Abstract
Catheter ablation has been widely used to manage recurrent atrial and ventricular arrhythmias. It has been established that contrast-enhanced magnetic resonance can accurately characterize the myocardium. In this review, we summarize the role of cardiac magnetic resonance in identification of arrhythmogenic substrates, and the potential utility of cardiac magnetic resonance for catheter ablation of complex atrial and ventricular arrhythmias.
Introduction
Catheter ablation is a well-established therapeutic strategy for patients with recurrent arrhythmia. Scar related sustained monomorphic ventricular tachycardia (VT) is most commonly seen after myocardial infarction. Although implantable cardioverter defibrillators offer the best protection against sudden cardiac death, catheter ablation for VT suppression is occasionally required. Atrial fibrillation (AF), the most common cardiac arrhythmia, is also amenable to catheter ablation. Together, these arrhythmias offer the most complex and gratifying challenges for electrophysiologists today.
Successful ablation requires the correct identification of underlying critical arrhythmogenic substrates. In the commonly used technique of electroanatomic mapping, substrates are identified indirectly, by collecting local voltage amplitudes as a surrogate of the state of nearby myocardium. This method is time consuming, lacks sensitivity for scar substrates deep to the surface being mapped, and lacks specificity for scar especially in the setting of poor catheter contact or thinner myocardium. Therefore, development of improved strategies to define arrhythmogenic scar substrates is warranted.
Cardiovascular magnetic resonance (CMR) is a noninvasive imaging modality with high contrast resolution that lacks ionized radiation. CMR has been used extensively in the recent decade due to its ability to characterize cardiac anatomy and function. As validated histopathologically, CMR can visualize fibrosis by delayed imaging of gadolinium-based contrast agents that accumulate within the extracellular space and have slower washout from scar than from healthy myocardium (1).
Today, with the help of evolving mapping technologies, CMR images can be merged with electrograms derived from the electrophysiologic study, thus creating an anatomic roadmap for the electrophysiologist (2). Myocardial scar, the most common substrate for reentrant arrhythmias, can be easily displayed by late gadolinium enhanced (LGE) CMR (3). Incorporation of the LGE derived scar anatomy shortens the procedure time devoted to substrate identification and enables ventricular tachycardia (VT) ablation in the setting of hemodynamic instability that precludes conventional electrophysiologic mapping and dense point-by-point voltage mapping. However, the technique of LGE CMR promises to do more than that. A thorough understanding of the physiologic conduction characteristics associated with various anatomic scar substrates may improve patient selection for arrhythmia therapies and facilitate ablation even in cases that are amenable to conventional electrophysiologic mapping.
In this review, we summarize the role of CMR in prediction of arrhythmogenic substrates, focusing on two arrhythmias: Scar related VT and atrial fibrillation (AF).
Ventricular tachycardia
Despite significant advances in technology and therapeutics; cardiac disease is still the leading cause of mortality in the industrialized world. Many deaths are attributable to ventricular arrhythmia, especially in patients with structural heart disease. Scar related sustained monomorphic VT is a common arrhythmia after myocardial infarction. Although mortality is best prevented with implantable defibrillators in such patients, many require catheter ablation for VT suppression. Due to continuing improvements in electrophysiology techniques, catheter ablation is now a validated option for treatment of scar related VT to reduce the morbidity associated with structural heart disease. A crucial step for successful VT ablation is detailed characterization of the underlying arrhythmogenic substrate.
Josephson and colleagues’ early fundamental studies showed that surgical resection of critical regions of the endocardium and subendocardium could terminate sustained VT (4,5). Post-myocardial infarct related VTs that were resistant to medical therapy, were successfully treated by resecting fibrotic endocardial tissue and infarct border zone regions. Patients that underwent such daring procedures were VT free during a follow-up period of 6 to 24 months (5). These findings inspired the consequent histopathological studies, which evaluated the resected endocardium and subendocardium specimens as a potential substrate for VT (6,7).
Fenoglio et al. defined the morphologic characteristics of arrhythmogenic substrates derived from surgical resections of the endocardium in patients with recurrent VT (6). In the resected specimens, bundles of viable myocardial fibers within dense fibrous tissue extended to the margins of the surgical resection. The authors concluded that the abnormal structure and arrangement of the surviving cardiac fibers in the endocardium might be the critical substrate for VT. A following study by de Bakker et al. included subjects with sustained VT, to describe the electrophysiologic and histologic findings in the resected endocardium of patients with previous myocardial infarction (7). The electrophysiologic signs of arrhythmogenic substrates such as fractionated electrograms and slow conduction were detected in areas where viable muscle fibers and fibrous tissue were mixed heterogeneously; and where muscle fibers were organized and isolated by connective tissue. Presystolic activity was located intramurally and subendocardially, supporting the concept that reentry occurred via isolated bundles of surviving myocytes within the infarct and the larger subendocardial muscle mass.
After validation of LGE for identification of scarred myocardium, numerous studies have sought to define VT substrates noninvasively (2,3). Peri-infarct zones are located between normal and infarcted tissue, surrounding the core scar. They appear as regions of intermediate intensity on LGE CMR, thus also referred to as gray or heterogeneous zones. Although some intermediate intensity regions may represent volume averaging of adjacent regions with dense scar and viable tissue, histological studies have verified that intermediate intensity regions usually represent a mixture of viable myocardium and scar (8). It has been shown that the extent of the heterogeneous zone is associated with spontaneous and inducible VT, and predicts mortality after myocardial infarction (9–11). In a study that enrolled 235 ischemic and nonischemic cardiomyopathy patients, the extent of the heterogeneous zone was independently associated with appropriate implantable defibrillator shocks for ventricular arrhythmias or cardiac death (12). Additionally, Perez-David et al. showed that post-infarction VT conducting channels were associated with heterogeneous zones detected by LGE CMR (13).
To understand the pathophysiologic basis of these observations, Estner et al. used LGE CMR in animal studies to show that heterogeneous zones were located at successfully ablated VT sites, and that incomplete ablation of these zones was associated with VT recurrence (14). Ashikaga et al. also studied swine hearts and defined arrhythmogenic substrates by using LGE CMR. Critical VT sites were identified as viable myocardial fibers adjacent to the scar tissue in the peri-infarct region (15). Other studies based on electroanatomic substrate mapping, have found that successful ablation of scar related VTs can be performed from the scar border zone (16). Animal based CMR studies and previous surgical studies indicate that the infarct border contains viable tissue, and close contact of normal and abnormal conduction pathways as well as viable and fibrotic tissue interactions may form the VT substrate (6–8,14,15).
