Summary
Despite acute respiratory and chronic respiratory and gastro-intestinal complications, most infants and children with a history of oesophageal atresia / trachea-oesophageal fistula [OA/TOF] can expect to live a fairly normal life. Close multidisciplinary medical and surgical follow-up can identify important co-morbidities whose treatment can improve symptoms and optimize pulmonary and nutritional outcomes. This article will discuss the aetiology, classification, diagnosis and treatment of congenital TOF, with an emphasis on post-surgical respiratory management, recognition of early and late onset complications, and long-term clinical outcomes.
Keywords: tracheo-oesophageal fistula, oesophageal atresia, vocal cord abnormalities, tracheomalacia, chronic lung disease, gastro-oesophageal reflux disease, esophageal dysmotility, dysphagia
INTRODUCTION
Congenital tracheo-oesophageal fistula (TOF) is a relatively common anomaly of the foregut that is associated with acute and chronic respiratory and digestive complications. A TOF consists of an abnormal connection, or fistula tract, between the trachea and the oesophagus, and usually occurs with oesophageal atresia (OA), a congenital malformation in which the upper oesophagus terminates in a blind-ending pouch. Though surgical repair of TOF leads to clinical improvement, some degrees of respiratory, feeding, and gastrointestinal co-morbidities often persist. The clinical presentation, diagnosis, and severity of TOF are variable, and depend in part on the classification type.
Epidemiology and Classification
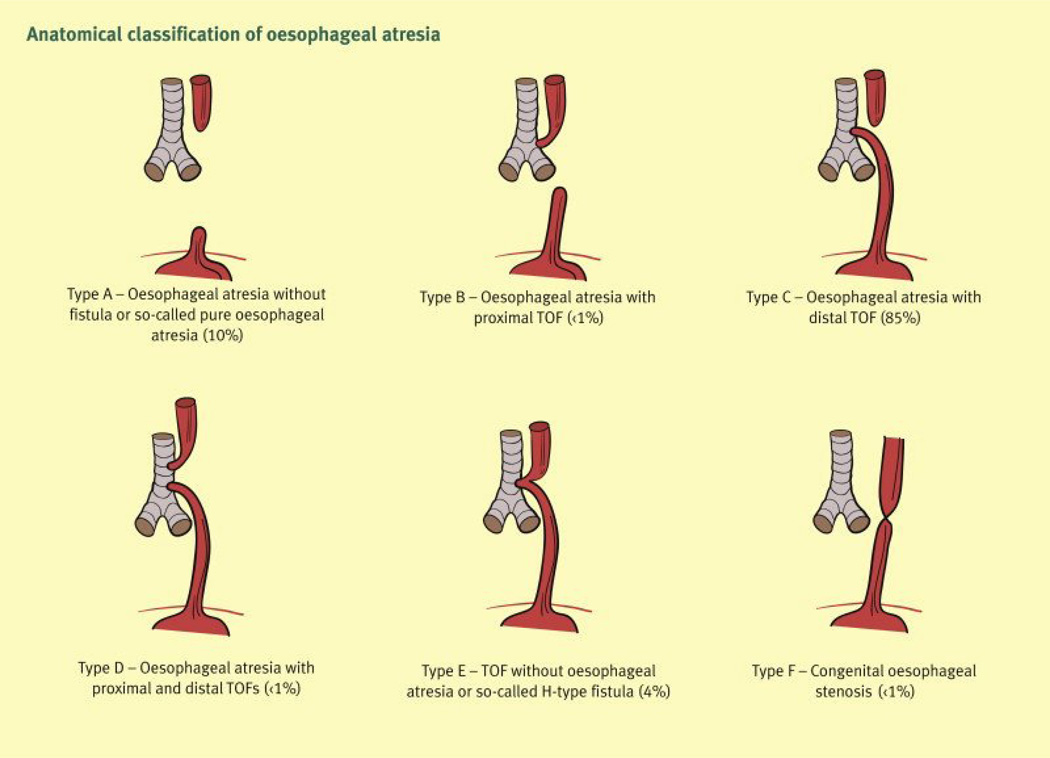
Tracheo-oesophageal fistulae occur along a spectrum of severity. The majority of TOF (90–93%) occur in association with OA; the latter is the most common congenital malformation of the oesophagus.1–3 The estimated incidence of OA/TOF is 1 in 3,500 births4–6 with males having a slight increased incidence, depending on the classification type.7 TOF and OA/TOF are classified according to anatomic features [Figure 1]. Type A refers to isolated OA, type B to OA with proximal TOF, type C to OA with distal TOF (most common: 85%), type D to OA with proximal and distal TOF, type E (also called “H-type”) to isolated TOF without OA ,( four to seven percent)3,8, and type F to congenital oesophageal stenosis.3
Figure 1.
Anatomical classification of oesophageal atresia and tracheo-oesophageal fistula.
Reproduced with permission.3
PATHOGENESIS
Foetal Development
OA/TOF develops early in foetal life. The embryologic foregut differentiates into the primitive lung and oesophagus between 22–23 days, and from there, a tracheal/lung bud develops. First an epithelial, then a mesenchymal septum forms to fully separate the respiratory and oesophageal tracts. The division of the respiratory and oesophageal tracts is complete by the time the embryo is six to seven weeks old.9 In OA/TOF, it is thought that defective epithelial-mesenchymal interactions during early embryologic development leads to improper branching of the lung bud, resulting in a fistula tract between the trachea and oesophagus.10
Genetic and Environmental Factors
The aetiology of OA/TOF is likely multifactorial, with environmental, genetic, and possibly epigenetic factors involved in its pathogenesis. The familial recurrence rate is low (one percent), and most cases are likely the result of de novo mutations.11 Genetically identical monozygotic twins have a 50% chance of sharing the OA phenotype, while genetically non-identical dizygotic twins have half that risk. Other contributing factors, including environmental exposures, may also be important.12 Studies of mouse and rat models and also tissue from humans with OA/TOF have implicated a defect in the Sonic Hedgehog (Shh) signalling pathway and its downstream effectors (including FOXf1), which contribute to epithelial/mesenchymal signalling.13 Recently, a microdeletion that encompasses the FOX transcription gene cluster at 16q24.1, which affects foregut and lung development, has been implicated,13 and other genes specific to defined genetic syndromes have also been identified.14 Some proposed environmental factors that have been associated with OA/TOF include maternal first trimester exposure to exogenous sex hormones,15 methimazole,16 pesticides and herbicides,17 diabetes,18 and an unknown infection.19 One case-control study reported a slight increased risk of OA/TOF with maternal smoking or alcohol use during pregnancy,17 but a much larger study did not support this association.20 Currently, the role of environmental factors in the development of OA/TOF is not completely understood.
