Abstract
Background and objectives
Mineralocorticoid receptor antagonism reduces morbidity and mortality in patients with heart failure, but the safety of these drugs in patients receiving dialysis is unclear. This study evaluated whether hyperkalemia and/or hypotension limited the use of eplerenone, a selective mineralocorticoid receptor antagonist, in hemodialysis patients.
Design, setting, participants, & measurements
This was a randomized controlled trial of prevalent patients receiving hemodialysis at five Canadian centers. Participants were randomly allocated to 13 weeks of eplerenone titrated to 50 mg daily (n=77) or a matching placebo (n=77). The primary outcome was permanent discontinuation of the drug because of hyperkalemia or hypotension. Secondary outcomes included hyperkalemia, hypotension, and cardiovascular events.
Results
Seventy-five eplerenone-treated patients and 71 placebo-treated patients were included in the per protocol population. The primary outcome occurred in three patients (4.0%) in the eplerenone group and two (2.8%) in the placebo group, for an absolute risk difference of 1.2 percentage points (95% confidence interval, −4.7 to 7.1 percentage points). Eplerenone was interpreted as noninferior to placebo with respect to the primary outcome (i.e., a discontinuation rate for these reasons >10% was excluded). In the eplerenone group, nine patients (11.7%) developed hyperkalemia (potassium level >6.5 mEq/L), compared with two patients (2.6%) in the placebo group (relative risk, 4.5; 95% confidence interval, 1.0 to 20.2). There was no significant effect on predialysis or postdialysis BP.
Conclusion
Eplerenone increased the risk of hyperkalemia but did not result in an excess need to permanently discontinue the drug. Further trials are required to determine whether mineralocorticoid receptor antagonism improves cardiovascular outcomes in patients receiving long-term dialysis.
Introduction
Despite technical advances in dialysis, the outcomes for patients with ESRD are poor. Observational studies suggest cardiac remodeling arises from chronic pressure-volume overload in patients who require dialysis (1). Subsequently, cardiomyopathy and congestive heart failure develop and lead to cardiac arrhythmias and eventually cardiac arrest (2,3). This is of particular importance for patients who require dialysis, as cardiovascular disease accounts for 40% of their deaths (4).
Aldosterone is implicated in cardiac remodeling and heart failure (5–7). Furthermore, mineralocorticoid receptor antagonism (MRA) in patients with heart failure who do not have ESRD prevents cardiovascular death, hospitalizations, and changes in cardiac morphology (8–11). However, MRA is used infrequently in dialysis patients (12). Given the effect of MRA on serum potassium in patients without ESRD and the capacity of patients with ESRD to develop severe hyperkalemia, uncertainty regarding the effect of MRA on serum potassium in patients with ESRD may limit its use.
We conducted the Pilot trial of Hemodialyis patients undergoing Aldosterone antagoniSm with Eplerenone (PHASE), a placebo-controlled, multicenter, randomized controlled trial to inform the safety and tolerability of eplerenone, a selective MRA, in patients undergoing hemodialysis. For eplerenone to be a useful therapy and to be studied in a large randomized controlled trial, we postulated that it must be reasonably tolerated. We therefore defined PHASE to test the noninferiority of eplerenone to placebo for the outcome of permanent discontinuation of the study drug due to hyperkalemia or hypotension.
Materials and Methods
Design Overview
We conducted a parallel-group, randomized controlled trial to establish noninferiority of eplerenone compared with a matching placebo in patients who required long-term hemodialysis. The research ethics board at each participating institution reviewed and accepted the protocol, and all participants provided informed consent.
Setting and Participants
Recruitment occurred at five Canadian centers between April and September 2013. Adult patients who required hemodialysis for at least 90 days before enrollment and provided informed consent were eligible to participate. We excluded patients who had any of the following: documented hyperkalemia (potassium level >6.0 mEq/L) within the prior 4 weeks; hypotension, defined as a systolic BP <90 mmHg that required treatment, or a change in antihypertensive medications in the preceding 4 weeks; a previously documented sensitivity to eplerenone; ongoing use of spironolactone or eplerenone; pregnancy; or living-related transplant scheduled within the next 6 months.
Randomization and Intervention
Eligible participants were randomly allocated to eplerenone or placebo using a computerized web-based randomization system maintained by the coordinating center (the Population Health Research Institute). The randomization lists, stratified by center with randomly permuted block sizes, were generated by a statistician independent of the study. Participants, health care providers, study personnel, and outcome assessors were all blind to treatment allocation.