On the other hand, several studies point to the scar core on CMR as the substrate for VT. Desjardins et al. performed electroanatomic mapping and LGE CMR in patients post myocardial infarction. The authors found that critical VT cites were located predominantly within the core infarct region (17). Sasaki et al. from our group performed LGE CMR in patients with ischemic cardiomyopathy prior to catheter ablation for VT (18). In this study, all critical VT sites were located in regions with >25% scar transmurality, but central pathway sites were located in regions with >75% scar transmurality. Many sites that exhibited isolated potentials and were identified as central circuit sites via entrainment mapping resided in regions with 100% transmural scar by LGE CMR (Figure 1). These findings are in agreement with the early surgical work by Fenoglio et al. that defined the arrhythmogenic VT substrates as bundles of viable myocardial fibers within dense fibrous tissue (6). A recent article from Piers and colleagues also evaluated the characteristics of VT substrates with LGE CMR in ischemic and nonischemic patients who underwent VT ablation (19). Critical VT sites were associated with high scar transmurality and the majority of the critical VT sites were located within 5 mm distance from >75% transmural scar and from the core-borderzone transition. Central pathway sites had higher scar transmurality and signal intensity than the average of the entire scar. The findings of these studies suggest that the degree of transmurality and proximity to the scar core are indicators of arrhythmogenic substrates in patients with VT, and that central pathway sites tend to exist within relatively dense and transmural scar.
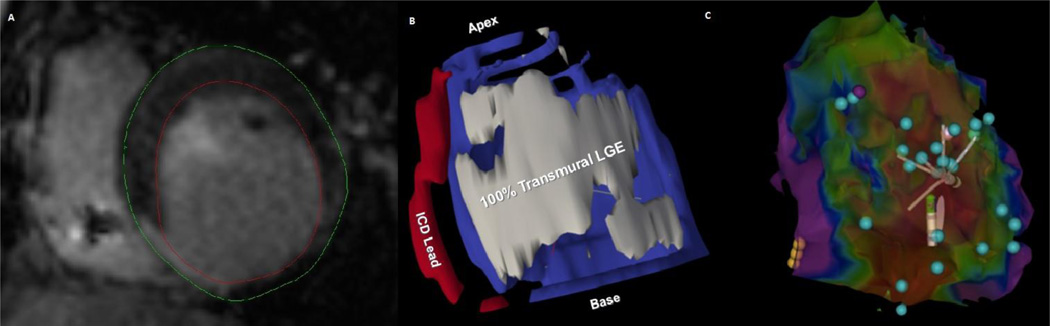
Figure 1.
The figure illustrates an ischemic inferior scar as a substrate for ventricular tachycardia. Panel A is a short axis LGE image showing the right ventricle with dark ICD lead susceptibility artifact in the cavity, and the left ventricle (LV) with a bright inferoseptal transmural infarct. The red and green lines were drawn as LV endocardial and epicardial contours, respectively. In Panel B, a segmented image is displayed which shows the 100% transmural infarction region (grey), LV myocardium (blue), and the ICD lead (red). Panel C is the corresponding electroanatomic map of the LV viewed from the inferior aspect. Red regions indicated voltage <0.1 mV and purple regions are healthy with voltage > 1.5 mV. The yellow balls indicate the position of the His bundle. Many isolated delayed potentials, suggesting channels with slow conduction (blue balls), were observed within 100% transmural scar regions on LGE. The basal lateral group of isolated potentials formed the central circuit sites for the clinical ventricular tachycardia in this patient. Ablation of all isolated potentials within the dense scar led to VT non-inducibility. The patient has had no further shocks over 12 months of follow-up to date.
Reentry is the mechanism responsible for the great majority of scar related VTs. Slow conduction, unidirectional block, and an initiating mechanism are the main requirements for reentry. In scar related VTs, fibrosis causes the electrophysiologic and anatomic alterations that yield the necessary environment for reentry. Dense fibrosis with minimal surviving bundles sets up the environment for unidirectional block and directs the arrhythmic circuit. Viable myocyte bundles along the infarct border-zone and within the scar form channels with slow conduction properties. With an initiating factor, the reentry cycle starts, passes through slow conduction channels, and completes the circuit around the dense scar (20). Therefore, both sets of studies described above are likely correct: portions of the heterogeneous border-zone are involved in VT circuits, most commonly as entrance or exit sites. Viable myocytes within the infarct core are also involved and form the slow conduction central circuit zones necessary for VT maintenance. The latter sites are harder to detect by the current resolution of CMR, but typically represent the more efficacious target for ablation.
Although there are conflicting results in the literature regarding the critical VT cites derived from LGE CMR, the basic reentry mechanisms suggest that both dense scar and infarct border zones contribute to the substrate for scar related VTs (9–11,16,17,21). In our opinion, fully transmural scar on LGE CMR often contains the critical substrate for VT circuits, where ablation is more effective and less detrimental to cardiac remodeling. Future availability of LGE sequences with higher resolution will likely enable more effective CMR substrate mapping for scar related VTs.
Electroanatomic Mapping for VT and Correlations with LGE CMR
Three-dimensional reconstructions of cardiac anatomy combined with electrophysiological information facilitate mapping and ablation procedures for VT. The integration of LGE CMR into electroanatomic mapping systems is particularly helpful in the presence of reentrant arrhythmias involving scar tissue. Initial studies have shown that three-dimensional scar segmentations can be obtained from LGE CMR and registered within electroanatomic mapping systems to guide VT ablation in patients with ischemic cardiomyopathy (22). Desjardins et al. confirmed these findings by reporting an integrated system that combines preprocedural scar mapping from LGE images and intraprocedural voltage mapping (17). Additionally, pre-procedural scar segmentation and incorporation into electroanatomic mapping systems can expedite the targeting of arrhythmogenic substrates in patients with non-ischemic cardiomyopathy, where the substrate is often deep to the surface and difficult to identify with surface voltage mapping (23).