Clinical and Syndromic Associations
Most cases of OA/TOF occur spontaneously, and though the defects may be isolated, at least 50% have one or more additional anatomic malformations.21–23 The most common associated anomalies are those within the VACTERL spectrum (V-vertebral anomalies, A-ano-rectal atresia, C-congenital heart lesions, TE-tracheo-oesophageal defects, and L-limb anomalies).21 Congenital heart defects are the most common comorbidity (13–34%).24 Some genetic syndromes that are associated with OA/TOF include CHARGE (C-coloboma, H- heart defects, A- choanal atresia, R- retardation of growth and/or development, G- genital and/or urinary defects, E- ear anomalies) syndrome, Feingold syndrome, AEG (anophthalmia-oesophageal-genital) syndrome, and X-linked Opitz syndrome.13,25 OA/TOF also occurs in association with trisomies, particularly 18 and 21.24 A causal mutation associated with a defined genetic syndrome of known aetiology can be identified in up to 11–12% of patients with OA/TOF.26, 11
CLINICAL AND DIAGNOSTIC FEATURES
Prenatal Diagnosis
Suggestive ultrasound findings are usually nonspecific when present, but the proportion of OA/TOF cases diagnosed prenatally has increased with time, and has been reported to be as high as 36%.27 Nevertheless, prenatal diagnosis remains challenging. OA/TOF is more likely to be diagnosed prenatally if gastrointestinal tract obstruction is present, and prenatal diagnosis is associated with increased clinical severity.28 Ultrasound features of OA with or without TOF can also include polyhydramnios and either a small stomach or absent, non-fluid filled stomach. Polyhydramnios is present in approximately 60% of pregnancies with OA/TOF,29 but polyhydramnios lacks specificity for OA/TOF, as it can be seen in many other conditions and is sometimes an incidental or benign finding. Only about one-third of cases of OA/TOF have both a small or absent fluid-filled stomach and polyhydramnios,29,30 but the presence of these findings combined is associated with a positive predictive value for OA of 39–56%.30–33 A normal-appearing fluid-filled stomach also occurs in some cases of OA/TOF because the fistula tract between the trachea and lower oesophagus may permit enough fluid passage to fill the stomach. Foetal MRI can be useful when there is suspicion of OA on prenatal ultrasound, and the presence of an upper oesophageal pouch on foetal MRI has a significant positive predictive value for OA/TOF. In foetuses suspected of OA/TOF, foetal MRI correctly ruled OA in or out in 78% pregnancies.34
Clinical Presentation
Infants with OA/TOF usually have clinically apparent symptoms before or during the first feed. Affected infants are unable to swallow saliva and may require excess suctioning for copious oral secretions. Symptoms should become apparent at the first oral feeding if not before, when the infant coughs, chokes, and may have emesis, cyanosis, and respiratory distress. Gastric distention may occur as a result of a TOF between the trachea and distal oesophagus. Passage of an oro-gastric tube soon after birth should be attempted in infants with a prenatal diagnosis of polyhydramnios or clinical symptoms suspicious for OA/TOF.32 Postnatal confirmation of OA occurs when there is failure to pass an orogastric tube past 9 or 10 cm from the mouth with the presence of a coiled tube in the oesophagus by radiography.32 Premature birth is also common in infants with OA/TOF. In a retrospective study from 43 children’s hospitals over a 13 year period, 37% of patients were born prematurely.35
Diagnosis of Isolated TOF
The diagnosis of an isolated TOF, also called a type E or H fistula can be challenging. Classic symptoms include coughing and choking spells with feeding, recurrent pneumonia, and intermittent abdominal distension related to aerophagia, but may be less pronounced than in cases with associated OA. Feeding symptoms may be reproduced during a clinical encounter and should disappear when feeds are given through a nasogastric tube.8,36 Though symptoms are usually present from birth, they are sometimes intermittent and may wax and wane in severity, which may contribute to the delay in diagnosis. A review of three case series showed that choking and cyanosis during feeding were the most common presenting signs (90–97%), followed by recurrent pneumonia (73–100%), then abdominal distention (33–44%) and that 50% were accompanied by at least one other anatomic anomaly.8,37–39 A single-centre study of twelve cases of isolated TOF over a 25 year period found that the age at diagnosis and repair ranged from four weeks to four years of age, and that two cases had received inpatient treatment for a pulmonary infection for one month before the suspicion of a TOF was raised.36 A more recent single-centre study of five cases consisted entirely of diagnoses made in infancy, with the median age at repair of 15 days [range one week to two months] of life.40 Rarely, diagnosis of a congenital isolated TOF occurs in adulthood.
Diagnosis of an isolated TOF is usually made by fluoroscopy with a contrast oesophagram and/or upper gastro-intestinal [GI] study. The intention to evaluate for a TOF should be noted in the radiology order, as radiology protocols may vary based on the indication. Traditionally, an oesophagram with a pull-back technique in which the lower oesophagus is filled and the catheter pulled upward has been used, but a simpler contrast swallow may also be diagnostic.41 If an isolated TOF is not seen through fluoroscopic examination and clinical suspicion remains, bronchoscopy should be performed under anaesthesia, as this sometimes identifies a fistula tract that was not seen with fluoroscopy. Methylene blue placed in the trachea combined with bronchoscopy and oesophageal endoscopy has also been used to determine the presence and location of a tracheo-oesophageal fistula.42
Additional Diagnostic Considerations Prior to Surgical Repair
Endoscopic airway evaluation prior to surgical repair of OA/TOF may reveal additional abnormalities that could otherwise have been overlooked, which has led some experts to recommend airway endoscopy routinely for all cases of OA/TOF prior to surgical repair.43,44 In one study of 88 infants with OA/TOF who underwent direct laryngotracheobronchoscopy prior to surgical repair, 20% were found to have additional airway defects. Defects included a second TOF site (17%), fistula from a main pulmonary bronchus (42%), laryngeal cleft (11%), and vallecular cyst (6%). Three subjects (17%) believed to have pure oesophageal atresia were found to have an accompanying TOF.44. Pre-operative endoscopy therefore may be useful for surgical planning. Pre-operative computed tomography scanning with three-dimensional airway reconstruction is not sensitive: scanning may miss up to 20% of fistula tracts45 and additional anomalies such as a laryngeal cleft or other airway anomalies, which are better detected by direct visualization (laryngoscopy/bronchoscopy).46 Therefore, computed tomography scanning is not routinely recommended as part of pre-operative evaluation, as the risks of radiation likely outweigh the potential benefits.45,46
Surgical Repair and Post-Operative Complications
The treatment for OA/TOF is surgical repair, and most patients do well post-operatively and have an excellent prognosis, with post-operative survival of 95–99% for isolated OA/TOF.12,27 Survival rates are lower in patients with multiple congenital anomalies, especially those with trisomies, who often have multiple major comorbidities. Treatment consists of TOF ligation. When an OA is present a primary surgical anastomosis of the oesophageal segments is performed when surgically appropriate; surgery usually takes place in the first few days of life. When there is a long gap OA (a large distance between upper and lower oesophageal pouches, e.g., greater than three to five centimetres),47 primary anastomosis may not be possible. In these cases, surgical repair may be more complicated and may require a staged approach with multiple surgeries, and sometimes partial oesophageal replacement with colon or stomach.48 A gastrostomy tube may need to be placed prior to surgical repair in order to support nutrition, particularly in cases when delayed or staged repair is required. Isolated TOF and some cases of OA/TOF may be candidates for thoracoscopic repair.49 Some post-operative complications that may occur in the short or long term include (reported by a single- tertiary care centre in the United States over two decades (227 infants)): symptomatic stricture (35%), anastomotic leak (16%), and recurrent TOF (3%).50 A French multi-centre registry of 307 infants with OA/TOF reported that 34% of patients experienced post-operative complications within the first year, including anastomotic stenosis requiring oesophageal dilation (22%), anastomotic leak (8%), and recurrent TOF (4%).51
Pulmonary and Related Comorbidities and Treatment
With markedly improved surgical and post-surgical care, the majority of infants born with OA/TOF will survive into adulthood. However, respiratory morbidities are common and may be significant [Table 1]. Pulmonary care of these infants and children involves managing comorbidities and preventing or minimizing damage to the lungs. Comorbidities that may impact the respiratory system are classified according to the following categories: 1) functional or structural anomalies of the upper airway (tracheomalacia, vocal cord problems, and others); 2) GI tract problems (gastro-oesophageal reflux disease (GORD), oesophageal dysmotility, oesophageal stricture, and others); 3) dysphagia and aspiration; and 4) lower airway abnormalities, including bronchomalacia and airway hyper-responsiveness. One study that examined outcomes of children following OA/TOF repair in 43 children’s hospitals found that 54.7% of children were readmitted within the first two years of life: 12.7% had been hospitalized for treatment of pneumonia, 11.7% had undergone anti-reflux surgery and 6.1% had a tracheostomy. 35 A French registry of all 307 OA/TOF patients born in 38 centres in France in 2008 and 2009 reported that 59% were readmitted to the hospital within the first year of life for respiratory (48%) or digestive (52%) reasons, with a mean number of hospitalizations per patient of 2.5. At the most recent clinic follow up (on average 12 months of life), 37% of these patients were experiencing active respiratory symptoms, 15% had dysphagia, and 12% had required anti-reflux surgery.51
Table 1.
