Abstract
Background
Schistosoma mansoni infection has been associated with an increased HIV prevalence in humans and SHIV incidence in primate models. We hypothesized that immune activation from this gastrointestinal mucosa infection would increase highly HIV-susceptible CD4 T cell subsets in the blood and the foreskin through common mucosal homing.
Methodology/Principal Findings
Foreskin tissue and blood were obtained from 34 HIV- and malaria-uninfected Ugandan men who volunteered for elective circumcision, 12 of whom were definitively positive for S. mansoni eggs in stool and 12 definitively negative for both S. mansoni eggs and worm antigen. Tissue and blood T cell subsets were characterized by flow cytometry and immunohistochemistry (IHC). Th17 and Th1 cells from both the blood and foreskin expressed higher levels of CCR5 and were more activated than other CD4 T cell subsets. S. mansoni-infected men had a higher frequency of systemic Th1 cells (22.9 vs. 16.5% of blood CD4 T cells, p<0.05), Th17 cells (2.3 vs. 1.5%, p<0.05), and Th22 cells (0.5 vs. 0.3%, p<0.01) than uninfected men. Additionally, Th17 cells in the blood of S. mansoni-infected men demonstrated enhanced function (28.1 vs. 16.3% producing multiple cytokines, p = 0.046). However, these immune alterations were not observed in foreskin tissue.
Conclusions/Significance
S. mansoni infection was associated with an increased frequency of highly HIV-susceptible Th1, Th17 and Th22 cell subsets in the blood, but these T cell immune differences did not extend to the foreskin. S. mansoni induced changes in T cell immunology mediated through the common mucosal immune system are not likely to increase HIV susceptibility in the foreskin.
Author Summary
Fishing communities in East Africa have a very high prevalence of HIV, and also high rates of other endemic infections such as malaria and the fluke Schistosoma mansoni. Genital infections are known to increase HIV susceptibility through the recruitment and activation of mucosal CD4 T cells to the site of HIV sexual exposure. These activated CD4 T cells are necessary for an effective host immune response but are also preferentially infected by HIV. We hypothesized that S. mansoni infection in the gut mucosa might increase recruitment and activation of HIV target cells at other mucosal sites, and thereby contribute to high HIV rates in fishing communities. We enrolled men from a fishing community in Uganda and examined the frequency of highly HIV-susceptible cell types in their blood and foreskin tissue (a main site of HIV acquisition in heterosexual men). We found that men with S. mansoni infection had a greater frequency of HIV target cells in their blood, but not their foreskin tissue, perhaps because foreskin cells did not express mucosal homing markers. It is possible that HIV target cells observed in the blood of S. mansoni-infected individuals may traffic to other mucosae, such as the vagina or gut, and so the possibility that S. mansoni infection increases risk at these sites should be explored.
Introduction
HIV continues to be a public health crisis, with 2.3 million new infections and 1.6 million HIV-related deaths in 2013. Most new infections (70%) occurred in sub-Saharan Africa (SSA), where the predominant mode of transmission is heterosexual sex [1]. Despite the high number of new cases of HIV, the likelihood of transmission during a single sexual exposure is low, and is almost always established by a single virus quasispecies out of multiple distinct strains in the transmitting partner [2]. This suggests that the genital mucosa presents a significant barrier to infection. The substantial heterogeneity in susceptibility between individuals [3, 4] may reflect differences in the availability of target cells in the genital mucosa [5, 6], and increased levels of genital immune activation may account for the much higher per-contact risk of acquisition after exposure in SSA [7, 8].
CD4 T cells expressing the chemokine receptor CCR5 are the predominant targets of HIV during initial infection [2, 9, 10], and specific CD4+ T helper (Th) subsets are particularly susceptible to HIV. Activated Th cells are more susceptible to infection [11–13], as are Th17 cells (defined by the production of IL-17 [14]), Th1 cells (produce IFNγ [15]) and Th22 cells (produce IL-22 in the absence of IL-17 or IFNγ [16–18]). Not only are these subsets more susceptible to HIV infection in vitro [19–22], but they are also selectively depleted early in HIV infection [21, 23–25], and are less frequent in HIV-exposed seronegative (HESN) men [26]. Th17 cells have the capacity to not only produce IL-17, but also other pro-inflammatory cytokines, including IL-22 and IFNγ [27, 28]. Polyfunctional Th17 cells are more susceptible to HIV infection in vitro than either Th1 cells or Th17 cells that produce IL-17 alone [20–22], and are rapidly depleted in early HIV infection [28]. The mucosal availability of these highly susceptible CD4 T cells may determine whether exposure to HIV results in infection [5].
In keeping with the role of these mucosal cell subsets in HIV susceptibility, their numbers are increased in the genital mucosa by sexually-transmitted infections (STIs) that enhance HIV risk, such as Herpes simplex virus type 2 (HSV-2) [29–34], even in the absence of clinically apparent ulceration [35]. Recent studies show that non-genital infections common to SSA, such as helminthic infections, promote systemic inflammation and CCR5 expression [36–40]. Whether immune activation from infections of the gastrointestinal mucosa, such as helminthic infections, would translate into genital immune alterations is not known, but immune stimulus at one mucosal surface often leads to T cell activation and recruitment at distal mucosal sites through the expression of common mucosal homing markers [41]. This may explain why Kenyan women without STIs nonetheless have an increased frequency of activated CD4 T cells in their cervical mucosa compared to their peers from the United States [8]. While helminthic infections were not tested for in this study, their high regional prevalence suggests a potential role in the mucosal immune activation observed in these otherwise healthy women.
HIV disproportionately affects fishing communities in SSA; incidence rates on the shores of Lake Victoria in Uganda approach 5%, while the national average is under 0.75% [42–44]. Helminthic infections are very common in these fishing communities, with over 60% of men aged 20–29 years infected with Schistosoma mansoni [45], a parasitic worm that causes a chronic gastrointestinal (GI) infection if untreated and has been associated with increased HIV prevalence [46]. During infection, cercariae (free-swimming larval stage) found in contaminated fresh water penetrate the skin and migrate through the circulation to the portal vein where they mature into adult worms. Females release approximately 300 eggs per day, which traverse the walls of the vein and intestine to be released into the gut lumen and excreted [47]. While the worms themselves evade immune destruction [48], eggs are highly antigenic and not only require a strong CD4 T cell response for efficient release [49, 50], but also incite the formation of CD4 T cell-containing granulomas in intestinal tissue [47]. The mechanism for the potential association between S. mansoni infection and HIV susceptibility is unclear, since S. mansoni does not directly involve the urogenital mucosa. This is in contrast to S. haematobium (uncommon in the Entebbe region [45]), which involves the urogenital mucosa and therefore alters mucosal HIV susceptibility directly at the site of sexual exposure [51–53]. However, S. mansoni infection does result in increased HIV target cells in the blood [54], and rhesus macaques infected with S. mansoni are 17-times more susceptible to SHIV [55].
Clinical trials of adult circumcision have demonstrated that the foreskin is a major site of HIV acquisition in uncircumcised heterosexual men [56–58]. We therefore sought to determine whether S. mansoni infection is associated with alterations in HIV target cells, both systemically, and at this relevant tissue site.
Methods
Study participants
Participants aged 18–49 years were recruited from the Uganda Virus Research Institute- International AIDS Vaccine Initiative (UVRI-IAVI) Voluntary HIV Counselling and Testing (VCT) Clinic in Kasenyi, Uganda, a fishing community on the shore of Lake Victoria. All men who expressed an interest in voluntary adult medical male circumcision were offered enrolment in the study. Exclusion criteria included current use of immunosuppressive therapy or infection with malaria. Men who had symptomatic genital infections were to be provided treatment and followed up weekly until resolution of symptoms, at which point they would be scheduled for circumcision. To avoid stigmatization, both HIV-infected and uninfected men were offered participation in this study, however, blood and foreskin samples were only analyzed for HIV-uninfected men. All participants were offered VCT, and HIV-infected men were referred to Wagagai Health Center (a private health facility at Kasenyi landing site) for treatment and care. Individuals who tested positive for schistosomiasis were treated at the clinic with single-dose praziquantel, 40 mg/kg body weight.
Ethics statement
All participants provided written informed consent, and ethical approval was obtained through the Institutional Review Boards at the Uganda Virus Research Institute and at the University of Toronto.
