Abstract
A theory-driven confirmatory approach comparing diathesis-stress and differential-susceptibility models of gene-environment (GxE) interactions was applied to examine whether 5-HTTLPR genotype moderated the effect of early maternal caregiving on autonomic nervous system (ANS) stress reactivity in 113 adolescents aged 13–17 years. Findings supported a differential-susceptibility, rather than diathesis-stress, framework. Carriers of one or more 5-HTTLPR short alleles (SS/SL carriers) reporting higher-quality caregiving exhibited approach ANS responses to a speech task, whereas those reporting lower-quality caregiving exhibited withdrawal ANS responses. Carriers of two 5-HTTLPR long alleles (LL carriers) were unaffected by caregiving. Findings suggest that 5-HTTLPR genotype and early caregiving in interaction are associated with ANS stress reactivity in adolescents in a “for better and for worse” fashion, and they demonstrate the promise of confirmatory methods for testing GxE interactions.
Keywords: Gene-environment interaction, differential-susceptibility, 5-HTTLPR
Physiological stress reactivity has consequences for emotional and physical health (Boyce et al., 2001; Lovallo, 2011). Early caregiving influences stress responsivity (Luecken & Lemery, 2004), and differences in physiological stress responses also are heritable (Mueller et al., 2012). Genetic predispositions may heighten susceptibility to the effect of caregiving on stress responses (Luecken & Lemery, 2004). Investigating gene-environment (GxE) interactions may elucidate how caregiving shapes stress reactivity.
Two competing hypotheses underlie most GxE research. The diathesis-stress hypothesis proposes that individuals with, versus without, a “vulnerability” allele are more susceptible to the negative impact of adverse environments (Monroe & Simons, 1991). However, in adaptive environments, the vulnerability allele does not affect functioning. The differential susceptibility hypothesis proposes that “susceptibility” alleles make individuals more malleable to environmental influence in general (Bakermans-Kranenburg & van IJzendoorn, 2006; Belsky, Bakermans-Kranenburg, & van IJzendoorn, 2007; Belsky & Pluess, 2009, 2013). Those most susceptible to the negative effects of adverse environments also respond most to the positive effects of supportive environments, responding in a “for better and for worse” (p. 300) manner, depending on the environment (Belsky et al., 2007; Ellis, Boyce, Belsky, Bakermans-Kranenburg, & van IJzendoorn, 2011).
Certain genetic polymorphisms appear to function more like susceptibility than vulnerability alleles (Belsky & Pluess, 2009, 2013), including the short (S) allele of 5-HTTLPR, a polymorphism in the serotonin transporter gene promoter. Compared to the long (L) allele, the S allele has been associated with reduced serotonin transporter protein availability and function (Homberg & Lesch, 2011). Consistent with a differential-susceptibility framework, some studies suggest SS/SL carriers function better than LL carriers under positive conditions and worse under negative conditions (Belsky & Pluess, 2009; Cicchetti & Rogosch, 2012; Taylor et al., 2006). Indeed, a recent meta-analysis documented such a GxE interaction for Caucasian youth (van IJzendoorn, Belsky & Bakermans-Kranenburg, 2012).
Mechanisms underlying differential-susceptibility effects remain limited. Most GxE research applying a differential-susceptibility framework has examined behavioral indicators of complex phenotypes (e.g., depression; Taylor et al., 2006). However, mechanisms related to differential-susceptibility operate at multiple levels of analysis, and growing evidence suggests that cognitive, physiological, and neural processes respond to the environment in a differential-susceptibility related fashion (Belsky & Pluess, 2013). Examining processes related to emotional reactivity is particularly relevant for GxE interactions involving 5-HTTLPR. The S allele is associated with heightened emotional reactivity that may be adaptive or maladaptive depending on the environment (Homberg & Lesch, 2011), and 5-HTTLPR interacts with caregiving to predict physiological stress reactivity in youth (Frigerio et al., 2009; Gilissen, Bakermans-Kranenburg, van IJzendoorn, & Linting, 2008). However, prior studies have lacked a theoretical framework for determining whether physiological processes respond to environmental influences in a differential-susceptibility related manner.
We addressed this limitation by investigating a potential differential-susceptibility related mechanism at the level of physiological processes. We examined autonomic nervous system (ANS) indicators that differentiate between approach and withdrawal responses to acute stress, as specified by the biopsychosocial model of challenge and threat (Blascovich, 2013; Mendes, McCoy, Major, & Blascovich, 2008). Challenge (approach) responses involve sympathetic nervous system activation, increased cardiac output (CO), and decreased vascular resistance. Threat (withdrawal) responses involve sympathetic activation, increased vascular resistance, and low CO reactivity (Mendes et al., 2008); such responses are maladaptive because vascular resistance reduces delivery of oxygenated blood to the brain and peripheral tissues to facilitate responses to acute stress. These differential ANS patterns permit an investigation of whether certain individuals respond to the environment in a “for better and for worse” manner. We previously found that adolescents exposed to child maltreatment exhibited a threat ANS stress response involving blunted CO and increased total peripheral resistance (TPR) reactivity (McLaughlin, Sheridan, Alves, & Mendes, 2014). Although the terms adaptive and maladaptive are frequently applied to challenge and threat responses, respectively, these refer to the consequences of specific ANS patterns following acute stress but not to the underlying developmental processes that generate these responses. From an evolutionarily-informed differential-susceptibility framework, exposure to adversity is thought to shift development toward strategies that are biologically adaptive under adverse conditions, even if they might compromise health (Belsky & Pluess, 2013). Considering the threat response solely as maladaptive fails to appreciate the pressures that led this response to develop. Indeed, threat responses resemble freezing (Mendes, Gray, Mendoza-Denton, Major, & Epel, 2007), which could be adaptive when escape is not possible in threatening situations.
