Abstract
Background and objectives
Lifetime risk estimates of CKD can be used effectively in public education campaigns. This study sought to estimate lifetime risk of incident CKD stage 3 and higher in Iceland for people without CKD by the age of 45 years.
Design, setting, participants, & measurements
This was a prospective cohort study with longitudinal creatinine measurements of residents in Reykjavik, Iceland, from 1967 to 2005. CKD was ascertained by two consecutive eGFR measurements <60 ml/min per 1.73 m2, development of treated kidney failure, one eGFR<60 ml/min per 1.73 m2 if the participant died before the next evaluation, or one eGFR<45 ml/min per 1.73 m2 if it was the last eGFR.
Results
Mean follow-up was 25 (SD 10) years. Of the study participants, 727 (19%) developed the outcome and 942 (24%) died first. By age 85 years, the lifetime risks for 45-year-old women and men without prevalent CKD were 35.8% (95% confidence interval [95% CI], 32.7 to 38.9) and 21.3% (95% CI, 18.7 to 23.8), respectively. Risk was higher in individuals with a lower eGFR, hypertension, and a higher body mass index. Lifetime risk for higher stages of CKD 3b and 4 were less common than stage 3a; by age 85 years, the lifetime risks for CKD stages 3a, 3b, and 4 in women were 38.5% (95% CI, 25.8 to 51.1), 19.4% (95% CI, 8.9 to 29.9), and 3.6% (95% CI, 2.2 to 5.0), respectively.
Conclusions
The lifetime risk of developing CKD stage 3 or higher is substantial, emphasizing the importance of strategies to prevent development of CKD throughout the course of life. Estimates are lower than reported using single estimates of GFR, emphasizing the importance of confirming estimates of reduced GFR in studies of CKD.
Keywords: CKD, GFR, epidemiology and outcomes, lifetime risk, elderly
Introduction
Earlier stages of CKD, even before the development of kidney failure, are associated with substantial morbidity and risk (1–6). Public awareness of CKD is necessary to identify modifiable risk factors to prevent kidney disease development and progression; however, awareness remains low (7). Lifetime risk estimates account for competing causes of death, thereby providing estimates of the absolute risk of developing a disease during one’s remaining lifespan, and these estimates can be used more effectively in public education campaigns.
Development of CKD is often asymptomatic and is usually identified by laboratory measurements. CKD is defined as a GFR<60 ml/min per 1.73 m2 or the presence of kidney damage persistent for >3 months or another measure of chronicity (8). In epidemiologic studies, serum creatinine is used to estimate the GFR, but it is not measured with sufficient frequency to replicate clinical practice, making it difficult to ascertain chronicity. Previous studies of lifetime risk used simulation methods based on cross-sectional data of CKD prevalence estimates using a single eGFR (9–11). In a community-based sample of white men and women beginning in middle age without CKD and followed until old age, we used longitudinal measurements of creatinine to estimate the lifetime risk of developing CKD ascertained by two measurements of GFR<60 ml/min per 1.73 m2.
Materials and Methods
Study Population
The Reykjavik Study is a prospective population-based cohort study of approximately 19,000 people (age 31–59 years) and was established in 1967 to investigate patterns of cardiovascular disease and its risk factors in Iceland (12). The cohort was divided randomly into six groups. The B group was designated for longitudinal follow-up and was invited to six visits (12); visits occurred every 3–7 years until 1996 (13) and subsequently as part of a follow-up study (the Age, Gene/Environment, Susceptibility [AGES] Reykjavik Study) between 2002 and 2005 (13). Participants from Reykjavik Study group B who attended at least two examinations, or who died after their first follow-up examination, and who had an eGFR>60 ml/min per 1.73 m2 at baseline or subsequently developed ESRD, were included in the current analysis.
Outcome
Our primary goal was to ascertain CKD stage 3 or higher, which would exclude participants with transient fluctuations in GFR. Our primary method to ascertain CKD used the following criteria: (1) two consecutive measurements of eGFR<60 ml/min per 1.73 m2, (2) development of treated kidney failure with dialysis or transplant (ESRD), (3) one measurement of eGFR<60 ml/min per 1.73 m2 if the participant died before the next measurement, and (4) one measurement of eGFR<45 ml/min per 1.73 m2 if it was the last eGFR. In sensitivity analyses, we tested alternative ascertainment methods varying one or more of these above parameters (Supplemental Table 1). In all, participants who developed ESRD were considered to have CKD, even if they developed it without documentation of eGFR<60 ml/min per 1.73 m2. The first method required two consecutive measurements of eGFR<60 ml/min per 1.73 m2 or one measurement of eGFR<60 ml/min per 1.73 m2 if it was at the last measurement of eGFR owing to death or lack of subsequent creatinine measurement. The second required eGFR<60 ml/min per 1.73 m2 on two occasions or eGFR<60 ml/min per 1.73 m2 on the first measurement if the participant died before the second measurement. The third required eGFR<45 ml/min per 1.73 m2 if it was at the last measurement of eGFR as a result of death or lack of subsequent creatinine measurement. The fourth required only one measurement of eGFR<60 ml/min per 1.73 m2 irrespective of the results of follow-up measurements and was used for comparison with previous reports.
We also evaluated the lifetime risk of CKD stages 3a, 3b, and 4 (eGFR of 45–59, 30–44, and <30 ml/min per 1.73 m2, respectively). Given the small number of patients who reached the later stages, we were not able to compare results using one or more values for eGFR, and we included all individuals with at least one measurement of eGFR<60 ml/min per 1.73 m2.
eGFR
Serum creatinine was assayed using end point or kinetic Jaffe reaction (Technicon Autoanalyzer I 1967–1986, SMA-6 1986–1988, Cobas-Mira analyzer 1988–2005) (14). Samples were not available from the Reykjavik Study for calibration to reference materials. Creatinine values at the AGES–Reykjavik Study visit (2002–2006) are traceable to National Institute of Standards and Technology creatinine standard reference material 909b. eGFR was calculated using the CKD in Epidemiology (CKD-EPI) study equation (15). We calculated the change in eGFR over time using a random-effects mixed model with random intercept and slope. We calculated the difference in eGFR slopes between men and women and per 5 years higher baseline age.
Statistical Analyses
Sex-specific lifetime risk was calculated using age as the time scale. The time was left truncated at entry into the study and right censored at death or end of follow-up, which accounts for the differing length of follow-up and initiation of the observation period at different ages. Lifetime risk was estimated from 45 years and then at 10-year intervals to 75 and at 80 years. When referring to risk of CKD in those without prevalent CKD at ages >45 years, we use the term cumulative risk. In sensitivity analyses, risk was estimated without adjustment for competing risk of death. The risk for incident CKD stage 3a and stage 3b was calculated by subtracting the cumulative risk of CKD stage 3a or higher and CKD stage 3b or higher, and CKD stage 3b or higher and CKD stage 4 or higher, respectively. The 95% confidence intervals (95% CIs) for these calculated cumulative risk estimates were derived using 2000 bootstrap resamples. Subgroup analyses according to baseline hypertension, body mass index (BMI), and smoking status as well as level of eGFR were performed. Analyses were performed using published SAS macro (16) and R (17) software.