Sample collection and diagnostic testing
After VCT, consenting participants completed a behavioural questionnaire and were scheduled for circumcision. HIV testing was performed according to the Uganda National Algorithm, consisting of two rapid tests (Alere Determine HIV-1/2, Abbott Laboratories, Matsudo-shi, Japan; and HIV 1/2 STAT-PAK Dipstick Assay, Chembio Diagnostic Systems, Medford, USA), with a third rapid (Unigold HIV, Trinity Biotech, Wicklow, Ireland) used for discordant results. Participants returned to provide stool and terminal urine samples (collection vials from VWR, Mississauga, Canada) for three consecutive days surrounding surgery (i.e. the day prior to surgery, the day of surgery, and the day after surgery). Urine was immediately separated, with 1.5ml reserved for Circulating Cathodic Antigen (CCA) detection (stored at 4°C until analysis later the same day) and the remainder preserved with 0.2% bleach for S. haematobium egg detection by light microscopy [59]. Screening for S. mansoni was performed using the Kato-Katz method [59], with three slides screened per stool sample. Presence of urine-CCA, which does not distinguish between species of schistosomes and therefore cannot differentiate S. mansoni from S. haematobium, was detected using the Rapid Medical Diagnostics Schistosomiasis Test (Rapid Medical Diagnostics; Pretoria, RSA) according to the manufacturer’s directions. Previous studies in Rakai have found the Kato Katz method on two stool samples (sensitivity = 98.6%) or rapid CCA detection in one urine sample (sensitivity = 91.7%, specificity = 75.0%) accurately detect S. mansoni infection [60]. For the purposes of statistical comparisons in this study, men were classified as schistosomiasis-positive if they were positive by both Urine-CCA and had eggs in a stool sample (S. mansoni) on at least one study visit. Men were considered to be schistosomiasis-negative if negative for both Urine-CCA and eggs at all study visits.
Circumcisions were performed at the Wagagai Health Center at Kasenyi, Entebbe, Uganda. Prior to surgery, a clinical officer performed a physical examination and collected 16ml of whole blood. Surgery was deferred and participants were treated if urethral discharge or genital ulceration were present. Foreskins were collected into RPMI 1640 media supplemented with: 10% heat-inactivated FBS, 10U/ml penicillin, 10μg/ml streptomycin, 250ng/ml amphotericin B, and 2mM L-Glutamine (all from Gibco, Invitrogen; Carlsbad, CA, USA; henceforth referred to as R10 medium). Blood and foreskin samples were transported to UVRI-IAVI laboratories for immediate processing: four sections of foreskin were snap frozen into cryomolds in Optimal Cutting Temperature (OCT) compound (Fisher Scientific, Toronto, Canada) for immunohistochemistry (IHC); and one large section was reserved for T cell isolation. An aliquot of whole blood was removed for malaria diagnostics, and the remainder was separated into plasma and PBMC fractions by density gradient centrifugation (Ficoll-Paque Plus; Amersham Biosciences; Uppsala, Sweden). Participants were confirmed to be negative for malaria using both the Cypress Diagnostics Malaria falciparum Rapid Test (Langdorp, Belgium) and by microscopy (Giemsa stained thick smear). HSV-2 serology was performed by ELISA (Herpes Simplex Type 2 IgG ELISA, Kalon Biological Ltd., Guildford, UK), as previously validated in Uganda [61]. PBMCs were reserved for flow cytometry.
T cell isolation from the foreskin
T cells were isolated from foreskin tissue as previously described [62]. Briefly, tissue was disrupted by a combination of mechanical and enzymatic digestion with 1.0ml of 500U/ml Collagenase Type I (Gibco, Invitrogen; Carlsbad, CA, USA) in the presence of 50U/ml of DNAse (Invitrogen). The resulting cell suspension was filtered through a 100μm cell strainer (BD Biosciences; Franklin Lakes, NJ USA) to remove any remaining undigested tissue. Filtered cells were allowed to rest under normal growth conditions (37°C, 5% CO2, humidified atmosphere) for 3–7 hours.
Characterization of T cell subsets
PBMC and foreskin mononuclear cell counts were determined by trypan blue exclusion. 1x106 PBMCs and 10-20x106 foreskin mononuclear cells (depending on yield) were plated in 500μl culture medium with 5μg/ml Brefeldin A (GolgiPlug, BD Biosciences) and fluorochrome-labelled CCR6 antibody (11A9; BD Biosciences). Cells were stimulated for 9 hours at 37°C with either: 0.5ng/ml phorbol-12-myristate-13-acetate (PMA) and 0.5μg/ml ionomycin; 2μg/ml Staphylococcal enterotoxin B (SEB); or vehicle (0.1% Dimethyl sulfoxide, DMSO; all from Sigma; St. Louis, MO, USA). After stimulation, samples were washed with cold 2% Foetal Bovine Serum (FBS) in phosphate buffered saline (PBS) and stained with LIVE/DEAD Fixable Aqua Dead Cell Stain Kit (Life Technologies, Burlington, Canada), washed and then stained with fluorochrome-labeled monoclonal antibodies specific for the following surface antigens: CD3 Qdot655 (S4.1), CD4 PE-Cy7 (SK3), CCR5 PE-Cy5 (2D7/CCR5), CD69 APC-Cy7 (FN50), HLA-DR PE-CF594 (G46-6), and CD38 AF700 (HIT2; all BD Biosciences). In a second aliquot of cells, cells were stained for homing integrin α4 FITC (9F10), β7 APC (FIB504; both BD) and Cutaneous Lymphocyte Antigen (CLA) PE (HECA-452, Miltenyi Biotec; San Diego, CA, USA) in addition to CD3 and CD4. Samples were then washed and permeabilized using the eBioscience fixation/permeabilization solution (eBiosciences; San Diego, CA, USA) and stained with fluorochrome-labeled monoclonal antibodies specific for the following intracellular cytokines: IFNγ V450 (B27; BD Biosciences), IL17A AF488 (eBio64DEC17; eBioscience), and IL22 PE (22URTI; eBioscience). Data were acquired using an LSRII flow cytometer (BD Biosciences).
CD3 density quantification by immunohistochemistry
All flow cytometry data were analyzed as relative proportions of CD3+ T cells. To determine if the overall density of CD3 T cells varied between men, as opposed to the relative abundance of specific subsets, the average number of T cells per mm2 of tissue was determined using IHC staining for CD3. OCT-cryopreserved tissues were sectioned to 5μm, fixed in acetone, and frozen for batch staining. At the time of staining, frozen sections were thawed and air-dried at room temperature. Sections were blocked with 10% normal goat serum and stained with rabbit anti-human CD3 antibody (Dako, Carpinteria, CA, USA) followed by goat anti-rabbit secondary antibody conjugated to AF647 (Life Technologies). Slides were then washed with PBS, counter stained with Hoechst DAPI, and air-dried. The number of CD3+ T cells per mm2 of tissue for each patient was derived from the average of four tissue sections: two from separate locations on the distal edge of the foreskin, and two from the proximal edge. A median of 6.10mm2 of foreskin tissue was analyzed by IHC per patient. Whole sections were scanned at 0.5μm/pixel using the TissueScope 4000 (Huron Technologies, Waterloo, Canada). Image analysis software (Definiens, München, Germany) was used to delineate the apical edge of the epidermis of the entire length of each section to a depth of 300μm (excluding artefacts or folds). CD3 cells were defined as nuclear DAPI fluorescence overlapping with, or directly adjacent to, AF647 fluorescence. The average density of CD3+ cells/mm2 of foreskin tissue was then multiplied by the percentage of CD3+ cells found to co-express CD4 by flow cytometry to calculate the average number of CD4 T cells per mm2 of foreskin tissue.
