Abstract
Background
While delirium has been increasingly recognized as a serious and potentially preventable source of morbidity and mortality for hospitalized older persons, its long-term implications are not well understood. The objective of this study is to determine the total 1-year health care costs associated with delirium.
Methods
Hospitalized patients aged 70 years and older who participated in a previous controlled clinical trial of a delirium prevention intervention at an academic medical center between 1995 and 1998 were followed for 1 year after discharge. Total inflation-adjusted healthcare costs were computed using data from Medicare administrative files, hospital billing records, and the Connecticut Long-Term Care Registry. Regression models were used to determine costs associated with delirium after adjusting for patient sociodemographic and clinical characteristics.
Results
During the index hospitalization, 109 (13%) patients developed delirium while 732 did not. Patients with delirium had significantly higher unadjusted healthcare costs than non-delirious patients and survived fewer days. After adjusting for pertinent demographic and clinical characteristics, average costs per day survived among patients with delirium were over two and a half times the costs among patients without delirium. Total cost estimates attributable to delirium ranged from $16,303 to $64,421 per patient, implying that the national burden of delirium on the health care system ranges from $38 billion to $152 billion each year.
Conclusions
The economic impact of delirium is substantial, rivaling the health care costs of falls and diabetes. These results highlight the need for increased efforts to mitigate this clinically significant and costly disorder.
Introduction
Delirium, characterized as an acute decline of cognition and attention, represents a common and severe problem for hospitalized older patients, with occurrence rates from 14–56% and hospital mortality rates from 25–33%.1, 2 The development of delirium has been associated with increased morbidity, persistent functional decline, increased nursing time per patient, higher per day hospital costs, increased length of hospital stay, higher rates of nursing home placement and increased mortality.3–6 Delirium often initiates a cascade of events that can include functional decline, caregiver burden, increased morbidity and mortality, and higher health care costs.3–5, 7–10 The problem of delirium in older hospitalized patients has assumed particular importance because patients aged 65 years and over currently account for more than 48% of all days of hospital care.11
Although the short-term implications of delirium have been well-documented, recent evidence 2–6, 8, 10, 12–17 suggests that delirium also has substantial long-term sequelae with significant implications for health care utilization and costs. However, previous studies of health care costs related to delirium have been limited to specific services (i.e., hospital length of stay, intensive care unit, or nursing home care). In an effort to document the broader economic and health care burden of delirium, the objective of this study is to determine the long-term direct health care costs associated with delirium. The current study provides a comprehensive cost estimate for all direct health care services from the index hospitalization through 1 year after discharge.
Methods
Sample
The study sample consisted of 841 individuals who participated in a controlled trial of a delirium prevention intervention at Yale-New Haven Hospital between 1995 and 1998. Details of the study are described elsewhere.18 Briefly, patients meeting the following criteria were enrolled: consecutive admissions to three non-intensive care general medical units, aged 70 or above, no evidence of delirium at admission, and at intermediate or high risk for delirium based on a previously developed risk model.19 Patients who could not participate in interviews (e.g., profound dementia, language barrier, profound aphasia, intubation, coma, or respiratory isolation), who had a terminal illness, who had a hospital stay of 48 hours or less, or who had prior enrollment in the study were excluded. Informed consent for participation and permission to acquire subsequent follow-up data was obtained from the patients, or from a proxy for those with substantial cognitive impairment, according to procedures approved by the institutional review board of the Yale University School of Medicine.
Delirium was ascertained daily during hospitalization using the Confusion Assessment Method,20, 21 with delirium defined by the presence of acute onset and fluctuating course, inattention, and either disorganized thinking or altered level of consciousness. Patients who developed delirium while hospitalized were identified, and all patients were followed for up to 1 year following discharge to determine health care service use and costs. Of the 919 subjects enrolled in the original trial,18 25 were excluded because they could not be linked to the Medicare files, 50 were excluded because they were enrolled in a Medicare managed care health maintenance organization and hence did not have detailed cost data, and 3 were excluded because they were missing cost data from the index hospitalization. Thus, the final study sample, which included both intervention and control subjects, consisted of 841 individuals.