Various studies have confirmed that low voltage corresponds to LGE CMR derived scar. Wijnmaalen et al. reported that in post-MI patients undergoing VT ablation, LGE CMR could be successfully integrated with electroanatomic mapping and that the scar information derived from LGE CMR is correlated with voltage mapping. Codreanu et al compared electroanatomic mapping with LGE CMR data for delineation of post-infarct scars in patients who underwent post-infarct VT ablation (24). This study confirmed that bipolar electroanatomic mapping could reliably differentiate scar from healthy tissue. We have also shown close associations between unipolar and bipolar voltage mapping and areas of scar identified by LGE-CMR in patients with both ischemic and non-ischemic cardiomyopathy (18, 25). These results, which generally apply to the left ventricle and interventricular septum, however, cannot be applied to the right ventricle. Right ventricular imaging with LGE CMR is challenging due to the thin walled structures and motion artifacts. It has been suggested that the inversion time required for optimal nulling of the myocardium differs between right and left ventricles (26). Partial-volume effects of bright blood next to right ventricular trabeculations may also lead to inaccurate measurements. In a study that compared LGE CMR and electroanatomic mapping in ARVD patients, LGE CMR was less sensitive than electroanatomic mapping in identifying right ventricular scar (27). Additionally, a recent study comparing LGE CMR with electroanatomic mapping in patients with right ventricular disease (ARVD and myocarditis) and right-sided VT showed that LGE CMR overlooked small scars and could not identify right ventricular substrates in the majority of cases (28).
Atrial fibrillation
Atrial fibrillation (AF) is the most common arrhythmia, reaching the highest prevalence in the elderly population. The high recurrence rate and associated morbidity and mortality of AF, as well as the ineffectiveness and side effects of antiarrhythmic drugs have resulted in efforts to develop new management strategies for AF. Today, catheter ablation is a commonly used option for patients with recurrent and symptomatic AF despite anti-arrhythmic drug therapy. Although the success of the procedure has been improved in recent years, post-ablation recurrence rates remain high. Selecting appropriate patients for the procedure is essential given the high recurrence rate and the possibility of ablation related complications.
AF is typically initiated from focal triggered activity within the pulmonary veins. Initial bursts of AF, lead to sustained episodes overtime as electrical alterations take form. Once AF is established, structural alterations occur throughout the atrium. However, the electrical and structural alterations that favor AF are not only caused by the presence of the arrhythmia but also by other co-morbidities that favor AF and may precede the arrhythmia. Electrical alterations include reduction of the action potential duration and refractory period, as well as myocardial voltage (29, 30). Structural alterations primarily consist of fibrosis, a suitable substrate for reentry. Fibrosis also causes inhomogeneity in atrial conduction and stabilizes the reentry waves, thus enabling AF perpetuation (31).
Frustaci and colleagues obtained endomyocardial atrial septal biopsies to examine histopathologic changes in patients with lone AF. They demonstrated abnormalities including inflammation, necrosis, and patchy fibrosis in all patients, suggesting that these histological abnormalities could serve as organic substrates for paroxysmal AF (32). Swartz et al. analyzed atrial tissue specimens that were resected during cardiac surgery from patients without prior AF (33). The authors observed greater atrial fibrosis in subjects with subsequent post-operative AF. Recently, Platonov and colleagues evaluated the extent of fibrosis in a post-mortem study (34). Histopathologic examination revealed that fibrosis of the left atrial myocardium and major atrial conduction pathways was significantly higher in patients with AF compared to those in the control group. Importantly, this association was independent of age. Additionally, patients with permanent AF had more extensive fibrosis than patients with paroxysmal AF.
Myocardial scar assessment by contrast enhanced CMR is a well-established method for the ventricle. Despite the current limitations in atrial wall imaging such as the thin wall thickness near the spatial resolution of CMR and irregular QRS complexes which challenge prospective ECG triggering techniques for imaging, recent advances in CMR technology have provided the opportunity for non-invasive assessment of atrial arrhythmogenic substrates. There is growing evidence that the presence and extent of the atrial LGE can be characterized by CMR (35), and that atrial LGE corresponds to regions with reduced bipolar voltage (36, 37).
Peters and colleagues initially reported the feasibility of atrial LGE CMR. The authors found no evidence of LGE in 23 AF patients who underwent CMR prior to ablation. Post-ablation, however, LGE was detected in all patients (38). Oakes and colleagues later examined left atrial LGE in patients with paroxysmal and persistent AF and compared the extent of LGE with invasive electroanatomic mapping (39). The regions with low voltage on invasive mapping matched regions of increased left atrial LGE. In healthy volunteers, the atrial myocardium revealed no LGE. In AF patients, the extent of LGE was strongly associated with persistent AF, and arrhythmia recurrence. Similarly, Spragg et al. from our group demonstrated that low-voltage areas by endocardial mapping were associated with left atrial LGE (36). The sensitivity and positive predictive value of LGE for identification of low voltage regions were 0.84 and 0.80, respectively. The specificity and negative predictive value LGE for identification of normal voltage regions were 0.68 and 0.73, respectively. In contrast, McGann and colleagues examined left atrial wall biopsies that were obtained during cardiac surgery and examined the association of histopathologic abnormalities with atrial LGE (40). Regions with left atrial LGE were associated with tissue fibrosis, while no LGE was seen in normal tissue. While stronger discriminative ability has been reported by the Utah group, our findings suggest that caution is warranted in accepting a direct association between atrial LGE and reduced voltage when using conventional LGE analysis methods (36). Additionally, reduced atrial bipolar voltage on invasive mapping may indicate thinner walls, inflammation, as well as poor catheter contact or a perpendicular bipole angle to the myocardial surface. Therefore, reduced voltage is not specific to fibrosis and additional studies of atrial LGE are warranted to properly denote the characteristics of LGE in association with various left atrial regions and processes.