Statistical analysis
Flow cytometry data was analyzed in FlowJo v.9.8.2 (Treestar; Ashland, OR, USA). Expression of surface markers was compared between Th subsets using the Friedman chi-square test; with post-hoc pairwise comparisons made using the Wilcoxon related samples rank test; Bonferroni adjusted p-values are reported. T cell populations were compared between S. mansoni-infected and uninfected men by Mann-Whitney U test. Analysis of Th17 cell polyfunctionality was performed using Boolean gating in FlowJo software, and polyfunctionality compared between S. mansoni-infected and uninfected men with a chi-squared distribution (1000 permutations of pies) using SPICE v5.35 software (Exon, National Institute of Allergy & Infectious Diseases)[63]. Post-hoc comparisons between functional Th17 subsets were made using related samples Wilcoxon rank test. Excel v.14.4.8 (Microsoft; Redmond, WA, USA) was used prior to statistical testing, and statistical tests were run using SPSS v.20.0 for Mac (IBM; New York, NY, USA). Based on previous foreskin T cell studies [26, 62, 64], a sample size of 34 men provided 80% power to detect a difference of one standard deviation in the proportion of either foreskin or blood CD4 T cells that were Th17 cells (α = 0.05).
Results
Study population
Participants consisted of 34 HIV-uninfected men living in fishing communities on the shores of Lake Victoria who were undergoing elective adult circumcision for HIV prevention. No participant tested positive for malaria, had a symptomatic genital infection, or was taking immunosuppressive therapy at the time of enrolment. As the immunopathology of schistosomiasis is the result of egg production, and not necessarily the presence of worms, we compared men with documented S. mansoni egg production on at least one of the three days surrounding surgery (n = 12; median 918 eggs per gram (epg); IQR 108–4644 epg) to control men who were free of both eggs and CCA in urine (an antigen originating from the gut of mature worms) for three consecutive days surrounding surgery (n = 12). No S. haematobium eggs were detected in any participant. Men who had detectable Urine-CCA but who were not shedding eggs (n = 10) were included graphically in the Th17 functional analysis, but were excluded from statistical analysis. S. mansoni-infected and control men were of similar age, HIV-risk taking behaviour (based on self-reported condom use, recent number of sexual partners, and transactional sex), and HSV-2 serostatus (Table 1). However, S. mansoni infection was associated with employment in the fishing industry (p = 0.04) and more frequent contact with lake water (p = 0.03).
Table 1. Participant demographics.
| S. mansoni | Uninfected | ||||
|---|---|---|---|---|---|
| (n = 12) | (n = 12) | p-value | |||
| Age (yrs) | 26.7 | (18–37) | 22.4 | (18–31) | ns |
| HSV-2 Seropositive (%) | 33.3 | 8.4 | ns | ||
| Sex Partners Last 6 Months (#) | 1.3 | (0–3) | 1.1 | (0–4) | ns |
| Using Condoms | |||||
| Never | 66.7 | 58.3 | |||
| Sometimes | 8.4 | 16.7 | ns | ||
| Always | 25.0 | 25.0 | |||
| Works in the fishing industry (%) | 58.3 | 0.0 | 0.037 | ||
| Contact with lake > weekly (%) | 58.3 | 8.4 | 0.027 | ||
| Every treated for schistosomiasis (%) | 16.7 | 8.4 | ns | ||
Characterization of HIV target cell populations in the foreskin and blood
We first characterized Th1, Th17, and Th22 cells, independent of S. mansoni infection status, to test our assumption that these three Th subsets are preferential HIV targets. We examined expression levels of markers known to be associated with HIV susceptibility, namely the HIV co-receptor CCR5 [10, 65] and activation markers CD69 [65, 66] and HLA-DR [11, 66–68] on these Th subsets in the blood and foreskin of all 34 men.
CCR5 expression levels were decreased after PMA/iono stimulation (median 1.4% CCR5+ of CD4 T cells after PMA/iono vs. 3.2% on unstimulated), and so we report expression levels on functional subsets defined using SEB stimulation. Gating strategy and representative plots of stimulated cells are shown in Fig 1. Th subset definitions were mutually exclusive and exhaustive: Th17 cells produced IL-17A, and either IFNγ or IL-22; Th1 cells produced IFNγ, but not IL-17A; Th22 cells produced IL-22, but not IL-17A or IFNγ; and “other CD4 T cells” (the comparator group) were Th cells that did not produce any of these three cytokines upon stimulation (Fig 1).
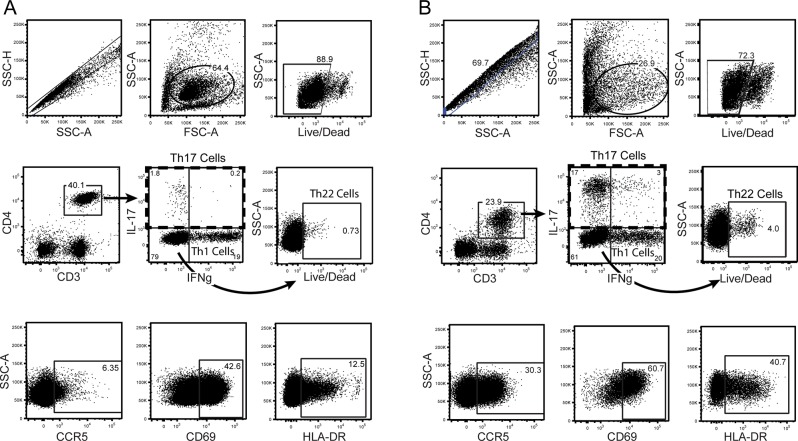
Fig 1. Flow cytometry gating strategy for Th subsets isolated from (A) the blood and (B) the foreskin.
Blood cells were isolated by density centrifugation, and foreskin cells by mechanical and enzymatic digestion of tissue. Dead cells and doublets were excluded. Lymphocytes were identified based on characteristic size and granularity in blood samples and gates applied to foreskin samples. CD4 T cells were identified by expression of CD3 and CD4. Th subsets among CD4 T cells were identified by cytokine production in response to non-specific stimuli (SEB shown). Gates for cytokine production were based on unstimulated samples. Th17 cells were identified by production of IL-17A; Th1 cells by production of IFNγ and not IL-17A; Th22 cells by production of IL-22 in the absence of either IFNγ or IL-17A. Representative plots showing expression of CCR5, CD69 and HLA-DR on CD4 T cells are also shown.
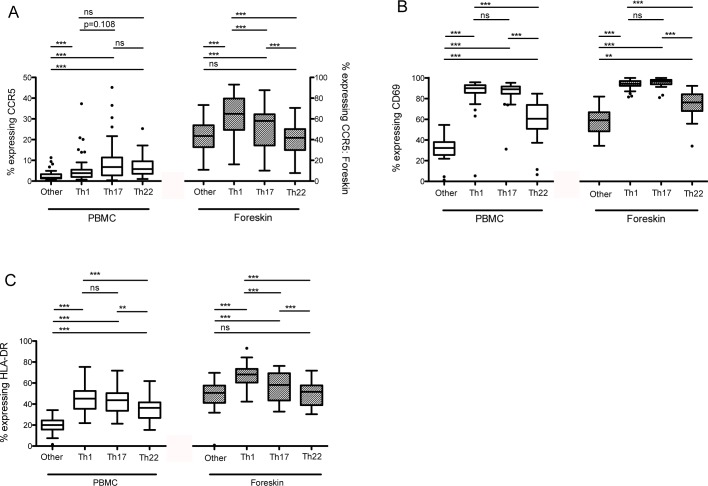
In the blood, all three Th subsets (Th1, Th17 and Th22) were more likely to express the HIV co-receptor CCR5 than other CD4 T cells (other CD4 T cells: 2.8%; all p<0.001; Fig 2A), although there were no significant differences between Th subsets (Th17: 10.8%; Th22: 7.1%; Th1: 6.3%). All three subsets also expressed higher levels of activation markers CD69 and HLA-DR compared to other CD4 T cells (other CD4 T cells: 31.1% CD69+, Fig 2B; and 20.0% HLA-DR+, Fig 2C; all p<0.001). Th17 and Th1 cells had substantially higher levels of both activation markers (87.9 and 86.0% CD69+, respectively; 43.4 and 45.9% HLA-DR+, respectively); Th22 cells were also more activated than other CD4 cells (60.5% CD69+ and 35.8% HLA-DR+, p<0.001), albeit less so that Th1 or Th17 cells (both p<0.001).
Fig 2. Differential expression of markers of HIV susceptibility on Th populations isolated from the blood and foreskin.