Sources of data
Information on patient demographic characteristics, comorbidities, and functional status were obtained from primary data collected during the controlled trial. Data on health care service use and costs, including inpatient, outpatient, nursing home, home health, rehabilitation, and other services, were obtained from Medicare Part A and B administrative claims files for these patients. Additional service use and cost data were obtained from Yale Medical Information Systems for the index hospitalization and subsequent readmissions to Yale-New Haven Hospital. Because Medicare nursing home coverage is limited to 100 days of care and information on stays beyond this limit may be inaccurate or missing, the Connecticut Long-Term Care Registry (LTCR) was used to supplement the Medicare files. The LTCR is a longitudinal database containing demographic, health status, and nursing home length of stay information (including dates of all nursing home admissions and discharges) for all Connecticut nursing facility resident stays.
Patient deaths were identified by telephone follow-up contacts at 1, 6, and 12-month periods, by daily obituary review, and by the Social Security Death Index. All deaths and dates of death were confirmed by at least 2 sources: review of medical records, death certificates, systematic obituary review, Medicare Enrollment and Claims files and/or National Death Index or Social Security databases.
Measures
Total health care costs for patients in the controlled trial were computed during the index hospitalization and through 1 year after discharge. For costs incurred during the index hospitalization, hospital charges were converted to costs using the hospital-specific cost-to-charge ratio. For all other services, costs were calculated using Medicare reimbursed amounts rather than charges, because reimbursed amounts are payments actually received by providers for their services and hence are a better measure of transaction prices than billed charges.22–24 For patients with unqualified nursing home days (i.e., days not reimbursed by Medicare because they exceed the 100 day limit), the number of additional days of care for these patients was determined from the Medicare records or LTCR, and costs for these days were imputed using the average daily cost of care associated with the nursing home in which the patient was admitted. Costs were adjusted for inflation using the medical care component of the consumer price index, and are reported in 2005 dollars.
Analyses
We used SAS software, version 9.1 for all analyses.25 We first compared unadjusted mean total costs across the delirium and non-delirium groups using a Wilcoxon test. Next, we calculated adjusted mean total costs using linear regression models. Independent variables in the model included whether the patient had delirium during the index hospitalization, patient age, race, gender, whether the patient received the delirium prevention intervention, Charlson comorbidity score, whether the patient had dementia, the number of impairments in activities of daily living, whether the patient died during the study period, and an interaction term of Charlson comorbidity score with whether the patient died during the study period. We explored other interaction terms as well, but the interaction of Charlson score and whether the patient died was the only interaction term that significantly improved the fit of the model. Because traditional ordinary least square regression is not appropriate for skewed data, costs were log-transformed before running the regression model, and adjusted average total costs were re-transformed to the non-log scale using the smearing estimator,26 after ascertaining that the log-scale residuals were homoscedastic.27
Because some patients died during the study period, costs may be right-censored. Moreover, if more patients with delirium die before the end of the study period than patients without delirium, the costs associated with delirium may be underestimated. To account for this potential bias, total direct health care costs were also modeled in 2 additional ways. First, total costs were divided by total days survived to derive an average cost per day survived. Adjusted costs per day survived were computed for patients with delirium and for those without delirium using the same regression model techniques described above, using average cost per day survived as the dependent variable. These adjusted average costs per day survived were then multiplied by the average number of days survived in each group to derive a total cost for each group. Standard errors of these total cost estimates were calculated using bootstrapping methods,28 and a t-test was used to compare costs across the delirium and non-delirium groups.