Results
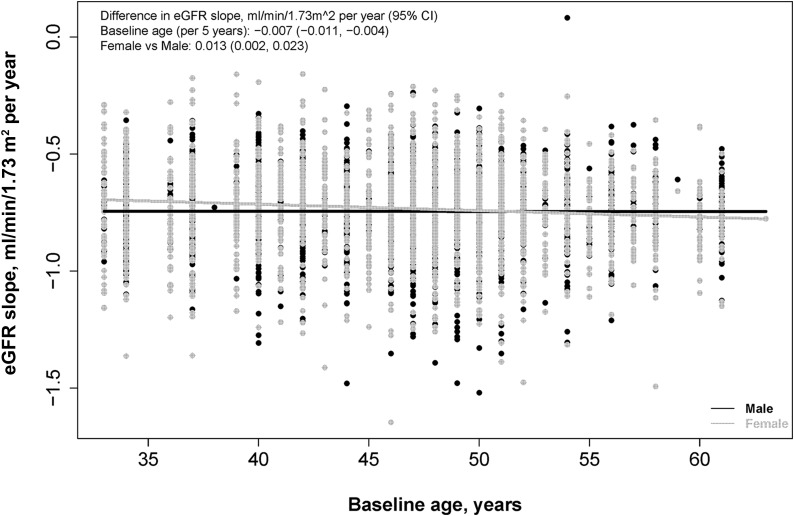
Of the 4560 Reykjavik Study group B participants, 3888 with an eGFR>60 ml/min per 1.73 m2 at baseline met the follow-up criteria. Participants excluded from the analysis were more likely to be current smokers and were more likely to have hypertension, proteinuria at baseline, and lower eGFR (Supplemental Table 2). Mean age at baseline was 46 (SD 7) years (Table 1). Women were less likely to be current smokers or have hypertension, and they had lower BMI, triglyceride, and eGFR levels compared with men. Men and women had similar rates of eGFR decline per year, at 0.74 (SD 0.17) and 0.73 (SD 0.19) ml/min per 1.73 m2 per year, respectively, and there was minimal variation by baseline age (Figure 1).
Table 1.
Clinical characteristics of the study population
| Characteristic | Men (n=1900, 48.9%) | Women (n=1988, 51.1%) | Total (n=3888) | P Value |
|---|---|---|---|---|
| Age (yr) | 47 (7) | 46 (7) | 46 (7) | 0.16 |
| Smoking status, n (%)a | ||||
| Never smoked | 363 (19.1) | 880 (44.3) | 1243 (32.0) | <0.001 |
| Past smoker | 330 (17.4) | 233 (11.7) | 563 (14.5) | |
| Current smoker | 1207 (63.5) | 875 (44.0) | 2082 (53.6) | |
| Education, n (%) | ||||
| Primary school | 621 (32.7) | 1065 (53.6) | 1686 (43.4) | <0.001 |
| Secondary | 777 (40.9) | 830 (41.8) | 1607 (41.3) | |
| College | 325 (17.1) | 60 (3.0) | 385 (9.9) | |
| University | 177 (9.3) | 33 (1.7) | 210 (5.4) | |
| Diabetes, n (%) | 33 (1.8) | 29 (1.5) | 62 (1.6) | 0.46 |
| Hypertension, n (%)b | 846 (44.5) | 792 (39.9) | 1638 (42.1) | 0.003 |
| History of cardiovascular disease, n (%) | 2 (0.1) | 0 (0.0) | 2 (0.1) | 0.15 |
| Cholesterol (mg/dl) | 256 (44) | 256 (48) | 256 (46) | 0.87 |
| Systolic BP (mmHg) | 135 (16) | 134 (19) | 134 (18) | 0.03 |
| Diastolic BP (mmHg) | 86 (10) | 85 (10) | 85 (10) | <0.001 |
| Triglycerides (mg/dl) | 97 (49) | 80 (34) | 87 (42) | <0.001 |
| Body mass index (kg/m2) | 26 (3) | 24 (4) | 25 (4) | <0.001 |
| Fasting blood sugar (mg/dl) | 84 (13) | 79 (11) | 81 (12) | <0.001 |
| Proteinuria, n (%)c | 30 (1.6) | 9 (0.5) | 39 (1.1) | 0.001 |
| eGFR (ml/min per 1.73 m2) | 92 (13) | 87 (13) | 89 (13) | <0.001 |
Data are given as the mean (SD) unless otherwise indicated.
Smoking was categorized as past smoker, current smoker, and never smoked based on self-report.
Hypertension was defined as systolic BP >140 mmHg or diastolic BP >90 mmHg or receiving hypertension treatment.
During the Reykjavik Study visits, urine protein was determined using reagent strip testing to provide semiquantitative results. Participants with 1+ or higher were considered to have proteinuria.
Figure 1.
Change in eGFR over time by baseline age for men and women. Slope of eGFR for each person was calculated from a random-effects mixed model with random intercept and slope. Difference in change in GFR by age was computed per 5 years higher baseline age. 95% CI, 95% confidence interval.
During a mean follow-up of 25 (SD 10) years, the median number of creatinine measurements was 5 (25th percentile to 75th percentile, 4–6); 727 (19%) participants developed CKD stage 3 or higher and 942 (24%) died without developing CKD stage 3 or higher. There were 19 ESRD events, with four participants not observed to have eGFR<60 ml/min per 1.73 m2 throughout the follow-up.
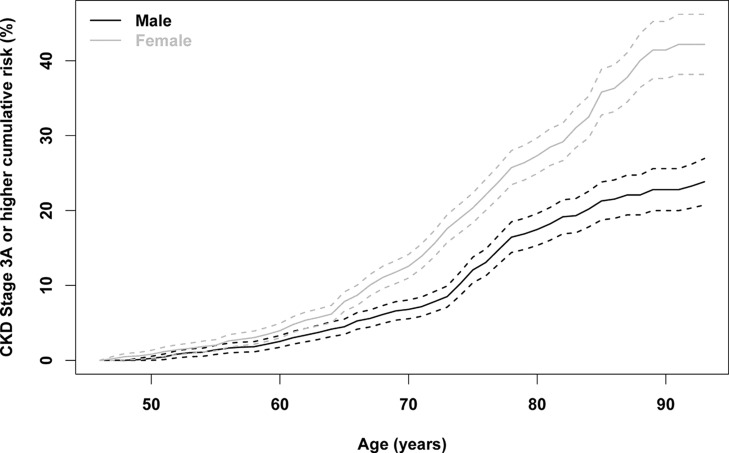
By age 85 years, the lifetime risk for developing CKD stage 3 or higher for a 45-year-old woman was 35.8% (95% CI, 32.7 to 38.9) (Figure 2, Table 2). The risk remains high for people who survive to older age without developing CKD. For example, the 5-year cumulative risk for an 80-year-old woman was 15.0% (95% CI, 11.0 to 19.0). Regardless of age, the lifetime risk of CKD stage 3 or higher is greater in women than in men. For example, the lifetime risk for a 45-year-old man was 21.3% (95% CI, 18.7 to 23.8). In sensitivity analyses, we tested alternative methods for the ascertainment of CKD (Supplemental Tables 1 and 3). Table 3 and Supplemental Figure 1 show that the lifetime risk for women varies between 27% (95% CI, 24.7 to 30.0) and 61.5% (95% CI, 58.7 to 64.2) by CKD definition. Similar differences were seen for men and for women.
