Abstract
Background and objectives
Endothelin A receptor antagonists (ERAs) decrease residual albuminuria in patients with diabetic kidney disease; however, their clinical utility may be limited by fluid retention. Consequently, the primary objective of this study was to identify predictors for ERA-induced fluid retention among patients with type 2 diabetes and CKD. A secondary objective was to determine if the degree of fluid retention necessarily correlated with the magnitude of albuminuria reduction in those patients receiving ERAs.
Design, setting, participants, & measurements
A post hoc analysis was conducted of the phase IIb atrasentan trials assessing albuminuria reduction in 211 patients with type 2 diabetes, urine albumin/creatinine ratios of 300–3500 mg/g, and eGFRs of 30–75 ml/min per 1.73 m2 who were randomly assigned to receive placebo (n=50) or atrasentan 0.75 mg/d (n=78) or 1.25 mg/d (n=83) for 12 weeks. Changes in body weight and hemoglobin (Hb) after 2 weeks of treatment were used as surrogate markers of fluid retention.
Results
Baseline predictors of weight gain after 2 weeks of atrasentan treatment were higher atrasentan dose, lower eGFR, higher glycated hemoglobin, higher systolic BP, and lower homeostatic metabolic assessment product. Higher atrasentan dose and lower eGFR also predicted decreases in Hb. There were no changes in B-type natriuretic peptide. There was no correlation between reduction in albuminuria after 2 weeks of atrasentan treatment and changes in body weight or Hb.
Conclusions
In the Reducing Residual Albuminuria in Subjects With Diabetes and Nephropathy With Atrasentan/JAPAN trials, atrasentan-associated fluid retention was more likely in patients with diabetes and nephropathy who had lower eGFR or received a higher dose of atrasentan. Finding that albuminuria reduction was not associated with changes in body weight and Hb suggests that the albuminuria-reducing efficacy of atrasentan is not impaired by fluid retention.
Keywords: atrasentan, diabetic nephropathy, albuminuria
Introduction
Endothelin A (ETA) receptor antagonists (ERAs) decrease residual albuminuria in patients with diabetic nephropathy, even in the setting of optimal therapy (including maximal tolerated labeled doses of renin-angiotensin system inhibitors) (1). Therefore, this class of agents, by further reducing proteinuria, may delay renal function deterioration and could have a positive effect on other cardiovascular outcomes.
A potentially limiting issue is that ERA administration is associated with fluid retention leading to weight gain, edema, and/or congestive heart failure (CHF). A phase III trial, A Randomised, Double Blind, Placebo Controlled, Parallel Group Study to Assess the Effect of the Endothelin Receptor Antagonist Avosentan on Time to Doubling of Serum Creatinine, End Stage Renal Disease or Death in Patients With Type 2 Diabetes Mellitus and Diabetic Nephropathy (ASCEND), using avosentan, an ETA-selective antagonist, in patients with stages 2–4 diabetic CKD was terminated early because of adverse cardiovascular events (including a 3-fold increased incidence of CHF) (2). This adverse event was particularly unfortunate given that avosentan induced an approximately 45% decrease in residual albuminuria. A phase II study involving 286 patients with diabetic nephropathy with a baseline creatinine clearance of approximately 80 ml/min found similar reductions in albuminuria between avosentan doses used in the ASCEND trial and doses that were 10-fold lower; there were no significant adverse events caused by fluid retention in the low-dose group (3). Given that ERA-induced fluid retention is likely dose-dependent (4), these studies raised the question of whether ERAs could exert antiproteinuric effects in patients with diabetic CKD at doses that did not cause clinically significant fluid retention and whether the degree of the antiproteinuric effect is coupled to the degree of fluid retention.
To better define patients at risk for ERA-induced fluid retention, we conducted a post hoc analysis of data from the combined Reducing Residual Albuminuria in Subjects With Diabetes and Nephropathy With Atrasentan (RADAR) trial and an identical trial conducted in Japan to determine which baseline parameters predict atrasentan-associated fluid retention in a population of patients with diabetic nephropathy, using weight gain and hemoglobin (Hb) as proxies for fluid retention. We then sought to determine if the degree of atrasentan-associated fluid retention and albuminuria reduction were related in these patients.