Few studies have directly compared predictions from diathesis-stress and differential-susceptibility models, but a recently-developed confirmatory approach permits direct testing of these models (Belsky, Pluess, & Widaman, 2013; Widaman et al., 2012). Using this approach, Belsky and colleagues (2013) found evidence for differential-susceptibility in predicting children’s social competence and behavioral problems from dopamine receptor D4 variants and childcare quality. We applied this theory-driven confirmatory approach to examine the role of 5-HTTLPR genotype and early maternal caregiving in shaping ANS reactivity in adolescents. We selected ANS measures that span positive and negative response patterns to detect whether individuals responded in a “for better and for worse” fashion. We also selected an early caregiving measure that incorporated supportive and neglectful parenting dimensions. Like some previous GxE studies (e.g., Taylor et al., 2006), caregiving was assessed retrospectively, and thus provides a conservative test of GxE hypotheses given potential retrospective reporting biases. We hypothesized that findings would support the differential-susceptibility model, such that SS/SL carriers would exhibit 1) a challenge response of increased CO and reduced TPR reactivity under higher-quality caregiving and 2) a threat response of blunted CO and increased TPR reactivity under lower-quality caregiving. We hypothesized that ANS reactivity in LL carriers would be less associated with caregiving than in SS/SL carriers.
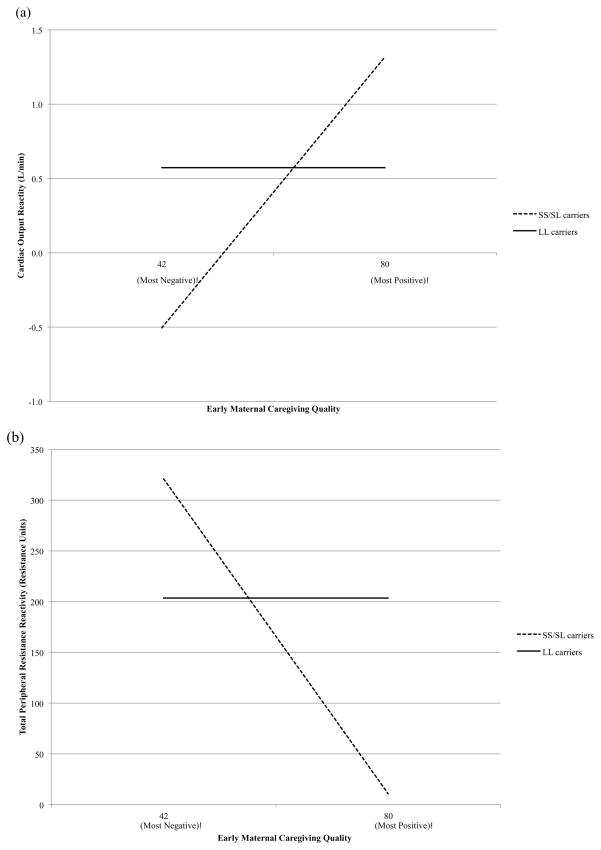
Figure 1.
Predicted values as a function of caregiving for carriers of one or more 5-HTTLPR short alleles (SS/SL carriers) and carriers of two 5-HTTLPR long alleles (LL carriers) based on the Strong Differential-Susceptibility model for (a) cardiac output and (b) total peripheral resistance reactivity during the speech.
Method
Participants
A community-based sample of 168 adolescents aged 13–17 was recruited in Boston and Cambridge, MA (see McLaughlin et al., 2014, for details). The analytic sample comprised 113 individuals with 5-HTTLPR genotype, caregiving, and physiological data. We excluded individuals with a heart murmur, severe cognitive impairment, or a pervasive developmental disorder (n=3), individuals taking medications that influence cardiovascular functioning (n=4), and individuals who did not complete the study (n=7). The sample was 58.4% female (n=66), with a mean age of 14.8 years (SD=1.4). Racial and ethnic composition was 38.9% White (n=44), 18.6% Black (n=21), 20.4% Hispanic (n=23), 8.0% Asian (n=9), and 14.2% Biracial or Other (n=16). Participants included in and excluded from analyses did not differ significantly on age, gender, or White/Non-White race, ps≥.30.
Procedure
Participants provided DNA samples prior to completing a five-minute baseline resting period during which physiological data were acquired. Adolescents completed the Childhood Experiences of Care and Abuse (CECA; Bifulco, Brown, & Harris, 1994) interview, which was used to assess caregiving. Parents/guardians provided informed consent; adolescents provided assent. Participants completed the Trier Social Stress Test (TSST), a widely used stress induction procedure (Kudielka, Hellhammer, & Kirschbaum, 2007). The TSST involves three five-minute periods: a speech preparation period, a speech, and a mental subtraction task in front of evaluators (see McLaughlin et al., 2014, for details). Electrocardiogram (ECG) and cardiac impedance were recorded continuously across each period; blood pressure was recorded during the first and fourth minutes of each period.