The second approach was to use a partitioned estimator to model total costs based on methods developed by Lin 29 and Bang and Tsiatis.30 The study period was divided into 1-month time intervals, and average total direct health care costs for patients with delirium and for patients without delirium were computed in each month among those individuals who survived to the end of that month. A Cox proportional hazards regression model was used to estimate fitted Kaplan-Meier estimators for surviving to the end of each month, and costs were summed across months using the Kaplan-Meier estimators as inverse weights. Bootstrapping methods 28 were again used to compute standard errors for the cost estimates, and a t-test was used to compare costs across the delirium and non-delirium groups.
All authors had full access to all the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis.
Results
Characteristics of the sample are presented in Table 1. Of the 841 individuals included in the study sample, 109 (13.0%) developed delirium during the index hospitalization. A higher proportion of patients with delirium were admitted from a nursing home, had comorbid dementia, or died during the study period compared to patients who did not develop delirium. Delirium patients also had more impairments in activities of daily living, higher Charlson and APACHE II scores, and lower MMSE scores. A lower proportion of patients who received the delirium prevention intervention developed delirium compared to patients who did not receive the intervention.
Table 1.
Baseline characteristics of patients in the sample *
| Measure | Total cohort (N=841) | Delirium group (N=109) | Non-delirium group (N=732) | p-value † |
|---|---|---|---|---|
| Age | 80.2 ± 6.4 | 81.7 ± 7.1 | 80.0 ± 6.3 | 0.02 |
| Male gender | 329 (39) | 41 (38) | 288 (39) | 0.73 |
| Non-white race | 104 (12) | 20 (18) | 84 (12) | 0.04 |
| Married | 302 (36) | 32 (29) | 270 (37) | 0.13 |
| Residence in nursing home prior to admission | 53 (6) | 12 (11) | 41 (6) | 0.03 |
| Education (years) | 11.1 ± 3.5 | 10.2 ± 3.3 | 11.2 ± 3.5 | 0.004 |
| Charlson | 3.0 ± 2.3 | 3.4 ± 2.4 | 2.9 ± 2.3 | 0.03 |
| APACHE II score (first 48 hours of admission) | 15.7 ± 4.1 | 17.0 ± 4.3 | 15.5 ± 4.0 | <.001 |
| Dementia | 110 (13) | 30 (28) | 80 (11) | <.001 |
| Number of ADL disabilities (prior to hospitalization) | 1.0 ± 1.7 | 2.0 ± 2.4 | 0.9 ± 1.6 | <.001 |
| MMSE score (at hospital admission) | 23.3 ± 4.9 | 19.8 ± 5.1 | 23.8 ± 4.6 | <.001 |
| Principal Diagnosis | ||||
| Pneumonia | 92 (11) | 10 (9) | 82 (11) | 0.53 |
| Chronic lung disease | 90 (11) | 6 (6) | 84 (11) | 0.06 |
| Congestive heart failure | 96 (11) | 17 (16) | 79 (11) | 0.14 |
| Ischemic heart attack | 72 (9) | 4 (4) | 68 (9) | 0.05 |
| Gastrointestinal disease | 111 (13) | 14 (13) | 97 (13) | 0.91 |
| Diabetes mellitus or metabolic disorder | 37 (4) | 6 (6) | 31 (4) | 0.55 |
| Cancer | 22 (3) | 4 (4) | 18 (2) | 0.46 |
| Cerebrovascular disease | 20 (2) | 4 (4) | 16 (2) | 0.34 |
| Renal failure | 17 (2) | 2 (2) | 15 (2) | 0.88 |
| Anemia | 12 (1) | 0 (0) | 12 (2) | 0.18 |
| Other | 272 (32) | 42 (39) | 230 (31) | 0.14 |
| Received the delirium prevention intervention | 413 (49) | 43 (39) | 370 (51) | 0.03 |
Values reported are N(%) or mean ± SD. APACHE II = Acute Physiology and Chronic Health Evaluation, ADL = Activities of Daily Living, MMSE = Mini Mental State Examination.
P-values are for comparison of the delirium and non-delirium groups.