Materials and Methods
Study Design and Protocol
Pooled data from the RADAR (NCT01356849) and Japan (NCT01424319) atrasentan (RADAR/JAPAN) trials, two identical phase IIb, randomized, double-blind, placebo-controlled, 12-week, multicenter studies were analyzed. The study design and primary results of these clinical trials have been reported (5). Briefly, participants who had type 2 diabetes and nephropathy, defined as a urinary albumin to creatinine ratio (UACR) of ≥300 and ≤3500 mg/g, an eGFR (CKD epidemiology collaboration formula) of 30–75 ml/min per 1.73 m2, and who were receiving a maximum tolerated labeled daily dose of a renin-angiotensin-aldosterone system inhibitor entered a 12-week treatment period with a follow-up visit 4 weeks after study drug discontinuation. Participants were randomly assigned to placebo, 0.75 mg/d atrasentan, or 1.25 mg/d atrasentan. The primary end point was the change in the UACR over time.
Measurements
For this post hoc analysis, changes in indices of fluid retention, including body weight and Hb, were analyzed as a function of baseline parameters and a function of change in the UACR. All measurements were performed at 2-week intervals. Laboratory parameters were measured in a central laboratory in the United States (Quest Diagnostics Clinical Trials, Valencia, CA) and Japan (BML, Saitama, Japan). Analysis primarily focused on changes in fluid retention at week 2 of atrasentan therapy to maximize detection of a direct effect of atrasentan on fluid retention.
Statistics
Analyses were performed using SAS software (version 9.2; SAS Institute, Cary, NC). Data are presented as mean±SD or median (first quartile, third quartile) for skewed variables. The natural logarithm of albuminuria was used to normalize its distribution. Log-transformed variables were used in all regression analyses. A backward selection multivariate linear and logistic regression was used to identify predictors of week 2 changes in body weight and Hb. For the logistic regression model predictors of ≥2 kg rise in body weight (upper quartile of change of combined atrasentan 0.75 and 1.25 mg/d arms) and Hb fall ≥1.3 g/dL (lower quartile of change of combined atrasentan 0.75 and 1.25 mg/d arms) were investigated. In both the linear and logistic regression model the following covariates were entered with stepwise backward adjustment: treatment assignment, age, sex, body weight, Hb, eGFR, albuminuria, systolic BP, log-transformed homeostatic metabolic assessment (HOMA) product, log-transformed B-type natriuretic peptide (BNP), thiazide, and loop diuretic use. Pearson correlations were calculated to assess associations between week 2 changes in log-transformed albuminuria and body weight and Hb. Changes in fluid retention were also analyzed in UACR responders (≥30% reduction from baseline) and UACR nonresponders (<30% reduction from baseline). A P value <0.05 was considered to indicate statistical significance.
Results
Baseline Characteristics
As previously described, there were no significant differences in baseline characteristics among the placebo, atrasentan 0.75 mg/d, and atrasentan 1.25 mg/d groups (Table 1) (5).
Table 1.
Baseline characteristics
| Characteristic | Placebo (n=50) | Atrasentan 0.75 mg/d (n=78) | Atrasentan 1.25 mg/d (n=83) |
|---|---|---|---|
| Age, y | 64.3±9.0 | 65.0±9.8 | 64.5±8.8 |
| Sex | |||
| Male | 40 (80) | 63 (81) | 57 (69) |
| Female | 10 (20) | 15 (19) | 26 (31) |
| SBP, mmHg | 136±14 | 138±14 | 136±15 |
| DBP, mmHg | 72±10 | 75±10 | 74±9 |
| eGFR, ml/min per 1.73 m2 | 49.3±13.3 | 47.9±14.6 | 50.6±13.6 |
| HbA1c, % | 7.4±1.3 | 7.5±1.5 | 7.7±1.4 |
| Weight, kg | 84.3±20.2 | 87.1±22.1 | 88.3±18.4 |
| Hemoglobin, g/dl | 12.7±1.8 | 12.9±1.5 | 12.9±1.8 |
| Hematocrit, % | 38.1±5.4 | 38.8±4.3 | 38.6±5.3 |
| Serum albumin, g/dl | 4.0±0.4 | 4.0±0.4 | 4.1±0.3 |
| BNP, median (Q1–Q3), pg/ml | 31.7 (18.3–93.0) | 33.0 (16.0–70.0) | 33.5 (14.0–64.0) |
| UACR, median (Q1–Q3), mg/g creatinine | 671 (410–1536) | 878 (515–1682) | 826 (481–1389) |
| RAS inhibitors | 50 (100) | 78 (100) | 83 (100) |
| Diuretics | |||
| Loop diuretics | 19 (38) | 29 (37) | 27 (33) |
| Thiazides | 29 (58) | 42 (54) | 43 (52) |
Values are mean±SD, n (%), or as otherwise indicated. SBP, systolic BP; DBP, diastolic BP; HbA1c, glycated hemoglobin; BNP, B-type natriuretic peptide; Q1, quartile 1; Q3, quartile 3; UACR, urinary albumin to creatinine ratio; RAS, renin-angiotensin system. Modified from reference 5, with permission.