Expression of (A) CCR5, (B) CD69, and (C) HLA-DR was measured on CD4 T cells isolated from the blood (PBMCs) and foreskin tissue using flow cytometry. “Other” refers to CD4+/CD3+ cells that do not produce IL-17A, IFNγ or IL-22. Expression of surface markers on Th subsets was compared using the Friedman chi-square test; post-hoc pairwise comparisons made using Wilcoxon related samples rank test and Bonferroni adjusted p-values are reported (*p<0.05; **p<0.01; ***p<0.001).
In the foreskin, Th1 and Th17 cells expressed significantly higher levels of CCR5 than other CD4 T cells (61.1% and 52.6% vs. 42.7%, both p<0.001; Fig 2A). In contrast to the blood, Th22 cells expressed no more CCR5 than other CD4 T cells (39.0 vs. 42.7%, ns). Also in contrast to the blood, foreskin Th1 cells expressed significantly more CCR5 than Th17 cells (p<0.001). Expression of HLA-DR on foreskin Th subsets followed a similar pattern, with Th1 cells expressing highest levels (69.4%; p<0.001 vs. other CD4 T cells and Th17 cells), followed by Th17 cells (59.1%, p<0.001 vs. other CD4 T cells), and Th22 cells having similar expression to other CD4 T cells (51.6% and 48.8%, respectively; Fig 2B). All three Th populations of interest had higher CD69 expression than other CD4 T cells (other CD4 T cells: 58.1%, all p<0.001; Fig 2B), with Th17 and Th1 cells having higher levels (both 94.0%) than Th22 cells (68.7%; p<0.001). In a separate flow cytometry panel, we assessed the expression of gut and skin homing markers on foreskin and blood CD4 T cells. We found the foreskin to be enriched for CD4 T cells expressing the skin homing marker CLA compared to the blood (59.8% of foreskin CD4 T cells vs. 18.2% of blood, p<0.001), but to have significantly reduced expression of the mucosal homing marker α4β7 (3.2% vs. 6.8%, p<0.001; S1 Fig).
HIV target cell proportional frequency and absolute number in S. mansoni-infected and uninfected men
S. mansoni-infected men had significantly higher frequencies of Th1 (22.9 vs. 16.5% of CD4 T cells, p<0.05; Fig 3A), Th17 (2.3 vs. 1.5%, p<0.05) and Th22 (0.5 vs. 0.3%, p<0.01) cells in their blood compared to uninfected men. However, these differences did not extend to the foreskin, since S. mansoni-infected and uninfected men had similar frequencies of susceptible Th populations in foreskin tissue. We also did not observe significant differences in overall CD4 T cell expression of CCR5, CD69 or HLA-DR between S. mansoni-infected and uninfected men, in either the blood or foreskin (Fig 3B). While flow cytometry provides the proportion (%) of CD3 T cells expressing markers of interest, it does not provide information on the total number of T cells present in the tissue. Using IHC to determine the number of CD3 T cells per mm2 of tissue, we found no statistically significant difference in the average density of foreskin CD3+ cells (262/mm2 (IQR 134-471/mm2) vs. 191/mm2 (IQR 125-533/mm2); p = 0.52) in S. mansoni-infected and uninfected men.
Fig 3. HIV target cells in the blood and foreskin of men infected with S. mansoni.
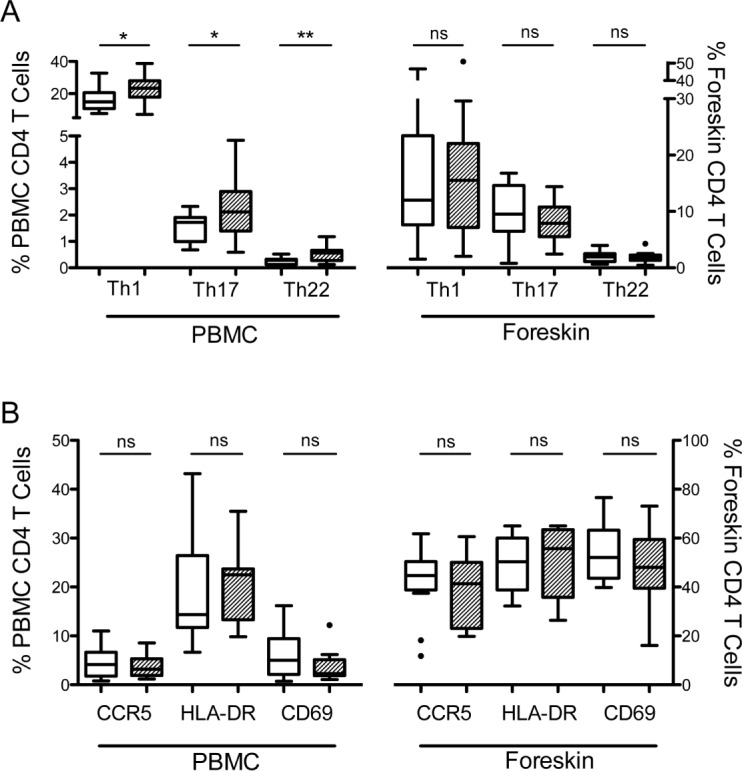
HIV target cells were identified by flow cytometry from cells isolated from the blood (PBMC) or foreskin, and were compared between men shedding S. mansoni eggs (hatched bars) and men who were free of Schistosoma infection (clear bars). (A) Proportions of CD4 T cells that are Th1, Th17 or Th22 cells. (B) Expression of markers of HIV susceptibility on CD4 T cells. T cell populations were compared between S. mansoni-infected and uninfected men by Mann-Whitney U test (*p<0.05; **p<0.01).
Impact of S. mansoni infection on Th17 cell function
We then went on to compare the functional capacity of Th17 cells, defined as their ability to co-produce IL-22 and/or IFNγ in addition to IL-17A, between S. mansoni-infected and uninfected men. Th17 cells from the blood of men infected with S. mansoni had a greater capacity to produce pro-inflammatory cytokines than those from uninfected men (Fig 4A, p = 0.046). Post-hoc analysis performing pairwise comparisons showed trends to increased numbers of triple cytokine producing cells (2.7 vs. 0.92%, p = 0.059) and IFNγ-producing Th17 cells (13.4 vs. 8.6%, p = 0.104), and significantly increased IL-22 producing Th17 cells (10.1 vs. 4.4%, p = 0.013; Fig 4B) in S. mansoni-infected men.
Fig 4. Functional capacity of Th17 cells isolated from the blood of men infected with S. mansoni.
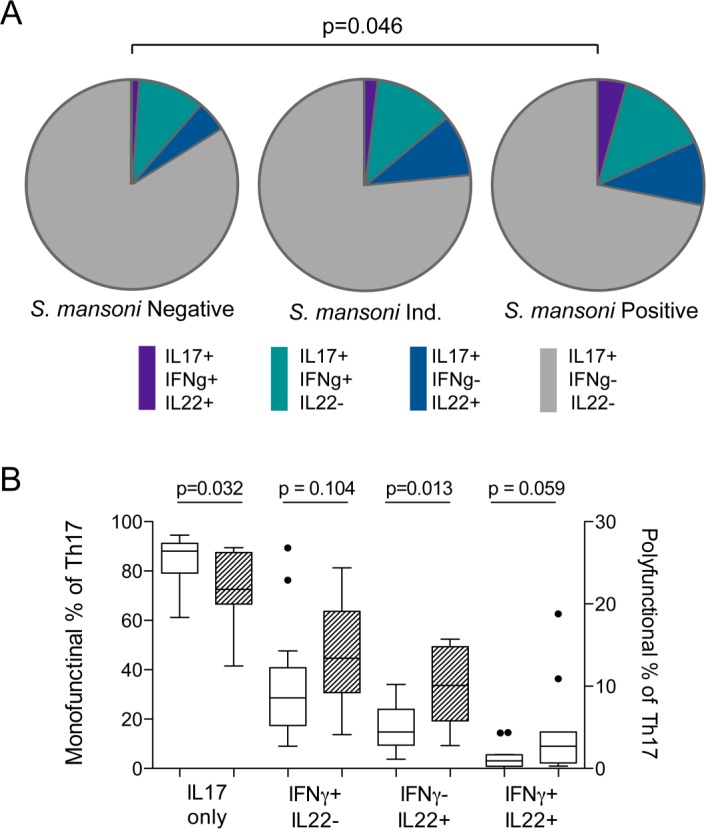
The ability of Th17 cells isolated from the blood to produce a second or third cytokine (IL-22 and/or IFNγ in addition to IL-17A) in response to non-specific stimulation (PMA/iono) was measured using flow cytometry. (A) Proportion of Th17 cells producing additional cytokines was compared between men infected with S. mansoni (shedding eggs), intermediate (presence of worms detected by Urine-CCA, but not shedding eggs), and uninfected men (no detection of worm antigen or eggs). Assessment of polyfunctionality was performed using SPICE v5.35 software with polyfunctionality compared between S. mansoni-infected and uninfected men using a chi-squared distribution (1000 permutations of pies)[63]. (B) Post-hoc comparison of individual functional subsets between S. mansoni-infected (hatched bars) and uninfected men (related samples Wilcoxon rank test).