As shown in Table 2, delirium patients survived an average of 256 days during the 1-year follow-up period, compared to 322 days for non-delirium patients, although this difference was not statistically significant (p=0.89). Despite the shorter survival time, total unadjusted health care costs were significantly higher for patients who developed delirium during the index hospitalization than for those without delirium ($69,498 ± 59,120 versus $47,958 ± 45,640, respectively; p<0.001). Total costs per day survived were also higher for patients with delirium than for those without, both among patients who died during the study period and among those who survived.
Table 2.
Unadjusted Survival and Cost Outcomes
| Measure | Total cohort (N=841) | Delirium group (N=109) | Non-delirium group (N=732) | p-value † |
|---|---|---|---|---|
| Died within 1 year, N (%) | 208 (25) | 47 (43) | 161 (22) | <.001 |
| Days of follow-up | ||||
| Mean ± SD | 313 ± 116 | 256 ± 157 | 322 ± 106 | 0.89 |
| Median | 369 | 369 | 369 | |
| Total health care costs* | ||||
| Mean ± SD | $50,745 ± $48,113 | $69,498 ± $59,120 | $47,958 ± $45,640 | <.001 |
| Median | $33,295 | $56,722 | $30,662 | |
| Total costs per day survived* | ||||
| All patients | ||||
| Mean ± SD | $256 ± $396 | $563 ± $774 | $211 ± $276 | <.001 |
| Median | $140 | $322 | $117 | |
| Patients who died during study period | ||||
| Mean ± SD | $461 ± $481 | $732 ± $773 | $382 ± $316 | 0.004 |
| Median | $332 | $471 | $287 | |
| Patients who survived during entire study period | ||||
| Mean ± SD | $104 ± $100 | $186 ± $122 | $95 ± $92 | <.001 |
| Median | $66 | $159 | $60 | |
Costs are adjusted for inflation and are reported in 2005 dollars.
P-values are for comparison of the delirium and non-delirium groups.
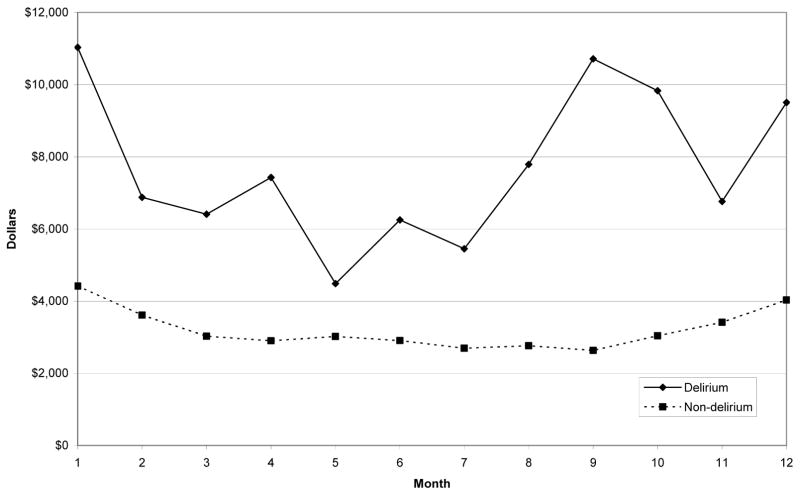
Results from the regression models showed that delirium patients had significantly higher costs than patients without delirium even after adjusting for relevant demographic and clinical characteristics. As expected, patients with higher Charlson scores, who had dementia, or who died during the follow-up period also had significantly higher total healthcare costs. Receipt of the delirium prevention intervention did not significantly affect costs. Adjusted total health care costs by month for the delirium and non-delirium groups based on the regression models are illustrated in Figure 1. Adjusted costs are higher for the delirium group in each month. The difference in adjusted total costs between the delirium and non-delirium groups is initially relatively large ($6,613 in the first month), then falls over time until about month 5, and then generally increases again through month 9.
Figure 1.