The Utah investigators have proposed a staging algorithm to categorize the extent of left atrial LGE (35). In their studies patients are classified into 4 groups according to the percentage of left atrial LGE: Stage I (<5%), stage II (>5% to <20%), stage III (>20% to <35%), and stage IV (>35%). Catheter ablation success was significantly associated with the extent of the left atrial LGE; no recurrence was observed in patients with stage I while the recurrence rate was as high as 96% among patients with stage IV atrial LGE. The extent of the enhancement was independent of comorbidities or AF type. Another study by the same research group defined the percentage of the left atrial LGE with a modified staging algorithm: Stage I (<10%), stage II (>10% to <20%), stage III (>20% to <30%), and stage IV (>30%) (40). The amount of left atrial LGE was strongly associated with AF ablation outcome, and recurrence rates were significantly higher in the setting of pre-procedural stage IV fibrosis. A multicenter prospective study by the same group has again confirmed these findings. The study observed a graded increase in recurrence rates among groups, ranging from 14% at stage I to 65% at stage IV after adjusting for potential confounders (41). These results suggest that left atrial LGE CMR may improve patient selection for AF catheter ablation.
Besides pre-procedural implications for catheter ablation, LGE CMR has also been proposed for integration with navigation systems and for scar recognition during the ablation procedure. We have developed a novel technique for displaying LGE, voltage, and atrial anatomy on the same image (36). Bisbal and colleagues also successfully integrated LGE CMR into electroanatomic mapping systems for ablation of recurrent AF (42). Vergara et al. later demonstrated the feasibility and safety of real-time CMR guidance, as well as visualization of lesion formation in a study conducted on animal models (43). Lesion locations determined by CMR were qualitatively associated with locations on ex vivo gross examination of the heart.
LGE CMR has been also used to assess ablation related scar during the follow-up period. The amount of post-ablation scar and complete circumferential scarring of the pulmonary veins has been associated with better clinical outcomes (44, 45). McGann and colleagues have reported reduced recurrence rates in patients with a greater degree of scar formation at 3 months post-ablation (46). In the same study, authors also performed contrast enhanced CMR immediately after ablation, and reported that ablation related lesions were heterogeneous, including hyper-enhanced and non-enhanced core regions which likely indicate micro-vascular obstruction. The non-enhanced lesions were associated with LGE at 3 months post-ablation.
The inverse association between ablation related LGE and AF recurrence might be explained by a direct association between incomplete isolation of the pulmonary veins and larger LGE gaps around the pulmonary vein antra. Badger et al. demonstrated that AF recurrences following ablation were associated with gaps between lesions that can be identified by atrial LGE. In their hands, these gaps were correlated with the recovery of electric conduction (45). Similarly, Bisbal et al. reported that all of the electrically reconnected sites had gaps on LGE and that gaps matched with reconnection sites in the majority of the pulmonary veins (42). In this study, LGE CMR was integrated into navigation system for guidance of the ablation procedure and led to reduced RF ablation, but not total procedure time. In contrast, we have found that while some electrical gaps correspond to gaps on LGE, the current resolution of atrial CMR is insufficient for identification of many pulmonary vein reconnection sites (36).
These findings confirm that contrast enhanced CMR is a potentially valuable tool for pre-procedural patient selection, guidance of ablation procedures, and post-ablation follow-up. In cases with extensive LGE, a more extensive ablation strategy in addition to isolation of the pulmonary veins may be necessary. In repeat procedures, the procedure might be guided by atrial LGE maps. Most importantly, atrial LGE CMR may allow improved patient selection so that unnecessary procedures are avoided and patients with little chance of procedural benefit are spared the costs and risks of AF ablation. However, additional studies are needed before such strategies are adopted for routine clinical practice.
Limitations of Atrial LGE CMR
Despite advances in LGE CMR technology over the past decade, left atrial imaging has several technical limitations, including motion blurring, flow artifacts and limited image resolution as well as variations in the scar threshold based on selected inversion time. The left atrium has thin walls and exhibits significant anatomical complexity. Also, adjacent structure enhancement (such as valve structure and aortic wall enhancement) on LGE imaging must be distinguished from left atrial myocardial enhancement.
Previous studies emphasized these limitations. Taclas et al compared RF ablation sites with location of post-procedural scar formation in the left atrium by LGE CMR (47). In 20% of the ablation sites, there was no correlation with LGE CMR derived scar. In the following years, Hunter et al. performed pre and post-ablation LGE CMR in 50 participants with paroxysmal AF (48). Patients underwent either RF or cryo balloon ablation. In the authors’ hands, sensitivity and specificity for detection of ablation lesions was 60% and 96%, respectively, on LGE imaging. Sensitivity was higher when the LGE image was fused onto a segmented atrial surface (88%). The authors concluded that atrial LGE imaging was not yet sufficiently accurate to identify ablation lesions. More recently, Harrison et al. reported a weak association between endocardial voltage and LGE CMR signal intensities. Moreover, LGE CMR could not reliably identify post-ablation gaps (49). Similarly, our group could not find an association between electrical gaps and LGE discontinuities in AF patients undergoing repeat pulmonary vein isolation (36). These findings indicate that gap sites might consist of viable myocardium that is too small to be detected with the current LGE CMR resolution.
Detecting pre-ablation enhancement is more challenging than detecting post-ablation enhancement since fibrosis tends to be diffuse with intensity overlaps with normal myocardium. Peters et al. evaluated ablation related left atrial scar in patients with AF and identified post-procedural scar formation with LGE CMR in all of the study participants; however, they could not detect enhancement in pre-ablation images (38). Similarly, a recent multi-center study showed that segmentation in pre-ablation images is more challenging than segmentation of the post-ablation images (50).
Many studies have used electroanatomic mapping as a surrogate of diseased atrial myocardium but there is not enough histological data regarding the accurate voltage threshold for identification of diseased atrial myocardium and dense fibrosis. Our group was the first to underscore the limitations in the discriminative indices of typical LGE image analysis for identification of low voltage regions in the left atrium (36). In a recent animal based study, unlike the widely accepted cut-off values, mean atrial bipolar voltage was found to be 0.6 mV in the middle of the ablation lesion immediately after ablation, and 0.3 mV at post-ablation follow-up (51). Further studies are warranted for tissue validation of appropriate voltage thresholds as well as validation of the atrial LGE technique.
Another major problem with left atrial scar imaging is the lack of standardized evaluation of the LGE CMR segmentation and quantification of the enhancement. Although many studies used techniques that have been validated for the ventricle, the use of such techniques for the left atrium has not been validated. Identification of a normal region of left atrial myocardium is required for using techniques typically applied to the ventricle (such as 2–3 standard deviation intensity thresholds above normal myocardium) and there is no reliable way to assess the location of normal left atrial myocardium with LGE CMR. Karim et al. compared different algorithms for segmentation of the left atrial scar imaging in a multi-center international study and measured the performance of the different algorithms on a common scale (50). The authors concluded that no algorithm is better than others, emphasizing the need for algorithmic developments in left atrial scar imaging to standardize the segmentation methods. Until such limitations are met, and standardized and generalizable protocols for image acquisition and analysis have been developed, it is hard to advocate the general clinical use of atrial LGE imaging for management of AF patients.