Measures
Early Caregiving
The CECA interview (Bifulco et al., 1994; Bifulco, Brown, Lillie, & Jarvis, 1997) is a well-validated early caregiving measure. Inter-rater reliability is excellent, and validation studies suggest high agreement between siblings’ reports (Bifulco et al., 1994; Bifulco et al., 1997; Brown, Craig, Harris, Handley, & Harvey, 2007). We measured early maternal caregiving with 16 items regarding the mother figure who raised the adolescent for the longest period before age 17. Negative caregiving items (e.g., “She was very critical of me”) were reverse-scored and summed with positive caregiving items (e.g., “She was concerned about my worries”) to index caregiving quality (α=.84; these items did not capture physical and sexual abuse). Higher scores indicated higher-quality caregiving. Range in the sample was 42–80; the possible range is 16–80.
Genotyping
Participants provided saliva samples for DNA collection using Oragene® kits (DNA Genotek, Ontario, Canada). DNA extraction and genotyping were performed at the Massachusetts General Hospital Psychiatric and Neurodevelopmental Genetics Unit Core Lab based on a previously modified published protocol (Taylor et al., 2006). Call rate for 5-HTTLPR was 98.8%. Based on meta-analytic findings from GxE interactions with 5-HTTLPR in youth (van IJzendoorn et al., 2012) and evidence that SS/SL carriers exhibit heightened stress sensitivity compared to LL carriers (Homberg & Lesch, 2011), we compared SS/SL vs. LL carriers.
Physiological Measures
Electrocardiogram (ECG) recordings were obtained with a Biopac ECG amplifier (Goleta, CA) using a modified Lead II configuration. Cardiac impedance recordings were obtained with a Bio-Impedance Technology model HIC-2500 impedance cardiograph (Chapel Hill, NC). A Colin Prodigy II oscillometric blood pressure machine (Colin Medical Instruments, San Antonio, TX) measured blood pressure recordings (see McLaughlin et al., 2014). CO for each minute was calculated as heart rate*stroke volume (SV; the amount of blood ejected from the heart on each cardiac cycle). We calculated TPR using the standard formula: (Mean Arterial Pressure/CO)*80 (Sherwood et al., 1990). Data were scored by two independent raters. SV differences greater than 5% were adjudicated by the second author. CO and TPR reactivity were calculated from the first minute of the baseline, speech preparation, speech, and math periods. Various physiological data points could not be scored due to faulty sensors or signal loss or noise (<8% of data), resulting in varying degrees of freedom for CO and TPR reactivity for the different TSST periods.
Analytic Approach
Using the confirmatory approach of Belsky et al. (2013) and Widaman et al., (2012), a priori testing of diathesis-stress and differential-susceptibility GxE interactions employed the following re-parameterized regression model:
D represents 5-HTTLPR genotype (0=LL carriers; 1=SS/SL carriers), and X represents early caregiving. C is the point on X where the regression lines for the gene groups cross. If C falls within the observed range of X, then the interaction is disordinal (supporting differential-susceptibility). If C falls at or beyond the most adaptive value on X, then the interaction is ordinal (supporting diathesis-stress; see Belsky et al., 2013, for details).
Four models were tested to evaluate strong vs. weak versions of differential-susceptibility and diathesis-stress. In the Strong Differential-Susceptibility model, C was estimated and B1, the slope for X for LL carriers, was constrained to zero. Fixing B1 to zero posits that LL carriers are unaffected by caregiving. The Strong Differential-Susceptibility has received prior support (Belsky et al., 2013), and was our preferred model. The Weak Differential-Susceptibility model posits that LL carriers are influenced by the environmental variable but to a lesser degree than SS/SL carriers; thus, C and B1 were estimated. The strong and weak versions of diathesis-stress were similar to those for differential-susceptibility except that, consistent with the diathesis-stress ordinal GxE interaction, C was fixed to the most adaptive value observed on X. Given meta-analytic evidence suggesting small-to-medium effect sizes for associations between environmental factors and developmental problems in youth for SS/SL carriers (van IJzendoorn et al., 2012), power was estimated a priori based on small-to-medium effect size, alpha of .05, and sample size of 113. Power was low (0.60); thus, this study is best viewed as a hypothesis-generating investigation.
Results
Descriptive Statistics
Participant characteristics are presented in Table 1. Genotype frequencies did not deviate from Hardy-Weinberg equilibrium, χ2(1)=1.54, p=.21. SS/SL and LL carriers did not differ in age, gender, White/Non-White race, baseline cardiac measures, or caregiving, ps>.22. No significant gene-environment correlation between SS/SL carrier status and caregiving was observed, r=.04, p=.67. As reported previously, the TSST resulted in significant sympathetic nervous system activation (McLaughlin et al., 2014), a requirement for testing the threat/challenge distinction (Mendes et al., 2008).
Table 1.
Participant characteristics based on 5-HTTLPR genotype (N=113)
|
5-HTTLPR Genotype
|
|||
|---|---|---|---|
| LL (n=39) | SS/SL (n=74) | ||
| % (n) | % (n) | ||
| Female | 51.3 (20) | 62.2 (46) | p=.27 |
| White | 35.9 (14) | 40.5 (30) | p=.63 |
| M (SD) | M (SD) | ||
| Age | 14.6 (1.4) | 15.0 (1.3) | p=.23 |
| Baseline cardiac output | 5.39 (1.95) | 5.55 (2.08) | p=.70 |
| Baseline total peripheral resistance | 1324.38 (569.59) | 1291.14 (512.25) | p=.75 |
| Early caregiving quality | 70.28 (7.12) | 68.50 (8.57) | p=.27 |
Note. LL=carriers of two 5-HTTLPR long alleles. SS/SL=carriers of one or more 5-HTTLPR short alleles.