Adjusted mean total health care costs by month
As illustrated in Table 3, adjusted total costs were significantly higher for the delirium group than for the non-delirium group. Total costs per day survived were over two and a half times higher for delirium patients compared to patients without delirium. In the model that ignores the right-censoring problem (method 1), costs for delirium patients were $16,303 higher than for non-delirium patients. Costs attributable to delirium were higher in the two models that accounted for the fact that the data were right-censored (methods 2 and 3), ranging from $60,516 to $64,421. Ninety-five percent of the difference in costs was due to inpatient and nursing home care.
Table 3.
Adjusted total 1-year health care costs *
| Measure | Delirium Group | Non-delirium Group | Difference (Delirium - Non- delirium) | p-value |
|---|---|---|---|---|
| Total costs per survival day | $461 ± $570 | $166 ± $195 | $295 | <.001 |
| Total costs, method 1† | $65,755 ± $58,247 | $49,452 ± $43,806 | $16,303 | 0.005 |
| Total costs, method 2 ‡ | $117,620 ± $109,530 | $53,199 ± $54,698 | $64,421 | <.001 |
| Total costs, method 3 § | $120,349 ± $181,274 | $59,833 ± $55,155 | $60,516 | <.001 |
Costs are adjusted for inflation and are reported in 2005 dollars.
Based on ordinary least square (OLS) regression model of log-transformed total costs.
Based on OLS regression model of log-transformed daily costs multiplied by average days survived.
Based on partitioned estimator of Bang and Tsiatis (2000).
Comment
This study documents the considerable direct health care costs associated with delirium in the United States. We estimate that delirium is responsible for between $60,516 and $64,421 in additional health care costs per delirious patient per year. Following Inouye et al.2 and assuming that delirium complicates hospital stays for 20% of the 11.8 million persons aged 65 and older who are hospitalized each year, our results imply that total direct 1-year health care costs attributable to delirium range from $143 billion to $152 billion nationally. These estimates are adjusted for the difference in survival time. Even using our most conservative estimate, which ignores the right-censoring problem, costs associated with delirium exceed $38 billion per year. Given that a number of effective interventions have been developed to prevent or treat delirium,18, 31–36 at least some of these costs may be avoidable.
We took great care not to underestimate costs associated with delirium due to more patients with delirium dying before the end of the study period than patients without delirium. However, costs may also be underestimated if patients with delirium die quietly, that is, without additional diagnostic or therapeutic intervention. To explore this possibility, we compared average daily costs for patients with and without delirium stratified by whether they survived the entire study period. Average daily costs were significantly higher for the delirium patients regardless of whether they died during the study period (Table 2). Even though we did not demonstrate the cost savings for delirious patients who die quickly in our secondary data analysis, this remains a possibility for a subset of patients which should be acknowledged and which may bias our results towards underestimating the costs associated with delirium.
National annual health care costs have been estimated for a number of conditions, including hip fracture ($7 billion),37 non-fatal falls ($19 billion),38 diabetes ($91.8 billion),39 and cardiovascular disease ($257.6 billion).40 While we acknowledge the difficulty and limitations in comparing across conditions due to differences in study methodology, diagnostic overlap, and shared comorbidities, our results suggest that the economic burden of delirium is substantial, even relative to other conditions.
The pattern of costs over time is interesting. As previous studies have shown,8, 10, 42–44 delirium increases hospital length of stay and costs, so the large initial costs associated with delirium are not surprising. The increased costs later in the period may be due to recurrence of delirium or terminal care costs, although more research is needed to explore the sources of these costs.
We included patients in the study sample who had received the delirium prevention intervention in order to have the largest possible sample size. While these patients had lower rates of delirium than patients in the control group, receipt of the delirium prevention intervention did not significantly affect costs in the multivariable models. To the extent that including these patients biases our results, we would argue that the bias would be conservative, because, if anything, delirium in the intervention group would have been anticipated to be less costly. Moreover, as a sensitivity analysis, when the sample was limited to just those usual care patients who did not receive the intervention, the costs associated with delirium were not substantially different (data not shown).