Discussion
The likelihood of infection after sexual exposure to HIV is heterogeneous and likely determined by availability of susceptible CD4 T cells at the site of exposure [6]. Activated CD4 T cells [11–13] and Th1, Th17 [19–24], and Th22 [25] subsets have been implicated as preferential targets of HIV, and their frequency at mucosal sites may be altered by co-infections. In keeping with the hypothesis that these Th cell subsets represent preferential HIV targets, they demonstrated an elevated expression of the HIV co-receptor CCR5, and were more likely to express activation markers CD69 and HLA-DR, markers associated with increased HIV cellular entry [65]. Not only did we find that S. mansoni infection was associated with an increased systemic (blood) frequency of Th1, Th17 and Th22 cells, but functional analysis of blood Th17 cells demonstrated that S. mansoni infection was associated with increased production of pro-inflammatory cytokines IL-22 and IFNγ, a characteristic associated with selective depletion during HIV infection [25]. However, the systemic immune impact of S. mansoni infection did not extend to the foreskin. Specifically, we found no difference in the frequency of Th1, Th17 or Th22 cells, or in immune activation or CCR5 expression, between the foreskins of S. mansoni-infected and uninfected men. Of note, Th subsets in the foreskin had a different pattern of CCR5, HLA-DR and CD69 expression compared to blood, with Th22 cells at this site having lower CCR5 expression and activation status than Th1/Th17 subsets.
Our observation of increased systemic CD4 T cell activation is consistent with the known immunopathology of S. mansoni. A robust CD4 T cell response is essential for egg excretion during S. mansoni infection, demonstrated by the observations that egg excretion does not occur in immunocompromised mice [69, 70] or humans [50]. Eggs may also become trapped in the tissue on their way to being excreted [71], and the development of granulomatous lesions around them is dependent on CD4 T cells [72, 73]. While early studies in mice attributed the immunopathology of schistosomiasis infection to IFNγ production and Th1 activation [74, 75], the identification of Th17 cells as a distinct Th subset [76] led to the observation that Th17 cells were integral to granuloma formation, and high levels of IL-17A were produced by granuloma cells [77, 78]. Our findings of increased frequencies of Th1 and Th17 cells in the blood of S. mansoni-infected men is consistent with these previous observations. To our knowledge Th22 cells have not been studied in the setting of S. mansoni infection, but since they play a role in the maintenance of intestinal epithelial integrity [18, 25, 79] we hypothesize that the increase we observed during S. mansoni infection may be in response to the inflammatory foci that form in the intestinal epithelium during egg excretion.
Th17 and Th1 cells are highly susceptible to HIV in vitro, with IFNγ-producing Th17 cells (elevated in S. mansoni-infected men in the current work) being particularly susceptible [20–22], and Th22 cells may also be more susceptible [25]. However, the increased frequency of the highly susceptible Th subsets that we observed in the blood of S. mansoni-infected men was not observed in foreskin tissue. Since the foreskin is the primary site of HIV acquisition in heterosexual Ugandan men, the potential impact of these systemic S. mansoni-associated immune changes on HIV susceptibility in heterosexual men is not clear. This is in keeping with two recent publications that found no association between S. mansoni infection and either HIV incidence [80] or prevalence [81] in Entebbe, Uganda. However, our findings do emphasize the need for tissue specific studies when assessing the potential impact of co-infections on HIV susceptibility. This need was further demonstrated by the different patterns of CCR5 expression observed on Th subsets in the blood compared to foreskin: while blood CCR5 expression would suggest that Th22 cells were more HIV-susceptible than Th1 cells, foreskin Th22 cells had relatively low CCR5 expression.
We had hypothesized that S. mansoni infection would alter HIV target cells in the foreskin through activation of the common mucosal immune system. Local gut epithelial damage occurs during S. mansoni egg excretion and would be expected to directly increase the frequency of HIV target cells at this site, likely explaining the increased susceptibility to SHIV seen in S. mansoni-infected primates after rectal but not systemic virus challenge [55]. However, we had speculated that the upregulation of common mucosal T cell homing molecules, such as the integrin α4β7 [82, 83], might also increase T cell activation and HIV target cell numbers at genital mucosal sites. One reason that we did not observe this may be that T cells in the foreskin expressed high levels of the skin homing marker CLA, but not α4β7.
This study had two limitations that should be considered. The first is the relatively small sample size. This study was designed to determine if a larger, prospective study of the effect of S. mansoni infection on foreskin immunology was warranted. It was powered to detect a difference of one standard deviation in Th populations between groups, and so more subtle chances in foreskin T cell immunology would be missed. However, the robust immune differences seen in the blood without consistent trends in the foreskin suggest that a larger sample size would not change our conclusions. A second limitation of this study was the lack of diagnostic testing for STIs other than HSV-2. Genital co-infections are associated with local mucosal immune activation [33], and therefore if they were overrepresented among control men (S. mansoni-uninfected), this would have contributed to the absence of S. mansoni-associated foreskin immune changes in this study. However, since there was a non-significant trend to increased HSV-2 prevalence in S. mansoni-infected men, it seems unlikely that these same men had significantly lower rates of other STIs. Furthermore, since HSV-2 infection is associated with T cell activation in the foreskin but not blood [35], it is not likely that inter-group differences in STI prevalence explain the presence of T cell immune alterations in the blood.
In conclusion, we found that S. mansoni infection in HIV-uninfected Ugandan men was associated with an increase in highly HIV-susceptible T cell subsets in the blood, but not the foreskin. Whether these immune changes would alter HIV susceptibility, given that they are limited to the blood compartment, is not clear. Future studies are warranted to determine if the systemic immune changes observed in this study extend to other mucosal sites of HIV acquisition, such as the cervicovaginal or gut mucosae.
Supporting Information
(DOC)
(A) Representative staining and (B) expression of Cutaneous Lymphocyte Antigen (CLA) or integrin α4β7 on CD4 T cells isolated from either the blood (clear bars) or foreskin tissue (hatched bars). Homing marker expression was compared between foreskin and blood cells by Wilcoxon related samples rank test (**p<0.01).
(TIF)
Acknowledgments
The authors would like to thank Kate Ochom, Edwig Nagabirwa, Roy Kyabangi, and Safina Ndyanabo from the Wagagai Health Center for assistance with sample collection; and also STTARR and the University Health Network Department of Pathology (both Toronto, Canada) for assistance with CD3 staining and microscopy.
Data Availability
All relevant data are within the paper and its Supporting Information files.
Funding Statement
Research was primarily funded by the Canadian Institutes of Health Research (http://www.cihr-irsc.gc.ca) grant numbers TMI-138656 and HBF-137700 (RK). Ontario HIV Treatment Network (www.ohtn.on.ca) provided funding through the University of Toronto Endowed Chair in HIV Research (RK salary support) and a Studentship Award (JLP salary support; grant number STU G708). This work was also partially funded by IAVI with the generous support of USAID and other donors; a full list of IAVI donors is available at www.iavi.org. The contents of this manuscript are the responsibility of the authors and do not necessarily reflect the views of USAID or the US Government. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.UNAIDS. Global Report: UNAIDS report on the global AIDS epidemic 2013. WHO Library Cataloguing-in-Publication Data: 2013.