Atrasentan-Associated Changes in Weight, BNP, and Hb
The incidences of pulmonary and peripheral edema in these patients have been previously reported (5) and are not discussed in detail herein; no serious adverse events related to fluid retention were associated with atrasentan treatment. Body weight increased by approximately 1 kg after 2 weeks of treatment compared with a decrease of approximately 1 kg in the placebo group (Table 2). Changes in diuretic dose were similar among treatment groups throughout the study (4%, 5%, and 8% for the placebo, 0.75 mg/d, and 1.25 mg/d groups, respectively). Weight declined by 1–2 kg during the 30-day recovery period in the atrasentan-treated groups, but did not change during this time in the placebo group. Hb decreased by approximately 1 g/dl in both atrasentan groups after 2 weeks of treatment, and these reductions persisted throughout the treatment period (Table 2). Hb normalized by 30 days after treatment discontinuation, suggesting that the atrasentan-associated decrease in Hb was caused by hemodilution. Despite the gain in weight in patients who received atrasentan, no significant change was observed in BNP (Table 2).
Table 2.
Changes over time in hemoglobin and body weight during treatment with placebo or atrasentan
| Variable | Time | Placebo | Atrasentan 0.75 mg/d | Atrasentan 1.25 mg/d | |||
|---|---|---|---|---|---|---|---|
| Week | n | Mean±SD or Median | n | Mean±SD or Median | n | Mean±SD or Median | |
| UACR, mg/g | Baseline | 50 | 671 | 78 | 878 | 83 | 826 |
| 2 | 48 | 696 | 75 | 573 | 82 | 515 | |
| 6 | 48 | 686 | 74 | 636 | 75 | 461 | |
| 12 | 48 | 797 | 70 | 521 | 69 | 470 | |
| Recovery | 45 | 737 | 68 | 1051 | 71 | 727 | |
| Hemoglobin, g/dl | Baseline | 50 | 12.7±1.8 | 78 | 12.9±1.5 | 83 | 12.9±1.8 |
| 2 | 47 | 12.5±1.9 | 74 | 12.0±1.5 | 80 | 11.8±1.8 | |
| 6 | 48 | 12.6±1.9 | 72 | 11.9±1.7 | 73 | 11.6±1.7 | |
| 12 | 47 | 12.6±2.1 | 69 | 11.8±1.6 | 68 | 11.8±1.8 | |
| Recovery | 26 | 12.3±1.9 | 39 | 12.4±1.5 | 47 | 12.5±1.9 | |
| BNP, pg/ml | Baseline | 50 | 31.7 | 77 | 33.0 | 82 | 33.5 |
| 12 | 47 | 35.0 | 66 | 33.1 | 67 | 37.0 | |
| Weight, kg | Baseline | 50 | 84.3±20.2 | 78 | 87.1±22.1 | 83 | 88.3±18.4 |
| 2 | 48 | 83.2±19.6 | 77 | 88.0±22.2 | 82 | 89.4±18.7 | |
| 6 | 48 | 83.3±19.6 | 74 | 87.3±21.8 | 75 | 89.6±19.0 | |
| 12 | 48 | 82.8±18.6 | 70 | 87.3±22.3 | 69 | 89.0±19.2 | |
| Recovery | 44 | 83±20 | 61 | 85±23 | 70 | 88±19 | |
UACR, urinary albumin to creatinine ratio; BNP, B-type natriuretic peptide.