Causes of Acute and Chronic Respiratory Symptoms in Infants and Children following OA/TOF Repair
| Conditions |
|---|
| Vocal cord paresis/paralysis |
| Dysphagia/aspiration |
| Aspiration of upper airway secretions |
| Tracheomalacia and/or bronchomalacia |
| Diverticulum of posterior trachea at site of TOF repair |
| Aberrant airway anatomy |
| Lower airway flow obstruction with or without bronchodilator response |
| Restrictive lung disease |
| Atelectasis related to poor mucociliary clearance/impaired cough |
| Aspiration pneumonia |
| Recurrent oesophageal stricture |
| Recurrent TEF |
| Late Diagnosis of Laryngeal Cleft |
| Eosinophilic esophagitis |
| Oesophageal foreign body impaction |
Upper Airway Disorders
Laryngeal Clefts
An association between laryngeal clefts and OA/TOF has been described by several authors in case reports and case series52–56 and was recently confirmed by a large, retrospective study from a tertiary care centre: of 161 cases diagnosed with laryngeal clefts, 12% were associated with a history of TOF, and aspiration was the presenting symptom 50% of the time.43 However, prospective endoscopic data is lacking, and the prevalence of laryngeal cleft associated with OA/TOF is not known. Diagnosis of a laryngeal cleft may be delayed by months to years after the surgical repair of TOF and thus requires a high index of suspicion; endoscopic evaluation for a laryngeal cleft should be considered in patients with a history of OA/TOF and recurrent wheezing, dysphagia/aspiration, or pneumonia.43 Alternatively, if a laryngeal cleft is diagnosed endoscopically around the time of primary OA/TOF surgery, early cleft repair may prevent subsequent lung damage and respiratory morbidity.43
Vocal Cord Abnormalities
Some infants are found to have unilateral or bilateral vocal cord paresis (hypomobility) or paralysis (immobility) following OA/TOF repair, and associated symptoms may be mild to severe. The true prevalence of vocal cord paresis or paralysis in OA/TOF is not known, as prospective data is lacking, and is likely higher than reported. Prevalence estimates have ranged from 3–20%, and the majority of patients were evaluated and subsequently diagnosed with endoscopy because of clinical symptoms. Symptoms of vocal cord paresis or paralysis include aphonia or dysphonia, a weak or hoarse cry, stridor, and coughing or choking with feeds. Vocal cord paralysis should be suspected in infants unable to be weaned off the ventilator or in infants with post-operative upper airway obstruction. Otherwise, symptoms may be episodic or persistent, may wax and wane in intensity, and may be misattributed to other common comorbidities of OA/TOF, including reflux and laryngotracheomalacia. Determining whether vocal cord paresis or paralysis is congenital or acquired can be challenging, as either may be the result of congenital malformations or may occur post-operatively after a recurrent laryngeal nerve injury during surgery or secondary to a prolonged or traumatic intubation. This has prompted some to recommend evaluation of vocal cord function via endoscopy prior to surgical repair of OA/TOF. Endoscopy with direct visualization should be performed in patients suspected of having vocal cord problems while the patient is either awake or lightly sedated in order to examine vocal cord motion. Infants found to have bilateral vocal cord paralysis may require long-term tracheostomy placement, particularly those who fail to wean from mechanical ventilation or have recurrent episodes of respiratory failure due to upper airway obstruction. Unilateral vocal cord paralysis and unilateral or bilateral vocal cord paresis are often managed expectantly and may resolve spontaneously. Those with vocal cord abnormalities are at heightened risk for swallowing dysfunction and aspiration, and swallowing ability should be evaluated.
Tracheomalacia and Tracheal Diverticula
The prevalence of tracheomalacia associated with OA/TOF is difficult to estimate based on a lack of prospective endoscopic data on large numbers of patients. Prevalence estimates from large studies have ranged from 5–15%, but these studies have primarily reported more severe cases. For example, one large study reported that 15% of cases of OA/TOF had tracheomalacia, but 87% of these required surgery (11 of 13 required tracheostomy and two required aortopexy).50 More likely, some degree of tracheomalacia is present in most cases of OA/TOF and only a subset of these cases is severe enough to warrant surgical attention. One small prospective study of patients who underwent multiple endoscopic evaluations demonstrated that 87% (21 of 27) had tracheomalacia, which was severe in three cases (14%). Tracheomalacia is related to weakness in the tracheal cartilage that allows the anterior and posterior walls of the trachea to collapse and touch upon expiration and coughing. Symptoms include feeding difficulty, a characteristic barking cough, expiratory stridor, and occasionally apnoeic/cyanotic spells. Tracheomalacia may contribute to ongoing respiratory symptoms that are unresponsive to medical treatment and may significantly impair clearance of respiratory secretions, placing infants and children at risk for significant morbidity and even hospitalization in the setting of respiratory infections. In addition, children with tracheomalacia also experience delayed or prolonged recovery from respiratory infections.