The treatment algorithm is shown in detail in Supplemental Table 1. After randomization, participants received a bottle of 25-mg eplerenone tablets or matching placebo. Participants were instructed to take one tablet per day. The study medication was increased to two tablets per day (eplerenone, 50 mg/d, or matching placebo) on day 7 if it was tolerated and the predialysis serum potassium remained <6 mEq/L. The dose was titrated down if the serum potassium concentrations exceeded 6 mEq/L or if clinically significant hypotension continued despite the minimum dose of 25 mg three times per week. Further titration was made at study visits 2, 3, and 7 weeks after randomization. Down-titration of the study medication could occur between study visits if severe hyperkalemia was detected through routine care. The study drug was held if sequential down-titration did not result in potassium concentrations ≤6 mEq/L or if the potassium concentration exceeded 6.5 mEq/L at any time.
Treating nephrologists could change the dialysate potassium concentration or use potassium-binding resins as clinically indicated. Dietitians were asked to review a low-potassium diet with patients who developed hyperkalemia as per the usual standard of care. Before adjusting the dose of the study medication, physicians were asked to reduce non–study drug antihypertensives that did not have a specific indication if patients developed nonserious hypotension.
Outcomes and Follow-up
Outcomes definitions are detailed in the Supplemental Material. Briefly, our primary outcome was the permanent discontinuation of study drug due to hyperkalemia or significant hypotension (during or between dialysis treatments). The study duration of 13 weeks was chosen because experience suggests most medication discontinuations occur within this time and changes in potassium and BP were expected to occur in much less time (13,14). The predialysis serum potassium concentrations were measured at the first dialysis session of the week 1, 2, 3, 7 and 13 weeks after randomization. Potassium was otherwise measured according to local standards (every 4–6 weeks) and as clinically indicated. Secondary outcomes included permanent discontinuation of study drug for any reason, adherence to the study drug prescription (defined as ≥80% of study drug taken every week for the duration of the study), significant hyperkalemia (defined as a serum potassium >6.5 mEq/L, with sensitivity analyses defining hyperkalemia as potassium level >6 mEq/L and >7 mEq/L), clinically significant hypotension (defined as a systolic BP <90 mmHg necessitating intervention), predialysis serum potassium concentrations, pre- and postdialysis BP, fatal and nonfatal cardiovascular events, and all-cause death.
Statistical Analyses
The sample size estimate was based on the primary outcome. We decided that a drug that was discontinued 10% more frequently than placebo because of drug-specific adverse effects would be unlikely to be clinically useful. Our noninferiority margin was therefore a 10–percentage point absolute risk difference. To exclude a 10–percentage point absolute risk difference with 80% power, assuming a 5% baseline risk of permanent discontinuation due to hyperkalemia or hypotension, we required 74 participants in each group for a one-sided α value of 0.025. We considered eplerenone noninferior if the 95% confidence interval (95% CI) of the absolute risk difference excluded the noninferiority margin. We expected that 5% of participants would discontinue medication in the first week because of nonspecific symptoms and nonadherence and therefore would not be included in the per protocol analysis. We therefore aimed to recruit 154 participants. Furthermore, this sample size allowed us >85% power to exclude at least a 15–percentage point absolute risk difference in all-cause discontinuations, where we assumed a rate of 10% in the control group. Sample size estimates were performed with PASS software, version 11 (Kaysville, UT).
Our primary study objective was to determine the safety of eplerenone in dialysis patients. Because intention-to-treat populations can reduce the ability to demonstrate effects of treatment, particularly in nonadherent groups, our primary analyses used a per protocol population. The per protocol population was defined as all patients who were taking any dose of medication at the week 1 study visit. The per protocol population was used for the primary analysis of permanent discontinuation due to hyperkalemia or hypotension and permanent discontinuation for any cause. We conducted sensitivity analyses using all patients in the groups to which they were randomly assigned (i.e., the intention-to-treat population) to ensure our findings were robust. For all other outcomes, we used the intention-to-treat population for the primary analysis.
All analyses were predetermined, and no adjustments were made for multiplicity of comparisons. Data are presented as mean±SD, median (25th–75th percentile), or number (percentage) as appropriate. Results are expressed as the eplerenone group relative to the placebo group. For the outcomes of permanent discontinuation and adherence, we calculated the absolute risk difference and corresponding 95% CI. For the outcomes of hyperkalemia, significant hypotension, fatal vascular events, nonfatal vascular events, and all-cause death, we calculated the relative risk and corresponding 95% CIs. To determine the effect of eplerenone on predialysis serum potassium concentrations and mean predialysis and mean postdialysis systolic BP, we used random-effects linear regression. In each model, individuals were considered a random effect and treatment group a fixed effect. The effect of eplerenone was not adjusted for baseline values in these models. We also used a random-effects linear regression model to determine the relationship between the dose of eplerenone and the predialysis potassium. In this dose-effect model, the dose of eplerenone the patient was taking at each follow-up was considered a time-varying exposure. Patients in the placebo group were considered to have received no eplerenone at any time point. Patients were considered to be random effects, and the analysis was adjusted for baseline serum potassium as a fixed effect and dialysate potassium as a time-varying fixed effect. All analyses were conducted with SAS software, version 9.2 (SAS Institute, Inc., Cary, NC).