Differential-Susceptibility vs. Diathesis-Stress Models
Results of the Strong and Weak Differential-Susceptibility and Diathesis-Stress models for cardiac output (CO) and total peripheral resistance (TPR) reactivity during the TSST periods are presented in Tables 2 and 3. In addition to examining estimates of the cross-over point C, support for a given model was based on R2 values; the model with the highest R2 value best represented the data.
Table 2.
Predicting cardiac output reactivity from caregiving and SS/SL carrier status
| Speech preparation (n=113) | ||||
|---|---|---|---|---|
| Parameter | Differential-Susceptibility | Diathesis-Stress | ||
| Strong | Weak | Strong | Weak | |
| B0 | 0.35 (0.17) [0.02, 0.68] | 0.34 (0.19) [−0.04, 0.73] | 0.51 (0.13) [0.26, 0.77] | 0.63 (0.17) [0.30, 0.96] |
| B1 | 0.00a | 0.004 (0.02) [−0.04, 0.05] | 0.00a | 0.02 (0.02) [−0.02, 0.06] |
| C | 68.78 (5.69) [57.51, 80.06] | 68.60 (6.52) [55.69, 81.52] | 80.00a | 80.00a |
| B3 | 0.04 (0.01) [0.01, 0.06] | 0.04 (0.01) [0.01, 0.06] | 0.02 (0.01) [0.000, 0.04] | 0.03 (0.01) [0.004, 0.05] |
| R2 | .056 | .056 | .035 | .046 |
| Fb | 0.03 | 2.39 | ||
| df | 1,109 | 1,110 | ||
| Fc | 1.20 | 1.10 | ||
| df | 2,109 | 1,109 | ||
| Speech (n=109) | ||||
| Parameter | Differential-Susceptibility | Diathesis-Stress | ||
|---|---|---|---|---|
| Strong | Weak | Strong | Weak | |
| B0 | 0.57 (0.18) [0.23, 0.92] | 0.48 (0.35) [−0.21, 1.17] | 0.87 (0.14) [0.59, 1.15] | 1.11 (0.18) [0.76, 1.47] |
| B1 | 0.00a | 0.01 (0.03) [−0.04, 0.06] | 0.00a | 0.04 (0.02) [0.003, 0.08] |
| C | 64.47 (4.67) [55.22, 73.72] | 62.61 (8.33) [46.10, 79.12] | 80.00a | 80.00a |
| B3 | 0.05 (0.02) [0.02, 0.08] | 0.05 (0.02) [0.02, 0.08] | 0.02 (0.01) [−0.001, 0.05] | 0.04 (0.01) [0.01, 0.06] |
| R2 | .095 | .097 | .033 | .073 |
| Fb | 0.23 | 7.29* | ||
| df | 1,105 | 1,106 | ||
| Fc | 3.73* | 2.79+ | ||
| df | 2,105 | 1,105 | ||
| Math task (n=109) | ||||
| Parameter | Differential-Susceptibility | Diathesis-Stress | ||
|---|---|---|---|---|
| Strong | Weak | Strong | Weak | |
| B0 | 0.41 (0.17) [0.08, 0.75] | 0.24 (0.69) [−1.13, 1.61] | 0.61 (0.13) [0.34, 0.87] | 0.77 (0.17) [0.44, 1.11] |
| B1 | 0.00a | 0.01 (0.02) [−0.04, 0.06] | 0.00a | 0.03 (0.02) [−0.01, 0.07] |
| C | 62.89 (7.90) [47.23, 78.56] | 56.77 (27.17) [2.89, 110.64] | 80.00a | 80.00a |
| B3 | 0.03 (0.01) [0.000, 0.06] | 0.03 (0.02) [0.000, 0.06] | 0.01 (0.01) [−0.01, 0.04] | 0.02 (0.01) [−0.004, 0.05] |
| R2 | .040 | .043 | .010 | .033 |
| Fb | 0.29 | 3.29+ | ||
| df | 1,105 | 1,106 | ||
| Fc | 1.78 | 1.03 | ||
| df | 2,105 | 1,105 | ||
Note. SS/SL= carriers of one or more 5-HTTLPR short alleles. Standard errors presented in parentheses; 95% confidence intervals presented in brackets.
Parameter constrained to reported value; standard error is not applicable.
Difference in R2 for model vs. Strong Differential-Susceptibility.
Difference in R2 for model vs. Weak Differential-Susceptibility.
p<.05,
p<.10
Table 3.