Most left atrial CMR studies have quantified the extent of LGE based on signal intensity, which is measured in “arbitrary units.” This technique, however, may not provide objective thresholds due to the effects of various parameters on contrast intensity measurement such as surface coil proximity, contrast dose, delay in time of image acquisition after contrast injection, patient hematocrit, glomerular filtration rate, and body mass index (52). Our group recently examined a normalized measure of intensity that may overcome these limitations (37). We defined the image intensity ratio as left atrial signal intensity divided by the mean atrial blood pool image intensity. We then examined the association of the image intensity ratio with invasive electroanatomic mapping. Each unit increase in image intensity ratio was associated with 91.3% decrease in intracardiac bipolar voltage. These results indicated that the image intensity ratio is a promising measure to detect atrial fibrosis and that it exhibits improved diagnostic indices for identification of low voltage regions when compared to conventional analytic methods for detection of LGE (Figure 2).
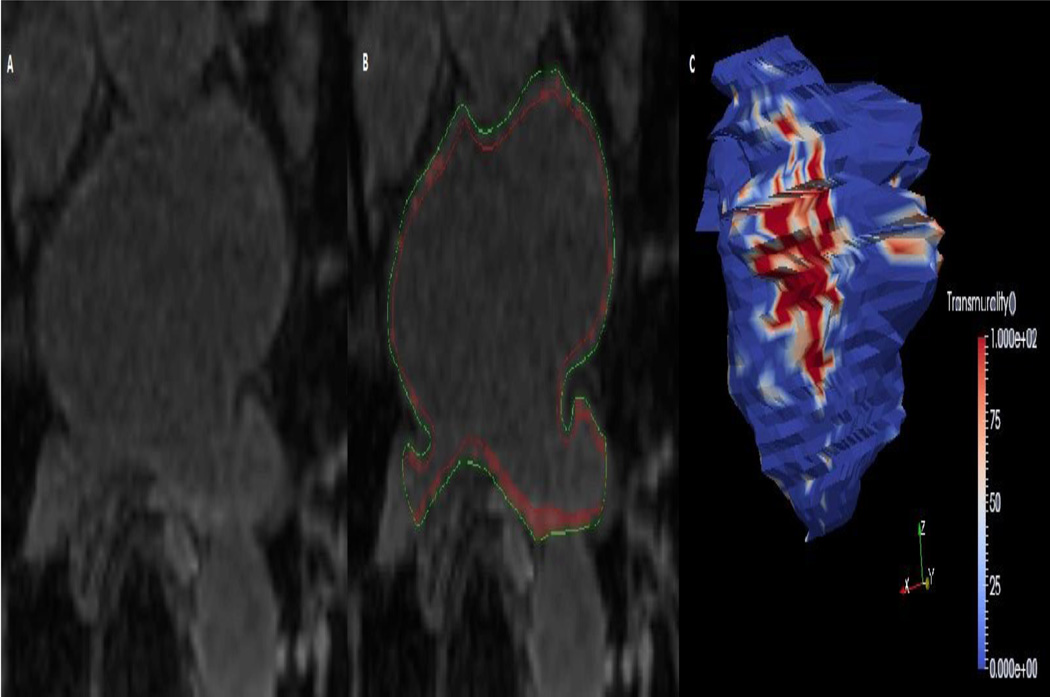
Figure 2.
The figure illustrates atrial LGE as a possible substrate for atrial fibrillation. Panel A is an example of an axial LGE image obtained at the level of the superior pulmonary veins. The manually drawn endocardial (red) and epicardial (green) contours in panel B allow the software to highlight high intensity regions, which indicate LGE. Panel C shows a three-dimensional reconstruction of the LA endocardium with projection of LGE transmurality.
Conclusion
The capability for non-invasive characterization of the atrial and ventricular myocardium is rapidly evolving. CMR enables detailed assessments of functional and structural characteristics without exposing the patient to radiation. The added costs and expertise required for appropriate image acquisition and analyses, as well as inadequate spatial resolution in the atrium, limit the routine use of CMR at present. The reproducibility and diagnostic ability of the atrial LGE CMR remains controversial. However, with improving techniques, accurate pre-procedural identification of the arrhythmogenic substrate by CMR may become an important adjunct for patient selection, procedural planning, and post-procedural evaluation.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Nakahara S, Vaseghi M, Ramirez RJ, Fonseca CG, Lai CK, Finn JP, et al. Characterization of myocardial scars: electrophysiological imaging correlates in a porcine infarct model. Heart Rhythm. 2011 Jul;8(7):1060–1067. doi: 10.1016/j.hrthm.2011.02.029. [DOI] [PubMed] [Google Scholar]
- 2.Wijnmaalen AP, van der Geest RJ, van Huls van Taxis CF, Siebelink HM, Kroft LJ, Bax JJ, et al. Head-to-head comparison of contrast-enhanced magnetic resonance imaging and electroanatomical voltage mapping to assess post-infarct scar characteristics in patients with ventricular tachycardias: real-time image integration and reversed registration. Eur Heart J. 2011 Jan;32(1):104–114. doi: 10.1093/eurheartj/ehq345. [DOI] [PubMed] [Google Scholar]
- 3.Gupta S, Desjardins B, Baman T, Ilg K, Good E, Crawford T, et al. Delayed-enhanced MR scar imaging and intraprocedural registration into an electroanatomical mapping system in post-infarction patients. JACC Cardiovasc Imaging. 2012 Feb;5(2):207–210. doi: 10.1016/j.jcmg.2011.08.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Josephson ME, Harken AH, Horowitz L. Endocardial excision: a new surgical technique for recurrent ventricular tachycardia. Circulation. 1979 Dec;60(7):1430–1439. doi: 10.1161/01.cir.60.7.1430. [DOI] [PubMed] [Google Scholar]
- 5.Guiraudon G, Fontaine G, Frank R, Escande G, Etievent P, Cabrol C. Encircling endocardial ventriculotomy: a new surgical treatment for life-threatening ventricular tachycardias resistant to medical treatment following myocardial infarction. Ann Thorac Surg. 1978 Nov;26(5):438–444. doi: 10.1016/s0003-4975(10)62923-2. [DOI] [PubMed] [Google Scholar]
- 6.Fenoglio JJ, Jr, Pham TD, Harken AH, Horowitz LN, Josephson ME, Wit AL. Recurrent sustained ventricular tachycardia: structure and ultrastructure of subendocardial regions in which tachycardia originates. Circulation. 1983 Sep;68(3):518–533. doi: 10.1161/01.cir.68.3.518. [DOI] [PubMed] [Google Scholar]
- 7.de Bakker JM, van Capelle FJ, Janse MJ, Wilde AA, Coronel R, Becker AE, et al. Reentry as a cause of ventricular tachycardia in patients with chronic ischemic heart disease: electrophysiologic and anatomic correlation. Circulation. 1988 Mar;77(3):589–606. doi: 10.1161/01.cir.77.3.589. [DOI] [PubMed] [Google Scholar]
- 8.Arheden H, Saeed M, Higgins CB, Gao DW, Ursell PC, Bremerich J, et al. Reperfused rat myocardium subjected to various durations of ischemia: estimation of the distribution volume of contrast material with echo-planar MR imaging. Radiology. 2000 May;215(2):520–528. doi: 10.1148/radiology.215.2.r00ma38520. [DOI] [PubMed] [Google Scholar]
