Abstract
Background and objectives
Disease-specific treatment options for autosomal dominant polycystic kidney disease are limited. Clinical intervention early in life is likely to have the greatest effect. In a 3-year randomized double-blind placebo-controlled phase 3 clinical trial, the authors recently showed that pravastatin decreased height-corrected total kidney volume (HtTKV) progression of structural kidney disease over a 3-year period. However, the underlying mechanisms have not been elucidated.
Design, setting, participants, & measurements
Participants were recruited nationally from July 2007 through October 2009. Plasma and urine samples collected at baseline, 18 months, and 36 months from 91 pediatric patients enrolled in the above-mentioned clinical trial were subjected to mass spectrometry–based biomarker analysis. Changes in biomarkers over 3 years were compared between placebo and pravastatin-treated groups. Linear regression was used to evaluate the changes in biomarkers with the percent change in HtTKV over 3 years.
Results
Changes in plasma concentrations of proinflammatory and oxidative stress markers (9- hydroxyoctadecadienoic acid, 13-hydroxyoctadecadienoic acid, and 15-hydroxyeicosatetraenoic acid [HETE]) over 3 years were significantly different between the placebo and pravastatin-treated groups, with the pravastatin group showing a lower rate of biomarker increase. Urinary 8-HETE, 9-HETE, and 11-HETE were positively associated with the changes in HtTKV in the pravastatin group.
Conclusions
Pravastatin therapy diminished the increase of cyclooxygenase- and lipoxygenase-derived plasma lipid mediators. The identified biomarkers and related molecular pathways of inflammation and endothelial dysfunction may present potential targets for monitoring of disease severity and therapeutic intervention of autosomal dominant polycystic kidney disease.
Keywords: autosomal dominant polycystic kidney disease, angiotensin-converting enzyme inhibitors, oxidative stress, pediatric nephrology, statins
Introduction
Autosomal dominant polycystic kidney disease (ADPKD) is the most common inherited kidney disease, occurring in one in 400 to one in 1000 live births (1). Approximately one half of affected patients develop ESRD by age 60 years. ADPKD is characterized by progressive cyst development and enlarged kidneys with multiple cysts. ADPKD is a genetic disease, and interventions provided early in life should have the highest success to inhibit its progression.
Our previous observational studies showed that children with ADPKD and hypertension have larger kidney volumes compared with their counterparts with normotension (2,3). There is a significant association between left ventricular mass index (LVMI) and renal volume in children with hypertension (4). We previously showed that angiotensin-converting enzyme inhibitors (ACEIs) reduced the decline of creatinine clearance and the increase in LVMI in pediatric ADPKD, but they did not affect progression of structural kidney disease (5).
Statins were shown to reduce the severity of PKD in heterozygous Han:Sprague-Dawley cystic rats (6,7) and to improve renal function in short-term studies in adults with ADPKD (8). Although the underlying mechanisms are not well understood, it has been proposed that these renoprotective effects are mediated by statin-related inhibition of G proteins, resulting in decreased cell proliferation (6).
On the basis of these findings, we designed a 3-year randomized double-blind placebo-controlled phase 3 clinical trial to determine the effect of pravastatin on kidney and cardiovascular disease progression in children and young adults with ADPKD who were receiving the ACEI lisinopril (9). The positive outcome of the study (10) supports early therapeutic intervention and pravastatin as an effective agent to slow the progression of ADPKD. However, the underlying mechanisms of this beneficial effect remain to be defined.
We and others have shown that adult patients with ADPKD have elevated levels of cardiovascular disease risk and oxidative stress markers compared with healthy individuals (11–15) (Figure 1). These as well as inflammatory bioactive lipid mediators were elevated early in the disease and were associated with the increase in total kidney volume (TKV) and decline in renal function (14,16,17) (Figure 1).
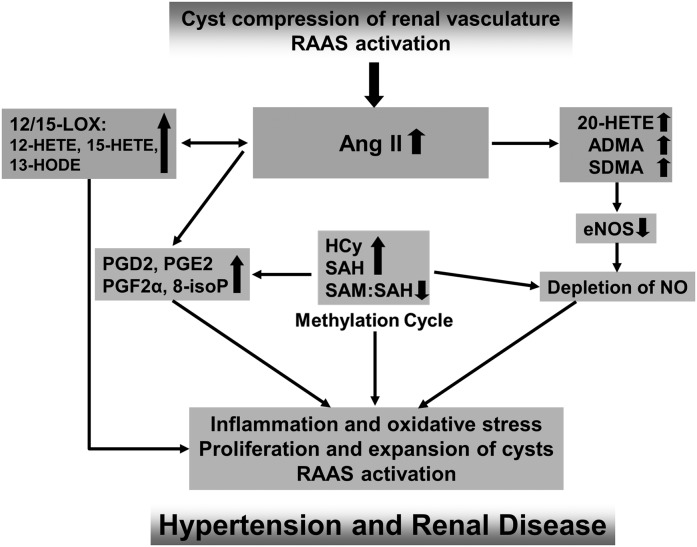
Figure 1.
Proposed mechanistic hypothesis based on the published data and the results of our studies in patients with ADPKD (11,16,44). A vicious cycle is created in which renal cysts activate the RAAS, and the resulting increase in AngII leads to (1) increased levels of eNOS inhibitor ADMA, which further depletes NO; (2) increased HCy and SAH levels within the methylation pathway and diminished methylation capacity (SAM/SAH); and (3) increased metabolism of AA, leading to generation of proinflammatory and oxidative stress–accelerating PGs as well as HETE and HODE. Oxidative stress, vascular inflammation, and endothelial dysfunction in turn augment the cyst expansion and activate the RAAS. 8-isoP, 8-isoprostane; ADMA, asymmetric dimethylarginine; ADPKD, autosomal dominant polycystic kidney disease; AngII, angiotensin II; eNOS, endothelial nitric oxide synthase; HCy, homocysteine; HETE, hydroxyeicosatetraenoic acid; HODE, hydroxyoctadecadienoic acid; LOX, lipoxygenase; NO, nitric oxide; RAAS, renin-angiotensin-aldosterone system; SAH, S-adenosyl homocysteine; SAM, S-adenosyl methionine; SDMA, symmetric dimethylarginine.