Predicting total peripheral resistance reactivity from caregiving and SS/SL carrier status
| Speech preparation (n=112) | ||||
|---|---|---|---|---|
| Parameter | Differential-Susceptibility | Diathesis-Stress | ||
| Strong | Weak | Strong | Weak | |
| B0 | 138.15 (41.94) [55.04, 221.27] | 130.10 (43.15) [44.57, 215.62] | 104.16 (32.54) [39.68, 168.64] | 86.75 (41.87) [3.77, 169.73] |
| B1 | 0.00a | 1.40 (5.99) [−10.48, 13.28] | 0.00a | −2.99 (4.51) [−11.94, 5.95] |
| C | 62.89 (11.30) [40.48, 85.29] | 64.52 (10.14) [44.42, 84.61] | 80.00a | 80.00a |
| B3 | −4.95 (3.61) [−12.10, 2.20] | −4.95 (3.62) [−12.13, 2.23] | −2.09 (2.84) [−7.71, 3.54] | −3.07 (3.21) [−9.43, 3.29] |
| R2 | .020 | .020 | .005 | .009 |
| Fb | 0.05 | 1.64 | ||
| df | 1,108 | 1, 109 | ||
| Fc | 0.84 | 1.24 | ||
| df | 2,108 | 1,108 | ||
| Speech (n=104) | ||||
| Parameter | Differential-Susceptibility | Diathesis-Stress | ||
|---|---|---|---|---|
| Strong | Weak | Strong | Weak | |
| B0 | 203.52 (40.22) [123.74, 283.30] | 205.32 (90.57) [25.63, 385.01] | 124.46 (32.14) [60.71, 188.21] | 71.45 (40.84) [−9.56, 152.46] |
| B1 | 0.00a | −0.13 (5.79) [−11.61, 11.35] | 0.00a | −8.89 (4.33) [−17.47, −0.30] |
| C | 56.39 (7.93) [40.66, 72.11] | 56.17 (12.81) [30.74, 81.59] | 80.00a | 80.00a |
| B3 | −8.17 (3.38) [−14.87, −1.46] | −8.17 (3.40) [−14.90, 1.43] | −1.83 (2.78) [−7.35, 3.69] | −4.78 (3.10) [−10.92, 1.36] |
| R2 | .089 | .089 | .004 | .044 |
| Fb | 0.001 | 9.43** | ||
| df | 1,100 | 1, 101 | ||
| Fc | 4.67* | 4.95* | ||
| df | 2,100 | 1,100 | ||
| Math task (n=106) | ||||
| Parameter | Differential-Susceptibility | Diathesis-Stress | ||
|---|---|---|---|---|
| Strong | Weak | Strong | Weak | |
| B0 | 139.87 (38.69) [63.14, 216.60] | 136.97 (38.37) [60.87, 213.06] | 104.61 (30.21) [44.71, 164.51] | 84.31 (38.94) [7.09, 161.53] |
| B1 | 0.00a | 0.85 (5.48) [−10.03, 11.72] | 0.00a | −3.43 (4.14) [−11.64, 4.78] |
| C | 66.30 (7.54) [51.35, 81.25] | 66.75 (7.15) [52.57, 80.93] | 80.00a | 80.00a |
| B3 | −6.49 (3.32) [−13.08, 0.11] | −6.49 (3.34) [−13.11, 0.14] | −3.52 (2.63) [−8.73, 1.70] | −4.64 (2.96) [−10.52, 1.23] |
| R2 | .036 | .037 | .017 | .023 |
| Fb | 0.02 | 2.09 | ||
| df | 1,102 | 1,103 | ||
| Fc | 1.05 | 1.41 | ||
| df | 2,102 | 1,102 | ||
Note. SS/SL= carriers of one or more 5-HTTLPR short alleles. Standard errors presented in parentheses; 95% confidence intervals presented in brackets.
Parameter constrained to reported value; standard error is not applicable.
Difference in R2 for model vs. Strong Differential-Susceptibility.
Difference in R2 for model vs. Weak Differential-Susceptibility.
p<.01,
p<.05
The Strong Differential-Susceptibility model received the strongest support for CO and TPR reactivity based on estimates of C and R2. Furthermore, for both outcomes, evidence for strong differential-susceptibility was greatest for reactivity during the speech. For CO reactivity to the speech, the estimate of C for the Strong Differential-Susceptibility model (64.47) was within the observed range of caregiving (42–80), and it fell near the mean (69.12). Moreover, the 95% confidence interval (CI) for C fell within the observed range of caregiving and did not include the most adaptive value (80). These results for CO reactivity to the speech suggested a disordinal interaction. Furthermore, the 95% CI for the slope of caregiving on CO reactivity during the speech for SS/SL carriers did not include zero. SS/SL carriers exhibited reduced CO reactivity (associated with a threat ANS response) to the speech under lower-quality caregiving but increased CO reactivity (associated with a challenge ANS response) to the speech under higher-quality caregiving (see Figure 1a). Associations between caregiving and CO reactivity among SS/SL carriers for speech preparation and math were in the same direction as for the speech, although the 95% CI for the slope of caregiving on CO reactivity during math included zero.
The Strong Differential-Susceptibility model explained 9.5% of the variance in CO reactivity during the speech. The four-parameter Weak Differential-Susceptibility model did not explain significantly more variance than the three-parameter Strong Differential-Susceptibility model (p=.63), supporting the more parsimonious version. The Strong Differential-Susceptibility Model accounted for more variance than both Diathesis-Stress models, and the Strong Diathesis-Stress model explained significantly less variance than the Strong Differential-Susceptibility model (p=.01). A formal significance test comparing the Weak Diathesis-Stress and Strong Differential-Susceptibility models was not possible because these models were not nested.