Results
Patients
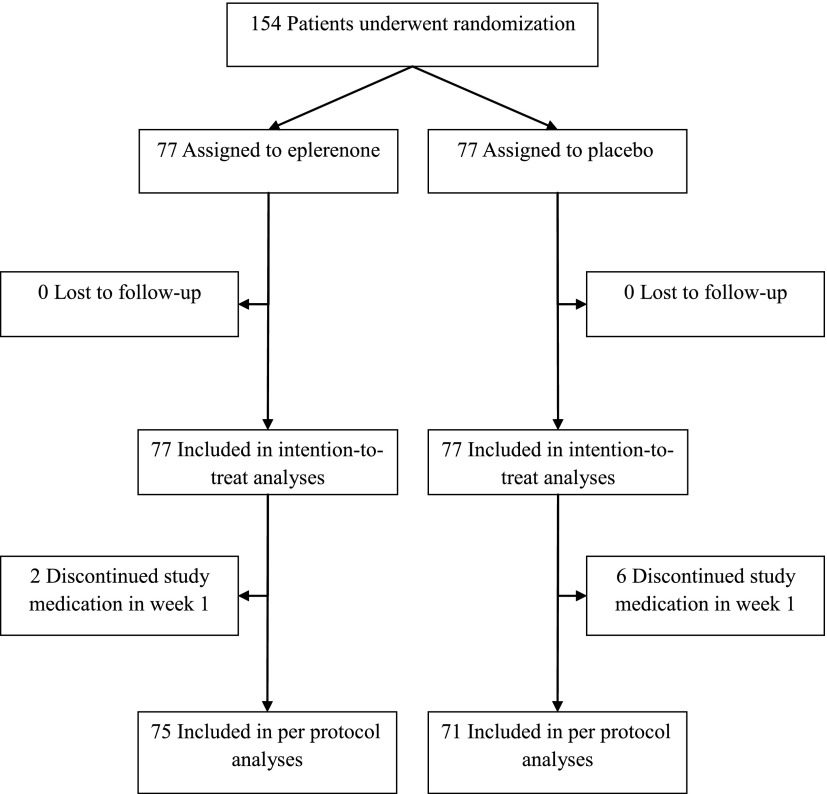
We randomly allocated 77 patients to the eplerenone group and 77 to the placebo group (Figure 1). No patients were lost to follow-up. The groups were similar with respect to baseline characteristics (Table 1).
Figure 1.
Participant flow diagram.
Table 1.
Patient characteristics at trial entry
| Characteristic | Eplerenone | Placebo |
|---|---|---|
| Age (yr) | 62.1±14.6 | 63.1±13.7 |
| Women, n (%) | 30 (39.0) | 28 (36.4) |
| Median dialysis vintage (yr) (25th–75th percentile) | 3 (2–5) | 3 (2–7) |
| Cause of ESRD, n (%) | ||
| Diabetes mellitus | 33 (42.9) | 35 (45.5) |
| Hypertension | 9 (11.7) | 5 (6.5) |
| GN | 20 (26.0) | 21 (27.3) |
| Polycystic kidney disease | 1 (1.3) | 3 (3.9) |
| Other | 14 (18.2) | 13 (16.9) |
| Comorbidities, n (%) | ||
| Diabetes mellitus | 40 (51.9) | 40 (51.9) |
| Coronary artery disease | 16 (20.8) | 13 (16.9) |
| Previous stroke | 4 (5.2) | 4 (5.2) |
| Peripheral vascular disease | 5 (6.5) | 9 (11.7) |
| Congestive heart failure | 8 (10.4) | 6 (7.8) |
| Predialysis SBP (mmHg) | 146±20 | 146±20 |
| Postdialysis SBP (mmHg) | 136±20 | 139±27 |
| Serum potassium (mEq/L) | 4.7±0.6 | 4.9±0.6 |
| Dialysate potassium (mEq/L) | 2.4±0.6 | 2.2±0.6 |
| Medications, n (%) | ||
| ACEI or ARB | 33 (42.9) | 32 (41.6) |
| β-blocker | 39 (50.6) | 39 (50.6) |
Values expressed with a plus/minus sign are the mean±SD. SBP, systolic BP; ACEI, angiotensin-converting enzyme inhibitor; ARB, angiotensin receptor blocker.