On the basis of this knowledge, we aimed to investigate whether the markers of endothelial dysfunction, inflammation, and oxidative stress identified in previous studies (11,13–16) (Figure 1) change in response to pravastatin therapy. This would qualify their use for monitoring of ADPKD disease progression and therapy efficacy. Plasma and urine samples from the above-mentioned clinical trial were used and biomarkers were correlated with changes in height-corrected total kidney volume (HtTKV).
Materials and Methods
Trial Design
For this study, we used plasma and urine samples collected during the referenced clinical trial (9,10). Eligible participants were aged 8–22 years and had ADPKD with a Schwartz eGFR>80 ml/min per 1.73 m2 (18).
Each participant was evaluated during a 2-day admission at the Clinical Translational Research Center at Children’s Hospital Colorado in Aurora, Colorado. Participants provided a detailed history and underwent a physical examination including growth parameters and Tanner staging (19–21). On the first night of admission, participants fasted for a 10-hour period and then blood was drawn for various analyses, including the biomarkers. Within 2 hours of collection, EDTA plasma tubes were frozen and stored at −70°C until analysis. Two 24-hour urine collections were obtained for creatinine, microalbumin, and eGFR determination (18). For biomarker analysis, urine from the second collection was used. Urine was stored at 4°C during the 24-hour collection; afterward, aliquots were made, frozen, and stored at −70°C until analysis. An additional centrifugation step (for 10 minutes at 400g) occurred before the liquid chromatography–mass spectrometry analysis. Twelve sitting BP measurements were obtained in the right arm (Dinamap Pro 300V2; GE Medical Systems Information Technology, Tampa, FL) and were related to published data for age-, sex-, and height-matched children (22). At the conclusion of the baseline visit, research participants were randomized to receive pravastatin or placebo for the remainder of the 3-year study. The standard dosage of pravastatin was based on age as follows: 20 mg daily (8–12 years) or 40 mg daily (13–22 years). All participants were treated with the ACEI lisinopril (10). At 18 and 36 months after the initial visit, participants returned for repeated admission, during which time they underwent the same study procedures described above (10).
The study protocol was approved and routinely reviewed by the Colorado Multiple Institutional Review Board. This clinical trial was registered at ClinicalTrials.gov (identifier NCT00456365). The study was in full compliance with the principles of the Declaration of Helsinki and its amendments. All patients and/or guardians gave written informed assent or consent as applicable.
Concentrations of biomarkers in healthy individuals were obtained from the literature (as referenced). Because of the unavailability of normal ranges for urinary hydroxyeicosatetraenoic acid (HETE) compounds, we determined them in house in the spot urine of 15 healthy adult volunteers (10 men and five women, also normalized to creatinine).
Analyses of Abdominal Magnetic Resonance Imaging
DICOM images were deidentified and TKV was measured by stereology (23,24) (Analyze 9.0; Mayo Foundation, Biomedical Imaging Resource Core, Rochester, MN) by a single analyst who had no knowledge of the patient’s status. To account for normal growth in children, TKV was corrected for height.
Since ACEIs prevented the decrease in creatinine clearance and the increase in LVMI, but they did not affect kidney volume growth (5), change in HtTKV was chosen as the primary correlation parameter. Furthermore, HtTKV serves as a better predictor than baseline age, serum creatinine, BUN, urinary albumin, or monocyte chemotactic protein-1 excretion for the onset of renal insufficiency (25,26).
Measurement of Biomarkers
Endothelial Dysfunction Markers.
Endothelial dysfunction markers, including arginine, asymmetric dimethylarginine (ADMA), symmetric dimethylarginine (SDMA), citrulline, cysteine, homocysteine (HCy), methionine (Met), S-adenosylhomocysteine (SAH), and S-adenosylmethionine (SAM), were quantified in plasma using an HPLC–tandem mass spectrometry (MS/MS) assay (27). In brief, the API5000 mass spectrometer (AB Sciex, Concord, ON, Canada) was run in the positive electrospray ionization mode (ESI) using multiple reaction monitoring (MRM). The following ion transitions were used (mass/charge ratio [m/z]): arginine, 175.2→70.1; ADMA, 203.2→46.2; SDMA, 203.2→172.2; d7-ADMA (internal standard), 210.2 →77.2; cysteine, 122.0→75.9; d2-cysteine (internal standard), 124.0→77.9; citrulline, 176.4→159.1; HCy, 136.1→90.1; d4-HCy (internal standard), 140.1→94.1; Met, 150.1→104.0; d3-Met (internal standard), 153.1→107.0; SAM, 399.0→250.1; d3-SAM (internal standard), 402.0→136.2; SAH, 385.0→136.2; and d5-SAH (internal standard), 391.0→137.2.
Urinary PGs.
Urinary PGs were analyzed on an HPLC system interfaced with an API5000 tandem mass spectrometer operated in the negative ESI mode. The following ion transitions (MRM mode) were monitored for the study compounds: m/z 353.2→193.1 for 8-isoprostane, PGF2α, and 9α,11β-PGF2; m/z 351.2→189.2 for PGD2, Δ12-PGD2, and PGE2; m/z 333.4→233.2 for PGJ2; m/z 315.2→271.3 for 15d-PGJ2; and 438.2→235.1 for leukotriene E4. The following deuterated internal standards were used: 8-isoprostane-d4, PGF2α-d9, 9α,11β-PGF2-d4, PGD2-d9, PGE2-d9, 15d-PGJ2-d4, and leukotriene E4-d5 (Cayman Chemical Company, Ann Arbor, MI).
Bioactive Lipid Mediators.
Bioactive lipid mediators were analyzed in plasma and urine samples using a modification of a previously described HPLC–tandem mass spectrometry assay (16,28). The API5500 mass spectrometer was run in the positive ESI MRM mode. The following hydroxy-fatty acids were quantified: 9-hydroxyoctadecadienoic acid (HODE), 13-HODE, 8-HETE, 9-HETE, 12-HETE, 15-HETE, 20-HETE, and 15-hydroxyeicosapentaenoic acid. All compounds, including the internal standards, 12-HETE-d8, 20-HETE-d6, 9-HODE-d4, and 13-HODE-d4, were purchased from Cayman Chemicals (Ann Arbor, MI, USA).