Figure 2.
Lifetime risk of stages 3–5 CKD for men and women starting at age 45 years. CKD is defined as follows: (1) two consecutive measurements of eGFR<60 ml/min per 1.73 m2, (2) development of treated kidney failure with dialysis or transplant (ESRD), (3) one measurement of eGFR<60 ml/min per 1.73 m2 if the participant died before the next measurement, and (4) one measurement of eGFR<45 ml/min per 1.73 m2 if it was the last eGFR.
Table 2.
Lifetime risk for CKD stage 3 or higher by starting age, stratified by sex
| Baseline Age (yr) | Years from Baseline Age | ||||
|---|---|---|---|---|---|
| 5 | 10 | 20 | 30 | 40 | |
| Women | |||||
| 45 | 0.8 (0.3 to 1.3) | 2.0 (1.3 to 2.8) | 7.8 (6.6 to 9.1) | 20.4 (18.4 to 22.3) | 35.8 (32.7 to 38.9) |
| 55 | 2.0 (1.3 to 2.7) | 6.0 (4.9 to 7.1) | 18.9 (17.0 to 20.9) | 34.9 (31.7 to 38.0) | |
| 65 | 5.3 (4.2 to 6.5) | 14.1 (12.3 to 16.0) | 31.6 (28.3 to 34.9) | ||
| 75 | 10.0 (7.8 to 12.2) | 22.2 (18.4 to 26.0) | |||
| 80 | 15.0 (11.0 to 19.0) | 24.9 (19.1 to 30.7) | |||
| Men | |||||
| 45 | 0.2 (0 to 0.5) | 1.4 (0.8 to 2.0) | 4.5 (3.5 to 5.5) | 12.1 (10.4 to 13.8) | 21.3 (18.7 to 23.8) |
| 55 | 1.2 (0.7 to 1.7) | 3.2 (2.3 to 4.1) | 11.1 (9.4 to 12.8) | 20.7 (18.1 to 23.3) | |
| 65 | 2.7 (1.8 to 3.6) | 9.0 (7.3 to 10.7) | 19.8 (17.0 to 22.6) | ||
| 75 | 8.2 (6.0 to 10.3) | 13.9 (10.9 to 17.0) | |||
| 80 | 7.4 (4.5 to 10.4) | 10.4 (6.5 to 14.2) | |||
Cumulative risk is shown as a percentage (95% confidence interval). Lifetime risk estimates are adjusted for competing risk of death. Cumulative risk was not determined for missing cells because of the small number of events.
Table 3.
Lifetime risk for CKD stage 3 or higher using the four sensitivity definitions for CKD stage 3
| Age (yr) | Outcome | Years from Baseline Age | ||||
|---|---|---|---|---|---|---|
| 5 | 10 | 20 | 30 | 40 | ||
| Women | ||||||
| 45 | 1 | 0.8 (0.3 to 1.3) | 2.2 (1.4 to 3.0) | 8.6 (7.3 to 9.9) | 26.8 (24.6 to 29.0) | 50.7 (47.7 to 53.6) |
| 2 | 0.8 (0.3 to 1.3) | 2.0 (1.3 to 2.8) | 7.8 (6.5 to 9.1) | 19.1 (17.2 to 21.1) | 30.2 (27.3 to 33.1) | |
| 3 | 0.8 (0.3 to 1.3) | 1.9 (1.2 to 2.7) | 7.2 (6.0 to 8.5) | 17.1 (15.3 to 19.0) | 27.4 (24.7 to 30.0) | |
| 4 | 2.9 (2.0 to 3.9) | 6.5 (5.2 to 7.7) | 16.8 (15.0 to 18.5) | 39.0 (36.6 to 41.3) | 61.5 (58.7 to 64.2) | |
| 55 | 1 | 2.2 (1.5 to 2.9) | 6.6 (5.4 to 7.8) | 25.4 (23.2 to 27.6) | 50.1 (46.5 to 53.1) | |
| 2 | 2.0 (1.3 to 2.7) | 6.0 (4.8 to 7.1) | 17.7 (15.8 to 19.6) | 29.1 (26.1 to 32.0) | ||
| 3 | 1.7 (1.1 to 2.3) | 5.4 (4.4 to 6.5) | 15.6 (13.8 to 17.5) | 26.2 (23.5 to 28.9) | ||
| 4 | 4.6 (3.6 to 5.6) | 11.2 (9.7 to 12.7) | 35.5 (33.0 to 37.9) | 60.0 (57.0 to 63.0) | 68.9 (65.5 to 72.3) | |
| 65 | 1 | 6.8 (5.5 to 8.0) | 20.7 (18.6 to 22.9) | 47.9 (44.7 to 51.2) | ||
| 2 | 5.0 (3.9 to 6.1) | 12.8 (11.0 to 14.6) | 25.3 (22.2 to 28.4) | |||
| 3 | 4.4 (3.4 to 5.5) | 11.2 (9.5 to 12.8) | 22.7 (19.9 to 25.5) | |||
| 4 | 11.3 (9.6 to 13.0) | 28.5 (25.9 to 31.0) | 57.2 (53.8 to 60.6) | 67.8 (63.9 to 71.6) | ||
| 75 | 1 | 19.0 (16.1 to 22.0) | 37.7 (33.5 to 41.8) | |||
| 2 | 7.3 (5.3 to 9.2) | 15.6 (12.3 to 19.0) | ||||
| 3 | 7.1 (5.2 to 9.0) | 14.4 (11.3 to 17.4) | ||||
| 4 | 22.3 (18.8 to 25.8) | 44.5 (39.7 to 49.2) | 60.7 (54.9 to 66.5) | |||
| 80 | 1 | 25.6 (20.6 to 30.3) | 39.6 (33.4 to 45.8) | |||
| 2 | 10.0 (6.6 to 13.4) | |||||
| 3 | 8.8 (5.8 to 11.8) | 13.5 (9.4 to 17.7) | ||||
| 4 | 31.7 (26.0 to 37.5) | 50.2 (42.8 to 57.6) | ||||
| Men | ||||||
| 45 | 1 | 0.2 (0 to 0.5) | 1.4 (0.8 to 2.0) | 5.1 (4.0 to 6.2) | 15.6 (13.7 to 17.5) | 35.3 (32.4 to 38.3) |
| 2 | 0.2 (0 to 0.5) | 1.4 (0.8 to 2.0) | 4.4 (3.3 to 5.4) | 11.4 (9.8 to 13.1) | 17.4 (15.1 to 19.7) | |
| 3 | 0.2 (0 to 0.5) | 1.2 (0.6 to 1.7) | 3.6 (2.6 to 4.5) | 8.2 (6.8 to 9.6) | 13.9 (11.8 to 15.9) | |