Baseline Predictors of Response in Weight and Hb
Baseline predictors of weight gain after 2 weeks of atrasentan treatment included an atrasentan dose of 0.75 or 1.25 mg/d versus placebo, lower eGFR, higher glycated hemoglobin (HbA1c), higher systolic BP, and lower HOMA product (Table 3). Linear regression analysis showed that atrasentan 0.75 and 1.25 mg/d were associated with a 0.8 (95% confidence interval [95% CI], 0.3 to 1.3) kg and 1.3 (95% CI, 0.8 to 1.7) kg weight gain, respectively. For each 10 mL/min lower baseline eGFR, body weight was higher by 0.2 (0.1–0.3) kg. For each percentage lower HbA1c, body weight was higher by 0.2 (95% CI, 0.1 to 0.4) kg; and for each 10-mmHg lower baseline systolic BP, body weight was higher by 0.1 (95% CI 0.0 to 0.3) kg. Logistic regression analysis of factors predicting a ≥2 kg weight gain (upper quartile of distribution, Figure 1A) showed the odds for a ≥2 kg weight gain were 3.0 (95% CI, 1.0 to 8.5) and 6.6 (95% CI, 2.3 to 18.6) times higher for atrasentan 0.75 and 1.25 mg/d, respectively. Determinants in the logistic regression were similar to those in the linear regression model (Table 3).
Table 3.
Independent baseline predictors of changes in weight and hemoglobin after 2 weeks of atrasentan therapy
| Variable | Linear Regression | Logistic Regression | ||
|---|---|---|---|---|
| Coefficient (95% CI) | P Value | Odds Ratio (95% CI) | P Value | |
| Weight response (≥2 kg weight gain) | ||||
| Atrasentan (0.75 versus placebo) | 0.8 (0.3 to 1.3) | 0.001 | 3.0 (1.0 to 8.5) | 0.04 |
| Atrasentan (1.25 versus placebo) | 1.3 (0.8 to 1.7) | <0.001 | 6.6 (2.3 to 18.6) | <0.001 |
| eGFR (10 ml/min per 1.73 m2) | –0.2 (–0.3 to –0.1) | 0.002 | 0.7 (0.4 to 1.0) | 0.01 |
| HbA1c (%) | 0.2 (0.1 to 0.4) | 0.002 | 1.7 (1.3 to 2.3) | <0.001 |
| Systolic BP (10 mmHg) | 0.1 (0.0 to 0.3) | 0.05 | — | |
| HOMA product (log) | –0.2 (–0.4 to –0.1) | 0.01 | 0.7 (0.5 to 1.0) | 0.03 |
| Hemoglobin response (≥1.3 g/dl hemoglobin fall) | ||||
| Atrasentan (0.75 versus placebo) | –0.7 (–0.9 to –0.5) | <0.001 | — | |
| Atrasentan (1.25 versus placebo) | –1.0 (–1.2 to –0.8) | <0.001 | 5.6 (2.5 to 12.7) | <0.001 |
| eGFR (10 ml/min per 1.73 m2) | 0.1 (0.1 to 0.2) | <0.001 | 0.6 (0.2 to 0.9) | 0.001 |
| Hemoglobin (g/dl) | –0.1 (–0.1 to 0.0) | <0.001 | 1.4 (1.1 to 1.8) | 0.003 |
| Weight (10 kg) | 0.1 (0.0 to 0.1) | 0.01 | 0.9 (0.8 to 1.0) | 0.05 |
The following covariates were included in the initial backward selection model: treatment assignment, age, sex, body weight, hemoglobin, eGFR, albuminuria, systolic BP, eGFR, log-transformed HOMA product, log-transformed B-type natriuretic peptide, thiazide, and loop diuretic use. Systolic BP and atrasentan dose were not included in the final logistic regression models for body weight and hemoglobin, respectively. HbA1c, glycated hemoglobin; HOMA, homeostatic model assessment; 95% CI, 95% confidence interval.
Figure 1.
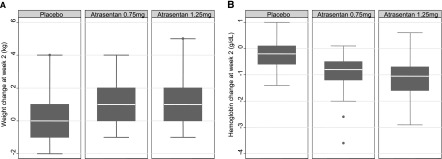
Distribution of changes in body weight and hemoglobin in subjects taking placebo, atrasentan 0.75 mg/d, or atrasentan 1.25 mg/d. Panel (A) shows changes in body weight and panel (B) shows changes in hemoglobin. Whiskers indicate all data points within 1.5 interquartile range of the upper and lower quartile range.