Urinary Metabolites.
Citrate, succinate, hippurate, uric acid, glucose, creatinine, α-ketoglutarate, and lactate were quantified in urine samples. The internal standard solution contained d6-glucose, d3-creatinine, d5-hippurate, and d4-succinate. All samples were analyzed on a positive/negative electrospray API4000 mass spectrometer (AB Sciex).
For more detail on the mass spectrometry methods, please refer to the Supplemental Material.
Statistical Analyses
All of the biomarkers were natural logarithm for analysis because the distributions were skewed. ANOVA was used to compare the change (Δ) in markers. Linear regression was used to evaluate the relationship of each biomarker with HtTKV. Three models were run for each marker: (1) unadjusted, (2) adjusted for age and sex, and (3) adjusted for age, sex, systolic BP, and log-transformed GFR. Although the original study was powered for a combined end point of 20% increase in LVMI, TKV, or urinary microalbumin excretion, this study was primarily exploratory. SPSS (version 21.0; SPSS, Chicago, IL) and SAS (version 9.3; SAS Institute, Cary, NC) software was used for the statistical analyses. A P value <0.05 was considered significant.
Results
Patient Characteristics
From 110 participants enrolled in the clinical trial, 101 plasma and 107 urine samples collected at baseline were available and were used for the biomarker analysis. The baseline characteristics are presented in Table 1. We randomized 56 patients to the pravastatin group and 54 patients to the placebo group. Ninety-one participants completed the 3-year study, resulting in an overall completion rate of 83%. From these, 88 plasma samples and 85 urine samples were available for the biomarker analysis, again without any preselection process.
Table 1.
Patient demographic, clinical characteristics, and biomarker concentrations at baseline visit
| Characteristic | Value |
|---|---|
| Age, yr | 15.6±3.7 |
| Female sex, % | 39 |
| Height, cm | 164.1±13.7 |
| Weight, kg | 63.5±20.3 |
| Urine microalbumin excretion, μg/min | 11.8 (6.6–24.1) |
| Left ventricular mass index, g/m2 | 53.7±11.4 |
| TKV, ml | 461.5 (341.5–656.5) |
| HtTKV, ml/m | 279.5 (211.5–378) |
| TKV/body surface area, ml/1.73 m2 | 465.5 (389.3–627) |
| Serum creatinine, mg/dl | 0.7 (0.5–0.8) |
| eGFR, ml/min per 1.73 m2 (18) | 144.2±36.7 |
| Systolic BP, mmHg | 122.0±10.1 |
| Diastolic BP, mmHg | 72.6±3.4 |
| Hematocrit, % | 40.4±3.4 |
| 24-h urine protein, g/d | 0.11 (0.08–0.16) |
| HDL cholesterol, mg/dl | 48.0±11.7 |
| LDL cholesterol, mg/dl | 86.4±22.4 |
| Total cholesterol, mg/dl | 147.7±28.5 |
Values are presented as the mean±SD or median (25th percentile to 75th percentile), unless otherwise indicated. TKV, total kidney volume; HtTKV, height-corrected total kidney volume.
Changes in Biomarker Concentrations over Time and Comparison with Healthy Individuals
Table 2 represents a summary of biomarker concentrations at baseline and after 3 years in either the placebo or pravastatin group compared with normal ranges reported in healthy individuals (adults and/or children, where available). Unfortunately, the majority of normal ranges for the investigated biomarkers have only been reported in healthy adult individuals. The mean age of our study participants was 16±4 years; thus, some variance in the comparison of the measured values and the reported adult normal range can be expected.
Table 2.
Changes in biomarker concentrations over 3 years in children and young adults with ADPKD
| Marker | Placebo and Pravastatin | Normal Range in Adults and Children | |
|---|---|---|---|
| Baseline | 36 mo | ||
| Methionine plasma, µM | 18.0±9.3 | 20.7±12.5 | 33±9 (49)a |
| 14.0±6.0 | 20.2±7.3 | 32±6 (50) | |
| 9-HODE plasma, ng/ml | 6.3±2.2 | 7.1±2.9 | 2.0±0.5 (51)a |
| 7.7±5.9 | 6.3±2.5 | ||
| 13-HODE plasma, ng/ml | 8.3±3.0 | 11.4±6.3 | 3.1±0.4 (51)a |
| 9.9±6.0 | 8.8±3.2 | ||
| 8-HETE plasma, pg/ml | 130±77 | 162±139 | 240±11 (51)a |
| 125±78 | 118±31 | ||
| 11-HETE plasma, pg/ml | 304±240 | 282±260 | 233±19 (51)a |
| 277±169 | 168±75 | ||
| 12-HETE plasma, ng/ml | 3.0±4.3 | 4.8±9.2 | 1.4±0.1 (51)a |
| 1.9±2.4 | 1.7±1.2 | ||
| 15-HETE plasma, pg/ml | 762±531 | 930±806 | 256±70 (51)a |
| 847±497 | 630±181 | ||
| 15-HEPE plasma, pg/ml | 57.0±27.9 | 48.5±22.0 | 63±6 (51)a |
| 73.5±42.8 | 47.7±15.2 | ||
| Citrate urine, µM/mmol creatinine | 207±172 | 158±140 | 203 (49–600) (52) |
| 156±161 | 173±203 | 184 (59–652) (53) | |
| PGF2α urine, ng/mmol creatinine | 244±208 | 160±134 | 106±17 (54)a |
| 209±287 | 186±165 | ||
| 9-HODE urine, ng/mmol creatinine | 93.9±56.5 | 77.0±42.8 | 30.2±28.8 |
| 101±115 | 77.9±41.9 | ||
| 13-HODE urine, ng/mmol creatinine | 200±342 | 131±169 | 61.5±82.5 |
| 169±201 | 135±146 | ||
| 8-HETE urine, ng/mmol creatinine | 20.0±50.8 | 13.0±17.8 | 15.2±23.3 (55) |
| 14.4±14.5 | 19.3±30.5 | 16.1±10.9 | |
| 9-HETE urine, ng/mmol creatinine | 21.5±66.1 | 16.8±15.5 | 24.1±17.9 |
| 17.7±20.4 | 21.3±26.9 | ||
| 11-HETE urine, ng/mmol creatinine | 14.2±29.9 | 7.8±6.9 | 17.4±10.2 |
| 10.9±9.3 | 11.2±18.5 | ||
| 11-HETE urine, ng/mmol creatinine | 690±3158 | 340±574 | 50.8±63.1 |
| 301±523 | 451±739 | ||
Biomarker concentrations measured in patients with ADPKD are presented as the mean±SD. Normal ranges of biomarker concentrations in healthy adults and children (where available) were adopted from the literature and are presented as the mean±SD, mean±SEM, or mean (25 percentile to 75 percentile). Normal ranges of urinary HODEs and HETEs were determined in house in the spot urine of 15 healthy adult volunteers (10 men and 5 women, also normalized to creatinine). ADPKD, autosomal dominant polycystic kidney disease; HODE, hydroxyoctadecadienoic acid; HETE, hydroxyeicosatetraenoic acid; HEPE, hydroxyeicosapentaenoic acid.