- 9.Roes SD, Borleffs CJ, van der Geest RJ, Westenberg JJ, Marsan NA, Kaandorp TA, et al. Infarct tissue heterogeneity assessed with contrast-enhanced MRI predicts spontaneous ventricular arrhythmia in patients with ischemic cardiomyopathy and implantable cardioverter-defibrillator. Circ Cardiovasc Imaging. 2009 May;2(3):183–190. doi: 10.1161/CIRCIMAGING.108.826529. [DOI] [PubMed] [Google Scholar]
- 10.Schmidt A, Azevedo CF, Cheng A, Gupta SN, Bluemke DA, Foo TK, et al. Infarct tissue heterogeneity by magnetic resonance imaging identifies enhanced cardiac arrhythmia susceptibility in patients with left ventricular dysfunction. Circulation. 2007 Apr 17;115(15):2006–2014. doi: 10.1161/CIRCULATIONAHA.106.653568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yan AT, Shayne AJ, Brown KA, Gupta SN, Chan CW, Luu TM, et al. Characterization of the peri-infarct zone by contrast-enhanced cardiac magnetic resonance imaging is a powerful predictor of post-myocardial infarction mortality. Circulation. 2006 Jul 4;114(1):32–39. doi: 10.1161/CIRCULATIONAHA.106.613414. [DOI] [PubMed] [Google Scholar]
- 12.Wu KC, Gerstenblith G, Guallar E, Marine JE, Dalal D, Cheng A, et al. Combined cardiac magnetic resonance imaging and C-reactive protein levels identify a cohort at low risk for defibrillator firings and death. Circ Cardiovasc Imaging. 2012 Mar;5(2):178–186. doi: 10.1161/CIRCIMAGING.111.968024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Perez-David E, Arenal A, Rubio-Guivernau JL, del Castillo R, Atea L, Arbelo E, et al. Noninvasive identification of ventricular tachycardia-related conducting channels using contrast-enhanced magnetic resonance imaging in patients with chronic myocardial infarction: comparison of signal intensity scar mapping and endocardial voltage mapping. J Am Coll Cardiol. 2011 Jan 11;57(2):184–194. doi: 10.1016/j.jacc.2010.07.043. [DOI] [PubMed] [Google Scholar]
- 14.Estner HL, Zviman MM, Herzka D, Miller F, Castro V, Nazarian S, et al. The critical isthmus sites of ischemic ventricular tachycardia are in zones of tissue heterogeneity, visualized by magnetic resonance imaging. Heart Rhythm. 2011 Dec;8(12):1942–1949. doi: 10.1016/j.hrthm.2011.07.027. [DOI] [PubMed] [Google Scholar]
- 15.Ashikaga H, Sasano T, Dong J, Zviman MM, Evers R, Hopenfeld B, et al. Magnetic resonance-based anatomical analysis of scar-related ventricular tachycardia: implications for catheter ablation. Circ Res. 2007;101:939–947. doi: 10.1161/CIRCRESAHA.107.158980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Verma A, Marrouche NF, Schweikert RA, Saliba W, Wazni O, Cummings J, et al. Relationship between successful ablation sites and the scar border zone defined by substrate mapping for ventricular tachycardia post-myocardial infarction. J Cardiovasc Electrophysiol. 2005 May;16(5):465–471. doi: 10.1046/j.1540-8167.2005.40443.x. [DOI] [PubMed] [Google Scholar]
- 17.Desjardins B, Crawford T, Good E, Oral H, Chugh A, Pelosi F, et al. Infarct architecture and characteristics on delayed enhanced magnetic resonance imaging and electroanatomic mapping in patients with postinfarction ventricular arrhythmia. Heart Rhythm. 2009 May;6(5):644–651. doi: 10.1016/j.hrthm.2009.02.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sasaki T, Miller CF, Hansford R, Yang J, Caffo BS, Zviman MM, et al. Myocardial structural associations with local electrograms: a study of postinfarct ventricular tachycardia pathophysiology and magnetic resonance-based noninvasive mapping. Circ Arrhythm Electrophysiol. 2012 Dec;5(6):1081–1090. doi: 10.1161/CIRCEP.112.970699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Piers SR, Tao Q, de Riva Silva M, Siebelink HM, Schalij MJ, van der Geest RJ, et al. CMR-based identification of critical isthmus sites of ischemic and nonischemic ventricular tachycardia. JACC Cardiovasc Imaging. 2014 Jul 9; doi: 10.1016/j.jcmg.2014.03.013. [DOI] [PubMed] [Google Scholar]
- 20.Raymond JM, Sacher F, Winslow R, Tedrow U, Stevenson WG. Catheter ablation for scar-related ventricular tachycardias. Curr Probl Cardiol. 2009 May;34(5):225–270. doi: 10.1016/j.cpcardiol.2009.01.002. [DOI] [PubMed] [Google Scholar]
- 21.Boyé P, Abdel-Aty H, Zacharzowsky U, Bohl S, Schwenke C, van der Geest RJ, et al. Prediction of life-threatening arrhythmic events in patients with chronic myocardial infarction by contrast-enhanced CMR. JACC Cardiovasc Imaging. 2011 Aug;4(8):871–879. doi: 10.1016/j.jcmg.2011.04.014. [DOI] [PubMed] [Google Scholar]
- 22.Andreu D, Berruezo A, Ortiz-Pérez JT, Silva E, Mont L, Borràs R, et al. Integration of 3D electroanatomic maps and magnetic resonance scar characterization into the navigation system to guide ventricular tachycardia ablation. Circ Arrhythm Electrophysiol. 2011 Oct;4(5):674–683. doi: 10.1161/CIRCEP.111.961946. [DOI] [PubMed] [Google Scholar]