| 4 | 0.5 (0.1 to 0.9) | 2.5 (1.7 to 3.3) | 8.6 (7.2 to 9.9) | 20.4 (18.3 to 22.4) | 40.0 (37.0 to 42.9) | |
| 55 | 1 | 1.4 (0.8 to 2.0) | 3.8 (2.9 to 4.8) | 14.8 (12.9 to 16.7) | 35.3 (32.3 to 38.3) | |
| 2 | 1.1 (0.6 to 1.6) | 3.1 (2.2 to 3.9) | 10.5 (8.8 to 12.1) | 16.6 (14.4 to 18.9) | ||
| 3 | 1.1 (0.6 to 1.6) | 2.5 (1.7 to 3.2) | 7.3 (5.9 to 8.7) | 13.2 (11.1 to 15.3) | ||
| 4 | 2.6 (1.8 to 3.4) | 6.5 (5.3 to 7.7) | 19.0 (17.0 to 21.1) | 39.9 (36.9 to 43.0) | 45.1 (41.8 to 48.4) | |
| 65 | 1 | 4.1 (3.0 to 5.2) | 12.5 (10.5 to 14.4) | 35.9 (32.6 to 39.3) | ||
| 2 | 2.7 (1.8 to 3.6) | 8.4 (6.7 to 10.0) | 15.4 (12.9 to 17.8) | |||
| 3 | 2.1 (1.3 to 2.9) | 5.4 (4.1 to 6.8) | 12.1 (9.8 to 14.3) | |||
| 4 | 5.5 (4.2 to 6.8) | 15.0 (12.9 to 17.2) | 40.0 (36.5 to 43.5) | 46.2 (42.4 to 50.1) | ||
| 75 | 1 | 14.7 (11.9 to 17.4) | 31.3 (27.3 to 35.4) | |||
| 2 | 6.4 (4.5 to 8.2) | 8.9 (6.4 to 11.3) | ||||
| 3 | 4.3 (2.8 to 5.9) | 8.3 (6.0 to 10.7) | ||||
| 4 | 16.1 (13.1 to 19.1) | 34.4 (30.1 to 38.7) | 42.9 (38.0 to 47.9) | |||
| 80 | 1 | 23.3 (18.6 to 28.0) | 32.0 (26.5 to 37.6) | |||
| 2 | 3.2 (1.2 to 5.3) | |||||
| 3 | 5.1 (2.7 to 7.5) | 7.8 (4.5 to 11.0) | ||||
| 4 | 25.7 (20.6 to 30.8) | 35.2 (29.3 to 41.2) | ||||
Risk is shown as the percentage (95% confidence interval). Risk was not determined for missing cells because of the small number of events. Lifetime risk estimates are adjusted for competing risk of death. Sensitivity is indicated as follows: 1, requires two consecutive measurements of eGFR<60 ml/min per 1.73 m2 or one measurement of eGFR<60 ml/min per 1.73 m2 if it was at the last measurement of eGFR due to death or lack of subsequent creatinine measurement or ESRD; 2, requires eGFR<60 ml/min per 1.73 m2 on two occasions or eGFR<60 ml/min per 1.73 m2 on the first measurement if the participant died before the second measurement or ESRD; 3, requires eGFR<60 ml/min per 1.73 m2 on two occasions or one eGFR<45 ml/min per 1.73 m2 if it was at the last measurement of eGFR due to death or lack of subsequent creatinine measurement or ESRD; and 4, requires only one measurement of eGFR<60 ml/min per 1.73 m2 irrespective of the results of follow-up measurements and was used for comparison with previous reports or ESRD. See Supplemental Table 1 for more details on the definitions of the four sensitivity definitions of CKD.
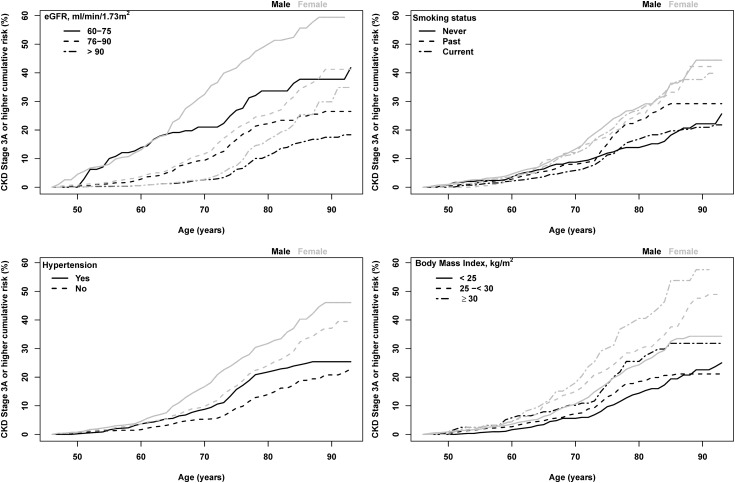
Failing to account for the competing risk of death led to a higher estimate of lifetime risk at long follow-up (Supplemental Table 4). With age, there is a greater effect with adjustment. For a woman in her 80s, the 10-year risk was 24.9% when adjusting for the competing risk of death; however, this risk is 33.3% if one does not adjust for the competing risk of death. As expected, the risk is lower for participants with higher levels of baseline eGFR (Figure 3, Supplemental Table 5). For example, a 45-year-old woman with an eGFR<75 ml/min per 1.73 m2 had a 55.7% (95% CI, 49.7 to 61.6) chance of developing CKD stage 3 or higher over the next 40 years, whereas a 45-year-old woman with an eGFR>90 ml/min per 1.73 m2 had a 25.4% (95% CI, 19.0 to 31.8) risk. Risk was higher among those with hypertension compared with those without as well as among those individuals with higher versus lower BMI (Figure 3, Supplemental Tables 6 and 7). In women, current smokers, past smokers, and those who never smoked all had similar risk (Figure 3, Supplemental Table 8). In men, past smokers had a higher risk than those who currently smoke or those who never smoked.
Figure 3.
Lifetime risk of stages 3–5 CKD for men and women starting at age 45 years by subgroup of eGFR (<75, 75–89, or ≥90), hypertension, body mass index (< 25, 25–<30, or ≥30 kg/m2), and smoking (current, past, or never).