Mean±SEM.
Children and young adults with ADPKD appeared to have lower plasma concentrations of Met compared with the healthy adults (at baseline; Table 2). Furthermore, plasma levels of proinflammatory and oxidative stress markers 9-HODE, 13-HODE, 12-HETE, and 15-HETE were elevated compared with those in healthy adults (at baseline); although they further increased in the placebo group, treatment with pravastatin led to their decline (Tables 2 and 3).
Table 3.
Difference in concentrations of plasma biomarkers between study groups (pravastatin versus placebo) over the 3-year study period compared with the baseline
| Variable (Δ) | Pravastatin | Placebo | P Value |
|---|---|---|---|
| Asymmetric dimethylarginine, µM | 0.07±0.09 | 0.03±0.10 | 0.09 |
| Arginine, µM | −9.49±27.36 | −9.73±31.15 | 0.97 |
| Citrulline, µM | 0.28±6.44 | 1.39±7.74 | 0.48 |
| Cysteine, µM | 2.91±30.22 | 14.47±30.25 | 0.08 |
| Homocysteine, µM | 0.57±8.20 | 2.83±6.30 | 0.17 |
| Methionine, µM | 6.58±9.44 | 3.19±13.31 | 0.18 |
| S-adenosyl methionine, nM | −2.55±37.76 | −0.78±31.65 | 0.84 |
| S-adenosyl homocysteine, nM | −4.41±6.09 | −4.03±6.60 | 0.74 |
| 9-HODE, ng/ml | −2.05±6.25 | 1.01±3.3 | 0.02 |
| 13-HODE, ng/ml | −1.82±6.84 | 3.32±6.82 | 0.004 |
| 8-HETE, ng/ml | −0.02±0.09 | 0.04±0.17 | 0.14 |
| 11-HETE, ng/ml | −0.13±0.18 | −0.01±0.38 | 0.12 |
| 12-HETE, ng/ml | −0.79±2.94 | 2.15±11.50 | 0.18 |
| 15-HETE, ng/ml | −0.28±0.55 | 0.18±1.04 | 0.04 |
| 20-HETE, ng/ml | −0.05±0.17 | 0.00±0.11 | 0.15 |
| 15-HEPE, ng/ml | −0.03±0.05 | −0.01±0.03 | 0.15 |
Data are presented as the mean±SD. HODE, hydroxyoctadecadienoic acid; HETE, hydroxyeicosatetraenoic acid; HEPE, hydroxyeicosapentaenoic acid.
In urine, citrate levels were in the same range as in healthy adults and declined in the placebo group, with no change in the pravastatin group (Table 2). Similarly, PGF2α declined more strongly in the patients receiving placebo compared with those taking pravastatin (Table 2). This decline, however, appeared to be toward normal levels reported in healthy adults (Table 2). By contrast, urinary 8-HETE, which was in the normal range at baseline, increased only in patients receiving pravastatin (Table 2).
Correlations of Plasma Biomarkers
When the percent change in HtTKV over 3 years was regressed against the log-transformed biomarker concentration, only the change in Met was positively associated with the change in HtTKV in the placebo group (unadjusted model, P=0.02). In the pravastatin group, 13-HODE was inversely associated with the change in HtTKV (Table 4).
Table 4.
Regression of percent change in HtTKV over 3 years versus change in plasma biomarker concentrations by study group (placebo and pravastatin)
| Variable (Δ) | Group | Model | Estimate ± SEM | 95% Confidence Limits | P Value |
|---|---|---|---|---|---|
| Methionine, µM | Placebo | Unadjusted | 0.64±0.25 | 0.14 to 1.1 | 0.01 |
| Model 1 | 0.45±0.27 | −0.10 to 1.0 | 0.11 | ||
| Model 2 | 0.45±0.28 | −0.12 to 1.0 | 0.12 | ||
| 15-HEPE, ng/ml | Placebo | Unadjusted | −49.4±19.0 | −89.6 to −9.1 | 0.02 |
| Model 1 | −45.3±20.4 | −89.0 to −1.6 | 0.04 | ||
| Model 2 | −48.0±18.3 | −87.9 to −8.0 | 0.02 | ||
| 13-HODE, ng/ml | Pravastatin | Unadjusted | −4.1±1.7 | −7.5 to −0.7 | 0.02 |
| Model 1 | −4.5±1.7 | −8.0 to −1.1 | 0.01 | ||
| Model 2 | −3.7±1.9 | −7.7 to −0.28 | 0.07 |
Results are presented as the estimate±SEM with 95% confidence limits. Only significantly different results are presented. Regression models included the following: model 1, adjusted for age and sex; and model 2, adjusted for age, sex, log-transformed GFR, and systolic BP. HtTKV, height-corrected total kidney volume; HEPE, hydroxyeicosapentaenoic acid; HODE, hydroxyoctadecadienoic acid.
Correlations of Urine Biomarkers
There were no significant differences in change in biomarker concentration between the pravastatin and placebo groups, using ANOVA.
Regression analysis of the percent change in HtTKV over 3 years in all patients versus the log-transformed biomarkers (unadjusted, model 1 adjusted for age and sex, and model 2 adjusted for age, sex, log-transformed GFR, and systolic BP) revealed significant inverse associations with citrate (estimate ± SEM, −0.004±0.002 mmol/mmol creatinine; 95% confidence limits, −0.01 to −0.001; P=0.02 for model 2) and PGF2α (estimate ± SEM, −0.003±0.001 µg/mmol creatinine; 95% confidence limits, −0.01 to −0.001; P=0.02 for model 2).