- 23.Bogun FM, Desjardins B, Good E, Gupta S, Crawford T, Oral H, et al. Delayed-enhanced magnetic resonance imaging in nonischemic cardiomyopathy: utility for identifying the ventricular arrhythmia substrate. J Am Coll Cardiol. 2009 Mar 31;53(13):1138–1145. doi: 10.1016/j.jacc.2008.11.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Codreanu A, Odille F, Aliot E, Marie PY, Magnin-Poull I, Andronache M, et al. Electroanatomic characterization of post-infarct scars comparison with 3-dimensional myocardial scar reconstruction based on magnetic resonance imaging. J Am Coll Cardiol. 2008 Sep 2;52(10):839–842. doi: 10.1016/j.jacc.2008.05.038. [DOI] [PubMed] [Google Scholar]
- 25.Sasaki T, Miller CF, Hansford R, Zipunnikov V, Zviman MM, Marine JE, et al. Impact of nonischemic scar features on local ventricular electrograms and scar-related ventricular tachycardia circuits in patients with nonischemic cardiomyopathy. Circ Arrhythm Electrophysiol. 2013 Dec;6(6):1139–1147. doi: 10.1161/CIRCEP.113.000159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Grosse-Wortmann L, Macgowan CK, Vidarsson L, Yoo SJ. Late gadolinium enhancement of the right ventricular myocardium: is it really different from the left? J Cardiovasc Magn Reson. 2008 May 8;10:20. doi: 10.1186/1532-429X-10-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Marra MP, Leoni L, Bauce B, Corbetti F, Zorzi A, Migliore F, et al. Imaging study of ventricular scar in arrhythmogenic right ventricular cardiomyopathy: comparison of 3D standard electroanatomical voltage mapping and contrast-enhanced cardiac magnetic resonance. Circ Arrhythm Electrophysiol. 2012 Feb;5(1):91–100. doi: 10.1161/CIRCEP.111.964635. [DOI] [PubMed] [Google Scholar]
- 28.Santangeli P, Hamilton-Craig C, Dello Russo A, Pieroni M, Casella M, Pelargonio G, et al. Imaging of scar in patients with ventricular arrhythmias of right ventricular origin: cardiac magnetic resonance versus electroanatomic mapping. J Cardiovasc Electrophysiol. 2011 Dec;22(12):1359–1366. doi: 10.1111/j.1540-8167.2011.02127.x. [DOI] [PubMed] [Google Scholar]
- 29.Wijffels MC, Kirchhof CJ, Dorland R, Allessie MA. Atrial fibrillation begets atrial fibrillation. A study in awake chronically instrumented goats. Circulation. 1995 Oct 1;92(7):1954–1968. doi: 10.1161/01.cir.92.7.1954. [DOI] [PubMed] [Google Scholar]
- 30.Boldt A, Wetzel U, Lauschke J, Weigl J, Gummert J, Hindricks G, et al. Fibrosis in left atrial tissue of patients with atrial fibrillation with and without underlying mitral valve disease. Heart. 2004;90:400–405. doi: 10.1136/hrt.2003.015347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Jalife J. Mechanisms of persistent atrial fibrillation. Curr Opin Cardiol. 2014 Jan;29(1):20–27. doi: 10.1097/HCO.0000000000000027. [DOI] [PubMed] [Google Scholar]
- 32.Frustaci A, Chimenti C, Bellocci F, Morgante E, Russo MA, Maseri A. Histological substrate of atrial biopsies in patients with lone atrial fibrillation. Circulation. 1997 Aug 19;96(4):1180–1184. doi: 10.1161/01.cir.96.4.1180. [DOI] [PubMed] [Google Scholar]
- 33.Swartz MF, Fink GW, Lutz CJ, Taffet SM, Berenfeld O, Vikstrom KL, et al. Left versus right atrial difference in dominant frequency, K(+) channel transcripts, and fibrosis in patients developing atrial fibrillation after cardiac surgery. Heart Rhythm. 2009 Oct;6(10):1415–1422. doi: 10.1016/j.hrthm.2009.06.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Platonov PG, Mitrofanova LB, Orshanskaya V, Ho SY. Structural abnormalities in atrial walls are associated with presence and persistency of atrial fibrillation but not with age. J Am Coll Cardiol. 2011 Nov 15;58(21):2225–2232. doi: 10.1016/j.jacc.2011.05.061. [DOI] [PubMed] [Google Scholar]
- 35.Mahnkopf C, Badger TJ, Burgon NS, Daccarett M, Haslam TS, Badger CT, et al. Evaluation of the left atrial substrate in patients with lone atrial fibrillation using delayed-enhanced MRI: implications for disease progression and response to catheter ablation. Heart Rhythm. 2010;7:1475–1481. doi: 10.1016/j.hrthm.2010.06.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Spragg DD, Khurram I, Zimmerman SL, Yarmohammadi H, Barcelon B, Needleman M, et al. Initial experience with magnetic resonance imaging of atrial scar and co-registration with electroanatomic voltage mapping during atrial fibrillation: success and limitations. Heart Rhythm. 2012 Dec;9(12):2003–2009. doi: 10.1016/j.hrthm.2012.08.039. [DOI] [PubMed] [Google Scholar]
- 37.Khurram IM, Beinart R, Zipunnikov V, Dewire J, Yarmohammadi H, Sasaki T, et al. Magnetic resonance image intensity ratio, a normalized measure to enable interpatient comparability of left atrial fibrosis. Heart Rhythm. 2014 Jan;11(1):85–92. doi: 10.1016/j.hrthm.2013.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Peters DC, Wylie JV, Hauser TH, Kissinger KV, Botnar RM, Essebag V, et al. Detection of pulmonary vein and left atrial scar after catheter ablation with three-dimensional navigator-gated delayed enhancemnt MR imaging: initial experience. Radiology. 2007 Jun;:243–243. 690–695. doi: 10.1148/radiol.2433060417. [DOI] [PubMed] [Google Scholar]