Because our primary intention was to examine the safety of eplerenone, we first focused on per protocol analyses, which are typically more sensitive to between-group differences and are often preferred for noninferiority hypotheses (15). The per protocol group (participants who continued receiving any dose of trial medication after 1 week) included 75 participants in the eplerenone group and 71 patients in the placebo group. In the eplerenone group, 97.4%, 89.6%, 84.2%, 84.2%, and 78.9% of participants were taking the study drug at weeks 1, 2, 3, 7, and 13, respectively. In the placebo group, 92.2%, 92.2%, 85.7%, 80.5%, and 80.3% were taking the study drug at weeks 1, 2, 3, 7, and 13, respectively (Supplemental Table 2). During the study, the odds of taking the study drug did not differ between the eplerenone and placebo groups (odds ratio, 0.96; 95% CI, 0.41 to 2.25). Sixty-one (79.2%) participants reported taking ≥80% of the prescribed dose at all visits compared with 60 (76.6%) in the placebo group (absolute risk difference, 1.3 percentage points; 95% CI, −11.7 to 14.3 percentage points).
Discontinuation
Discontinuation rates are reported in Table 2. In the eplerenone group, three (4.1%) participants permanently discontinued study medication because of hyperkalemia or hypotension compared with two patients in the placebo group (2.8%), with an absolute risk difference of 1.2 percentage points (95% CI, −4.7 to 7.1 percentage points). The upper bound of the 95% CI excluded 10 percentage points, the criterion for noninferiority. This was not materially altered by analyzing the intention-to-treat population (absolute risk difference, 1.3 percentage points; 95% CI, −4.3% to 6.9 percentage points).
Table 2.
Permanent discontinuations and adherence in eplerenone- and placebo-treated patients
| Adherence measure/Population | Eplerenone, n (%) | Placebo, n (%) | Risk Difference (95% CI) (percentage points) |
|---|---|---|---|
| Permanent discontinuation for hyperkalemia or hypotension | |||
| Per protocol | 3 (4.0) | 2 (2.8) | 1.2 (−4.7 to 7.1) |
| Intention-to-treat | 3 (3.9) | 2 (2.6) | 1.3 (−4.3 to 6.9) |
| Permanent discontinuation for any cause | |||
| Per protocol | 14 (18.7) | 9 (12.7) | 6.0 (−5.7 to 17.7) |
| Intention-to-treat | 16 (20.8) | 15 (19.5) | 1.3 (−11.4 to 14.0) |
| Took ≥80% of prescribed dose at every visit | |||
| Per protocol | 60 (80.0) | 59 (83.1) | −3.1 (−15.7 to 9.5) |
| Intention-to-treat | 61 (79.2) | 60 (77.9) | 1.3 (−11.7 to 14.3) |
The per protocol population included 75 eplerenone-treated patients and 71 placebo-treated patients while the intention-to-treat population included 77 patients in each group. 95% CI, 95% confidence interval.
Fourteen (18.7%) participants in the eplerenone group compared with nine (12.7%) in the placebo group permanently discontinued the study medication for any reason (absolute risk difference, 6.0 percentage points; 95% CI, −5.7 to 17.7 percentage points). Sensitivity analysis using the intention-to-treat population did not substantially differ from the per protocol analysis (absolute risk difference, 1.3%; 95% CI, −11.4 to 14.0 percentage points).
Hyperkalemia
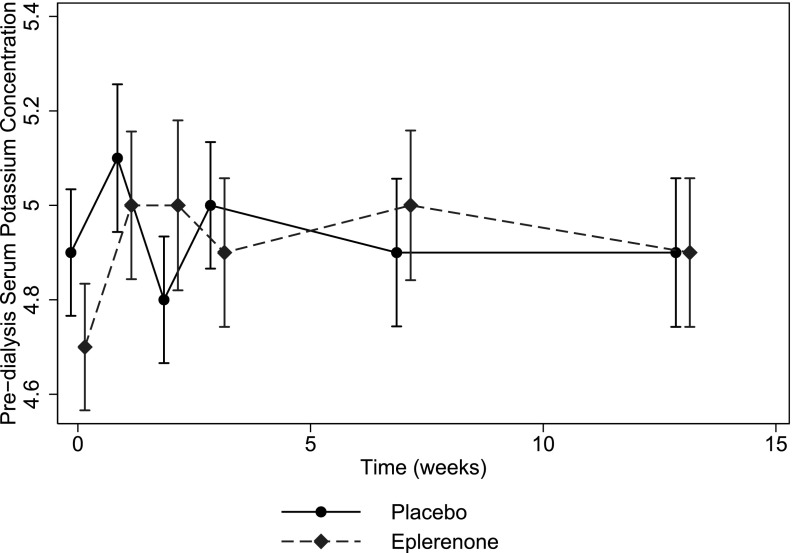
Rates of hyperkalemia are reported in Table 3. More patients in the eplerenone group experienced hyperkalemia with potassium level >6.5 mEq/L than in the placebo group (relative risk, 4.5; 95% CI, 1.0 to 20.2). Hyperkalemia with potassium level >7.0 mEq/L was experienced by four (5.2%) eplerenone-treated patients and no placebo-treated patients. In the eplerenone group, eight of nine patients developed hyperkalemia within 4 weeks of starting eplerenone, 50 mg daily; the ninth patient developed hyperkalemia at the last study visit after a missed dialysis session. In the placebo group, the two patients who developed hyperkalemia did so within 4 weeks of randomization. Mean predialysis serum potassium levels were increased by eplerenone by an average of 0.16 mEq/L (95% CI, 0.04 to 0.28 mEq/L) (Figure 2). There was a graded relationship between dose of eplerenone and serum potassium, but an increase in serum potassium was statistically significant only at an eplerenone dose of 50 mg daily (Table 4). There was no significant difference in prescribed dialysate potassium concentrations during follow-up (between-group difference, −0.05 mEq/L; 95% CI, −0.14 to 0.04 mEq/L), although 14 (18.7%) eplerenone-treated patients reduced their dialysate potassium concentration during the study compared with eight (11.0%) placebo-treated patients. No patients received long-term treatment with potassium exchange resins during the trial. Three (9.1%) patients in the eplerenone group and five (15.6%) patients in the placebo group had their angiotensin-converting enzyme inhibitor or angiotensin receptor blocker discontinued over the course of the trial.