The Strong Differential-Susceptibility Model was also the most strongly supported model for TPR reactivity, particularly during the speech (see Table 3). For this model, the 95% CI for C fell entirely within the observed range of caregiving and did not include the most adaptive value. Again, these results supported a disordinal GxE interaction. The 95% CI for the slope of caregiving on TPR reactivity during the speech for SS/SL carriers did not include zero. SS/SL carriers exhibited elevated TPR reactivity (associated with a threat ANS response) to the speech under lower-quality caregiving but reduced TPR reactivity (associated with a challenge ANS response) under higher-quality caregiving (see Figure 1b).
Population Stratification
Given the racial/ethnic diversity in our sample, we inferred underlying population structure from 40 ancestry-informative markers to address population stratification (i.e., the presence of systematic differences in allele frequencies as a function of subpopulations in the sample; Pritchard & Rosenberg, 1999). We ran a standard regression for detecting GxE interactions for CO and TPR reactivity to the speech, covarying the first two principal components from a principal components analysis of the ancestry-informative markers, along with their two-way interactions with 5-HTTLPR genotype and early caregiving (cf. Keller, 2014). Estimates of C remained within the observed range of caregiving with these covariates (C=65.31 for CO and 49.37 for TPR). White/Non-White race was not significantly associated with SS/SL carrier status, χ2(1)=0.23, p=.63, or CO or TPR, ps>.33, further suggesting that results were not due to confounding effects of race.
Discussion
Using a confirmatory, theory-driven approach, we demonstrated that the 5-HTTLPR S allele functioned as a marker of differential-susceptibility in predicting ANS stress reactivity. This is the first study to support a differential-susceptibility GxE interaction model in predicting ANS reactivity in adolescents using a recently-developed, theory-driven, confirmatory technique (Belsky et al., 2013; Widaman et al., 2012). Building on previous research (Belsky et al., 2013), we found support for the Strong Differential-Susceptibility model in predicting CO and TPR reactivity to the TSST speech from 5-HTTLPR and early maternal caregiving.
By examining ANS reactivity, our findings extend the work on 5-HTTLPR as a differential-susceptibility marker. Most differential-susceptibility-informed research has examined 5-HTTLPR as a predictor of behavioral markers of complex phenotypes (e.g., depression), but differential-susceptibility mechanisms are postulated to operate at multiple levels, including physiological processes (Belsky & Pluess, 2013). ANS reactivity represents a plausible intermediate phenotype linking 5-HTTLPR variation to individual differences in behavior. The S allele has been associated with increased emotional reactivity to environmental stimuli, which may have positive or negative consequences depending on the context (Homberg & Lesch, 2011). Our finding that SS/SL carriers exhibited differential ANS stress responses based on caregiving suggests a physiological mechanism underlying differences in emotional reactivity. Our estimates of the interaction cross-over point were consistent with differential-susceptibility for CO and TPR reactivity across all TSST periods but were most robust for reactivity to the speech. Previous studies have found public speaking tasks, including the TSST speech, to elicit particularly strong ANS reactivity (al’Absi et al., 1997; Kirschbaum, Pirke, Hellhammer, 1993). Our findings provide preliminary evidence that differential-susceptibility related differences in ANS reactivity in adolescents may be especially likely under interpersonally-salient evaluative conditions, although replication of these results in larger samples is needed. Our results differ somewhat from a study in children that found lowest stress reactivity to the TSST among LL carriers and secure parental attachment (Gilissen et al., 2008). However, Gilissen et al. (2008) did not consider differential-susceptibility when testing GxE interactions, and differences between the two studies, including environmental measures, physiological markers, and analytic approaches, make direct comparison difficult.
Despite these novel findings, we acknowledge several limitations. First, the cross-sectional design and retrospective reporting of caregiving preclude assessments of causality. Furthermore, although high validity of caregiving reports on the CECA interview has been documented in studies of siblings (e.g., Brown et al., 2007), retrospective recall of caregiving is a limitation. Prospective research is needed, particularly for elucidating directionality of effects. Second, although our caregiving measure incorporated positive and negative aspects, the observed range did not include the lowest possible caregiving scores. We hypothesize that findings would be more pronounced for even lower-quality caregiving, but research is required to test this prediction. Moreover, our measure captured maternal caregiving, and thus only partially reflects early caregiving experiences. Third, our sample size is relatively small for genetics studies and power was low; replication with larger samples is needed. However, we did not encounter issues with model non-convergence, which is an issue when interactions are absent or small (Widaman et al., 2012). Fourth, we were unable to examine the triallelic classification of 5-HTTLPR based on rs25531, a single nucleotide polymorphism that may modify a subset of L alleles, such that LG, but not LA, alleles function similarly to S alleles (Hu et al., 2005). By grouping LG and LA alleles together, lack of consideration of this triallelic classification of 5-HTTLPR would likely bias results toward the null. Moreover, in van IJzendoorn et al.’s (2012) meta-analysis of 5-HTTLPR moderation of environments on developmental outcomes in youth, biallelic vs. triallelic genotyping was not a significant moderator of effect size. Fifth, it is possible that another genetic marker in linkage disequilibrium with 5-HTTLPR accounted for our findings. We also did not consider variants other than 5-HTTLPR that could serve as differential-susceptibility markers. Sixth, population stratification is a concern given the racial/ethnic heterogeneity of our sample, but results were similar when we modeled underlying population structure using ancestry-informative markers, and race was not differentially related to 5-HTTLPR genotype, CO, or TPR. Further research using larger samples with mixed ancestry is needed to clarify whether these associations hold across race and ethnicity, especially given that 5-HTTLPR may be a differential-susceptibility marker primarily for Caucasian individuals (van IJzendoorn et al., 2012).