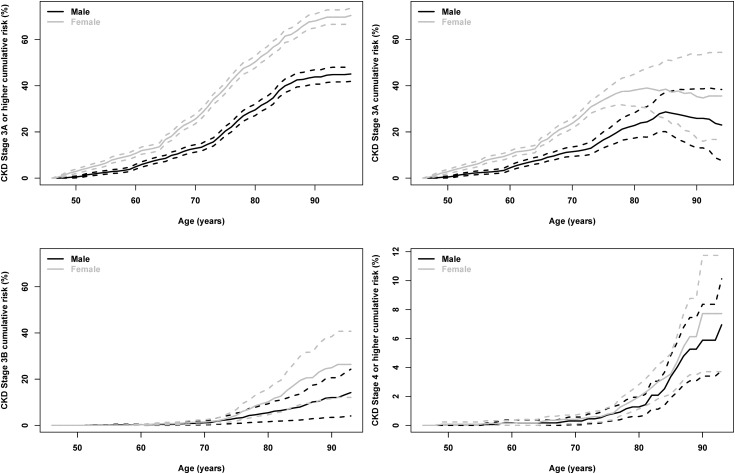
We also evaluated the lifetime risk for developing CKD stages 3a, 3b, or 4 (Figure 4, Table 4). A total of 1097, 290, and 70 participants developed these outcomes, respectively. By age 85 years, the lifetime risks for a 45-year-old woman to reach CKD stage 3a, 3b, or 4 were 38.5 (95% CI, 25.8 to 51.1), 19.4 (95% CI, 8.9 to 29.9), and 3.6 (95% CI, 2.2 to 5.0) respectively. Development of CKD stages 3b and 4 occurs later than CKD stage 3a; for example, in those have not developed CKD by age 65 years, the cumulative risk of CKD stage 3a remains substantial at 15.7 (95% CI, 14.0 to 17.5); however, risk was much lower for stage 3b or 4 (<1%). Similar to risks for stage 3 or higher, the lifetime risk of CKD stage 3b or higher is greater in women than in men and in people with lower levels of GFR at baseline compared with higher levels of GFR; as expected, this risk decreases in individuals with higher levels of GFR and in people without hypertension.
Figure 4.
Lifetime risk for incident CKD stage 3A or higher, stage 3A, stage 3B, and stage 4 or higher. CKD stage was defined using one measurement of GFR below the threshold. ESRD was included in CKD stage 3a or higher or stage 4.
Table 4.
Lifetime risk for incident CKD stages 3A, 3B, or 4 by starting age, stratified by sex
| Baseline Age (yr) | Years from Baseline Age | ||||
|---|---|---|---|---|---|
| 5 | 10 | 20 | 30 | 40 | |
| Stage 3A | |||||
| Women | |||||
| 45 | 2.8 (1.9 to 3.8) | 6.3 (5.1 to 7.6) | 15.7 (14.0 to 17.5) | 33.8 (30.3 to 37.4) | 38.5 (25.8 to 51.1) |
| 55 | 4.4 (3.4 to 5.3) | 10.3 (8.9 to 11.7) | 30.4 (28.0 to 32.7) | 36.7 (33.5 to 39.9) | |
| 65 | 10.4 (8.8 to 11.9) | 24.1 (21.7 to 26.5) | 33.8 (30.3 to 37.3) | ||
| 75 | 13.9 (10.7 to 17.2) | 23.2 (18.8 to 27.6) | |||
| 80 | 16.7 (11.5 to 21.9) | 21.7 (13.6 to 29.8) | |||
| Men | |||||
| 45 | 0.5 (0.1 to 0.9) | 2.4 (1.6 to 3.2) | 8.0 (6.5 to 9.5) | 16.6 (13.1 to 20.0) | 28.7 (20.1 to 37.2) |
| 55 | 2.2 (1.5 to 3.0) | 6.0 (4.8 to 7.2) | 15.2 (13.3 to 17.1) | 28.3 (25.3 to 31.3) | |
| 65 | 4.5 (3.3 to 5.8) | 11.3 (9.4 to 13.3) | 27.6 (24.2 to 31.0) | ||
| 75 | 12.1 (9.2 to 14.9) | 24.1 (19.9 to 28.3) | |||
| 80 | 18.2 (13.5 to 23.0) | 16.9 (10.8 to 23.0) | |||
| Stage 3B | |||||
| Women | |||||
| 45 | 0 (0 to 0) | 0.1 (0 to 0.2) | 0.7 (0.2 to 1.2) | 4.4 (1.9 to 6.9) | 19.4 (8.9 to 29.9) |
| 55 | 0.2 (0 to 0.4) | 0.7 (0.3 to 1.1) | 4.4 (3.3 to 5.5) | 19.7 (16.8 to 22.7) | |
| 65 | 0.8 (0.4 to 1.2) | 3.9 (2.8 to 4.9) | 20.0 (16.9 to 23.0) | ||
| 75 | 7.0 (5.2 to 8.8) | 18.1 (14.8 to 21.4) | |||
| 80 | 13.1 (9.6 to 16.6) | 21.5 (15.3 to 27.8) | |||
| Men | |||||
| 45 | 0 (0 to 0) | 0.1 (0 to 0.3) | 0.4 (0 to 0.8) | 3.1 (0.9 to 5.3) | 7.9 (2.3 to 13.6) |
| 55 | 0.2 (0 to 0.4) | 0.3 (0 to 0.6) | 3.1 (2.1 to 4) | 8.1 (6.0 to 10.2) | |
| 65 | 0.8 (0.3 to 1.3) | 3.1 (2.1 to 4.1) | 8.7 (6.5 to 10.9) | ||
| 75 | 3.3 (1.8 to 4.8) | 6.8 (4.4 to 9.2) | |||
| 80 | 4.3 (1.8 to 6.8) | 11.4 (6.5 to 16.2) | |||
| Stage 4 | |||||
| Women | |||||
| 45 | 0.1 (0 to 0.2) | 0.1 (0 to 0.2) | 0.3 (0 to 0.6) | 0.8 (0.3 to 1.2) | 3.6 (2.2 to 5.0) |
| 55 | 0.1 (0 to 0.2) | 0.2 (0 to 0.4) | 0.7 (0.3 to 1.1) | 3.5 (2.1 to 5) | |
| 65 | 0.1 (0 to 0.3) | 0.5 (0.1 to 0.9) | 3.5 (2.0 to 4.9) | ||
| 75 | 1.4 (0.6 to 2.2) | 3.2 (1.7 to 4.7) | |||
| 80 | 1.9 (0.6 to 3.3) | 7.0 (2.2 to 11.8) | |||
| Men | |||||
| 45 | 0 (0 to 0) | 0 (0 to 0) | 0.2 (0 to 0.4) | 0.7 (0.3 to 1.2) | 3.4 (1.9 to 4.8) |
| 55 | 0.2 (0 to 0.4) | 0.2 (0 to 0.4) | 0.7 (0.3 to 1.2) | 3.5 (2.0 to 5.0) | |
| 65 | 0.2 (0 to 0.4) | 0.6 (0.2 to 1.1) | 3.7 (2.0 to 5.4) | ||
| 75 | 0.7 (0.1 to 1.4) | 3.5 (1.7 to 5.4) | |||
| 80 | 3.2 (1.2 to 5.2) | 7.0 (3.4 to 10.6) | |||
Cumulative risk is shown as the percentage (95% confidence interval). Lifetime risk estimates are adjusted for competing risk of death. Cumulative risk was not determined for missing cells because of the small number of events.