A posterior tracheal wall diverticulum at the site of prior TOF repair has been reported in some patients who were evaluated for persistent respiratory symptoms.57 Some have proposed that this is a post-operative abnormality that may become larger and more symptomatic with time. A tracheal or laryngeal diverticulum or pouch may contribute to pooling of oral secretions and/or recurrent pneumonia, and clinical improvement has been reported in some patients who have undergone diverticulum repair.57,58
Feeding/Swallowing and Gastrointestinal Problems
Swallowing Dysfunction
A common cause of respiratory symptoms in infants and children following surgical repair of TOF is swallowing dysfunction, or dysphagia, and symptoms range from mild to severe. There are several factors which predispose infants and children to aspiration post OA/TOF repair, including: 1) inability to protect the airway during swallowing as with vocal cord paresis or paralysis; 2) oesophageal dysmotility and reflux; and 3) pooling of upper airway secretions from oesophageal dysmotility, tracheal or oesophageal strictures, or a tracheal diverticulum/pouch. Prior to initiating full oral feeding following OA/TOF surgery, a swallowing evaluation should be performed by a specialist to assess the safety of oral feeds and to determine strategies to avoid or minimize aspiration of feeds. Some infants and children also experience feeding aversion and oral feeding refusal due to GORD, oesophageal strictures, or oesophageal dysmotility. In these cases or cases of inadequate oral intake a gastrostomy tube may be required in order to facilitate adequate growth.2 Approximately half of children with OA/TOF have weights below the 25th percentile during the first five years of life. This seems to improve with age, as only one-third had weights below the 25th percentile at age ten,59 and another study reported that 76% of adults with history of OA/TOF repair in infancy were at or above the 50th percentile for weight.
Symptoms of dysphagia remain very common in children and adults after OA/TOF repair, and up to half of adults report occasional dysphagia.60,61 As a result, children and adults who are post OA/TOF repair often use strategies to improve swallowing. These may include chewing slowly, avoiding meat, and drinking fluids to promote bolus propulsion down the oesophagus. Due to abnormal oesophageal motility and/or stricture at the site of repair, impaction of food in the oesophagus can occur and may require extraction. Food impaction can cause severe respiratory distress and can be life-threatening, especially in small children with tracheomalacia. In one study, the mean age of foreign body extraction in children with OA/TOF was 25 months.62 Despite ongoing abnormalities in oesophageal motility, in many patients GI symptoms and dysphagia become less frequent and less severe with increasing age.63,64 In one study from Germany, 85% of ten year olds who had a history of OA/TOF repair reported that eating was always or usually pleasurable.65
Reflux and/or Oesophagitis
GORD is very common in children with OA/TOF and can contribute to respiratory symptoms. All infants with OA have abnormal oesophageal peristalsis and are at risk for significant dysmotility.3 One study of 61 children who underwent surgical repair of OA and distal TOF and then subsequent endoscopy with 18 hour pH-monitoring repeated within the first year and again at 3, 5, and 10 years post-operatively reported that nearly half (46%) were diagnosed with significant GORD (either requiring anti-reflux surgery, biopsy with moderate or severe oesophagitis, or meeting pH-monitoring criteria). GORD may become more clinically apparent over time, as the number of children diagnosed with significant GORD more than doubled from six months to one year post-operatively, and only one patient experienced spontaneous resolution.66 Many infants and children require medical management, but anti-reflux surgery may also be required. The French multicentre study of 301 patients reported that 22% of patients required gastrostomy feedings at one year follow up, and most of these were placed around the time of birth for surgical reasons. In the same study, 12% of patients required anti-reflux surgery at median age of 164 days.51
GORD may be a lifelong problem for patients with a history of OA/TOF repair. A study of adults who underwent OA repair in infancy found that 32% had symptoms of GORD and 59% met manometric and/or pH criteria.67 Another study found that 63% of adults reported symptoms of GORD and that both reflux esophagitis and Barrett’s oesophagus were common findings on endoscopy.68 GORD may have a negative impact on pulmonary function: adolescents with a history of TOF who had severe GORD also had lower FEV1 on spirometry compared with those with a history of TOF who did not have severe GORD.69 GORD may exacerbate respiratory symptoms, especially if accompanied by dysphagia, and should be considered as a possible contributing factor in patients with a history of OA/TOF who are experiencing frequent chest infections (pneumonia, persistent cough, etc.). Eosinophilic esophagitis has also been described in association with OA/TOF and should be considered in patients who still have significant reflux symptoms, dysphagia, or recurrent strictures despite standard GORD treatment.70,71
Lower Airway Abnormalities
Structural and functional airway abnormalities are common in infants and young children post OA/TOF repair. In addition to tracheomalacia and bronchomalacia, less common anatomic abnormalities may include ectopic or absent bronchus and congenital bronchial stenosis.72 Evaluation of the airways via flexible bronchoscopy can help identify these problems in infants and children before primary OA/TOF repair or in those with persistent respiratory symptoms post OA/TOF surgery. Tracheomalacia and bronchomalacia can exacerbate GORD, impair airway clearance, and lead to atelectasis and plugging of the small airways. Impaired airway clearance can lead to more frequent or prolonged chest infections, and in severe cases permanent lung damage, such as bronchiectasis, if not addressed.
Airway Clearance
Respiratory symptoms with illnesses may be particularly severe in children with tracheo-bronchomalacia due to inspiratory and/or expiratory airway collapsibility. This impairs the effectiveness of coughing, which leads to difficulty clearing airway secretions; airway collapsibility may also result in respiratory distress and increased work of breathing.73 In addition, some children with tracheomalacia respond adversely to smooth muscle relaxation caused by beta agonists, leading to a worsening of airflow limitation.74 This may be clinically apparent in children with tracheo-bronchomalacia as a paradoxical increase in wheezing or stridor (depending on the location of the airway malacia) or increased respiratory distress; however, it may not result in overt symptoms. Providers should keep this in mind if using bronchodilators in patients known or suspected to have tracheo-bronchomalacia. Infants and children with tracheo-bronchomalacia may experience significant respiratory distress and require non-invasive positive pressure ventilation during periods of respiratory illness to maintain the patency of collapsible airways and to avoid episodes of oxygen desaturation and atelectasis. In addition, secondary bacterial infections may occur more frequently in children post OA/TOF repair due to impaired mucociliary clearance. Therefore, respiratory culture and antibiotic treatment should be considered when respiratory symptoms last greater than two weeks following a viral illness.