Citrate and PGF2α urine concentrations were inversely associated with the change in HtTKV over 3 years in the placebo group, whereas 8-HETE, 9-HETE, and 11-HETE were positively associated with the change in HtTKV in the pravastatin group (Table 5).
Table 5.
Regression of percent change in HtTKV versus change in urine biomarkers by study group (placebo and pravastatin)
| Variable | Group | Model | Estimate ± SEM | 95% Confidence Limits | P Value |
|---|---|---|---|---|---|
| Citrate, mmol/mmol creatinine | Placebo | Unadjusted | −0.007±0.004 | −0.02 to 0.001 | 0.09 |
| Model 1 | −0.008±0.004 | −0.02 to −0.004 | 0.04 | ||
| Model 2 | −0.008±0.004 | −0.02 to −0.003 | 0.04 | ||
| PGF2α, µg/mmol creatinine | Placebo | Unadjusted | −0.007±0.003 | −0.01 to −0.001 | 0.03 |
| Model 1 | −0.006±0.003 | −0.01 to −0.001 | 0.03 | ||
| Model 2 | −0.006±0.003 | −0.01 to −0.001 | 0.03 | ||
| 8-HETE, µg/mmol creatinine | Pravastatin | Unadjusted | 0.14±0.06 | 0.01 to 0.27 | 0.03 |
| Model 1 | 0.17±0.06 | 0.05 to 0.29 | 0.01 | ||
| Model 2 | 0.18±0.06 | 0.06 to 0.30 | 0.01 | ||
| 9-HETE, µg/mmol creatinine | Pravastatin | Unadjusted | 0.15±0.07 | 0.01 to 0.29 | 0.03 |
| Model 1 | 0.22±0.06 | 0.09 to 0.35 | 0.001 | ||
| Model 2 | 0.23±0.06 | 0.11 to 0.36 | <0.001 | ||
| 11-HETE, µg/mmol creatinine | Pravastatin | Unadjusted | 0.18±0.11 | −0.05 to 0.40 | 0.11 |
| Model 1 | 0.23±0.10 | 0.01 to 0.44 | 0.04 | ||
| Model 2 | 0.26±0.10 | 0.04 to 0.47 | 0.02 |
Results are presented as the estimate±SEM with 95% confidence limits. Regression models included the following: model 1, adjusted for age and sex; and model 2, adjusted for age, sex, log-transformed GFR, and systolic BP. HtTKV, height-corrected total kidney volume; HETE, hydroxyeicosatetraenoic acid.
Discussion
ADPKD is the fourth most common cause of ESRD. The disease course and risk factors for ADPKD progression have not been well described in large cohorts of children. Because the structural disease starts early and renal function is maintained within the normal range for some decades (5,29–31), identifying risk markers early in the course might provide the basis for effective intervention and minimization of complications in patients with ADPKD.
Experimental evidence in rats has shown that statins decrease the severity of functional and structural disease in ADPKD (6,7,32). In adult patients with ADPKD, 4-week administration of simvastatin improved renal blood flow and GFR (8). Moreover, statins are beneficial for endothelial dysfunction (33–35), which is known to occur early in patients with ADPKD, even before the onset of hypertension (36).
Therefore, we designed a 3-year randomized study to examine the effects of statin treatment on kidney volume growth, LVMI, and urinary microalbumin excretion in children and young adults with ADPKD (9). The results showed that the percent increase in HtTKV adjusted for age, sex, and hypertension status over the 3-year period was significantly decreased with pravastatin (10).
Plasma concentrations of 9-HODE and 13-HODE, which are both metabolites of linoleic acid through oxidation by 15-lipoxygenase (LOX) and cyclooxygenases (COXs) into 13-HODE (37–39) and by COX into 9-HODE (37), and 12-HETE and 15-HETE (12-LOX/15-LOX metabolites of AA) were elevated in children and young adults with ADPKD compared with the levels reported in healthy adults. This confirms our findings as well as results of other studies, showing that adult patients with ADPKD have higher levels of these bioactive lipids compared with healthy individuals and that their concentrations are associated with increased TKV and/or decreased renal function (14,16). These COX and LOX metabolites have been shown to directly function as key mediators of angiotensin II–induced renin inhibition (40,41). They have also been shown to activate the peroxisome proliferator–activated receptor γ, known to be implicated in cyst growth (42).
Furthermore, our results showed that changes in plasma levels of proinflammatory and oxidative stress markers 9-HODE, 13-HODE, and 15-HETE over 3 years were significantly different between the placebo and pravastatin groups, with the pravastatin group showing a decline and, thus, improvement, compared with the placebo group. Because the renin-angiotensin-aldosterone system is suspected to be a major contributor to cyst growth in both animals and humans with ADPKD (43,44), the ability of pravastatin to reduce the plasma concentrations of these COX and LOX metabolites over 3 years could represent a potential molecular mechanism by which pravastatin reduces cyst growth. This hypothesis is further supported by a separate mechanistic study, in which lovastatin was successful in reducing the 13-HODE and 12-HETE plasma levels that were significantly elevated in cystic Cy/+ Han:Sprague-Dawley rats (7).
Changes in urinary citrate and PGF2α were negatively associated with the percent change in HtTKV over 3 years in the placebo group. Pravastatin treatment seemed to counteract the citrate decline that was also previously shown to occur in patients with ADPKD during disease progression (45). In regard to PGF2α, both patient groups showed lower urinary levels at 3 years than at the baseline.
Furthermore, we identified urinary 8-HETE, 9-HETE, and 11-HETE to be positively associated with the change in HtTKV in the pravastatin group. This is an interesting finding because the mean levels of plasma 8-HETE and 11-HETE were lower in the pravastatin group than in the placebo group after 3 years (corrected for baseline). A possible explanation is that pravastatin therapy leads to an enhanced clearance and elimination of these mediators, causing a reduction in their concentrations in blood and potentially in kidney tissue. Supporting this conclusion was an observation made in cystic rats, in which lovastatin treatment decreased (albeit not significantly) kidney tissue concentrations of 8-HETE, 9-HETE, and 12-HETE, which were significantly higher in the kidneys of cystic rats (7).