Discussion
Lifetime risk estimates are easily understood by health care professionals and the general public, are effective in public education campaigns, and assist clinicians and policy makers in predicting future burden of disease, screening strategies, and design of trials and observational studies. In other diseases, statements such as “one in three will develop diabetes” or “one in two men and one in three women will develop cardiovascular disease” provide powerful messages to all audiences (16,18,19). In this study of serial eGFR determinations in a community-based cohort, we showed that for participants without CKD starting in middle age, the lifetime risk of CKD stage 3 or higher is comparable to diabetes and cardiovascular disease, with estimates of 36% for women (approximately one in three) and 21% for men (approximately one in five). The higher risk for women persisted at all ages and in all subgroups tested. The lifetime risk of higher stages of CKD is less frequent but importantly occurs primarily in very elderly individuals.
Assessing CKD in large population-based prospective studies is challenging. Unlike diseases such as atrial fibrillation or myocardial infarction, the development of CKD may not be associated with symptoms and relies on frequent laboratory tests to capture its inception. In addition, the ascertainment of CKD relies on evidence of chronicity because some kidney diseases are acute and GFR can return to normal levels and creatinine may be affected by factors other than GFRs, which also fluctuate. In clinical practice, it is straightforward to assess chronicity by repeating laboratory tests. In epidemiologic studies, assessment of chronicity is difficult; thus, most of the literature on the prevalence and prognosis associated with CKD is based on one measurement. We utilized the advantage of our longitudinal data and used two measurements of eGFR to ascertain CKD in our primary definition. However, our data cannot replicate clinical practice entirely. In order to capture persons who developed CKD late in the course of the study, our sensitivity definitions included persons with one measurement of eGFR<60 ml/min per 1.73 m2 if they died after the measurement or persons with one measurement of eGFR<45 ml/min per 1.73 m2 at their visit, recognizing that it is less common to have acute changes in GFR to <45 ml/min per 1.73 m2 that return to normal levels. Our primary definition as well as these two sensitivity definitions led to substantially lower estimated lifetime risk for CKD compared with the two other sensitivity definitions, which use only one measurement of eGFR<60 ml/min per 1.73 m2 or include all patients who reached GFR<60 ml/min per 1.73 m2 at their last visit.
Two recent articles estimated the lifetime risk of stages 3–5 CKD based on a single eGFR and mathematical simulation from cross-sectional data. The results are not displayed in a comparable manner, so it is difficult to make direct comparisons; however, both appear to be higher (9,11). In one study, the lifetime risks for 40-year-old white women and men were 66% and 56% for stages 3–5 CKD, respectively (9). In the second study, the lifetime risk of stage 3a CKD for 30-year-old white men and women was 49% (11). Both of these studies used simulation tools that resulted in different but higher estimates than our current analysis, suggesting that incorporating only one value for eGFR may have overestimated lifetime risk and prevalence of CKD. Our results also support other studies, which demonstrate that confirmation eGFRs<60 ml/min per 1.73 m2 provide better estimates of prognosis than one value alone (20,21).
In contrast with the reported higher incidence of ESRD for men, we observed that lifetime risk of CKD stage 3 or higher was higher in women at all ages (22). Grams et al. showed similar differences using United States data, suggesting that these data are not specific to the Icelandic population (9). The difference may be attributable to faster progression to ESRD in men, increased survival in women, inaccuracies in estimating equations, or lower levels of normal GFR in women. In support of the first factor, some studies (23), although not all (24), have shown a lower prevalence of albuminuria associated with low eGFR in women than men. Our data do not show significant differences in lifetime risk of stage 4 CKD between men and women. Second, data from a large meta-analysis showed that men have a higher risk for mortality than women at levels of eGFR>60 ml/min per 1.73 m2, supporting the hypothesis that this sex difference in lifetime risk for stage 3 CKD may be due in part to increased survival in women (9,23). Our sensitivity analyses in which we did not account for the competing risk of death suggests that increased survival may contribute to this difference but does not explain it all. Third, inaccuracy of estimating equations is likely less of a concern. Fan et al. reported that the CKD-EPI equation has slight (but nonsignificant) overestimation of GFR in women in this population compared with men, which would have led to a lower lifetime risk of CKD, not higher as was observed (25). Finally, most other studies that use eGFR to ascertain CKD or establish reference values for GFR in the general population, including studies from Iceland, have also shown higher prevalence of CKD or lower levels of GFR in women (14,15,24,26,27). In our study, women had an eGFR that was approximately 5 ml/min per 1.73 m2 lower than men at baseline. Data such as these raise the possibility that sex-specific thresholds for CKD may be needed; however, these thresholds would need to be reconciled with other data that suggest no difference between men and women in risk for ESRD by level of GFR (23).
We observed that the lifetime risk for CKD stage 3 or higher is greater at older age. Indeed, CKD stages 3b and 4 are rare until late life; even then, these stages are substantially less common than stage 3a. This is consistent with prior reports of prevalence of CKD in older populations (28). Some have interpreted the high rate of CKD in elderly individuals to indicate that low GFR in elderly patients is part of normal aging and does not reflect a pathologic state (29), and others suggest that a fixed definition for CKD across all ages is not appropriate (30,31). However, data from a large meta-analysis demonstrate that adverse outcomes related to level of eGFR and albuminuria are present in older adults and that absolute risk is in fact higher in older adults compared with younger adults (32).
Strengths of this study are the well characterized population-based cohort with >25 years of follow-up and longitudinal measurements of serum creatinine obtained at set time points from midlife to late life as well as mortality outcomes. Ascertainment of CKD using two values of eGFR allows for greater confidence that the estimates are not subject to acute effects on kidney function or serum creatinine. There are also several limitations. First, changes in the creatinine assay over time could have led to small changes in eGFR and therefore influenced those determined to have CKD stage 3. However, the serum creatinine assay at the last time points was calibrated to reference standards. Second, serum creatinine measurements were only available every 3–7 years and some participants were excluded because they did not have sufficient measurements performed. Third, we did not have data on urinary albumin excretion for either ascertainment of CKD or evaluation of differences in lifetime risk of CKD based on level of albuminuria. The urine dipstick data we had were not sensitive enough to identify those with the earliest stages of kidney disease. Fourth, our results may not be generalizable to other populations or other races other than European whites. Fifth, lifetime risk estimates are likely to be higher in blacks or populations with higher prevalence of diabetes or obesity; thus, we may be underestimating the risk in these groups. Finally, lifetime risks for specific stages may be overestimates because they were derived using only one measurement of GFR.