Baseline predictors of Hb change after 2 weeks of atrasentan treatment included an atrasentan dose of 0.75 or 1.25 mg/d versus placebo, eGFR, Hb, and weight (Table 3). Logistic regression analysis of factors predicting a ≥1.3 g/dl fall in Hb (upper quartile of distribution of combined atrasentan 0.75 and 1.25 mg arms, Figure 1B) showed an increase in the odds of 5.6 (95% CI, 2.5 to 12.7) fold with atrasentan 1.25 mg versus placebo (but no significant association with 0.75 mg) and 0.6 (95% CI, 0.2 to 0.9) fold for each 10 ml/min lower baseline eGFR. Small but significant associations with baseline weight and Hb were also observed. Baseline BNP was not associated with changes in body weight or Hb.
Correlation Between Changes in UACR and Changes in Weight and Hb
We did not observe a correlation between changes in UACR and body weight at week 2 in subjects taking placebo or atrasentan 0.75 or 1.25 mg/d (Table 4). Examination of R2 revealed that a very small percentage (0.5%–7%) of the variability in the albuminuria response in patients taking atrasentan is explained by the changes in weight. Changes in UACR as a function of body weight were also analyzed after 12 weeks of atrasentan treatment. As for the 2-week data, no correlation was detected between changes in UACR and body weight (data not shown). However, similar to the 2-week data, analysis of R2 revealed that only 0.8% of the variability in the albuminuria response was accounted for by the response in weight.
Table 4.
Correlations between changes in hemoglobin and weight with urinary albumin to creatinine ratio change after 2 weeks of placebo or atrasentan treatment
| Variable | Pearson Correlation | R2 |
|---|---|---|
| Placebo | ||
| Δ Hemoglobin (g/dl) | −0.05 | <0.01 |
| Δ Body weight (kg) | −0.06 | <0.01 |
| Atrasentan 0.75 mg/d | ||
| Δ Hemoglobin (g/dl) | 0.14 | 0.02 |
| Δ Body weight (kg) | −0.26 | 0.07 |
| Atrasentan 1.25 mg/d | ||
| Δ Hemoglobin (g/dl) | 0.18 | 0.04 |
| Δ Body weight (kg) | −0.07 | <0.01 |
No correlation was observed between changes in UACR and Hb after 2 weeks of atrasentan treatment in subjects taking placebo, atrasentan 0.75 mg/d, or atrasentan 1.25 mg/d (Table 4). Analysis of R2 reveals that 2%–3% of the variability in albuminuria response in patients taking atrasentan is explained by the changes in Hb. Similarly, changes in the UACR after 12 weeks of atrasentan treatment were not correlated with decreases in Hb (data not shown); however, examination of R2 shows that a small amount (4%–6.9%) of the variability in the albuminuria response was accounted for by the response in Hb.
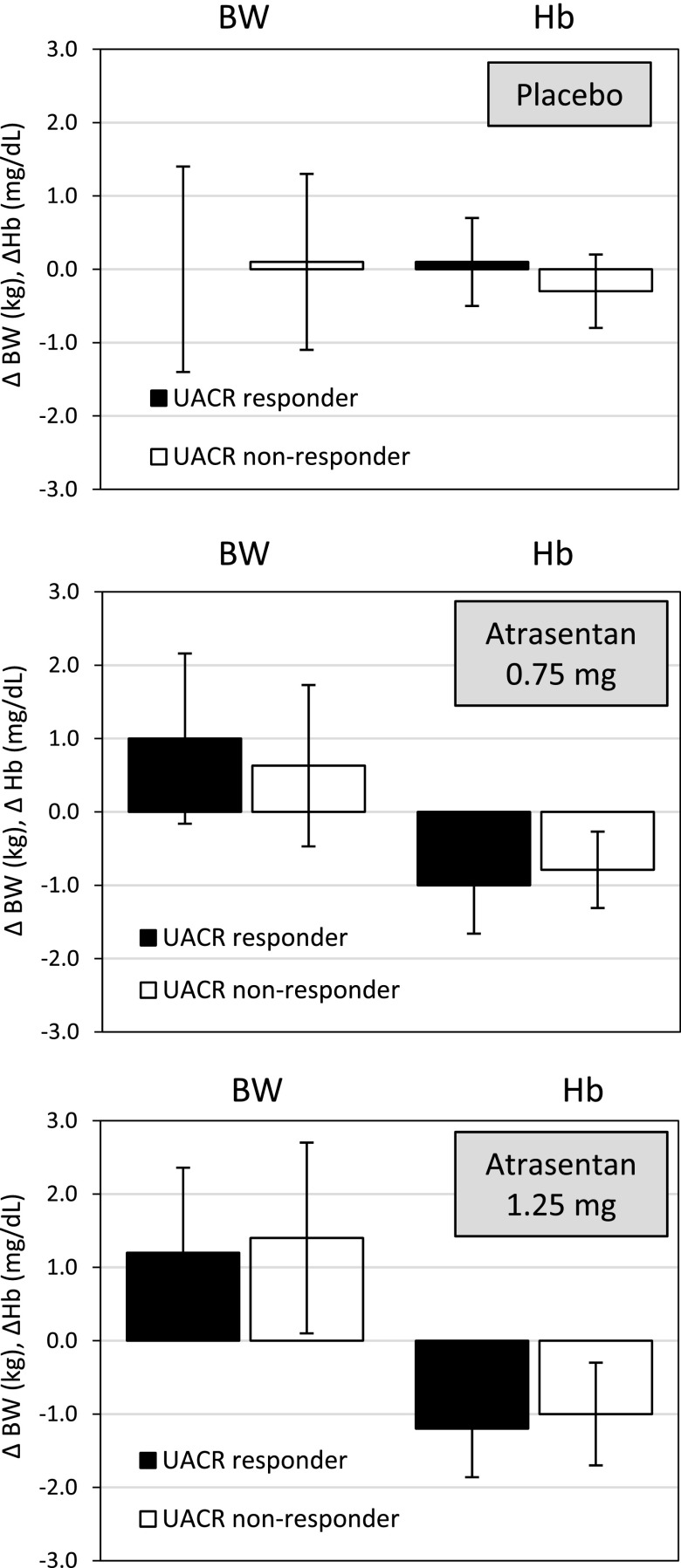
Changes in body weight or Hb were compared between UACR responders (≥30% reduction in the UACR) and nonresponders (<30% reduction in the UACR) after 2 weeks of atrasentan treatment. No difference was detected between UACR responders and nonresponders in changes in body weight or Hb in subjects receiving placebo or the atrasentan 0.75 or 1.25 mg/d doses (Figure 2).