Pulmonary Function and Asthma
Respiratory symptoms and pulmonary function test abnormalities are common post OA/TOF repair and may persist into late childhood and adult life. Wheezing and doctor-diagnosed asthma are relatively common and exceed the prevalence of the general population.75 Malmstrom and colleagues reported the results of spirometry in 25 patients post OA/TOF repair (mean age 13.7 years). They reported that 35% had a restrictive pattern while 30% had an obstructive pattern.76 Using a self-reported questionnaire, 41% of patients reported current respiratory symptoms. Of the patients who reported respiratory symptoms, 52% had a history of wheezing or pneumonia. Testing of these patients revealed that bronchial reactivity and atopy were common, with 78% of those tested showing bronchial hyper-responsiveness to inhaled histamine and 58% with positive skin testing.76 A similar study performed in Finnish adults with a history of OA/TOF repair (mean age 36) showed that 37% reported current wheeze and 52% reported recurrent respiratory infections. The prevalence of atopy was 37%, comparable to the general population, but the prevalence of bronchial hyper-responsiveness was 41%, comparable to other studies of OA/TOF, but much higher than the general population in Finland (17%).75
Restrictive lung disease is common post OA/TOF repair, and there are multiple potential predisposing factors. These include congenital vertebral or chest wall abnormalities (i.e., scoliosis or post-operative rib fusions), surgical trauma, aspiration, and/or recurrent chest infections.77 A study that used pulmonary function testing (including spirometry and lung volumes) in 14 children post OA/TOF repair (7–12 years of age) found a mean reduction in thoracic gas volume, FVC, and FEV1 of two standard deviations below predicted.78 The majority of children in this study reported current cough and dysphagia. In contrast, other studies have reported normal pulmonary function tests in children and adults post OA/TOF repair.61 One small study found that children post OA/TOF had reduced exercise duration compared with controls.79 However, another study found no difference in exercise tolerance between children with OA/TOF and controls with GORD alone, however the children with history of OA/TOF repair had significantly lower TLC and FVC, evidence of restrictive lung disease.69 Using the forced oscillation technique, Harrison and colleagues reported that children (mean age 7.6 years) post OA/TOF repair had higher airway resistance and lower reactance at six hertz than controls. Children post OA/TOF repair in this study also had significantly lower FVC and FEV1 compared to healthy controls, and children with a history of anti-reflux surgery had diminished lung function compared to children without a history of this surgery.80 This could be the result of severe reflux and subsequent lung damage, or could be secondary to factors about the surgery.
CONCLUSION
Most infants and children with OA/TOF experience clinical improvement and survive into adulthood. While some co-morbidities may be lifelong, many patients experience overall symptomatic and clinical improvement with increasing age. Infancy and early childhood can be a vulnerable period in which children with history of OA/TOF repair experience significant morbidity. Though any of the following problems may be lifelong in some individuals, the reported prevalence of significant dysphagia, respiratory infections, choking, GORD , and growth impairment generally decreases after the first five years of life, and many patients experience clinical improvement.59 This period coincides with a critical window for lung growth and alveolar development for all children; therefore, providers should be mindful that frequent or serious chest infections or ongoing aspiration of feeds or oral secretions may result in permanent damage and significantly impair lung growth. We emphasize the importance of regular outpatient follow-up with pulmonary specialists in children with severe or persistent respiratory symptoms and in those suspected of having impaired airway clearance or other airway abnormalities. We recommend close and expectant management for respiratory symptoms of wheezing, cough, and infection, with treatment based on symptoms and a low threshold for further investigations such as flexible bronchoscopy, especially when respiratory symptoms are persistent, frequent, or severe.
Educational Aims.
To present the epidemiology of tracheo-oesophageal fistula, including incidence, genetic basis, and association with genetic syndromes and other anomalies.
To present the classification, types, and diagnosis of tracheo-oesophageal fistula.
To discuss medical treatment and short and long-term clinical outcomes with an emphasis on pulmonary treatment and outcomes.
Practice Points.
Comorbidities related to the airways, gastrointestinal tract, and feeding should be anticipated and evaluated early.
We recommend upper and lower airway endoscopy be performed early, either before primary surgical repair, or soon after if respiratory symptoms occur, in order to prevent lung damage from unaddressed comorbidities. Infants who have had pre-operative airway endoscopy may still require post-operative endoscopy, as some abnormalities may occur or worsen.
Multidisciplinary communication and coordination of care is important. Joint endoscopy should be considered in cases of unexplained or persistent pulmonary symptoms with participation of multiple subspecialists who can examine the upper and lower airways and gastrointestinal tract.
Patients with respiratory symptoms should be followed by pulmonologists and obtain regular clinical assessments and pulmonary function tests.
Future Directions for clinical research.
Longitudinal, prospective data from large numbers of patients post OA/TOF repair, including endoscopic evaluations pre- and post-operatively to better estimate the frequency and degree of tracheomalacia and other associated upper and lower airway comorbidities are needed.
The effectiveness of various pulmonary and reflux treatment regimens should be evaluated.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosures/Conflicts of interest: none
References
- 1.Flint PW, Haughey BH, Lund V, et al. Cummings Otolaryngology. 6th ed. Elsevier; 2015. Pediatric Tracheal Anomalies; pp. 3171–3183. [Google Scholar]
- 2.Goyal A, Jones MO, Couriel JM, Losty PD. Oesophageal atresia and tracheo-oesophageal fistula. Archives of disease in childhood Fetal and neonatal edition. 2006;91:F381–F384. doi: 10.1136/adc.2005.086157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hosie GP, Short M. Oesophageal atresia. Paediatric Surgery I. 2009;28:38–42. [Google Scholar]