Lifetime risk of CKD is substantial in both men and women and the risk for the development of CKD remains high even as one ages. However, estimates are lower than has been presented using single estimates of GFR, emphasizing the importance of confirming reduced GFR in studies of CKD. Strategies to prevent development of CKD throughout the course of life are needed, and these strategies should include education of patients and providers as to the risk factors for CKD and should focus on primary prevention of such risk factors.
Disclosures
L.I. and A.S.L. report funding to Tufts Medical Center for research and contracts with the National Institutes of Health, the National Kidney Foundation, Pharmalink AB, and Gilead Sciences, as well as a provisional patent filed August 15, 2014 (Precise Estimation of GFR from Multiple Biomarkers; licensing under negotiations). L.I. also reports a consulting agreement with Otsuka. T.H. is employed by the National Institute on Aging.
Supplementary Material
Acknowledgments
This study was supported by the National Institutes of Health (Grant R01-DK082447), the National Institute on Aging (Contract N01AG12100), the Icelandic Heart Association (Hjartavernd), and the Icelandic Parliament (Althingi). The funding sources were not required to approve publication of the finished manuscript.
Preliminary results of this research were presented in abstract form at the Annual Meeting of the American Society of Nephrology, held November 3–8, 2012, in San Diego, California.
Footnotes
Published online ahead of print. Publication date available at www.cjasn.org.
See related editorial, “Lifetime Risk of CKD: What Does It Really Mean?,” on pages 1504–1506.
This article contains supplemental material online at http://cjasn.asnjournals.org/lookup/suppl/doi:10.2215/CJN.00180115/-/DCSupplemental.
References
- 1.Matsushita K, van der Velde M, Astor BC, Woodward M, Levey AS, de Jong PE, Coresh J, Gansevoort RT, Chronic Kidney Disease Prognosis Consortium : Association of estimated glomerular filtration rate and albuminuria with all-cause and cardiovascular mortality in general population cohorts: A collaborative meta-analysis. Lancet 375: 2073–2081, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.van der Velde M, Matsushita K, Coresh J, Astor BC, Woodward M, Levey A, de Jong P, Gansevoort RT, van der Velde M, Matsushita K, Coresh J, Astor BC, Woodward M, Levey AS, de Jong PE, Gansevoort RT, Levey A, El-Nahas M, Eckardt KU, Kasiske BL, Ninomiya T, Chalmers J, Macmahon S, Tonelli M, Hemmelgarn B, Sacks F, Curhan G, Collins AJ, Li S, Chen SC, Hawaii Cohort KP, Lee BJ, Ishani A, Neaton J, Svendsen K, Mann JF, Yusuf S, Teo KK, Gao P, Nelson RG, Knowler WC, Bilo HJ, Joosten H, Kleefstra N, Groenier KH, Auguste P, Veldhuis K, Wang Y, Camarata L, Thomas B, Manley T, Chronic Kidney Disease Prognosis Consortium : Lower estimated glomerular filtration rate and higher albuminuria are associated with all-cause and cardiovascular mortality. A collaborative meta-analysis of high-risk population cohorts. Kidney Int 79: 1341–1352, 2011 [DOI] [PubMed] [Google Scholar]
- 3.Gansevoort RT, Matsushita K, van der Velde M, Astor BC, Woodward M, Levey AS, de Jong PE, Coresh J, Chronic Kidney Disease Prognosis Consortium : Lower estimated GFR and higher albuminuria are associated with adverse kidney outcomes. A collaborative meta-analysis of general and high-risk population cohorts. Kidney Int 80: 93–104, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Astor BC, Matsushita K, Gansevoort RT, van der Velde M, Woodward M, Levey AS, Jong PE, Coresh J, Astor BC, Matsushita K, Gansevoort RT, van der Velde M, Woodward M, Levey AS, de Jong PE, Coresh J, El-Nahas M, Eckardt KU, Kasiske BL, Wright J, Appel L, Greene T, Levin A, Djurdjev O, Wheeler DC, Landray MJ, Townend JN, Emberson J, Clark LE, Macleod A, Marks A, Ali T, Fluck N, Prescott G, Smith DH, Weinstein JR, Johnson ES, Thorp ML, Wetzels JF, Blankestijn PJ, van Zuilen AD, Menon V, Sarnak M, Beck G, Kronenberg F, Kollerits B, Froissart M, Stengel B, Metzger M, Remuzzi G, Ruggenenti P, Perna A, Heerspink HJ, Brenner B, de Zeeuw D, Rossing P, Parving HH, Auguste P, Veldhuis K, Wang Y, Camarata L, Thomas B, Manley T, Chronic Kidney Disease Prognosis Consortium : Lower estimated glomerular filtration rate and higher albuminuria are associated with mortality and end-stage renal disease. A collaborative meta-analysis of kidney disease population cohorts. Kidney Int 79: 1331–1340, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mahmoodi BK, Matsushita K, Woodward M, Blankestijn PJ, Cirillo M, Ohkubo T, Rossing P, Sarnak MJ, Stengel B, Yamagishi K, Yamashita K, Zhang L, Coresh J, de Jong PE, Astor BC, Chronic Kidney Disease Prognosis Consortium : Associations of kidney disease measures with mortality and end-stage renal disease in individuals with and without hypertension: A meta-analysis. Lancet 380: 1649–1661, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Foster MC, Rawlings AM, Marrett E, Neff D, Willis K, Inker LA, Coresh J, Selvin E: Cardiovascular risk factor burden, treatment, and control among adults with chronic kidney disease in the United States. Am Heart J 166: 150–156, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Plantinga LC, Boulware LE, Coresh J, Stevens LA, Miller ER, 3rd, Saran R, Messer KL, Levey AS, Powe NR: Patient awareness of chronic kidney disease: Trends and predictors. Arch Intern Med 168: 2268–2275, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Inker LA, Astor BC, Fox CH, Isakova T, Lash JP, Peralta CA, Kurella Tamura M, Feldman HI: KDOQI US commentary on the 2012 KDIGO clinical practice guideline for the evaluation and management of CKD. Am J Kidney Dis 63: 713–735, 2014 [DOI] [PubMed] [Google Scholar]