Figure 2.
Changes in BW and Hb in UACR responders (defined as ≥30% reduction in UACR at week 2) and UACR nonresponders (<30% reduction in UACR at week 2) to placebo, atrasentan 0.75 mg/d, or atrasentan 1.25 mg/d. Error bars represent the SD. BW, body weight; Hb, hemoglobin; UACR, urinary albumin to creatinine ratio.
Discussion
This post hoc analysis of the RADAR/JAPAN trials identified baseline predictors of atrasentan-associated fluid retention. Atrasentan dose and lower eGFR predicted atrasentan-induced fluid retention as assessed by both surrogates of fluid retention: body weight and Hb. The degree of the antialbuminuric effect of atrasentan was not correlated with the degree of fluid retention irrespective of when the data were analyzed as continuous parameters or when the UACR was dichotomized as more or less than a 30% change. (The 30% UACR reduction value was chosen on the basis of previous studies [6–8] and is used in the phase III trial [Study of Diabetic Nephropathy with Atrasentan] to select responders/nonresponders.) These findings suggest that the antialbuminuric effect of atrasentan does not depend on the degree of fluid retention.
The finding that a higher atrasentan dose predicted fluid retention is in agreement with previous studies, which showed that ERAs cause dose-dependent fluid retention (1,4). In contrast, the finding that a lower eGFR predicts atrasentan-induced fluid retention appears to conflict with a post hoc analysis of the ASCEND trial wherein avosentan-treated patients who developed CHF had a higher eGFR than placebo-treated patients who developed CHF (2). However, within-group analysis of these data shows that avosentan-treated patients who developed CHF tended to have a lower eGFR compared with avosentan-treated patients who had no CHF. Although the safe lower limit of the eGFR for ERA therapy is not defined, these findings suggest that strict monitoring of fluid retention is appropriate in patients with impaired renal function.
Atrasentan-induced weight gain was associated with higher baseline levels of systolic BP (in the linear regression analysis only) or HbA1c and lower baseline HOMA product; whether poorer hemodynamic and metabolic control per se increases the propensity for atrasentan-related fluid retention or is a marker for other underlying responsible factors is unknown. It should be noted that statin use and lower baseline serum cholesterol levels increased avosentan-related CHF risk in a post hoc analysis of the ASCEND trial (2). The reasons for this latter relationship and why atrasentan-associated fluid retention was not associated with these parameters are unknown.