- 39.Oakes RS, Badger TJ, Kholmovski EG, Akoum N, Burgon NS, Fish EN, et al. Detection and quantification of left atrial structural remodeling with delayed-enhancement magnetic resonance imaging in patients with atrial fibrillation. Circulation. 2009 Apr 7;119(13):1758–1767. doi: 10.1161/CIRCULATIONAHA.108.811877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.McGann C, Akoum N, Patel A, Kholmovski E, Revelo P, Damal K, et al. Atrial fibrillation ablation outcome is predicted by left atrial remodeling on MRI. Circ Arrhythm Electrophysiol. 2014 Feb;7(1):23–30. doi: 10.1161/CIRCEP.113.000689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Marrouche NF, Wilber D, Hindricks G, Jais P, Akoum N, Marchlinski F, et al. Association of atrial tissue fibrosis identified by delayed enhancement MRI and atrial fibrillation catheter ablation: the DECAAF study. JAMA. 2014 Feb 5;311(5):498–506. doi: 10.1001/jama.2014.3. [DOI] [PubMed] [Google Scholar]
- 42.Bisbal F, Guiu E, Cabanas-Grandío P, Berruezo A, Prat-Gonzalez S, Vidal B, et al. CMR-Guided approach to localize and ablate gaps in repeat AF ablation procedure. JACC Cardiovasc Imaging. 2014 Jul;7(7):653–663. doi: 10.1016/j.jcmg.2014.01.014. [DOI] [PubMed] [Google Scholar]
- 43.Vergara GR, Vijayakumar S, Kholmovski EG, Blauer JJ, Guttman MA, Gloschat C, et al. Real-time magnetic resonance imaging-guided radiofrequency atrial ablation and visualization of lesion formation at 3 Tesla. Heart Rhythm. 2011 Feb;8(2):295–303. doi: 10.1016/j.hrthm.2010.10.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Peters DC, Wylie JV, Hauser TH, Nezafat R, Han Y, Woo JJ, et al. Recurrence of atrial fibrillation correlates with the extent of post-procedural late gadolinium enhancement: a pilot study. JACC Cardiovasc Imaging. 2009 Mar;2(3):308–316. doi: 10.1016/j.jcmg.2008.10.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Badger TJ, Daccarett M, Akoum NW, Adjei-Poku YA, Burgon NS, Haslam TS, et al. Evaluation of left atrial lesions after initial and repeat atrial fibrillation ablation: lessons learned from delayed-enhancement MRI in repeat ablation procedures. Circ Arrhythm Electrophysiol. 2010 Jun;3(3):249–259. doi: 10.1161/CIRCEP.109.868356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.McGann C, Kholmovski E, Blauer J, Vijayakumar S, Haslam T, Cates J, et al. Dark regions of no-reflow on late gadolinium enhancement magnetic resonance imaging result in scar formation after atrial fibrillation ablation. J Am Coll Cardiol. 2011 Jul 5;58(2):177–185. doi: 10.1016/j.jacc.2011.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Taclas JE, Nezafat R, Wylie JV, Josephson ME, Hsing J, Manning WJ, et al. Relationship between intended sites of RF ablation and post-procedural scar in AF patients, using late gadolinium enhancement cardiovascular magnetic resonance. Heart Rhythm. 2010 Apr;7(4):489–496. doi: 10.1016/j.hrthm.2009.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hunter RJ, Jones DA, Boubertakh R, Malcolme-Lawes LC, Kanagaratnam P, Juli CF, Davies DW, Peters NS, Baker V, Earley MJ, Sporton S, Davies LC, Westwood M, Petersen SE, Schilling RJ. Diagnostic accuracy of cardiac magnetic resonance imaging in the detection and characterization of left atrial catheter ablation lesions: a multicenter experience. J Cardiovasc Electrophysiol. 2013 Apr;24(4):396–403. doi: 10.1111/jce.12063. [DOI] [PubMed] [Google Scholar]
- 49.Harrison JL, Sohns C, Linton NW, Karim R, Williams SE, Rhode KS, Gill J, Cooklin M, Rinaldi CA, Wright M, Schaeffter T, Razavi RS, O'Neill MD. Repeat Left Atrial Catheter Ablation: Cardiac Magnetic Resonance Prediction of Endocardial Voltage and Gaps in Ablation Lesion Sets. Circ Arrhythm Electrophysiol. 2015 Jan 15; doi: 10.1161/CIRCEP.114.002066. [DOI] [PubMed] [Google Scholar]
- 50.Karim R, Housden RJ, Balasubramaniam M, Chen Z, Perry D, Uddin A, et al. Evaluation of current algorithms for segmentation of scar tissue from late gadolinium enhancement cardiovascular magnetic resonance of the left atrium: an open-access grand challenge. J Cardiovasc Magn Reson. 2013 Dec 20;15:105. doi: 10.1186/1532-429X-15-105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Harrison JL, Jensen HK, Peel SA, Chiribiri A, Grondal AK, Bloch LO, Pedersen SF, Bentzon JF, Kolbitsch C, Karim R, Williams SE, Linton NW, Rhode KS, Gill J, Cooklin M, Aldo RC, Wright M, Kim WY, Schaeffter T, Razavi RS, O’Neill MD. Cardiac magnetic resonance and electroanatomical mapping of acute and chronic atrial ablation injury: a histological validation study. Eur Heart J. 2014;35:1484–1495. doi: 10.1093/eurheartj/eht560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Knowles BR, Batchelor PG, Parish V, Ginks M, Plein S, Razavi R, et al. Pharmacokinetic modeling of delayed gadolinium enhancement in the myocardium. Mag Res Med. 2008;60:1524–1530. doi: 10.1002/mrm.21767. [DOI] [PubMed] [Google Scholar]