Although previous studies have demonstrated the increased hospital and nursing home costs associated with delirium,5, 43, 44 this study is the first to document the costs associated with delirium across such a wide range of services (inpatient, intensive care unit, emergency room, outpatient, nursing home, home health, rehabilitation, and other services) and over such a long period of time. While the study has a number of strengths, such as the availability of detailed clinical information and comprehensive service use and cost data from multiple sources, some limitations of the analysis deserve comment. First, although our cost estimates are adjusted for a number of patient sociodemographic and clinical characteristics, there may be residual confounding due to inherent differences between the delirium and non-delirium groups that might affect costs. However, we believe that any bias introduced by such residual confounding would be small because we are able to include a number of detailed clinical measures in our models. Second, cost estimates are derived from a single site only, and hence the generalizability of the results may be limited. In addition, cost estimates include direct health care costs only, and do not take into account important indirect costs associated with caregiver burden or reduced quality of life. Finally, follow-up was truncated at one year; therefore, any costs associated with delirium that accrue more than 1 year after discharge are not included.
Despite these limitations, it is clear that the economic burden of delirium is substantial. It is our hope that these results draw attention to delirium as a serious condition with significant long-term clinical and economic implications. Future research will need to focus on the specific sources of the increased health care costs associated with delirium. Given that the condition is costly, increasing in magnitude with the aging population, and potentially preventable, increased efforts to prevent, detect and treat delirium are urgently needed.
Acknowledgments
Grant support was provided in part by grants R01AG12551, R21AG025193, and K24AG00949 (Dr. Inouye) and R21AG026566 (Dr. Marcantonio) from the National Institute on Aging.
The study was partially funded by grants from the National Institute on Aging. The sponsor did not participate in the design and conduct of the study; in the collection, management, analysis and interpretation of the data; or in the preparation, review or approval of the manuscript. All authors had full access to all of the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis.
References
- 1.Pandharipande P, Jackson J, Ely EW. Delirium: acute cognitive dysfunction in the critically ill. Curr Opin Crit Care. 2005;11:360–368. doi: 10.1097/01.ccx.0000170503.76528.4b. [DOI] [PubMed] [Google Scholar]
- 2.Inouye SK, Schlesinger MJ, Lydon TJ. Delirium: a symptom of how hospital care is failing older persons and a window to improve quality of hospital care. Am J Med. 1999;106:565–573. doi: 10.1016/s0002-9343(99)00070-4. [DOI] [PubMed] [Google Scholar]
- 3.Cole MG, Primeau FJ. Prognosis of delirium in elderly hospital patients. CMAJ. 1993;149:41–46. [PMC free article] [PubMed] [Google Scholar]
- 4.Inouye SK, Rushing JT, Foreman MD, Palmer RM, Pompei P. Does delirium contribute to poor hospital outcomes? A three-site epidemiologic study. J Gen Intern Med. 1998;13:234–242. doi: 10.1046/j.1525-1497.1998.00073.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Leslie DL, Zhang Y, Bogardus ST, Holford TR, Leo-Summers LS, Inouye SK. Consequences of preventing delirium in hospitalized older adults on nursing home costs. J Am Geriatr Soc. 2005;53:405–409. doi: 10.1111/j.1532-5415.2005.53156.x. [DOI] [PubMed] [Google Scholar]