Table 3.
Number of patients who experienced secondary outcomes
| Outcome | Eplerenone (n=77) | Placebo (n=77) | Relative Risk (95% CI) |
|---|---|---|---|
| Hyperkalemia | |||
| Defined as >6 mEq/L | 19 (24.7) | 14 (18.2) | 1.4 (0.7 to 2.5) |
| Defined as >6.5 mEq/L | 9 (11.7) | 2 (2.6) | 4.5 (1.0 to 20.2) |
| Defined as >7 mEq/L | 4 (5.2) | 0 (0) | NA |
| Significant hypotension | 16 (20.8) | 14 (18.2) | 1.1 (0.6 to 2.2) |
| Deaths | – | – | – |
| Vascular | 1 (1.3) | 2 (2.6) | 0.5 (0.0 to 5.4) |
| Any cause | 1 (1.3) | 2 (2.6) | 0.5 (0.0 to 5.4) |
| Nonfatal vascular event | 3 (3.9) | 4 (5.2) | 0.8 (0.2 to 3.2) |
| Fatal or nonfatal vascular event | 4 (5.2) | 6 (7.8) | 0.7 (0.2 to 2.3) |
All analyses use the intention-to-treat population. 95% CI, 95% confidence interval; NA, not applicable.
Figure 2.
Mean predialysis serum potassium over time in the placebo (circles and solid lines) and eplerenone (diamonds and dashed line) groups in the intention-to-treat population. Vertical lines represent 95% confidence intervals around the mean serum potassium level at each time point.
Table 4.
Dose-effect relationship between eplerenone and change in serum potassium from baseline
| Dose | Change in Serum Potassium Compared with Baseline, mEq/L (95% CI) |
|---|---|
| 0 mg/d | Referent |
| 25 mg three times per week | −0.25 (-0.54–0.05) |
| 25 mg/d | 0.05 (-0.09–0.20) |
| 50 mg/d | 0.21 (0.08–0.34) |
Analysis was controlled for dialysate potassium concentration and baseline serum potassium using mixed-effects regression with eplerenone dose included as a time-varying fixed effect variable and patient included as a random effect. 95% CI, 95% confidence interval.
BP
Sixteen (20.8%) participants in the eplerenone group and 14 (18.2%) in the placebo group experienced clinically significant hypotension (relative risk, 1.1; 95% CI, 0.6 to 2.2). Systolic BP did not significantly differ either before dialysis (between-group difference, −2.0 mmHg; 95% CI, −6.0 to 2.1 mmHg) or after dialysis (between-group difference, −1.5 mmHg; 95% CI, −5.3 to 2.3 mmHg) throughout the trial. Target weights were similar between the groups throughout the trial (mean difference, −0.01 kg; 95% CI, −0.36 to 0.34 kg). The median number of antihypertensive medications at study end was 2 in both groups, with a median reduction of 0 medications in both the eplerenone and placebo groups.
Cardiovascular Events and Safety
Cardiovascular events are summarized in Table 4. Using an intention-to-treat analysis, we observed no significant differences in nonfatal cardiovascular events, cardiovascular deaths, the composite of fatal and nonfatal cardiovascular events, or all-cause deaths.
Discussion
In this pilot randomized controlled trial, eplerenone was noninferior to placebo in terms of permanent discontinuation for hyperkalemia and hypotension and had similar rates of all-cause permanent discontinuation and adherence. Hyperkalemia occurred more frequently in the eplerenone group at full dose of the medication. These data suggest that MRA with eplerenone may be used safely in dialysis patients but that predialysis potassium needs to be monitored, particularly during the first 4 weeks after initiating therapy, and that MRA may not be suitable for patients with a history of difficult-to-control serum potassium concentrations.