- 4.Robb A, Lander A. Oesophageal atresia and tracheo-oesophageal fistula. Paediatric II. 2007;25:283–286. [Google Scholar]
- 5.Depaepe A, Dolk H, Lechat MF. The epidemiology of tracheo-oesophageal fistula and oesophageal atresia in Europe. EUROCAT Working Group. Archives of disease in childhood. 1993;68:743–748. doi: 10.1136/adc.68.6.743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Robert E, Mutchinick O, Mastroiacovo P, et al. An international collaborative study of the epidemiology of esophageal atresia or stenosis. Reproductive toxicology (Elmsford, NY) 1993;7:405–421. doi: 10.1016/0890-6238(93)90085-l. [DOI] [PubMed] [Google Scholar]
- 7.Torfs CP, Curry CJ, Bateson TF. Population-based study of tracheoesophageal fistula and esophageal atresia. Teratology. 1995;52:220–232. doi: 10.1002/tera.1420520408. [DOI] [PubMed] [Google Scholar]
- 8.Crabbe DC. Isolated tracheo-oesophageal fistula. Paediatric respiratory reviews. 2003;4:74–78. [PubMed] [Google Scholar]
- 9.Kluth D, Fiegel H. The embryology of the foregut. Seminars in pediatric surgery. 2003;12:3–9. doi: 10.1053/spsu.2003.50003. [DOI] [PubMed] [Google Scholar]
- 10.Crisera CA, Grau JB, Maldonado TS, Kadison AS, Longaker MT, Gittes GK. Defective epithelial-mesenchymal interactions dictate the organogenesis of tracheoesophageal fistula. Pediatric surgery international. 2000;16:256–261. doi: 10.1007/s003830050740. [DOI] [PubMed] [Google Scholar]
- 11.Brosens E, Ploeg M, van Bever Y, et al. Clinical and etiological heterogeneity in patients with tracheo-esophageal malformations and associated anomalies. European journal of medical genetics. 2014;57:440–452. doi: 10.1016/j.ejmg.2014.05.009. [DOI] [PubMed] [Google Scholar]
- 12.Schulz AC, Bartels E, Stressig R, et al. Nine new twin pairs with esophageal atresia: a review of the literature and performance of a twin study of the disorder. Birth defects research Part A, Clinical and molecular teratology. 2012;94:182–186. doi: 10.1002/bdra.22879. [DOI] [PubMed] [Google Scholar]
- 13.Shaw-Smith C. Genetic factors in esophageal atresia, tracheo-esophageal fistula and the VACTERL association: roles for FOXF1 and the 16q24.1 FOX transcription factor gene cluster, and review of the literature. European journal of medical genetics. 2010;53:6–13. doi: 10.1016/j.ejmg.2009.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Brunner HG, van Bokhoven H. Genetic players in esophageal atresia and tracheoesophageal fistula. Current opinion in genetics & development. 2005;15:341–347. doi: 10.1016/j.gde.2005.04.010. [DOI] [PubMed] [Google Scholar]
- 15.Nora JJ, Nora AH, Perinchief AG, Ingram JW, Fountain AK, Peterson MJ. Letter: Congenital abnormalities and first-trimester exposure to progestagen/oestrogen. Lancet. 1976;1:313–314. doi: 10.1016/s0140-6736(76)91455-0. [DOI] [PubMed] [Google Scholar]
- 16.Di Gianantonio E, Schaefer C, Mastroiacovo PP, et al. Adverse effects of prenatal methimazole exposure. Teratology. 2001;64:262–266. doi: 10.1002/tera.1072. [DOI] [PubMed] [Google Scholar]
- 17.Felix JF, van Dooren MF, Klaassens M, Hop WC, Torfs CP, Tibboel D. Environmental factors in the etiology of esophageal atresia and congenital diaphragmatic hernia: results of a case-control study. Birth defects research Part A, Clinical and molecular teratology. 2008;82:98–105. doi: 10.1002/bdra.20423. [DOI] [PubMed] [Google Scholar]
- 18.Oddsberg J, Lu Y, Lagergren J. Maternal diabetes and risk of esophageal atresia. Journal of pediatric surgery. 2010;45:2004–2008. doi: 10.1016/j.jpedsurg.2010.06.008. [DOI] [PubMed] [Google Scholar]
- 19.Kyyronen P, Hemminki K. Gastro-intestinal atresias in Finland in 1970–79, indicating time-place clustering. Journal of epidemiology and community health. 1988;42:257–265. doi: 10.1136/jech.42.3.257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Oddsberg J, Jia C, Nilsson E, Ye W, Lagergren J. Maternal tobacco smoking, obesity, and low socioeconomic status during early pregnancy in the etiology of esophageal atresia. Journal of pediatric surgery. 2008;43:1791–1795. doi: 10.1016/j.jpedsurg.2008.02.058. [DOI] [PubMed] [Google Scholar]
- 21.Keckler SJ, St Peter SD, Valusek PA, et al. VACTERL anomalies in patients with esophageal atresia: an updated delineation of the spectrum and review of the literature. Pediatric surgery international. 2007;23:309–313. doi: 10.1007/s00383-007-1891-0. [DOI] [PubMed] [Google Scholar]
- 22.La Placa S, Giuffre M, Gangemi A, et al. Esophageal atresia in newborns: a wide spectrum from the isolated forms to a full VACTERL phenotype? Italian journal of pediatrics. 2013;39:45. doi: 10.1186/1824-7288-39-45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chittmittrapap S, Spitz L, Kiely EM, Brereton RJ. Oesophageal atresia and associated anomalies. Archives of disease in childhood. 1989;64:364–368. doi: 10.1136/adc.64.3.364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Shaw-Smith C. Oesophageal atresia, tracheo-oesophageal fistula, and the VACTERL association: review of genetics and epidemiology. Journal of medical genetics. 2006;43:545–554. doi: 10.1136/jmg.2005.038158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Felix JF, Tibboel D, de Klein A. Chromosomal anomalies in the aetiology of oesophageal atresia and tracheo-oesophageal fistula. European journal of medical genetics. 2007;50:163–175. doi: 10.1016/j.ejmg.2006.12.004. [DOI] [PubMed] [Google Scholar]
- 26.de Jong EM, Felix JF, de Klein A, Tibboel D. Etiology of esophageal atresia and tracheoesophageal fistula: "mind the gap". Current gastroenterology reports. 2010;12:215–222. doi: 10.1007/s11894-010-0108-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pedersen RN, Calzolari E, Husby S, Garne E. Oesophageal atresia: prevalence, prenatal diagnosis and associated anomalies in 23 European regions. Archives of disease in childhood. 2012;97:227–232. doi: 10.1136/archdischild-2011-300597. [DOI] [PubMed] [Google Scholar]
- 28.Sparey C, Jawaheer G, Barrett AM, Robson SC. Esophageal atresia in the Northern Region Congenital Anomaly Survey, 1985–1997: prenatal diagnosis and outcome. American journal of obstetrics and gynecology. 2000;182:427–431. doi: 10.1016/s0002-9378(00)70234-1. [DOI] [PubMed] [Google Scholar]
- 29.Pretorius DH, Drose JA, Dennis MA, Manchester DK, Manco-Johnson ML. Tracheoesophageal fistula in utero. Twenty-two cases. Journal of ultrasound in medicine : official journal of the American Institute of Ultrasound in Medicine. 1987;6:509–513. doi: 10.7863/jum.1987.6.9.509. [DOI] [PubMed] [Google Scholar]