The mechanisms underlying the effect of pravastatin on structural kidney disease in pediatric ADPKD require further elucidation. Our results suggest that statins might have an effect on the metabolism of polyunsaturated fatty acids, via regulation of COX and LOX activities, resulting in reduced production of proinflammatory and endothelial dysfunction–inducing bioactive lipids. This in turn might alter cell proliferation and signal transduction pathways (46–48), changing the formation and expansion of kidney cysts in ADPKD. The next step is to investigate whether the adult ADPKD population could also benefit from statins early in the course of their disease, especially before a detectable decline in GFR.
It should be noted that this study uses a unique cohort of children with well defined ADPKD, with normal creatinine clearance and low frequency of left ventricular hypertrophy, who participated in a study of pravastatin effect on disease progression (9,10). Long-term stability data exceeding 12 months were not available for any of the measured biomarkers; however, the majority of the biomarkers were stable at −70°C or below for 12 months. In addition, regression analysis without adjustments for multiple comparisons leaves a risk of type I and type II statistical errors.
In conclusion, our findings provide a rationale for further evaluation of the potential clinical value of these markers as predictive and monitoring diagnostic tools in longitudinal prospective clinical studies of patients with ADPKD. Furthermore, the clinical outcome strongly suggests that early intervention in ADPKD may be warranted.
Disclosures
None.
Supplementary Material
Acknowledgments
The authors thank the research participants and the individuals and families who have committed their time and effort to improving medical care for all children with ADPKD.
This research was supported by the National Institutes of Health, the National Center for Research Resources, and the Zell Family Foundation.
Footnotes
Published online ahead of print. Publication date available at www.cjasn.org.
This article contains supplemental material online at http://cjasn.asnjournals.org/lookup/suppl/doi:10.2215/CJN.11331114/-/DCSupplemental.
References
- 1.Parfrey PS, Bear JC, Morgan J, Cramer BC, McManamon PJ, Gault MH, Churchill DN, Singh M, Hewitt R, Somlo S, Reeders ST: The diagnosis and prognosis of autosomal dominant polycystic kidney disease. N Engl J Med 323: 1085–1090, 1990 [DOI] [PubMed] [Google Scholar]
- 2.Fick-Brosnahan GM, Tran ZV, Johnson AM, Strain JD, Gabow PA: Progression of autosomal-dominant polycystic kidney disease in children. Kidney Int 59: 1654–1662, 2001 [DOI] [PubMed] [Google Scholar]
- 3.Brosnahan GM: Volume progression in polycystic kidney disease. N Engl J Med 355: 733, author reply 733–734, 2006 [DOI] [PubMed] [Google Scholar]
- 4.Cadnapaphornchai MA, McFann K, Strain JD, Masoumi A, Schrier RW: Increased left ventricular mass in children with autosomal dominant polycystic kidney disease and borderline hypertension. Kidney Int 74: 1192–1196, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cadnapaphornchai MA, McFann K, Strain JD, Masoumi A, Schrier RW: Prospective change in renal volume and function in children with ADPKD. Clin J Am Soc Nephrol 4: 820–829, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zafar I, Tao Y, Falk S, McFann K, Schrier RW, Edelstein CL: Effect of statin and angiotensin-converting enzyme inhibition on structural and hemodynamic alterations in autosomal dominant polycystic kidney disease model. Am J Physiol Renal Physiol 293: F854–F859, 2007 [DOI] [PubMed] [Google Scholar]
- 7.Klawitter J, Zafar I, Klawitter J, Pennington AT, Klepacki J, Gitomer BY, Schrier RW, Christians U, Edelstein CL: Effects of lovastatin treatment on the metabolic distributions in the Han:SPRD rat model of polycystic kidney disease. BMC Nephrol 14: 165, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.van Dijk MA, Kamper AM, van Veen S, Souverijn JH, Blauw GJ: Effect of simvastatin on renal function in autosomal dominant polycystic kidney disease. Nephrol Dial Transplant 16: 2152–2157, 2001 [DOI] [PubMed] [Google Scholar]
- 9.Cadnapaphornchai MA, George DM, Masoumi A, McFann K, Strain JD, Schrier RW: Effect of statin therapy on disease progression in pediatric ADPKD: Design and baseline characteristics of participants. Contemp Clin Trials 32: 437–445, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cadnapaphornchai MA, George DM, McFann K, Wang W, Gitomer B, Strain JD, Schrier RW: Effect of pravastatin on total kidney volume, left ventricular mass index, and microalbuminuria in pediatric autosomal dominant polycystic kidney disease. Clin J Am Soc Nephrol 9: 889–896, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Klawitter J, Reed-Gitomer BY, McFann K, Pennington A, Klawitter J, Abebe KZ, Klepacki J, Cadnapaphornchai MA, Brosnahan G, Chonchol M, Christians U, Schrier RW: Endothelial dysfunction and oxidative stress in polycystic kidney disease. Am J Physiol Renal Physiol 307: F1198–F1206, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Meijer E, Boertien WE, Nauta FL, Bakker SJ, van Oeveren W, Rook M, van der Jagt EJ, van Goor H, Peters DJ, Navis G, de Jong PE, Gansevoort RT: Association of urinary biomarkers with disease severity in patients with autosomal dominant polycystic kidney disease: A cross-sectional analysis. Am J Kidney Dis 56: 883–895, 2010 [DOI] [PubMed] [Google Scholar]