- 9.Grams ME, Chow EK, Segev DL, Coresh J: Lifetime incidence of CKD stages 3-5 in the United States. Am J Kidney Dis 62: 245–252, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Muzaale AD, Massie AB, Wang MC, Montgomery RA, McBride MA, Wainright JL, Segev DL: Risk of end-stage renal disease following live kidney donation. JAMA 311: 579–586, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hoerger TJ, Simpson SA, Yarnoff BO, Pavkov ME, Ríos Burrows N, Saydah SH, Williams DE, Zhuo X: The future burden of CKD in the United States: A simulation model for the CDC CKD Initiative. Am J Kidney Dis 65: 403–411, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sigurdsson E, Thorgeirsson G, Sigvaldason H, Sigfusson N: Unrecognized myocardial infarction: epidemiology, clinical characteristics, and the prognostic role of angina pectoris. The Reykjavik Study. Ann Intern Med 122: 96–102, 1995 [DOI] [PubMed] [Google Scholar]
- 13.Harris TB, Launer LJ, Eiriksdottir G, Kjartansson O, Jonsson PV, Sigurdsson G, Thorgeirsson G, Aspelund T, Garcia ME, Cotch MF, Hoffman HJ, Gudnason V: Age, Gene/Environment Susceptibility-Reykjavik Study: Multidisciplinary applied phenomics. Am J Epidemiol 165: 1076–1087, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Viktorsdottir O, Palsson R, Andresdottir MB, Aspelund T, Gudnason V, Indridason OS: Prevalence of chronic kidney disease based on estimated glomerular filtration rate and proteinuria in Icelandic adults. Nephrol Dial Transplant 20: 1799–1807, 2005 [DOI] [PubMed] [Google Scholar]
- 15.Levey AS, Stevens LA, Schmid CH, Zhang YL, Castro AF, 3rd, Feldman HI, Kusek JW, Eggers P, Van Lente F, Greene T, Coresh J, CKD-EPI (Chronic Kidney Disease Epidemiology Collaboration) : A new equation to estimate glomerular filtration rate. Ann Intern Med 150: 604–612, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Beiser A, D’Agostino RB, Sr, Seshadri S, Sullivan LM, Wolf PA: Computing estimates of incidence, including lifetime risk: Alzheimer’s disease in the Framingham Study. The Practical Incidence Estimators (PIE) macro. Stat Med 19: 1495–1522, 2000 [DOI] [PubMed] [Google Scholar]
- 17.R Core Development Team : R: A Language and Environment for Statistical Computing, Vienna, Austria, R Foundation for Statistical Computing, 2014 [Google Scholar]
- 18.Narayan KM, Boyle JP, Thompson TJ, Sorensen SW, Williamson DF: Lifetime risk for diabetes mellitus in the United States. JAMA 290: 1884–1890, 2003 [DOI] [PubMed] [Google Scholar]
- 19.Lloyd-Jones DM, Larson MG, Beiser A, Levy D: Lifetime risk of developing coronary heart disease. Lancet 353: 89–92, 1999 [DOI] [PubMed] [Google Scholar]
- 20.Lambers Heerspink HJ, Tighiouart H, Sang Y, Ballew S, Mondal H, Matsushita K, Coresh J, Levey AS, Inker LA: GFR decline and subsequent risk of established kidney outcomes: A meta-analysis of 37 randomized controlled trials. Am J Kidney Dis 64: 860–866, 2014 [DOI] [PubMed] [Google Scholar]
- 21.Manns BJ, Hodsman A, Zimmerman DL, Mendelssohn DC, Soroka SD, Chan C, Jindal K, Klarenbach S: Canadian Society of Nephrology commentary on the 2009 KDIGO Clinical Practice Guideline for the Diagnosis, Evaluation, and Treatment of CKD-Mineral and Bone Disorder (CKD-MBD). Am J Kidney Dis 55: 800–812, 2010 [DOI] [PubMed] [Google Scholar]
- 22.Collins AJ, Foley RN, Herzog C, Chavers B, Gilbertson D, Ishani A, Kasiske B, Liu J, Mau LW, McBean M, Murray A, St Peter W, Guo H, Gustafson S, Li Q, Li S, Li S, Peng Y, Qiu Y, Roberts T, Skeans M, Snyder J, Solid C, Wang C, Weinhandl E, Zaun D, Arko C, Chen SC, Dalleska F, Daniels F, Dunning S, Ebben J, Frazier E, Hanzlik C, Johnson R, Sheets D, Wang X, Forrest B, Constantini E, Everson S, Eggers P, Agodoa L: US Renal Data System 2010 Annual Data Report. Am J Kidney Dis 57[Suppl 1]: A8–, e1–e526., 2011 [DOI] [PubMed] [Google Scholar]
- 23.Nitsch D, Grams M, Sang Y, Black C, Cirillo M, Djurdjev O, Iseki K, Jassal SK, Kimm H, Kronenberg F, Oien CM, Levey AS, Levin A, Woodward M, Hemmelgarn BR, Chronic Kidney Disease Prognosis Consortium : Associations of estimated glomerular filtration rate and albuminuria with mortality and renal failure by sex: A meta-analysis. BMJ 346: f324, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.van den Brand JA, van Boekel GA, Willems HL, Kiemeney LA, den Heijer M, Wetzels JF: Introduction of the CKD-EPI equation to estimate glomerular filtration rate in a Caucasian population. Nephrol Dial Transplant 26: 3176–3181, 2011 [DOI] [PubMed] [Google Scholar]
- 25.Fan L, Levey AS, Gudnason V, Eiriksdottir G, Andresdottir MB, Gudmundsdottir H, Indridason OS, Palsson R, Mitchell G, Inker LA: Comparing GFR estimating equations using cystatin C and creatinine in elderly individuals [published online ahead of print December 19, 2014]. J Am Soc Nephrol 10.1681/ASN.2014060607 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wetzels JF, Kiemeney LA, Swinkels DW, Willems HL, den Heijer M: Age- and gender-specific reference values of estimated GFR in Caucasians: The Nijmegen Biomedical Study. Kidney Int 72: 632–637, 2007 [DOI] [PubMed] [Google Scholar]
- 27.Wesson L: Physiology of the Human Kidney, New York, Grune & Stratton, 1969 [Google Scholar]
- 28.Semba RD, Fink JC, Sun K, Cappola AR, Dalal M, Crasto C, Ferrucci L, Fried LP: Serum fibroblast growth factor-23 and risk of incident chronic kidney disease in older community-dwelling women. Clin J Am Soc Nephrol 7: 85–91, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Glassock RJ, Winearls C: Ageing and the glomerular filtration rate: Truths and consequences. Trans Am Clin Climatol Assoc 120: 419–428, 2009 [PMC free article] [PubMed] [Google Scholar]
- 30.Delanaye P, Schaeffner E, Ebert N, Cavalier E, Mariat C, Krzesinski JM, Moranne O: Normal reference values for glomerular filtration rate: What do we really know? Nephrol Dial Transplant 27: 2664–2672, 2012 [DOI] [PubMed] [Google Scholar]
- 31.Glassock RJ: Con: Thresholds to define chronic kidney disease should not be age dependent. Nephrol Dial Transplant 29: 774–779, discussion 779–782, 2014 [DOI] [PubMed] [Google Scholar]
- 32.Hallan SI, Matsushita K, Sang Y, Mahmoodi BK, Black C, Ishani A, Kleefstra N, Naimark D, Roderick P, Tonelli M, Wetzels JF, Astor BC, Gansevoort RT, Levin A, Wen CP, Coresh J, Chronic Kidney Disease Prognosis Consortium : Age and association of kidney measures with mortality and end-stage renal disease. JAMA 308: 2349–2360, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.