- 30.Choudhry M, Boyd PA, Chamberlain PF, Lakhoo K. Prenatal diagnosis of tracheo-oesophageal fistula and oesophageal atresia. Prenatal diagnosis. 2007;27:608–610. doi: 10.1002/pd.1745. [DOI] [PubMed] [Google Scholar]
- 31.de Jong EM, de Haan MA, Gischler SJ, et al. Pre- and postnatal diagnosis and outcome of fetuses and neonates with esophageal atresia and tracheoesophageal fistula. Prenatal diagnosis. 2010;30:274–279. doi: 10.1002/pd.2466. [DOI] [PubMed] [Google Scholar]
- 32.Houben CH, Curry JI. Current status of prenatal diagnosis, operative management and outcome of esophageal atresia/tracheo-esophageal fistula. Prenatal diagnosis. 2008;28:667–675. doi: 10.1002/pd.1938. [DOI] [PubMed] [Google Scholar]
- 33.Stringer MD, McKenna KM, Goldstein RB, Filly RA, Adzick NS, Harrison MR. Prenatal diagnosis of esophageal atresia. Journal of pediatric surgery. 1995;30:1258–1263. doi: 10.1016/0022-3468(95)90480-8. [DOI] [PubMed] [Google Scholar]
- 34.Ethun CG, Fallon SC, Cassady CI, et al. Fetal MRI improves diagnostic accuracy in patients referred to a fetal center for suspected esophageal atresia. Journal of pediatric surgery. 2014;49:712–715. doi: 10.1016/j.jpedsurg.2014.02.053. [DOI] [PubMed] [Google Scholar]
- 35.Sulkowski JP, Cooper JN, Lopez JJ, et al. Morbidity and mortality in patients with esophageal atresia. Surgery. 2014;156:483–491. doi: 10.1016/j.surg.2014.03.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Karnak I, Senocak ME, Hicsonmez A, Buyukpamukcu N. The diagnosis and treatment of H-type tracheoesophageal fistula. Journal of pediatric surgery. 1997;32:1670–1674. doi: 10.1016/s0022-3468(97)90503-0. [DOI] [PubMed] [Google Scholar]
- 37.Andrassy RJ, Ko P, Hanson BA, Kubota E, Hays DM, Mahour GH. Congenital tracheoesophageal fistula without esophageal atresia. A 22 year experience. American journal of surgery. 1980;140:731–733. doi: 10.1016/0002-9610(80)90105-1. [DOI] [PubMed] [Google Scholar]
- 38.Beasley SW, Myers NA. The diagnosis of congenital tracheoesophageal fistula. Journal of pediatric surgery. 1988;23:415–417. doi: 10.1016/s0022-3468(88)80437-8. [DOI] [PubMed] [Google Scholar]
- 39.Crabbe DC, Kiely EM, Drake DP, Spitz L. Management of the isolated congenital tracheo-oesophageal fistula. European journal of pediatric surgery : official journal of Austrian Association of Pediatric Surgery [et al] = Zeitschrift fur Kinderchirurgie. 1996;6:67–69. doi: 10.1055/s-2008-1066473. [DOI] [PubMed] [Google Scholar]
- 40.Ng J, Antao B, Bartram J, Raghavan A, Shawis R. Diagnostic difficulties in the management of H-type tracheoesophageal fistula. Acta radiologica (Stockholm, Sweden : 1987) 2006;47:801–805. doi: 10.1080/02841850600854902. [DOI] [PubMed] [Google Scholar]
- 41.Laffan EE, Daneman A, Ein SH, Kerrigan D, Manson DE. Tracheoesophageal fistula without esophageal atresia: are pull-back tube esophagograms needed for diagnosis? Pediatric radiology. 2006;36:1141–1147. doi: 10.1007/s00247-006-0269-0. [DOI] [PubMed] [Google Scholar]
- 42.Deng ZH, Yan ZL, Yin Y, et al. [Multi-disciplinary hybrid therapy for tracheoesophageal fistula in children: analysis of 4 cases] Zhonghua er ke za zhi Chinese journal of pediatrics. 2012;50:568–570. [PubMed] [Google Scholar]
- 43.Fraga JC, Adil EA, Kacprowicz A, et al. The association between laryngeal cleft and tracheoesophageal fistula: Myth or reality? The Laryngoscope. 2014 doi: 10.1002/lary.24804. [DOI] [PubMed] [Google Scholar]
- 44.Sharma N, Srinivas M. Laryngotracheobronchoscopy prior to esophageal atresia and tracheoesophageal fistula repair--its use and importance. Journal of pediatric surgery. 2014;49:367–369. doi: 10.1016/j.jpedsurg.2013.09.009. [DOI] [PubMed] [Google Scholar]
- 45.Mahalik SK, Sodhi KS, Narasimhan KL, Rao KL. Role of preoperative 3D CT reconstruction for evaluation of patients with esophageal atresia and tracheoesophageal fistula. Pediatric surgery international. 2012;28:961–966. doi: 10.1007/s00383-012-3111-9. [DOI] [PubMed] [Google Scholar]
- 46.Garge S, Rao KL, Bawa M. The role of preoperative CT scan in patients with tracheoesophageal fistula: a review. Journal of pediatric surgery. 2013;48:1966–1971. doi: 10.1016/j.jpedsurg.2013.06.010. [DOI] [PubMed] [Google Scholar]
- 47.Al-Shanafey S, Harvey J. Long gap esophageal atresia: an Australian experience. Journal of pediatric surgery. 2008;43:597–601. doi: 10.1016/j.jpedsurg.2007.12.001. [DOI] [PubMed] [Google Scholar]
- 48.Tovar JA, Fragoso AC. Current controversies in the surgical treatment of esophageal atresia. Scandinavian journal of surgery : SJS : official organ for the Finnish Surgical Society and the Scandinavian Surgical Society. 2011;100:273–278. doi: 10.1177/145749691110000407. [DOI] [PubMed] [Google Scholar]
- 49.Rothenberg SS. Thoracoscopic repair of esophageal atresia and tracheo-esophageal fistula in neonates: evolution of a technique. Journal of laparoendoscopic & advanced surgical techniques Part A. 2012;22:195–199. doi: 10.1089/lap.2011.0063. [DOI] [PubMed] [Google Scholar]
- 50.Engum SA, Grosfeld JL, West KW, Rescorla FJ, Scherer LR., 3rd Analysis of morbidity and mortality in 227 cases of esophageal atresia and/or tracheoesophageal fistula over two decades. Archives of surgery (Chicago, Ill : 1960) 1995;130:502–508. doi: 10.1001/archsurg.1995.01430050052008. discussion 8-9. [DOI] [PubMed] [Google Scholar]
- 51.Schneider A, Blanc S, Bonnard A, et al. Results from the French National Esophageal Atresia register: one-year outcome. Orphanet journal of rare diseases. 2014;9:206. doi: 10.1186/s13023-014-0206-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Leboulanger N, Garabedian EN. Laryngo-tracheo-oesophageal clefts. Orphanet journal of rare diseases. 2011;6:81. doi: 10.1186/1750-1172-6-81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Walner DL, Stern Y, Collins M, Cotton RT, Myer CM., 3rd Does the presence of a tracheoesophageal fistula predict the outcome of laryngeal cleft repair? Archives of otolaryngology--head & neck surgery. 1999;125:782–784. doi: 10.1001/archotol.125.7.782. [DOI] [PubMed] [Google Scholar]
- 54.Eriksen C, Zwillenberg D, Robinson N. Diagnosis and management of cleft larynx. Literature review and case report. The Annals of otology, rhinology, and laryngology. 1990;99:703–708. doi: 10.1177/000348949009900907. [DOI] [PubMed] [Google Scholar]
- 55.Glossop LP, Smith RJ, Evans JN. Posterior laryngeal cleft: an analysis of ten cases. International journal of pediatric otorhinolaryngology. 1984;7:133–143. doi: 10.1016/s0165-5876(84)80037-3. [DOI] [PubMed] [Google Scholar]