- 13.Menon V, Rudym D, Chandra P, Miskulin D, Perrone R, Sarnak M: Inflammation, oxidative stress, and insulin resistance in polycystic kidney disease. Clin J Am Soc Nephrol 6: 7–13, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wang D, Strandgaard S, Borresen ML, Luo Z, Connors SG, Yan Q, Wilcox CS: Asymmetric dimethylarginine and lipid peroxidation products in early autosomal dominant polycystic kidney disease. Am J Kidney Dis 51: 184–191, 2008 [DOI] [PubMed] [Google Scholar]
- 15.Wang D, Iversen J, Strandgaard S: Endothelium-dependent relaxation of small resistance vessels is impaired in patients with autosomal dominant polycystic kidney disease. J Am Soc Nephrol 11: 1371–1376, 2000 [DOI] [PubMed] [Google Scholar]
- 16.Klawitter J, Klawitter J, McFann K, Pennington AT, Abebe KZ, Brosnahan G, Cadnapaphornchai MA, Chonchol M, Gitomer B, Christians U, Schrier RW: Bioactive lipid mediators in polycystic kidney disease. J Lipid Res 55: 1139–1149, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Parikh CR, Dahl NK, Chapman AB, Bost JE, Edelstein CL, Comer DM, Zeltner R, Tian X, Grantham JJ, Somlo S: Evaluation of urine biomarkers of kidney injury in polycystic kidney disease. Kidney Int 81: 784–790, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Schwartz GJ, Brion LP, Spitzer A: The use of plasma creatinine concentration for estimating glomerular filtration rate in infants, children, and adolescents. Pediatr Clin North Am 34: 571–590, 1987 [DOI] [PubMed] [Google Scholar]
- 19.Marshall WA, Tanner JM: Growth and physiological development during adolescence. Annu Rev Med 19: 283–300, 1968 [DOI] [PubMed] [Google Scholar]
- 20.Marshall WA, Tanner JM: Variations in pattern of pubertal changes in girls. Arch Dis Child 44: 291–303, 1969 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Marshall WA, Tanner JM: Variations in the pattern of pubertal changes in boys. Arch Dis Child 45: 13–23, 1970 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.National High Blood Pressure Education Program Working Group on High Blood Pressure in Children and Adolescents : The fourth report on the diagnosis, evaluation, and treatment of high blood pressure in children and adolescents. Pediatrics 114[Suppl 4th Report]: 555–576, 2004 [PubMed] [Google Scholar]
- 23.Cadnapaphornchai MA, Masoumi A, Strain JD, McFann K, Schrier RW: Magnetic resonance imaging of kidney and cyst volume in children with ADPKD. Clin J Am Soc Nephrol 6: 369–376, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chapman AB, Guay-Woodford LM, Grantham JJ, Torres VE, Bae KT, Baumgarten DA, Kenney PJ, King BF, Jr, Glockner JF, Wetzel LH, Brummer ME, O’Neill WC, Robbin ML, Bennett WM, Klahr S, Hirschman GH, Kimmel PL, Thompson PA, Miller JP, Consortium for Radiologic Imaging Studies of Polycystic Kidney Disease cohort : Renal structure in early autosomal-dominant polycystic kidney disease (ADPKD): The Consortium for Radiologic Imaging Studies of Polycystic Kidney Disease (CRISP) cohort. Kidney Int 64: 1035–1045, 2003 [DOI] [PubMed] [Google Scholar]
- 25.Chapman AB, Bost JE, Torres VE, Guay-Woodford L, Bae KT, Landsittel D, Li J, King BF, Martin D, Wetzel LH, Lockhart ME, Harris PC, Moxey-Mims M, Flessner M, Bennett WM, Grantham JJ: Kidney volume and functional outcomes in autosomal dominant polycystic kidney disease. Clin J Am Soc Nephrol 7: 479–486, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rahbari-Oskoui F, Mittal A, Mittal P, Chapman A: Renal relevant radiology: Radiologic imaging in autosomal dominant polycystic kidney disease. Clin J Am Soc Nephrol 9: 406–415, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Klepacki J, Brunner N, Schmitz V, Klawitter J, Christians U, Klawitter J: Development and validation of an LC-MS/MS assay for the quantification of the trans-methylation pathway intermediates S-adenosylmethionine and S-adenosylhomocysteine in human plasma. Clin Chim Acta 421: 91–97, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Masoodi M, Mir AA, Petasis NA, Serhan CN, Nicolaou A: Simultaneous lipidomic analysis of three families of bioactive lipid mediators leukotrienes, resolvins, protectins and related hydroxy-fatty acids by liquid chromatography/electrospray ionisation tandem mass spectrometry. Rapid Commun Mass Spectrom 22: 75–83, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Franz KA, Reubi FC: Rate of functional deterioration in polycystic kidney disease. Kidney Int 23: 526–529, 1983 [DOI] [PubMed] [Google Scholar]
- 30.Gabow PA, Johnson AM, Kaehny WD, Kimberling WJ, Lezotte DC, Duley IT, Jones RH: Factors affecting the progression of renal disease in autosomal-dominant polycystic kidney disease. Kidney Int 41: 1311–1319, 1992 [DOI] [PubMed] [Google Scholar]
- 31.Peters DJ, Breuning MH: Autosomal dominant polycystic kidney disease: Modification of disease progression. Lancet 358: 1439–1444, 2001 [DOI] [PubMed] [Google Scholar]
- 32.Gile RD, Cowley BD, Jr, Gattone VH, 2nd, O’Donnell MP, Swan SK, Grantham JJ: Effect of lovastatin on the development of polycystic kidney disease in the Han:SPRD rat. Am J Kidney Dis 26: 501–507, 1995 [DOI] [PubMed] [Google Scholar]
- 33.O’Driscoll G, Green D, Taylor RR: Simvastatin, an HMG-coenzyme A reductase inhibitor, improves endothelial function within 1 month. Circulation 95: 1126–1131, 1997 [DOI] [PubMed] [Google Scholar]
- 34.Laufs U, La Fata V, Plutzky J, Liao JK: Upregulation of endothelial nitric oxide synthase by HMG CoA reductase inhibitors. Circulation 97: 1129–1135, 1998 [DOI] [PubMed] [Google Scholar]