- 6.Leslie DL, Zhang Y, Holford TR, Bogardus ST, Leo-Summers LS, Inouye SK. Premature death associated with delirium at 1-year follow-up. Arch Intern Med. 2005;165:1657–1662. doi: 10.1001/archinte.165.14.1657. [DOI] [PubMed] [Google Scholar]
- 7.Francis J, Kapoor WN. Prognosis after hospital discharge of older medical patients with delirium. J Am Geriatr Soc. 1992;40:601–606. doi: 10.1111/j.1532-5415.1992.tb02111.x. [DOI] [PubMed] [Google Scholar]
- 8.Levkoff SE, Evans DA, Liptzin B, et al. Delirium. The occurrence and persistence of symptoms among elderly hospitalized patients. Arch Intern Med. 1992;152:334–340. doi: 10.1001/archinte.152.2.334. [DOI] [PubMed] [Google Scholar]
- 9.Murray AM, Levkoff SE, Wetle TT, et al. Acute delirium and functional decline in the hospitalized elderly patient. J Gerontol. 1993;48:M181–186. doi: 10.1093/geronj/48.5.m181. [DOI] [PubMed] [Google Scholar]
- 10.O’Keeffe S, Lavan J. The prognostic significance of delirium in older hospital patients. J Am Geriatr Soc. 1997;45:174–178. doi: 10.1111/j.1532-5415.1997.tb04503.x. [DOI] [PubMed] [Google Scholar]
- 11.A Profile of Older Americans: 2001. Washington, DC: Administration on Aging, U.S. Department of Health and Human Services; 2001. pp. 1–13. [Google Scholar]
- 12.Rockwood K. The occurrence and duration of symptoms in elderly patients with delirium. J Gerontol. 1993;48:M162–166. doi: 10.1093/geronj/48.4.m162. [DOI] [PubMed] [Google Scholar]
- 13.Inouye SK. The dilemma of delirium: clinical and research controversies regarding diagnosis and evaluation of delirium in hospitalized elderly medical patients. Am J Med. 1994;97:278–288. doi: 10.1016/0002-9343(94)90011-6. [DOI] [PubMed] [Google Scholar]
- 14.Inouye SK. Delirium and cognitive decline: does delirium lead to dementia? In: Fillit HM, Butler RN, editors. Cognitive Decline: Strategies for Prevention. London: Greenwich Medical Media; 1997. pp. 85–107. [Google Scholar]
- 15.Rockwood K, Cosway S, Carver D, Jarrett P, Stadnyk K, Fisk J. The risk of dementia and death after delirium. Age Ageing. 1999;28:551–556. doi: 10.1093/ageing/28.6.551. [DOI] [PubMed] [Google Scholar]
- 16.Dolan MM, Hawkes WG, Zimmerman SI, et al. Delirium on hospital admission in aged hip fracture patients: prediction of mortality and 2-year functional outcomes. J Gerontol A Biol Sci Med Sci. 2000;55:M527–534. doi: 10.1093/gerona/55.9.m527. [DOI] [PubMed] [Google Scholar]
- 17.McCusker J, Cole M, Dendukuri N, Belzile E, Primeau F. Delirium in older medical inpatients and subsequent cognitive and functional status: a prospective study. CMAJ. 2001;165:575–583. [PMC free article] [PubMed] [Google Scholar]
- 18.Inouye SK, Bogardus ST, Jr, Charpentier PA, et al. A multicomponent intervention to prevent delirium in hospitalized older patients. N Engl J Med. 1999;340:669–676. doi: 10.1056/NEJM199903043400901. [DOI] [PubMed] [Google Scholar]
- 19.Inouye SK, Viscoli CM, Horwitz RI, Hurst LD, Tinetti ME. A predictive model for delirium in hospitalized elderly medical patients based on admission characteristics. Ann Intern Med. 1993;119:474–481. doi: 10.7326/0003-4819-119-6-199309150-00005. [DOI] [PubMed] [Google Scholar]