Despite these limitations, this study has several notable strengths. Incorporation of data across multiple methodologies and levels of analysis, inclusion of environmental and outcome variables covering a wide range of functioning, use of a well-established theoretical model to distinguish between acutely adaptive and maladaptive patterns of physiological reactivity (Blascovich, 2013), and use of a confirmatory theory-driven analytic approach make this work a novel contribution to the literature.
In sum, our findings indicate that SS/SL carriers exhibit relatively adaptive ANS acute stress responses in the presence of supportive early caregiving and relatively maladaptive responses in the presence of lower-quality caregiving. Differential ANS responses to stress may be one mechanism by which the early environment contributes to subsequent health, and 5-HTTLPR genotype may influence who is most susceptible to early experience.
Acknowledgments
This research was funded by grants from the National Institutes of Health (K01-MH092526 to Dr. McLaughlin; K01-MH092555 to Dr. Sheridan; T32-DA031099 sponsoring Dr. Walsh).
References
- al’Absi M, Bongard S, Buchanan T, Pincomb GA, Licinio J, Lovallo WR. Cardiovascular and neuroendocrine adjustment to public speaking and mental arithmetic stressors. Psychophysiology. 1997;34:266–275. doi: 10.1111/j.1469-8986.1997.tb02397.x. [DOI] [PubMed] [Google Scholar]
- Bakermans-Kranenburg MJ, van IJzendoorn MH. Gene-environment interaction of the dopamine D4 receptor (DRD4) and observed maternal insensitivity predicting externalizing behavior in preschoolers. Developmental Psychobiology. 2006;48:406–409. doi: 10.1002/dev.20152. [DOI] [PubMed] [Google Scholar]
- Belsky J, Bakermans-Kranenburg MJ, van IJzendoorn MH. For better and for worse: Differential susceptibility to environmental influences. Current Directions in Psychological Science. 2007;16:300–304. doi: 10.1111/j.1467-8721.2007.00525.x. [DOI] [Google Scholar]
- Belsky J, Pluess M. Beyond diathesis stress: Differential susceptibility to environmental influences. Psychological Bulletin. 2009;135:885–908. doi: 10.1037/a0017376. [DOI] [PubMed] [Google Scholar]
- Belsky J, Pluess M. Beyond risk, resilience, and dysregulation: Phenotypic plasticity and human development. Development and Psychopathology. 2013;25:1243–1261. doi: 10.1017/S095457941300059X. [DOI] [PubMed] [Google Scholar]
- Belsky J, Pluess M, Widaman KF. Confirmatory and competitive evaluation of alternative gene-environment interaction hypotheses. Journal of Child Psychology and Psychiatry. 2013;54:1135–1143. doi: 10.1111/jcpp.12075. [DOI] [PubMed] [Google Scholar]
- Bifulco A, Brown GW, Harris TO. Childhood Experiences of Care and Abuse (CECA): A retrospective interview measure. Journal of Child Psychology and Psychiatry. 1994;35:1419–1435. doi: 10.1111/j.1469-7610.1994.tb01284.x. [DOI] [PubMed] [Google Scholar]
- Bifulco A, Brown GW, Lillie A, Jarvis J. Memories of childhood neglect and abuse: Corroboration in a series of sisters. Journal of Child Psychology and Psychiatry. 1997;38:365–374. doi: 10.1111/j.1469-7610.1997.tb01520.x. [DOI] [PubMed] [Google Scholar]
- Blascovich J. Challenge and threat. In: Elliot AJ, editor. Handbook of approach and avoidance motivation. New York, NY: Psychology Press; 2013. pp. 431–446. [Google Scholar]
- Boyce WT, Quas J, Alkon A, Smider NA, Essex MJ, Kupfer DJ. Autonomic reactivity and psychopathology in middle childhood. The British Journal of Psychiatry. 2001;179:144–150. doi: 10.1192/bjp.179.2.144. [DOI] [PubMed] [Google Scholar]
- Brown GW, Craig TKJ, Harris TO, Handley RV, Harvey AL. Retrospective measures of early maltreatment and depressive episodes using the Childhood Experience of Care and Abuse (CECA) instrument—A life-course study of adult chronic depression—2. Journal of Affective Disorders. 2007;103:217–224. doi: 10.1016/j.jad.2007.06.003. [DOI] [PubMed] [Google Scholar]
- Cicchetti D, Rogosch FA. Gene x environment interaction and resilience: Effects of child maltreatment and serotonin, corticotropin releasing hormone, dopamine, and oxytocin genes. Development and Psychopathology. 2012;24:411–427. doi: 10.1017/S0954579412000077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellis BJ, Boyce WT, Belsky J, Bakermans-Kranenburg MJ, van IJzendoorn MH. Differential susceptibility to the environment: An evolutionary-neurodevelopmental theory. Development and Psychopathology. 2011;23:7–28. doi: 10.1017/S0954579410000611. [DOI] [PubMed] [Google Scholar]
- Frigerio A, Ceppi E, Rusconi M, Giorda R, Raggi ME, Fearon P. The role played by the interaction between genetic factors and attachment in the stress response in infancy. Journal of Child Psychology and Psychiatry. 2009;50:1513–1522. doi: 10.1111/j.1469-7610.2009.02126.x. [DOI] [PubMed] [Google Scholar]