Several other trials also suggested MRA can be used safely in dialysis patients (16–23). Four trials found a small but statistically significant rise in predialysis serum potassium with spironolactone treatment (18,19,22,23). However, hyperkalemia did not limit MRA use in any of the trials. Furthermore, an eight-patient pre-post study of eplerenone, 25 mg twice daily, showed no effect of eplerenone on serum potassium (24). The safety demonstrated in these trials should be interpreted in the context of the trials’ limitations, however. Most of the trials were small (ranging from eight to 300 patients) with limited power to detect an increased rate of hyperkalemia. These trials also tended to enroll patients with a serum potassium level <5 mEq/L before randomization. It would therefore take large changes in serum potassium to reach a potentially dangerous threshold. These studies thus may not be generalizable to a large proportion of dialysis patients with less well-controlled serum potassium. The potassium-monitoring protocol was unclear in some trials, and significant hyperkalemia may have been missed. Finally, several trials had significant losses to follow-up (up to 23%), making the accuracy of their estimates of hyperkalemia less reliable. Furthermore, health care providers should be aware of the risk of hyperkalemia with MRA given recent trials suggesting that angiotensin-converting enzyme inhibitors may cause excess hyperkalemia and serious adverse events in dialysis patients (25,26).
Recent trials demonstrate potential benefits with MRA in dialysis patients. For example, spironolactone improved left ventricular hypertrophy in 158 patients undergoing peritoneal dialysis and decreased carotid intimal thickness in 53 patients receiving hemodialysis (18,23). More important, spironolactone, 25 mg daily, in 309 Japanese hemodialysis patients reduced cardiovascular events by 60% (20). These benefits are consistent with findings suggesting that aldosterone may mediate left ventricular remodeling in dialysis recipients and that adequate antagonism of aldosterone’s effects may be of benefit (5,27–29). Although the results of these trials are promising, whether MRA improves patient-important outcomes for dialysis patients remains uncertain because of the studies’ limitations (insufficient numbers of outcome events, baseline differences between groups, and high losses to follow-up/discontinuation rates).
PHASE has several notable strengths; it specifically examined the effects of MRA on serum potassium, discontinuation rates, and adherence, and was powered to detect differences in permanent discontinuation and adherence that would preclude widespread use of MRA in dialysis patients. Our study also included a broad spectrum of patients from multiple centers in Canada, thereby improving the generalizability of our results.
Our results must also be considered in light of their limitations. PHASE was conducted only in hemodialysis patients; thus, its generalizability to peritoneal dialysis patients is limited. However, a recent randomized controlled trial of 158 peritoneal dialysis patient by Ito and colleagues suggest that a similar daily dose of spironolactone is safe over 2 years of study (18). Furthermore, peritoneal dialysis patients tend to have fewer issues with hyperkalemia compared with hemodialysis patients, suggesting that MRA should be at least as safe in these patients (30). Our trial lasted only 13 weeks, and we measured adherence through self-report. The true rate of permanent discontinuation and adherence may vary over longer durations of follow-up or alternate methods of measuring adherence. Furthermore, we did not collect information on the degree of residual renal function, which may modify the effects of eplerenone on the risk of hyperkalemia, and our trial was too small to reliably assess these subgroup effects. Whether preserved renal function would increase this risk (by providing an MRA-sensitive mechanism) or reduce this risk (by increasing the total potassium excretion) is unclear. Therefore, although MRA appears generally safe, insufficient data are available to warrant its general use for cardiovascular protection in patients receiving dialysis.
Mineralocorticoid receptor antagonism with eplerenone increases the risk of serious hyperkalemia but does not result in significantly more discontinuation of the drug. These results suggest that eplerenone may be safe to use in appropriate patients and with conscientious monitoring of predialysis potassium. However, whether MRA with eplerenone reduces cardiovascular events remains uncertain. Larger studies are required.
Disclosures
M.W. received research funding for this study from Pfizer through the Investigator Initiated Research program at Pfizer.
Supplementary Material
Acknowledgments
M.W. is supported by a New Investigator Award from the Kidney Research Scientist Core Education and National Training (KRESCENT) Program.
This trial was funded by the Canadian Institutes of Health Research, the Canadian Network and Centre for Trials Internationally, the Canadian Kidney Knowledge Translation and Generation Network, and the Pfizer Investigator Initiated Research program. No funder had any role in the design, conduct, or reporting of the trial.
Footnotes
Published online ahead of print. Publication date available at www.cjasn.org.
This article contains supplemental material online at http://cjasn.asnjournals.org/lookup/suppl/doi:10.2215/CJN.12371214/-/DCSupplemental.
