patients with schizophrenia. Front. Hum. Neurosci. 2011;5:127. doi: 10.3389/fnhum.2011.00127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roska B., Meister M. The retina dissects the visual scene into distinct features. In: Chalupa L., Werner J.S., editors. The New Visual Neurosciences. MIT Press; Cambridge, MA: 2014. [Google Scholar]
- Saidha S., Sotirchos E.S., Oh J., Syc S.B., Seigo M.A., Shiee N. Relationships between retinal axonal and neuronal measures and global central nervous system pathology in multiple sclerosis. JAMA Neurol. 2013;70:34–43. doi: 10.1001/jamaneurol.2013.573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Satue M., Garcia-Martin E., Fuertes I., Otin S., Alarcia R., Herrero R. Use of Fourier-domain OCT to detect retinal nerve fiber layer degeneration in Parkinson's disease patients. Eye (Lond.) 2013;27:507–514. doi: 10.1038/eye.2013.4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Satue M., Seral M., Otin S., Alarcia R., Herrero R., Bambo M.P. Retinal thinning and correlation with functional disability in patients with Parkinson's disease. Br. J. Ophthalmol. 2014;98:350–355. doi: 10.1136/bjophthalmol-2013-304152. [DOI] [PubMed] [Google Scholar]
- Schechter I., Butler P.D., Silipo G., Zemon V., Javitt D.C. Magnocellular and parvocellular contributions to backward masking dysfunction in schizophrenia. Schizophr. Res. 2003;64:91–101. doi: 10.1016/s0920-9964(03)00008-2. [DOI] [PubMed] [Google Scholar]
- Schechter I., Butler P.D., Jalbrzikowski M., Pasternak R., Saperstein A.M., Javitt D.C. A new dimension of sensory dysfunction: stereopsis deficits in schizophrenia. Biol. Psychiatry. 2006;60:1282–1284. doi: 10.1016/j.biopsych.2006.03.064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schiffman J., Maeda J.A., Hayashi K., Michelsen N., Sorensen H.J., Ekstrom M. Premorbid childhood ocular alignment abnormalities and adult schizophrenia-spectrum disorder. Schizophr. Res. 2006;81:253–260. doi: 10.1016/j.schres.2005.08.008. [DOI] [PubMed] [Google Scholar]
- Schubert E.W., Henriksson K.M., Mcneil T.F. A prospective study of offspring of women with psychosis: visual dysfunction in early childhood predicts schizophrenia-spectrum disorders in adulthood. Acta Psychiatr. Scand. 2005;112:385–393. doi: 10.1111/j.1600-0447.2005.00584.x. [DOI] [PubMed] [Google Scholar]
- Sedighi B., Shafa M.A., Abna Z., Ghaseminejad A.K., Farahat R., Nakhaee N. Association of cognitive deficits with optical coherence tomography changes in multiple sclerosis patients. J. Mult. Scler. 2014;1:117. [Google Scholar]
- Shalev I., Moffitt T.E., Wong T.Y., Meier M.H., Houts R.M., Ding J. Retinal vessel caliber and lifelong neuropsychological functioning: retinal imaging as an investigative tool for cognitive epidemiology. Psychol. Sci. 2013;24:1198–1207. doi: 10.1177/0956797612470959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheremata S., Chen Y. Co-administration of atypical antipsychotics and antidepressants disturbs contrast detection in schizophrenia. Schizophr. Res. 2004;70:81–89. doi: 10.1016/j.schres.2003.09.005. [DOI] [PubMed] [Google Scholar]
- Shuwairi S.M., Cronin-Golomb A., Mccarley R.W., O'donnell B.F. Color discrimination in schizophrenia. Schizophr. Res. 2002;55:197–204. doi: 10.1016/s0920-9964(01)00180-3. [DOI] [PubMed] [Google Scholar]
- Sikorski B.L., Malukiewicz G., Stafiej J., Lesiewska-Junk H., Raczynska D. The diagnostic function of OCT in diabetic maculopathy. Mediat. Inflamm. 2013;2013:434560. doi: 10.1155/2013/434560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silverstein S.M., Keane B.P. Perceptual organization in schizophrenia: plasticity and state-related change. Learn. Percept. 2009;1:229–261. [Google Scholar]