- 35.Wassmann S, Laufs U, Bäumer AT, Müller K, Ahlbory K, Linz W, Itter G, Rösen R, Böhm M, Nickenig G: HMG-CoA reductase inhibitors improve endothelial dysfunction in normocholesterolemic hypertension via reduced production of reactive oxygen species. Hypertension 37: 1450–1457, 2001 [DOI] [PubMed] [Google Scholar]
- 36.Kocaman O, Oflaz H, Yekeler E, Dursun M, Erdogan D, Demirel S, Alisir S, Turgut F, Mercanoglu F, Ecder T: Endothelial dysfunction and increased carotid intima-media thickness in patients with autosomal dominant polycystic kidney disease. Am J Kidney Dis 43: 854–860, 2004 [DOI] [PubMed] [Google Scholar]
- 37.Baer AN, Costello PB, Green FA: Stereospecificity of the hydroxyeicosatetraenoic and hydroxyoctadecadienoic acids produced by cultured bovine endothelial cells. Biochim Biophys Acta 1085: 45–52, 1991 [DOI] [PubMed] [Google Scholar]
- 38.Kühn H: Biosynthesis, metabolization and biological importance of the primary 15-lipoxygenase metabolites 15-hydro(pero)XY-5Z,8Z,11Z,13E-eicosatetraenoic acid and 13-hydro(pero)XY-9Z,11E-octadecadienoic acid. Prog Lipid Res 35: 203–226, 1996 [DOI] [PubMed] [Google Scholar]
- 39.Fang X, Kaduce TL, Spector AA: 13-(S)-hydroxyoctadecadienoic acid (13-HODE) incorporation and conversion to novel products by endothelial cells. J Lipid Res 40: 699–707, 1999 [PubMed] [Google Scholar]
- 40.Xu ZG, Yuan H, Lanting L, Li SL, Wang M, Shanmugam N, Kato M, Adler SG, Reddy MA, Natarajan R: Products of 12/15-lipoxygenase upregulate the angiotensin II receptor. J Am Soc Nephrol 19: 559–569, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Reddy MA, Adler SG, Kim YS, Lanting L, Rossi J, Kang SW, Nadler JL, Shahed A, Natarajan R: Interaction of MAPK and 12-lipoxygenase pathways in growth and matrix protein expression in mesangial cells. Am J Physiol Renal Physiol 283: F985–F994, 2002 [DOI] [PubMed] [Google Scholar]
- 42.Yu K, Bayona W, Kallen CB, Harding HP, Ravera CP, McMahon G, Brown M, Lazar MA: Differential activation of peroxisome proliferator-activated receptors by eicosanoids. J Biol Chem 270: 23975–23983, 1995 [DOI] [PubMed] [Google Scholar]
- 43.Barrett BJ, Foley R, Morgan J, Hefferton D, Parfrey P: Differences in hormonal and renal vascular responses between normotensive patients with autosomal dominant polycystic kidney disease and unaffected family members. Kidney Int 46: 1118–1123, 1994 [DOI] [PubMed] [Google Scholar]
- 44.Chapman AB, Johnson A, Gabow PA, Schrier RW: The renin-angiotensin-aldosterone system and autosomal dominant polycystic kidney disease. N Engl J Med 323: 1091–1096, 1990 [DOI] [PubMed] [Google Scholar]
- 45.Torres VE, Grantham JJ, Chapman AB, Mrug M, Bae KT, King BF, Jr, Wetzel LH, Martin D, Lockhart ME, Bennett WM, Moxey-Mims M, Abebe KZ, Lin Y, Bost JE, Consortium for Radiologic Imaging Studies of Polycystic Kidney Disease (CRISP) : Potentially modifiable factors affecting the progression of autosomal dominant polycystic kidney disease. Clin J Am Soc Nephrol 6: 640–647, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kostapanos MS, Liberopoulos EN, Elisaf MS: Statin pleiotropy against renal injury. J Cardiometab Syndr 4: E4–E9, 2009 [DOI] [PubMed] [Google Scholar]
- 47.Gomez SI, Mihos CG, Pineda AM, Santana O: The pleiotropic effects of the hydroxy-methyl-glutaryl-CoA reductase inhibitors in renal disease. Int J Nephrol Renovasc Dis 7: 123–130, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.D’Amico G: Statins and renal diseases: From primary prevention to renal replacement therapy. J Am Soc Nephrol 17[Suppl 2]: S148–S152, 2006 [DOI] [PubMed] [Google Scholar]
- 49.Psychogios N, Hau DD, Peng J, Guo AC, Mandal R, Bouatra S, Sinelnikov I, Krishnamurthy R, Eisner R, Gautam B, Young N, Xia J, Knox C, Dong E, Huang P, Hollander Z, Pedersen TL, Smith SR, Bamforth F, Greiner R, McManus B, Newman JW, Goodfriend T, Wishart DS: The human serum metabolome. PLoS One 6: e16957, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.James SJ, Cutler P, Melnyk S, Jernigan S, Janak L, Gaylor DW, Neubrander JA: Metabolic biomarkers of increased oxidative stress and impaired methylation capacity in children with autism. Am J Clin Nutr 80: 1611–1617, 2004 [DOI] [PubMed] [Google Scholar]
- 51.Quehenberger O, Armando AM, Brown AH, Milne SB, Myers DS, Merrill AH, Bandyopadhyay S, Jones KN, Kelly S, Shaner RL, Sullards CM, Wang E, Murphy RC, Barkley RM, Leiker TJ, Raetz CR, Guan Z, Laird GM, Six DA, Russell DW, McDonald JG, Subramaniam S, Fahy E, Dennis EA: Lipidomics reveals a remarkable diversity of lipids in human plasma. J Lipid Res 51: 3299–3305, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Bouatra S, Aziat F, Mandal R, Guo AC, Wilson MR, Knox C, Bjorndahl TC, Krishnamurthy R, Saleem F, Liu P, Dame ZT, Poelzer J, Huynh J, Yallou FS, Psychogios N, Dong E, Bogumil R, Roehring C, Wishart DS: The human urine metabolome. PLoS One 8: e73076, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Guneral F, Bachmann C: Age-related reference values for urinary organic acids in a healthy Turkish pediatric population. Clin Chem 40: 862–866, 1994 [PubMed] [Google Scholar]
- 54.Godard C, Vallotton MB, Favre L: Urinary prostaglandins, vasopressin, and kallikrein excretion in healthy children from birth to adolescence. J Pediatr 100: 898–902, 1982 [DOI] [PubMed] [Google Scholar]
- 55.Dreisbach AW, Smith SV, Kyle PB, Ramaiah M, Amenuke M, Garrett MR, Lirette ST, Griswold ME, Roman RJ: Urinary CYP eicosanoid excretion correlates with glomerular filtration in African-Americans with chronic kidney disease. Prostaglandins Other Lipid Mediat 113-115: 45–51, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.