References
- 1.Harnett JD, Kent GM, Barre PE, Taylor R, Parfrey PS: Risk factors for the development of left ventricular hypertrophy in a prospectively followed cohort of dialysis patients. J Am Soc Nephrol 4: 1486–1490, 1994 [DOI] [PubMed] [Google Scholar]
- 2.Foley RN, Curtis BM, Randell EW, Parfrey PS: Left ventricular hypertrophy in new hemodialysis patients without symptomatic cardiac disease. Clin J Am Soc Nephrol 5: 805–813, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Foley RN: Clinical epidemiology of cardiac disease in dialysis patients: Left ventricular hypertrophy, ischemic heart disease, and cardiac failure. Semin Dial 16: 111–117, 2003 [DOI] [PubMed] [Google Scholar]
- 4.Foley RN, Collins AJ: End-stage renal disease in the United States: An update from the United States Renal Data System. J Am Soc Nephrol 18: 2644–2648, 2007 [DOI] [PubMed] [Google Scholar]
- 5.Sato A, Funder JW, Saruta T: Involvement of aldosterone in left ventricular hypertrophy of patients with end-stage renal failure treated with hemodialysis. Am J Hypertens 12: 867–873, 1999 [DOI] [PubMed] [Google Scholar]
- 6.Brilla CG, Maisch B: Regulation of the structural remodelling of the myocardium: from hypertrophy to heart failure. Eur Heart J 15[Suppl D]: 45–52, 1994 [DOI] [PubMed] [Google Scholar]
- 7.Brilla CG, Matsubara LS, Weber KT: Antifibrotic effects of spironolactone in preventing myocardial fibrosis in systemic arterial hypertension. Am J Cardiol 71: 12A–16A, 1993 [DOI] [PubMed] [Google Scholar]
- 8.Pitt B, Zannad F, Remme WJ, Cody R, Castaigne A, Perez A, Palensky J, Wittes J, Randomized Aldactone Evaluation Study Investigators : The effect of spironolactone on morbidity and mortality in patients with severe heart failure. N Engl J Med 341: 709–717, 1999 [DOI] [PubMed] [Google Scholar]
- 9.Pitt B, Remme W, Zannad F, Neaton J, Martinez F, Roniker B, Bittman R, Hurley S, Kleiman J, Gatlin M, Eplerenone Post-Acute Myocardial Infarction Heart Failure Efficacy and Survival Study Investigators : Eplerenone, a selective aldosterone blocker, in patients with left ventricular dysfunction after myocardial infarction. N Engl J Med 348: 1309–1321, 2003 [DOI] [PubMed] [Google Scholar]
- 10.Pitt B, Reichek N, Willenbrock R, Zannad F, Phillips RA, Roniker B, Kleiman J, Krause S, Burns D, Williams GH: Effects of eplerenone, enalapril, and eplerenone/enalapril in patients with essential hypertension and left ventricular hypertrophy: The 4E-left ventricular hypertrophy study. Circulation 108: 1831–1838, 2003 [DOI] [PubMed] [Google Scholar]
- 11.Pitt B, Pfeffer MA, Assmann SF, Boineau R, Anand IS, Claggett B, Clausell N, Desai AS, Diaz R, Fleg JL, Gordeev I, Harty B, Heitner JF, Kenwood CT, Lewis EF, O’Meara E, Probstfield JL, Shaburishvili T, Shah SJ, Solomon SD, Sweitzer NK, Yang S, McKinlay SM, TOPCAT Investigators : Spironolactone for heart failure with preserved ejection fraction. N Engl J Med 370: 1383–1392, 2014 [DOI] [PubMed] [Google Scholar]
- 12.Frankenfield DL, Weinhandl ED, Powers CA, Howell BL, Herzog CA, St Peter WL: Utilization and costs of cardiovascular disease medications in dialysis patients in Medicare Part D. Am J Kidney Dis 59: 670–681, 2012 [DOI] [PubMed] [Google Scholar]
- 13.Berry C, McMurray JJV: Serious adverse events experienced by patients with chronic heart failure taking spironolactone. Heart 85: E8, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Teo KK, Pfeffer M, Mancia G, O’Donnell M, Dagenais G, Diaz R, Dans A, Liu L, Bosch J, Joseph P, Copland I, Jung H, Pogue J, Yusuf S, Aliskiren Prevention of Later Life Outcomes trial Investigators : Aliskiren alone or with other antihypertensives in the elderly with borderline and stage 1 hypertension: The APOLLO trial. Eur Heart J 35: 1743–1751, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jones B, Jarvis P, Lewis JA, Ebbutt AF: Trials to assess equivalence: The importance of rigorous methods. BMJ 313: 36–39, 1996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gross E, Rothstein M, Dombek S, Juknis HI: Effect of spironolactone on blood pressure and the renin-angiotensin-aldosterone system in oligo-anuric hemodialysis patients. Am J Kidney Dis 46: 94–101, 2005 [DOI] [PubMed] [Google Scholar]