- 2. Keele BF, Giorgi EE, Salazar-Gonzalez JF, Decker JM, Pham KT, Salazar MG, et al. Identification and characterization of transmitted and early founder virus envelopes in primary HIV-1 infection. Proc Natl Acad Sci U S A. 2008;105(21):7552–7. Epub 2008/05/21. 10.1073/pnas.0802203105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Hladik F, McElrath MJ. Setting the stage: host invasion by HIV. Nat Rev Immunol. 2008;8(6):447–57. Epub 2008/05/13. 10.1038/nri2302 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Yi TJ, Shannon B, Prodger J, McKinnon L, Kaul R. Genital immunology and HIV susceptibility in young women. Am J Reprod Immunol. 2013;69 Suppl 1:74–9. 10.1111/aji.12035 [DOI] [PubMed] [Google Scholar]
- 5. Talbert-Slagle K, Atkins KE, Yan KK, Khurana E, Gerstein M, Bradley EH, et al. Cellular superspreaders: an epidemiological perspective on HIV infection inside the body. PLoS Pathog. 2014;10(5):e1004092 10.1371/journal.ppat.1004092 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. McKinnon LR, Kaul R. Quality and quantity: mucosal CD4+ T cells and HIV susceptibility. Curr Opin HIV AIDS. 2012;7(2):195–202. 10.1097/COH.0b013e3283504941 [DOI] [PubMed] [Google Scholar]
- 7. Boily MC, Baggaley RF, Wang L, Masse B, White RG, Hayes RJ, et al. Heterosexual risk of HIV-1 infection per sexual act: systematic review and meta-analysis of observational studies. Lancet Infect Dis. 2009;9(2):118–29. Epub 2009/01/31. 10.1016/S1473-3099(09)70021-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Cohen CR, Moscicki A-B, Scott ME, Ma Y, Shiboski S, Bukusi E, et al. Increased levels of immune activation in the genital tract of healthy young women from sub-Saharan Africa. Aids. 2010;24(13):2069–74. 10.1097/QAD.0b013e32833c323b [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Li Q, Estes JD, Schlievert PM, Duan L, Brosnahan AJ, Southern PJ, et al. Glycerol monolaurate prevents mucosal SIV transmission. Nature. 2009;458(7241):1034–8. 10.1038/nature07831 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Salazar-Gonzalez JF, Salazar MG, Keele BF, Learn GH, Giorgi EE, Li H, et al. Genetic identity, biological phenotype, and evolutionary pathways of transmitted/founder viruses in acute and early HIV-1 infection. J Exp Med. 2009;206(6):1273–89. Epub 2009/06/03. 10.1084/jem.20090378 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Zhang Z. Sexual Transmission and Propagation of SIV and HIV in Resting and Activated CD4+ T Cells. Science. 1999;286(5443):1353–7. [DOI] [PubMed] [Google Scholar]
- 12. Zhang ZQ. Roles of substrate availability and infection of resting and activated CD4+ T cells in transmission and acute simian immunodeficiency virus infection. Proceedings of the National Academy of Sciences. 2004;101(15):5640–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Saba E, Grivel JC, Vanpouille C, Brichacek B, Fitzgerald W, Margolis L, et al. HIV-1 sexual transmission: early events of HIV-1 infection of human cervico-vaginal tissue in an optimized ex vivo model. Mucosal Immunol. 2010;3(3):280–90. Epub 2010/02/12. 10.1038/mi.2010.2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Akdis M, Palomares O, van de Veen W, van Splunter M, Akdis CA. TH17 and TH22 cells: a confusion of antimicrobial response with tissue inflammation versus protection. J Allergy Clin Immunol. 2012;129(6):1438–49; quiz50-1. Epub 2012/06/05. 10.1016/j.jaci.2012.05.003 [DOI] [PubMed] [Google Scholar]
- 15. Szabo SJ, Kim ST, Costa GL, Zhang X, Fathman CG, Glimcher LH. A novel transcription factor, T-bet, directs Th1 lineage commitment. Cell. 2000;100(6):655–69. Epub 2000/04/13. [DOI] [PubMed] [Google Scholar]
- 16. Trifari S, Kaplan CD, Tran EH, Crellin NK, Spits H. Identification of a human helper T cell population that has abundant production of interleukin 22 and is distinct from T(H)-17, T(H)1 and T(H)2 cells. Nat Immunol. 2009;10(8):864–71. 10.1038/ni.1770 [DOI] [PubMed] [Google Scholar]
- 17. Duhen T, Geiger R, Jarrossay D, Lanzavecchia A, Sallusto F. Production of interleukin 22 but not interleukin 17 by a subset of human skin-homing memory T cells. Nat Immunol. 2009;10(8):857–63. 10.1038/ni.1767 [DOI] [PubMed] [Google Scholar]
- 18. Eyerich S, Eyerich K, Pennino D, Carbone T, Nasorri F, Pallotta S, et al. Th22 cells represent a distinct human T cell subset involved in epidermal immunity and remodeling. J Clin Invest. 2009;119(12):3573–85. 10.1172/JCI40202 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Alvarez Y, Tuen M, Shen G, Nawaz F, Arthos J, Wolff MJ, et al. Preferential HIV infection of CCR6+ Th17 cells is associated with higher virus receptor expression and lack of CCR5 ligands. J Virol. 2013. Epub 2013/08/02. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Dillon SM, Manuzak JA, Leone AK, Lee EJ, Rogers LM, McCarter MD, et al. HIV-1 infection of human intestinal lamina propria CD4+ T cells in vitro is enhanced by exposure to commensal Escherichia coli. J Immunol. 2012;189(2):885–96. Epub 2012/06/13. 10.4049/jimmunol.1200681 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. El Hed A, Khaitan A, Kozhaya L, Manel N, Daskalakis D, Borkowsky W, et al. Susceptibility of Human Th17 Cells to Human Immunodeficiency Virus and Their Perturbation during Infection. The Journal of Infectious Diseases. 2010;201(6):843–54. 10.1086/651021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Gosselin A, Monteiro P, Chomont N, Diaz-Griffero F, Said EA, Fonseca S, et al. Peripheral blood CCR4+CCR6+ and CXCR3+CCR6+CD4+ T cells are highly permissive to HIV-1 infection. J Immunol. 2010;184(3):1604–16. 10.4049/jimmunol.0903058 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Prendergast A, Prado JG, Kang Y-H, Chen F, Riddell LA, Luzzi G, et al. HIV-1 infection is characterized by profound depletion of CD161+ Th17 cells and gradual decline in regulatory T cells. Aids. 2010;24(4):491–502. 10.1097/QAD.0b013e3283344895 [DOI] [PubMed] [Google Scholar]
- 24. Cecchinato V, Trindade CJ, Laurence A, Heraud JM, Brenchley JM, Ferrari MG, et al. Altered balance between Th17 and Th1 cells at mucosal sites predicts AIDS progression in simian immunodeficiency virus-infected macaques. Mucosal Immunol. 2008;1(4):279–88. 10.1038/mi.2008.14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Kim CJ, Nazli A, Rojas OL, Chege D, Alidina Z, Huibner S, et al. A role for mucosal IL-22 production and Th22 cells in HIV-associated mucosal immunopathogenesis. Mucosal Immunol. 2012. Epub 2012/08/03. [DOI] [PubMed] [Google Scholar]
- 26. Prodger JL, Hirbod T, Kigozi G, Nalugoda F, Reynolds SJ, Galiwango R, et al. Immune correlates of HIV exposure without infection in foreskins of men from Rakai, Uganda. Mucosal Immunol. 2014;7(3):634–44. 10.1038/mi.2013.83 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Wilson NJ, Boniface K, Chan JR, McKenzie BS, Blumenschein WM, Mattson JD, et al. Development, cytokine profile and function of human interleukin 17-producing helper T cells. Nat Immunol. 2007;8(9):950–7. [DOI] [PubMed] [Google Scholar]
- 28. Kim CJ, McKinnon LR, Kovacs C, Kandel G, Huibner S, Chege D, et al. Mucosal Th17 cell function is altered during HIV infection and is an independent predictor of systemic immune activation. J Immunol. 2013;191(5):2164–73. 10.4049/jimmunol.1300829 [DOI] [PubMed] [Google Scholar]