- Silverstein S.M., Keane B.P. Perceptual organization impairment in schizophrenia and associated brain mechanisms: review of research from 2005 to 2010. Schizophr. Bull. 2011;37:690–699. doi: 10.1093/schbul/sbr052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silverstein S.M., Kovacs I., Corry R., Valone C. Perceptual organization, the disorganization syndrome, and context processing in chronic schizophrenia. Schizophr. Res. 2000;43:11–20. doi: 10.1016/s0920-9964(99)00180-2. [DOI] [PubMed] [Google Scholar]
- Silverstein S.M., Berten S., Essex B., Kovacs I., Susmaras T., Little D.M. An fMRI examination of visual integration in schizophrenia. J. Integr. Neurosci. 2009;8:175–202. doi: 10.1142/s0219635209002113. [DOI] [PubMed] [Google Scholar]
- Silverstein S.M., Berten S., Essex B., All S.D., Kasi R., Little D.M. Perceptual organization and visual search processes during target detection task performance in schizophrenia, as revealed by fMRI. Neuropsychologia. 2010;48:2886–2893. doi: 10.1016/j.neuropsychologia.2010.05.030. [DOI] [PubMed] [Google Scholar]
- Silverstein S.M., All S.D., Kasi R., Berten S., Essex B., Lathrop K.L. Increased fusiform area activation in schizophrenia during processing of spatial frequency-degraded faces, as revealed by fMRI. Psychol. Med. 2010;40:1159–1169. doi: 10.1017/S0033291709991735. [DOI] [PubMed] [Google Scholar]
- Silverstein S.M., Keane B.P., Barch D.M., Carter C.S., Gold J.M., Kovacs I. Optimization and validation of a visual integration test for schizophrenia research. Schizophr. Bull. 2012;38:125–134. doi: 10.1093/schbul/sbr141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silverstein S.M., Wang Y., Keane B.P. Cognitive and neuroplasticity mechanisms by which congenital or early blindness may confer a protective effect against schizophrenia. Front. Psychol. 2012;3:624. doi: 10.3389/fpsyg.2012.00624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silverstein S.M., Wang Y., Roche M.W. Base rates, blindness, and schizophrenia. Front. Psychol. 2013;4:157. doi: 10.3389/fpsyg.2013.00157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silverstein S., Keane B., Papathomas T., Lathrop K., Kourtev H., Feigenson K. Processing of spatial-frequency altered faces in schizophrenia: effects of illness phase and duration. PLoS One. 2014;9:e114642. doi: 10.1371/journal.pone.0114642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siris S.G. Depression in schizophrenia: perspective in the era of "atypical" antipsychotic agents. Am. J. Psychiatry. 2000;157:1379–1389. doi: 10.1176/appi.ajp.157.9.1379. [DOI] [PubMed] [Google Scholar]
- Skottun B.C., Skoyles J.R. Contrast sensitivity and magnocellular functioning in schizophrenia. Vis. Res. 2007;47:2923–2933. doi: 10.1016/j.visres.2007.07.016. [DOI] [PubMed] [Google Scholar]
- Slaghuis W.L. Contrast sensitivity for stationary and drifting spatial frequency gratings in positive- and negative-symptom schizophrenia. J. Abnorm. Psychol. 1998;107:49–62. doi: 10.1037//0021-843x.107.1.49. [DOI] [PubMed] [Google Scholar]
- Smith D., Pantelis C., Mcgrath J., Tangas C., Copolov D. Ocular abnormalities in chronic schizophrenia: clinical implications. Aust. N. Z. J. Psychiatr. 1997;31:252–256. doi: 10.3109/00048679709073828. [DOI] [PubMed] [Google Scholar]
- Souza V.B., Moura Filho F.J., Souza F.G., Rocha C.F., Furtado F.A., Goncalves T.B. Cataract occurrence in patients treated with antipsychotic drugs. Rev. Bras. Psiquiatr. 2008;30:222–226. doi: 10.1590/s1516-44462008000300008. [DOI] [PubMed] [Google Scholar]
- Stan A.D., Lewis D.A. Altered cortical GABA neurotransmission in schizophrenia: insights into novel therapeutic strategies. Curr. Pharm. Biotechnol. 2012;13:1557–1562. doi: 10.2174/138920112800784925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teranishi T., Negishi K., Kato S. Dopamine modulates S-potential amplitude and dye-coupling between external horizontal cells in carp retina. Nature. 1983;301:243–246. doi: 10.1038/301243a0. [DOI] [PubMed] [Google Scholar]