- 17.Hussain S, Dreyfus DE, Marcus RJ, Biederman RWW, McGill RL: Is spironolactone safe for dialysis patients? Nephrol Dial Transplant 18: 2364–2368, 2003 [DOI] [PubMed] [Google Scholar]
- 18.Ito Y, Mizuno M, Suzuki Y, Tamai H, Hiramatsu T, Ohashi H, Ito I, Kasuga H, Horie M, Maruyama S, Yuzawa Y, Matsubara T, Matsuo S, Nagoya Spiro Study Group : Long-term effects of spironolactone in peritoneal dialysis patients. J Am Soc Nephrol 25: 1094–1102, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Matsumoto Y, Kageyama S, Yakushigawa T, Arihara K, Sugiyama T, Mori Y, Sugiyama H, Ohmura H, Shio N: Long-term low-dose spironolactone therapy is safe in oligoanuric hemodialysis patients. Cardiology 114: 32–38, 2009 [DOI] [PubMed] [Google Scholar]
- 20.Matsumoto Y, Mori Y, Kageyama S, Arihara K, Sugiyama T, Ohmura H, Yakushigawa T, Sugiyama H, Shimada Y, Nojima Y, Shio N: Spironolactone reduces cardiovascular and cerebrovascular morbidity and mortality in hemodialysis patients. J Am Coll Cardiol 63: 528–536, 2014 [DOI] [PubMed] [Google Scholar]
- 21.Saudan P, Mach F, Perneger T, Schnetzler B, Stoermann C, Fumeaux Z, Rossier M, Martin PY: Safety of low-dose spironolactone administration in chronic haemodialysis patients. Nephrol Dial Transplant 18: 2359–2363, 2003 [DOI] [PubMed] [Google Scholar]
- 22.Taheri S, Mortazavi M, Shahidi S, Pourmoghadas A, Garakyaraghi M, Seirafian S, Eshaghian A, Ghassami M: Spironolactone in chronic hemodialysis patients improves cardiac function. Saudi J Kidney Dis Transpl 20: 392–397, 2009 [PubMed] [Google Scholar]
- 23.Vukusich A, Kunstmann S, Varela C, Gainza D, Bravo S, Sepulveda D, Cavada G, Michea L, Marusic ET: A randomized, double-blind, placebo-controlled trial of spironolactone on carotid intima-media thickness in nondiabetic hemodialysis patients. Clin J Am Soc Nephrol 5: 1380–1387, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Shavit L, Neykin D, Lifschitz M, Slotki I: Effect of eplerenone on blood pressure and the renin-angiotensin-aldosterone system in oligo-anuric chronic hemodialysis patients—a pilot study. Clin Nephrol 76: 388–395, 2011 [DOI] [PubMed] [Google Scholar]
- 25.Agarwal R, Sinha AD, Pappas MK, Abraham TN, Tegegne GG: Hypertension in hemodialysis patients treated with atenolol or lisinopril: A randomized controlled trial. Nephrol Dial Transplant 29: 672–681, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Knoll GA, Sahgal A, Nair RC, Graham J, van Walraven C, Burns KD: Renin-angiotensin system blockade and the risk of hyperkalemia in chronic hemodialysis patients. Am J Med 112: 110–114, 2002 [DOI] [PubMed] [Google Scholar]
- 27.Iitake K, Kimura T, Matsui K, Ota K, Shoji M, Inoue M, Yoshinaga K: Effect of haemodialysis on plasma ADH levels, plasma renin activity and plasma aldosterone levels in patients with end-stage renal disease. Acta Endocrinol (Copenh) 110: 207–213, 1985 [DOI] [PubMed] [Google Scholar]
- 28.Kohagura K, Higashiuesato Y, Ishiki T, Yoshi S, Ohya Y, Iseki K, Takishita S: Plasma aldosterone in hypertensive patients on chronic hemodialysis: Distribution, determinants and impact on survival. Hypertens Res 29: 597–604, 2006 [DOI] [PubMed] [Google Scholar]
- 29.Vasan RS, Evans JC, Benjamin EJ, Levy D, Larson MG, Sundstrom J, Murabito JM, Sam F, Colucci WS, Wilson PW: Relations of serum aldosterone to cardiac structure: Gender-related differences in the Framingham Heart Study. Hypertension 43: 957–962, 2004 [DOI] [PubMed] [Google Scholar]
- 30.Yasuda G, Shibata K, Takizawa T, Ikeda Y, Tokita Y, Umemura S, Tochikubo O: Prevalence of constipation in continuous ambulatory peritoneal dialysis patients and comparison with hemodialysis patients. Am J Kidney Dis 39: 1292–1299, 2002 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.