This analysis did not find a significant correlation between fluid retention and the albuminuria-lowering effect of atrasentan. Although such analysis has not been previously conducted, our conclusions are supported by the previous finding of relatively comparable degrees of avosentan-induced albuminuria reduction in patients with diabetes with >300 mg/d albuminuria (mean UACR decreases of 21% with 5 mg/d and 30% with 50 mg/d) despite marked differences in the incidence of fluid retention (12% with 5 mg/d, and 32% with 50 mg/d) (3). Similarly, in a smaller phase IIa trial with 89 patients with diabetes and nephropathy, atrasentan at 0.75 and 1.75 mg/d reduced albuminuria comparably in the face of 3-fold greater incidence of edema in the higher-dose group (9). Although this study was not designed to determine the mechanisms by which atrasentan causes fluid retention, consideration of the potential mechanisms responsible for the fluid-retaining and antiproteinuric effects of ERAs suggests plausible explanations for how these two actions can be separated and may exhibit differential dose responsiveness. The antiproteinuric effect of atrasentan has a rapid onset, reverses within 30 days of drug discontinuation, and is associated with a modest decrease in BP (5,9), suggesting, at least in part, a hemodynamic effect. In contrast, recent studies have strongly linked ERA-induced fluid retention to blockade of natriuretic ETA receptors in the nephron in general, and the collecting duct in particular (10,11). Therefore, the fluid-retaining effect of ERAs is most likely related to direct effects on renal tubular sodium transport, whereas the antiproteinuric effect of ERAs is likely associated with actions on the vasculature and/or glomerulus. Finally, it could be anticipated that ERA mitigation of proteinuria per se would favor renal fluid excretion; however, ERAs (particularly when used in higher doses than in this analysis) could still promote fluid retention through a separate effect on tubule Na and water reabsorption.
In summary, this study found that higher atrasentan dose, lower eGFR, higher baseline BP, and poorer baseline metabolic control were associated with atrasentan-induced fluid retention, as assessed by changes in body weight and Hb. Atrasentan-associated fluid retention did not correlate with changes in albuminuria.
Disclosures
Donald Kohan consults for AbbVie and has received grant support from the National Institutes of Health (NIH). Fan Fan Hou consults for AbbVie and Astellas. Hirofumi Makino is a consultant for AbbVie, Astellas, and Teijin; receives speaker honoraria from Astellas, Boehringer Ingelheim, Chugai, Daiichi Sankyo, Dainippon Sumitomo, Kyowa Hakko Kirin, MSD, Novartis, Pfizer, Takeda, and Tanabe Mitsubishi; and receives grant support from Astellas, Boehringer Ingelheim, Daiichi Sankyo, Dainippon Sumitomo, Kyowa Hakko Kirin, Mochida, MSD, Novartis, Novo Nordisk, Pfizer, Takeda, and Tanabe Mitsubishi. Dalane Kitzman is a consultant for AbbVie, Relypsa, Regeneron, Weststat, DC Devices, and GlaxoSmithKline; has received grant support from Novartis; and owns stock in Gilead Sciences. Ricardo Correa-Rotter consults for AbbVie, Amgen, and FMC; has provided lectures for Astra-Zeneca, Amgen, and Janssen; and has received grant support from Fibrogen, Pfizer, and Amgen. Sheldon Tobe consults for AbbVie. Hans-Henrik Parving consults for AbbVie. Dick de Zeeuw consults for and received honoraria (to employer) from AbbVie, Astellas, AstraZeneca, Bristol-Myers Squibb, Chemocentryx, Johnson & Johnson, Hemocue, MSD, Novartis, Reata, Takeda, and Vitae. Hiddo Lambers Heerspink consults for and received honoraria (to employer) from AbbVie, Astellas, Reata, and Vitae. Robert Toto is a consultant to AbbVie, Amgen, Boehringer Ingelheim, Stealth Peptides, Bristol-Myers Squibb, Celgene, ZS Pharma, and Relypsa and receives grant support from Ardelyx and the NIH. Vlado Perkovic consults for AbbVie, Astellas, Bristol-Myers Squibb, Boehringer Ingelheim, Eli Lilly, GlaxoSmithKline, and Janssen; has received lecture fees or grant support from Baxter, Boehringer Ingelheim, Merck, and Pfizer; and his institution has held clinical trial contracts with AbbVie, Roche, Janssen, Servier, and Novartis. Giuseppe Remuzzi has consultancy agreements with AbbVie, Alexion Pharmaceuticals, Bayer Healthcare, Reata Pharmaceuticals, Novartis Pharma, AstraZeneca, Otsuka Pharmaceutical Europe, and Concert Pharmaceuticals—no personal remuneration was accepted; compensations are paid to his institution for research and educational activities. Blai Coll, Dennis Andress, and John Brennan are employees of AbbVie and may own stock or stock options.