- 29. Koelle DM, Corey L, Burke RL, Eisenberg RJ, Cohen GH, Pichyangkura R, et al. Antigenic specificities of human CD4+ T-cell clones recovered from recurrent genital herpes simplex virus type 2 lesions. J Virol. 1994;68(5):2803–10. Epub 1994/05/01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Horbul JE, Schmechel SC, Miller BR, Rice SA, Southern PJ. Herpes simplex virus-induced epithelial damage and susceptibility to human immunodeficiency virus type 1 infection in human cervical organ culture. PLoS ONE. 2011;6(7):e22638 Epub 2011/08/06. 10.1371/journal.pone.0022638 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Zhu J, Hladik F, Woodward A, Klock A, Peng T, Johnston C, et al. Persistence of HIV-1 receptor-positive cells after HSV-2 reactivation is a potential mechanism for increased HIV-1 acquisition. Nat Med. 2009;15(8):886–92. Epub 2009/08/04. 10.1038/nm.2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Rebbapragada A, Wachihi C, Pettengell C, Sunderji S, Huibner S, Jaoko W, et al. Negative mucosal synergy between Herpes simplex type 2 and HIV in the female genital tract. Aids. 2007;21(5):589–98. Epub 2007/02/23. [DOI] [PubMed] [Google Scholar]
- 33. Johnson KE, Redd AD, Quinn TC, Collinson-Streng AN, Cornish T, Kong X, et al. Effects of HIV-1 and Herpes Simplex Virus Type 2 Infection on Lymphocyte and Dendritic Cell Density in Adult Foreskins from Rakai, Uganda. Journal of Infectious Diseases. 2011;203(5):602–9. 10.1093/infdis/jiq091 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Freeman EE, Weiss HA, Glynn JR, Cross PL, Whitworth JA, Hayes RJ. Herpes simplex virus 2 infection increases HIV acquisition in men and women: systematic review and meta-analysis of longitudinal studies. AIDS. 2006;20(1):73–83. [DOI] [PubMed] [Google Scholar]
- 35. Prodger JL, Gray R, Kigozi G, Nalugoda F, Galiwango R, Nehemiah K, et al. Impact of asymptomatic Herpes simplex virus-2 infection on T cell phenotype and function in the foreskin. AIDS. 2012;26(10):1319–22. 10.1097/QAD.0b013e328354675c [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Kaul R, Cohen CR, Chege D, Yi TJ, Tharao W, McKinnon LR, et al. Biological factors that may contribute to regional and racial disparities in HIV prevalence. Am J Reprod Immunol. 2011;65(3):317–24. Epub 2011/01/13. 10.1111/j.1600-0897.2010.00962.x [DOI] [PubMed] [Google Scholar]
- 37. Pala P, Gomez-Roman VR, Gilmour J, Kaleebu P. An African perspective on mucosal immunity and HIV-1. Mucosal Immunol. 2009;2(4):300–14. 10.1038/mi.2009.23 [DOI] [PubMed] [Google Scholar]
- 38. Mazigo HD, Nuwaha F, Wilson S, Kinung'hi SM, Morona D, Waihenya R, et al. Epidemiology and interactions of Human Immunodeficiency Virus—1 and Schistosoma mansoni in sub-Saharan Africa. Infectious diseases of poverty. 2013;2(1):2 10.1186/2049-9957-2-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Secor WE. Interactions between schistosomiasis and infection with HIV-1. Parasite immunology. 2006;28(11):597–603. [DOI] [PubMed] [Google Scholar]
- 40. Clerici M, Butto S, Lukwiya M, Saresella M, Declich S, Trabattoni D, et al. Immune activation in africa is environmentally-driven and is associated with upregulation of CCR5. Italian-Ugandan AIDS Project. AIDS. 2000;14(14):2083–92. [DOI] [PubMed] [Google Scholar]
- 41. Staats HF, Montgomery SP, Palker TJ. Intranasal immunization is superior to vaginal, gastric, or rectal immunization for the induction of systemic and mucosal anti-HIV antibody responses. AIDS Res Hum Retroviruses. 1997;13(11):945–52. [DOI] [PubMed] [Google Scholar]
- 42. Seeley J, Nakiyingi-Miiro J, Kamali A, Mpendo J, Asiki G, Abaasa A, et al. High HIV incidence and socio-behavioral risk patterns in fishing communities on the shores of Lake Victoria, Uganda. Sex Transm Dis. 2012;39(6):433–9. 10.1097/OLQ.0b013e318251555d [DOI] [PubMed] [Google Scholar]
- 43. Kiwanuka N, Ssetaala A, Nalutaaya A, Mpendo J, Wambuzi M, Nanvubya A, et al. High incidence of HIV-1 infection in a general population of fishing communities around Lake Victoria, Uganda. PLoS One. 2014;9(5):e94932 10.1371/journal.pone.0094932 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. 2011 Uganda AIDS Indicator Survey (UAIS). Ministry of Health; 2012. [Google Scholar]
- 45. Kabatereine NB, Brooker S, Tukahebwa EM, Kazibwe F, Onapa AW. Epidemiology and geography of Schistosoma mansoni in Uganda: implications for planning control. Tropical medicine & international health: TM & IH. 2004;9(3):372–80. [DOI] [PubMed] [Google Scholar]
- 46. Downs JA, van Dam GJ, Changalucha JM, Corstjens PL, Peck RN, de Dood CJ, et al. Association of Schistosomiasis and HIV infection in Tanzania. The American journal of tropical medicine and hygiene. 2012;87(5):868–73. 10.4269/ajtmh.2012.12-0395 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Pearce EJ, MacDonald AS. The immunobiology of schistosomiasis. Nat Rev Immunol. 2002;2(7):499–511. [DOI] [PubMed] [Google Scholar]
- 48. McSorley HJ, Maizels RM. Helminth infections and host immune regulation. Clin Microbiol Rev. 2012;25(4):585–608. 10.1128/CMR.05040-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Doenhoff MJ. A role for granulomatous inflammation in the transmission of infectious disease: schistosomiasis and tuberculosis. Parasitology. 1997;115 Suppl:S113–25. [DOI] [PubMed] [Google Scholar]
- 50. Karanja DM, Colley DG, Nahlen BL, Ouma JH, Secor WE. Studies on schistosomiasis in western Kenya: I. Evidence for immune-facilitated excretion of schistosome eggs from patients with Schistosoma mansoni and human immunodeficiency virus coinfections. The American journal of tropical medicine and hygiene. 1997;56(5):515–21. [DOI] [PubMed] [Google Scholar]
- 51. Kleppa E, Ramsuran V, Zulu S, Karlsen GH, Bere A, Passmore JA, et al. Effect of female genital schistosomiasis and anti-schistosomal treatment on monocytes, CD4+ T-cells and CCR5 expression in the female genital tract. PLoS One. 2014;9(6):e98593 10.1371/journal.pone.0098593 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Kleppa E, Klinge KF, Galaphaththi-Arachchige HN, Holmen SD, Lillebo K, Onsrud M, et al. Schistosoma haematobium infection and CD4+ T-cell levels: a cross-sectional study of young South African women. PLoS One. 2015;10(3):e0119326 10.1371/journal.pone.0119326 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Jourdan PM, Holmen SD, Gundersen SG, Roald B, Kjetland EF. HIV target cells in Schistosoma haematobium-infected female genital mucosa. The American journal of tropical medicine and hygiene. 2011;85(6):1060–4. 10.4269/ajtmh.2011.11-0135 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Secor WE, Shah A, Mwinzi PM, Ndenga BA, Watta CO, Karanja DM. Increased density of human immunodeficiency virus type 1 coreceptors CCR5 and CXCR4 on the surfaces of CD4(+) T cells and monocytes of patients with Schistosoma mansoni infection. Infect Immun. 2003;71(11):6668–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Chenine AL, Shai-Kobiler E, Steele LN, Ong H, Augostini P, Song R, et al. Acute Schistosoma mansoni infection increases susceptibility to systemic SHIV clade C infection in rhesus macaques after mucosal virus exposure. PLoS Negl Trop Dis. 2008;2(7):e265 10.1371/journal.pntd.0000265 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Gray RH, Kigozi G, Serwadda D, Makumbi F, Watya S, Nalugoda F, et al. Male circumcision for HIV prevention in men in Rakai, Uganda: a randomised trial. The Lancet. 2007;369(9562):657–66. [DOI] [PubMed] [Google Scholar]
- 57. Bailey R, Moses S, Parker C, Agot K, Maclean I, Krieger J, et al. Male circumcision for HIV prevention in young men in Kisumu, Kenya: a randomised controlled trial. The Lancet. 2007;369(9562):643–56. [DOI] [PubMed] [Google Scholar]