- 56.Helardot PG, Bargy F, Manach Y, Grapin C. [Laryngo-tracheo-esophageal cleft associated with missed atresia] Chirurgie pediatrique. 1985;26:112–113. [PubMed] [Google Scholar]
- 57.Shah AR, Lazar EL, Atlas AB. Tracheal diverticula after tracheoesophageal fistula repair: case series and review of the literature. Journal of pediatric surgery. 2009;44:2107–2111. doi: 10.1016/j.jpedsurg.2009.04.036. [DOI] [PubMed] [Google Scholar]
- 58.Johnson LB, Cotton RT, Rutter MJ. Management of symptomatic tracheal pouches. International journal of pediatric otorhinolaryngology. 2007;71:527–531. doi: 10.1016/j.ijporl.2006.10.024. [DOI] [PubMed] [Google Scholar]
- 59.Little DC, Rescorla FJ, Grosfeld JL, West KW, Scherer LR, Engum SA. Long-term analysis of children with esophageal atresia and tracheoesophageal fistula. Journal of pediatric surgery. 2003;38:852–856. doi: 10.1016/s0022-3468(03)00110-6. [DOI] [PubMed] [Google Scholar]
- 60.Somppi E, Tammela O, Ruuska T, et al. Outcome of patients operated on for esophageal atresia: 30 years' experience. Journal of pediatric surgery. 1998;33:1341–1346. doi: 10.1016/s0022-3468(98)90003-3. [DOI] [PubMed] [Google Scholar]
- 61.Kovesi T, Rubin S. Long-term complications of congenital esophageal atresia and/or tracheoesophageal fistula. Chest. 2004;126:915–925. doi: 10.1378/chest.126.3.915. [DOI] [PubMed] [Google Scholar]
- 62.Zigman A, Yazbeck S. Esophageal foreign body obstruction after esophageal atresia repair. Journal of pediatric surgery. 2002;37:776–778. doi: 10.1053/jpsu.2002.32285. [DOI] [PubMed] [Google Scholar]
- 63.Dutta HK, Grover VP, Dwivedi SN, Bhatnagar V. Manometric evaluation of postoperative patients of esophageal atresia and tracheo-esophageal fistula. European journal of pediatric surgery : official journal of Austrian Association of Pediatric Surgery [et al] = Zeitschrift fur Kinderchirurgie. 2001;11:371–376. doi: 10.1055/s-2001-19718. [DOI] [PubMed] [Google Scholar]
- 64.Chetcuti P, Myers NA, Phelan PD, Beasley SW. Adults who survived repair of congenital oesophageal atresia and tracheo-oesophageal fistula. BMJ (Clinical research ed) 1988;297:344–346. doi: 10.1136/bmj.297.6644.344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Lacher M, Froehlich S, von Schweinitz D, Dietz HG. Early and long term outcome in children with esophageal atresia treated over the last 22 years. Klinische Padiatrie. 2010;222:296–301. doi: 10.1055/s-0030-1249610. [DOI] [PubMed] [Google Scholar]
- 66.Koivusalo A, Pakarinen MP, Rintala RJ. The cumulative incidence of significant gastrooesophageal reflux in patients with oesophageal atresia with a distal fistula--a systematic clinical, pH-metric, and endoscopic follow-up study. Journal of pediatric surgery. 2007;42:370–374. doi: 10.1016/j.jpedsurg.2006.10.010. [DOI] [PubMed] [Google Scholar]
- 67.Orringer MB, Kirsh MM, Sloan H. Long-term esophageal function following repair of esophageal atresia. Annals of surgery. 1977;186:436–443. doi: 10.1097/00000658-197710000-00005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Taylor AC, Breen KJ, Auldist A, et al. Gastroesophageal reflux and related pathology in adults who were born with esophageal atresia: a long-term follow-up study. Clinical gastroenterology and hepatology : the official clinical practice journal of the American Gastroenterological Association. 2007;5:702–706. doi: 10.1016/j.cgh.2007.03.012. [DOI] [PubMed] [Google Scholar]
- 69.Peetsold MG, Heij HA, Nagelkerke AF, Deurloo JA, Gemke RJ. Pulmonary function impairment after trachea-esophageal fistula: a minor role for gastro-esophageal reflux disease. Pediatric pulmonology. 2011;46:348–355. doi: 10.1002/ppul.21369. [DOI] [PubMed] [Google Scholar]
- 70.Oliveira C, Zamakhshary M, Marcon P, Kim PC. Eosinophilic esophagitis and intermediate esophagitis after tracheoesophageal fistula repair: a case series. Journal of pediatric surgery. 2008;43:810–814. doi: 10.1016/j.jpedsurg.2007.12.018. [DOI] [PubMed] [Google Scholar]
- 71.Dhaliwal J, Tobias V, Sugo E, et al. Eosinophilic esophagitis in children with esophageal atresia. Diseases of the esophagus : official journal of the International Society for Diseases of the Esophagus / ISDE. 2014;27:340–347. doi: 10.1111/dote.12119. [DOI] [PubMed] [Google Scholar]
- 72.Usui N, Kamata S, Ishikawa S, et al. Anomalies of the tracheobronchial tree in patients with esophageal atresia. Journal of pediatric surgery. 1996;31:258–262. doi: 10.1016/s0022-3468(96)90010-x. [DOI] [PubMed] [Google Scholar]
- 73.Carden KA, Boiselle PM, Waltz DA, Ernst A. Tracheomalacia and tracheobronchomalacia in children and adults: an in-depth review. Chest. 2005;127:984–1005. doi: 10.1378/chest.127.3.984. [DOI] [PubMed] [Google Scholar]
- 74.Panitch HB, Keklikian EN, Motley RA, Wolfson MR, Schidlow DV. Effect of altering smooth muscle tone on maximal expiratory flows in patients with tracheomalacia. Pediatric pulmonology. 1990;9:170–176. doi: 10.1002/ppul.1950090309. [DOI] [PubMed] [Google Scholar]
- 75.Sistonen S, Malmberg P, Malmstrom K, et al. Repaired oesophageal atresia: respiratory morbidity and pulmonary function in adults. The European respiratory journal. 2010;36:1106–1112. doi: 10.1183/09031936.00153209. [DOI] [PubMed] [Google Scholar]
- 76.Malmstrom K, Lohi J, Lindahl H, et al. Longitudinal follow-up of bronchial inflammation, respiratory symptoms, and pulmonary function in adolescents after repair of esophageal atresia with tracheoesophageal fistula. The Journal of pediatrics. 2008;153:396–401. doi: 10.1016/j.jpeds.2008.03.034. [DOI] [PubMed] [Google Scholar]
- 77.Sistonen SJ, Pakarinen MP, Rintala RJ. Long-term results of esophageal atresia: Helsinki experience and review of literature. Pediatric surgery international. 2011;27:1141–1149. doi: 10.1007/s00383-011-2980-7. [DOI] [PubMed] [Google Scholar]
- 78.Agrawal L, Beardsmore CS, MacFadyen UM. Respiratory function in childhood following repair of oesophageal atresia and tracheoesophageal fistula. Archives of disease in childhood. 1999;81:404–408. doi: 10.1136/adc.81.5.404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Zaccara A, Felici F, Turchetta A, et al. Physical fitness testing in children operated on for tracheoesophageal fistula. Journal of pediatric surgery. 1995;30:1334–1337. doi: 10.1016/0022-3468(95)90498-0. [DOI] [PubMed] [Google Scholar]
- 80.Harrison J, Martin J, Crameri J, Robertson CF, Ranganathan SC. Lung function in children with repaired tracheo-oesophageal fistula using the forced oscillation technique. Pediatric pulmonology. 2010;45:1057–1063. doi: 10.1002/ppul.21282. [DOI] [PubMed] [Google Scholar]