- Tian T., Zhu X.H., Liu Y.H. Potential role of retina as a biomarker for progression of Parkinson's disease. Int. J. Ophthalmol. 2011;4:433–438. doi: 10.3980/j.issn.2222-3959.2011.04.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tibber M.S., Anderson E.J., Bobin T., Antonova E., Seabright A., Wright B. Visual surround suppression in schizophrenia. Front. Psychol. 2013;4:88. doi: 10.3389/fpsyg.2013.00088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toledo J., Sepulcre J., Salinas-Alaman A., Garcia-Layana A., Murie-Fernandez M., Bejarano B. Retinal nerve fiber layer atrophy is associated with physical and cognitive disability in multiple sclerosis. Mult. Scler. 2008;14:906–912. doi: 10.1177/1352458508090221. [DOI] [PubMed] [Google Scholar]
- Uhlhaas P.J., Millard I., Muetzelfeldt L., Curran H.V., Morgan C.J. Perceptual organization in ketamine users: preliminary evidence of deficits on night of drug use but not 3 days later. J. Psychopharmacol. 2007;21:347–352. doi: 10.1177/0269881107077739. [DOI] [PubMed] [Google Scholar]
- Van Schouwenburg M., Aarts E., Cools R. Dopaminergic modulation of cognitive control: distinct roles for the prefrontal cortex and the basal ganglia. Curr. Pharm. Des. 2010;16:2026–2032. doi: 10.2174/138161210791293097. [DOI] [PubMed] [Google Scholar]
- Van Tonder G. Informing through an imperfect retina. In: Albertazzi L., Van Tonder G., Vishwanath D., editors. Perception Beyond Inference. MIT Press; Cambridge, MA: 2010. [Google Scholar]
- Viertio S., Laitinen A., Perala J., Saarni S.I., Koskinen S., Lonnqvist J. Visual impairment in persons with psychotic disorder. Soc. Psychiatry Psychiatr. Epidemiol. 2007;42:902–908. doi: 10.1007/s00127-007-0252-6. [DOI] [PubMed] [Google Scholar]
- Vollmer-Larsen A., Handest P., Parnas J. Reliability of measuring anomalous experience: the Bonn Scale for the Assessment of Basic Symptoms. Psychopathology. 2007;40:345–348. doi: 10.1159/000106311. [DOI] [PubMed] [Google Scholar]
- Warner R., Laugharne J., Peet M., Brown L., Rogers N. Retinal function as a marker for cell membrane omega-3 fatty acid depletion in schizophrenia: a pilot study. Biol. Psychiatry. 1999;45:1138–1142. doi: 10.1016/s0006-3223(98)00379-5. [DOI] [PubMed] [Google Scholar]
- Xin D., Bloomfield S.A. Effects of nitric oxide on horizontal cells in the rabbit retina. Vis. Neurosci. 2000;17:799–811. doi: 10.1017/s0952523800175133. [DOI] [PubMed] [Google Scholar]
- Yamamori H., Hashimoto R., Fujita Y., Numata S., Yasuda Y., Fujimoto M. Changes in plasma d-serine, l-serine, and glycine levels in treatment-resistant schizophrenia before and after clozapine treatment. Neurosci. Lett. 2014;582:93–98. doi: 10.1016/j.neulet.2014.08.052. [DOI] [PubMed] [Google Scholar]
- Yazulla S., Studholme K.M. Volume transmission of dopamine may modulate light-adaptive plasticity of horizontal cell dendrites in the recovery phase following dopamine depletion in goldfish retina. Vis. Neurosci. 1995;12:827–836. doi: 10.1017/s0952523800009391. [DOI] [PubMed] [Google Scholar]
- Yoon J.H., Maddock R.J., Rokem A., Silver M.A., Minzenberg M.J., Ragland J.D. GABA concentration is reduced in visual cortex in schizophrenia and correlates with orientation-specific surround suppression. J. Neurosci. 2010;30:3777–3781. doi: 10.1523/JNEUROSCI.6158-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshitsugu K., Yamada K., Toyota T., Aoki-Suzuki M., Minabe Y., Nakamura K. A novel scale including strabismus and 'cuspidal ear' for distinguishing schizophrenia patients from controls using minor physical anomalies. Psychiatry Res. 2006;145:249–258. doi: 10.1016/j.psychres.2005.10.013. [DOI] [PubMed] [Google Scholar]