Supplementary Material
Acknowledgments
We greatly appreciate the participation of the many individuals in these studies and the efforts of all of the investigators.
This study was supported by AbbVie.
An abstract containing data from this study was accepted for presentation at the American Society of Nephrology Kidney Week, November 11–16, 2014, in Philadelphia, Pennsylvania.
The sponsor was involved in the design of the study, in the collection and analysis of the data, and in writing the report. All authors had access to study results, and the lead author vouches for the accuracy and completeness of the data reported. The lead author had the final decision to submit the publication.
Footnotes
Published online ahead of print. Publication date available at www.cjasn.org.
This article contains supplemental material online at http://cjasn.asnjournals.org/lookup/suppl/doi:10.2215/CJN.00570115/-/DCSupplemental.
References
- 1.Kohan DE, Barton M: Endothelin and endothelin antagonists in chronic kidney disease. Kidney Int 86: 896–904, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mann JF, Green D, Jamerson K, Ruilope LM, Kuranoff SJ, Littke T, Viberti G, ASCEND Study Group : Avosentan for overt diabetic nephropathy. J Am Soc Nephrol 21: 527–535, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wenzel RR, Littke T, Kuranoff S, Jürgens C, Bruck H, Ritz E, Philipp T, Mitchell A, SPP301 (Avosentan) Endothelin Antagonist Evaluation in Diabetic Nephropathy Study Investigators : Avosentan reduces albumin excretion in diabetics with macroalbuminuria. J Am Soc Nephrol 20: 655–664, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Barton M, Kohan DE: Endothelin antagonists in clinical trials: Lessons learned. Contrib Nephrol 172: 255–260, 2011 [DOI] [PubMed] [Google Scholar]
- 5.de Zeeuw D, Coll B, Andress D, Brennan JJ, Tang H, Houser M, Correa-Rotter R, Kohan D, Lambers Heerspink HJ, Makino H, Perkovic V, Pritchett Y, Remuzzi G, Tobe SW, Toto R, Viberti G, Parving HH: The endothelin antagonist atrasentan lowers residual albuminuria in patients with type 2 diabetic nephropathy. J Am Soc Nephrol 25: 1083–1093, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Imai E, Haneda M, Chan JC, Yamasaki T, Kobayashi F, Ito S, Makino H: Reduction and residual proteinuria are therapeutic targets in type 2 diabetes with overt nephropathy: A post hoc analysis (ORIENT-proteinuria). Nephrol Dial Transplant 28: 2526–2534, 2013 [DOI] [PubMed] [Google Scholar]
- 7.Xie D, Hou FF, Fu BL, Zhang X, Liang M: High level of proteinuria during treatment with renin-angiotensin inhibitors is a strong predictor of renal outcome in nondiabetic kidney disease. J Clin Pharmacol 51: 1025–1034, 2011 [DOI] [PubMed] [Google Scholar]
- 8.de Zeeuw D, Remuzzi G, Parving HH, Keane WF, Zhang Z, Shahinfar S, Snapinn S, Cooper ME, Mitch WE, Brenner BM: Proteinuria, a target for renoprotection in patients with type 2 diabetic nephropathy: Lessons from RENAAL. Kidney Int 65: 2309–2320, 2004 [DOI] [PubMed] [Google Scholar]
- 9.Kohan DE, Pritchett Y, Molitch M, Wen S, Garimella T, Audhya P, Andress DL: Addition of atrasentan to renin-angiotensin system blockade reduces albuminuria in diabetic nephropathy. J Am Soc Nephrol 22: 763–772, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Stuart D, Chapman M, Rees S, Woodward S, Kohan DE: Myocardial, smooth muscle, nephron, and collecting duct gene targeting reveals the organ sites of endothelin A receptor antagonist fluid retention. J Pharmacol Exp Ther 346: 182–189, 2013 [DOI] [PubMed] [Google Scholar]
- 11.Stuart D, Rees S, Woodward SK, Koesters R, Strait KA, Kohan DE: Disruption of the endothelin A receptor in the nephron causes mild fluid volume expansion. BMC Nephrol 13: 166, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.