- 58. Auvert B, Taljaard D, Lagarde E, Sobngwi-Tambekou J, Sitta R, Puren A. Randomized, Controlled Intervention Trial of Male Circumcision for Reduction of HIV Infection Risk: The ANRS 1265 Trial. PLoS Medicine. 2005;2(11). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. WHO. Basic laboratory methods in mediacal parasitology In: Organization WH, editor. WHO Library Cataloguing in Publication Data: Macmillan/Clays; 1991. [Google Scholar]
- 60. Lamberton PH, Kabatereine NB, Oguttu DW, Fenwick A, Webster JP. Sensitivity and specificity of multiple Kato-Katz thick smears and a circulating cathodic antigen test for Schistosoma mansoni diagnosis pre- and post-repeated-praziquantel treatment. PLoS Negl Trop Dis. 2014;8(9):e3139 10.1371/journal.pntd.0003139 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Gamiel JL, Tobian AAR, Laeyendecker OB, Reynolds SJ, Morrow RA, Serwadda D, et al. Improved Performance of Enzyme-Linked Immunosorbent Assays and the Effect of Human Immunodeficiency Virus Coinfection on the Serologic Detection of Herpes Simplex Virus Type 2 in Rakai, Uganda. Clinical and Vaccine Immunology. 2008;15(5):888–90. 10.1128/CVI.00453-07 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Prodger JL, Gray R, Kigozi G, Nalugoda F, Galiwango R, Hirbod T, et al. Foreskin T-cell subsets differ substantially from blood with respect to HIV co-receptor expression, inflammatory profile, and memory status. Mucosal Immunol. 2012;5(2):121–8. Epub 2011/11/18. 10.1038/mi.2011.56 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Roederer M, Nozzi JL, Nason MC. SPICE: exploration and analysis of post-cytometric complex multivariate datasets. Cytometry Part A: the journal of the International Society for Analytical Cytology. 2011;79(2):167–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Prodger JL, Hirbod T, Gray R, Kigozi G, Nalugoda F, Galiwango R, et al. HIV Infection in Uncircumcised Men Is Associated With Altered CD8 T-cell Function But Normal CD4 T-cell Numbers in the Foreskin. J Infect Dis. 2014;209(8):1185–94. 10.1093/infdis/jit644 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Joag VR, McKinnon LR, Liu J, Kidane ST, Yudin MH, Nyanga B, et al. Identification of preferential CD4 T-cell targets for HIV infection in the cervix. Mucosal Immunol. 2015. [DOI] [PubMed] [Google Scholar]
- 66. Card CM, Rutherford WJ, Ramdahin S, Yao X, Kimani M, Wachihi C, et al. Reduced cellular susceptibility to in vitro HIV infection is associated with CD4+ T cell quiescence. PLoS ONE. 2012;7(9):e45911 Epub 2012/10/03. 10.1371/journal.pone.0045911 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Biancotto A, Iglehart SJ, Vanpouille C, Condack CE, Lisco A, Ruecker E, et al. HIV-1 induced activation of CD4+ T cells creates new targets for HIV-1 infection in human lymphoid tissue ex vivo. Blood. 2008;111(2):699–704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Meditz AL, Haas MK, Folkvord JM, Melander K, Young R, McCarter M, et al. HLA-DR+ CD38+ CD4+ T lymphocytes have elevated CCR5 expression and produce the majority of R5-tropic HIV-1 RNA in vivo. J Virol. 2011;85(19):10189–200. 10.1128/JVI.02529-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Doenhoff MJ, Hassounah O, Murare H, Bain J, Lucas S. The schistosome egg granuloma: immunopathology in the cause of host protection or parasite survival? Trans R Soc Trop Med Hyg. 1986;80(4):503–14. [DOI] [PubMed] [Google Scholar]
- 70. Doenhoff MJ, Pearson S, Dunne DW, Bickle Q, Lucas S, Bain J, et al. Immunological control of hepatotoxicity and parasite egg excretion in Schistosoma mansoni infections: stage specificity of the reactivity of immune serum in T-cell deprived mice. Trans R Soc Trop Med Hyg. 1981;75(1):41–53. [DOI] [PubMed] [Google Scholar]
- 71. Pearce EJ. Priming of the immune response by schistosome eggs. Parasite immunology. 2005;27(7–8):265–70. [DOI] [PubMed] [Google Scholar]
- 72. Lammie PJ, Linette GP, Phillips SM. Characterization of Schistosoma mansoni antigen-reactive T cell clones that form granulomas in vitro. J Immunol. 1985;134(6):4170–5. [PubMed] [Google Scholar]
- 73. Phillips SM, Lammie PJ. Immunopathology of granuloma formation and fibrosis in schistosomiasis. Parasitology today. 1986;2(11):296–302. [DOI] [PubMed] [Google Scholar]
- 74. Rutitzky LI, Hernandez HJ, Stadecker MJ. Th1-polarizing immunization with egg antigens correlates with severe exacerbation of immunopathology and death in schistosome infection. Proc Natl Acad Sci U S A. 2001;98(23):13243–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Rutitzky LI, Mirkin GA, Stadecker MJ. Apoptosis by neglect of CD4+ Th cells in granulomas: a novel effector mechanism involved in the control of egg-induced immunopathology in murine schistosomiasis. J Immunol. 2003;171(4):1859–67. [DOI] [PubMed] [Google Scholar]
- 76. Harrington LE, Hatton RD, Mangan PR, Turner H, Murphy TL, Murphy KM, et al. Interleukin 17-producing CD4+ effector T cells develop via a lineage distinct from the T helper type 1 and 2 lineages. Nat Immunol. 2005;6(11):1123–32. Epub 2005/10/04. [DOI] [PubMed] [Google Scholar]
- 77. Rutitzky LI, Lopes da Rosa JR, Stadecker MJ. Severe CD4 T cell-mediated immunopathology in murine schistosomiasis is dependent on IL-12p40 and correlates with high levels of IL-17. J Immunol. 2005;175(6):3920–6. [DOI] [PubMed] [Google Scholar]
- 78. Rutitzky LI, Stadecker MJ. Exacerbated egg-induced immunopathology in murine Schistosoma mansoni infection is primarily mediated by IL-17 and restrained by IFN-gamma. Eur J Immunol. 2011;41(9):2677–87. 10.1002/eji.201041327 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Sonnenberg GF, Fouser LA, Artis D. Border patrol: regulation of immunity, inflammation and tissue homeostasis at barrier surfaces by IL-22. Nat Immunol. 2011;12(5):383–90. 10.1038/ni.2025 [DOI] [PubMed] [Google Scholar]
- 80. Ssetaala A, Nakiyingi-Miiro J, Asiki G, Kyakuwa N, Mpendo J, Van Dam GJ, et al. Schistosoma mansoni and HIV acquisition in fishing communities of Lake Victoria, Uganda: a nested case-control study. Tropical medicine & international health: TM & IH. 2015;20(9):1190–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Sanya RE, Muhangi L, Nampijja M, Nannozi V, Nakawungu PK, Abayo E, et al. Schistosoma mansoni and HIV infection in a Ugandan population with high HIV and helminth prevalence. Tropical medicine & international health: TM & IH. 2015;20(9):1201–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. McKinnon LR, Nyanga B, Chege D, Izulla P, Kimani M, Huibner S, et al. Characterization of a human cervical CD4+ T cell subset coexpressing multiple markers of HIV susceptibility. J Immunol. 2011;187(11):6032–42. Epub 2011/11/04. 10.4049/jimmunol.1101836 [DOI] [PubMed] [Google Scholar]
- 83. Mora JR, Bono MR, Manjunath N, Weninger W, Cavanagh LL, Rosemblatt M, et al. Selective imprinting of gut-homing T cells by Peyer's patch dendritic cells. Nature. 2003;424(6944):88–93. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(DOC)
(A) Representative staining and (B) expression of Cutaneous Lymphocyte Antigen (CLA) or integrin α4β7 on CD4 T cells isolated from either the blood (clear bars) or foreskin tissue (hatched bars). Homing marker expression was compared between foreskin and blood cells by Wilcoxon related samples rank test (**p<0.01).
(TIF)
Data Availability Statement
All relevant data are within the paper and its Supporting Information files.