- Gilissen R, Bakermans-Kranenburg MJ, van IJzendoorn MH, Linting M. Electrodermal reactivity during the Trier Social Stress Test for Children: Interaction between the serotonin transporter polymorphism and children’s attachment representation. Developmental Psychobiology. 2008;50:615–625. doi: 10.1002/dev.20314. [DOI] [PubMed] [Google Scholar]
- Homberg JR, Lesch KP. Looking on the bright side of serotonin transporter gene variation. Biological Psychiatry. 2011;69:513–519. doi: 10.1016/j.biopsych.2010.09.024. [DOI] [PubMed] [Google Scholar]
- Hu X, Oroszi G, Chun J, Smith TL, Goldman D, Schuckit MA. An expanded evaluation of the relationship of four alleles to the level of response to alcohol and the alcoholism risk. Alcoholism: Clinical and Experimental Research. 2005;29:8–16. doi: 10.1097/01.ALC.0000150008.68473.62. [DOI] [PubMed] [Google Scholar]
- Keller MC. Gene x environment interaction studies have not properly controlled for potential confounders: The problem and the (simple) solution. Biological Psychiatry. 2014;75:18–24. doi: 10.1016/j.biopsych.2013.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirschbaum C, Pirke KM, Hellhammer DH. The ‘Trier Social Stress Test’ - a tool for investigating psychobiological stress responses in a laboratory setting. Neuropsychobiology. 1993;28:76–81. doi: 10.1159/000119004. [DOI] [PubMed] [Google Scholar]
- Kudielka BM, Hellhammer DH, Kirschbaum C. Ten years of research with the Trier Social Stress Test-revisited. In: Harmon-Jones E, Winkielman P, editors. Social neuroscience: Integrating biological and psychological explanations of social behavior. New York, NY: Guilford Press; 2007. pp. 56–83. [Google Scholar]
- Lovallo WR. Do low levels of stress reactivity signal poor states of health? Biological Psychiatry. 2011;86:121–128. doi: 10.1016/j.biopsych.2010.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luecken LJ, Lemery KS. Early caregiving and physiological stress responses. Clinical Psychology Review. 2004;24:171–191. doi: 10.1016/j.cpr.2004.01.003. [DOI] [PubMed] [Google Scholar]
- McLaughlin KA, Sheridan MA, Alves S, Mendes WB. Child maltreatment and autonomic nervous system reactivity to psychosocial stress: Identifying maladaptive reactivity patterns using the biopsychosocial model of challenge and threat. Psychosomatic Medicine. 2014 doi: 10.1097/PSY.0000000000000098. Advance online publication. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mendes WB, Gray H, Mendoza-Denton R, Major B, Epel ES. Why egalitarianism might be good for your health: Physiological thriving during intergroup interactions. Psychological Science. 2007;18:991–998. doi: 10.1111/j.1467-9280.2007.02014.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mendes WB, McCoy S, Major B, Blascovich J. How attributional ambiguity shapes physiological and emotional responses to social rejection and acceptance. Journal of Personality and Social Psychology. 2008;94:278–291. doi: 10.1037/0022-3514.94.2.278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monroe SM, Simons AD. Diathesis-stress theories in the context of life stress research: Implications for the depressive disorders. Psychological Bulletin. 1991;110:406–425. doi: 10.1037//0033-2909.110.3.406. [DOI] [PubMed] [Google Scholar]
- Mueller A, Strahler J, Armbruster D, Lesch KP, Brocke B, Kirschbaum C. Genetic contributions to acute autonomic stress responsiveness in children. International Journal of Psychophysiology. 2012;83:302–308. doi: 10.1016/j.ijpsycho.2011.11.007. [DOI] [PubMed] [Google Scholar]
- Pritchard JK, Rosenberg NA. Use of unlinked genetic markers to detect population stratification in association studies. American Journal of Human Genetics. 1999;65:220–228. doi: 10.1086/302449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sherwood A, Allen MT, Fahrenberg J, Kelsey RM, Lovallo WR, van Dooren LJP. Methodological guidelines for impedance cardiography. Psychophysiology. 1990;27:1–23. doi: 10.1111/j.1469-8986.1990.tb02171.x. [DOI] [PubMed] [Google Scholar]
- Taylor SE, Way BM, Welch WT, Hilmert CJ, Lehman BJ, Eisenberger NI. Early family environment, current adversity, the serotonin transporter promoter polymorphism, and depressive symptomatology. Biological Psychiatry. 2006;60:671–676. doi: 10.1016/j.biopsych.2006.04.019. [DOI] [PubMed] [Google Scholar]
- van IJzendoorn MH, Belsky J, Bakermans-Kranenburg MJ. Serotonin transporter genotype 5HTTLPR as a marker of differential susceptibility? A meta-analysis of child and adolescent gene-by-environment studies. Translational Psychiatry. 2012;2:e147. doi: 10.1038/tp.2012.73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Widaman KF, Helm JL, Castro-Schilo L, Pluess M, Stallings MC, Belsky J. Distinguishing ordinal and disordinal interactions. Psychological Methods. 2012;17:615–622. doi: 10.1037/a0030003. [DOI] [PMC free article] [PubMed] [Google Scholar]