- 20.Inouye SK, van Dyck CH, Alessi CA, Balkin S, Siegal AP, Horwitz RI. Clarifying confusion: the confusion assessment method. A new method for detection of delirium. Ann Intern Med. 1990;113:941–948. doi: 10.7326/0003-4819-113-12-941. [DOI] [PubMed] [Google Scholar]
- 21.Marcantonio ER, Michaels M, Resnick NM. Diagnosing delirium by telephone. J Gen Intern Med. 1998;13:621–623. doi: 10.1046/j.1525-1497.1998.00185.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Berndt ER, Bir A, Busch SH, Frank RG, Normand SL. The medical treatment of depression, 1991–1996: productive inefficiency, expected outcome variations, and price indexes. J Health Econ. 2002;21:373–396. doi: 10.1016/s0167-6296(01)00132-1. [DOI] [PubMed] [Google Scholar]
- 23.Leslie DL, Rosenheck RA. Shifting to Outpatient Care? Mental Health Care Use and Cost Under Private Insurance. Am J Psy. 1999;156:1250–1257. doi: 10.1176/ajp.156.8.1250. [DOI] [PubMed] [Google Scholar]
- 24.Leslie DL, Rosenheck RA. Changes in Inpatient Mental Health Utilization and Cost in a Privately Insured Population: 1993–1995. Med Care. 1999;37:457–468. doi: 10.1097/00005650-199905000-00005. [DOI] [PubMed] [Google Scholar]
- 25.SAS Institute I. SAS. 9.1 ed. Cary, NC: SAS Institute, Inc; [Google Scholar]
- 26.Duan N. Smearing estimate: a nonparametric retransformation method. J Am Stat Assoc. 1983;78:605–610. [Google Scholar]
- 27.Manning WG, Mullahy J. Estimating log models: to transform or not to transform? J Health Econ. 2001;20:461–494. doi: 10.1016/s0167-6296(01)00086-8. [DOI] [PubMed] [Google Scholar]
- 28.Efron B, Tibshirani R. An Introduction to the Bootstrap. New York, NY: Chapman & Hall; 1993. [Google Scholar]
- 29.Lin DY, Feuer EJ, Etzioni R, Wax Y. Estimating medical costs from incomplete follow-up data. Biometrics. 1997;53:419–434. [PubMed] [Google Scholar]
- 30.Bang H, Tsiatis AA. Median regression with censored cost data. Biometrics. 2002;58:643–649. doi: 10.1111/j.0006-341x.2002.00643.x. [DOI] [PubMed] [Google Scholar]
- 31.Marcantonio ER, Flacker JM, Wright RJ, Resnick NM. Reducing delirium after hip fracture: a randomized trial. J Am Geriatr Soc. 2001;49:516–522. doi: 10.1046/j.1532-5415.2001.49108.x. [DOI] [PubMed] [Google Scholar]
- 32.Milisen K, Foreman MD, Abraham IL, et al. A nurse-led interdisciplinary intervention program for delirium in elderly hip-fracture patients. J Am Geriatr Soc. 2001;49:523–532. doi: 10.1046/j.1532-5415.2001.49109.x. [DOI] [PubMed] [Google Scholar]
- 33.Ruth M, Locsin R. The effect of music listening on acute confusion and delirium in elders undergoing elective hip and knee surgery. J Clin Nurs. 2004;13:91–96. doi: 10.1111/j.1365-2702.2004.01048.x. [DOI] [PubMed] [Google Scholar]
- 34.Naughton BJ, Saltzman S, Ramadan F, Chadha N, Priore R, Mylotte JM. A multifactorial intervention to reduce prevalence of delirium and shorten hospital length of stay. J Am Geriatr Soc. 2005;53:18–23. doi: 10.1111/j.1532-5415.2005.53005.x. [DOI] [PubMed] [Google Scholar]
- 35.Tabet N, Hudson S, Sweeney V, et al. An educational intervention can prevent delirium on acute medical wards. Age Ageing. 2005;34:152–156. doi: 10.1093/ageing/afi031. [DOI] [PubMed] [Google Scholar]
- 36.Lundstrom M, Edlund A, Karlsson S, Brannstrom B, Bucht G, Gustafson Y. A multifactorial intervention program reduces the duration of delirium, length of hospitalization, and mortality in delirious patients. J Am Geriatr Soc. 2005;53:622–628. doi: 10.1111/j.1532-5415.2005.53210.x. [DOI] [PubMed] [Google Scholar]
- 37.Haentjens P, Lamraski G, Boonen S. Costs and consequences of hip fracture occurrence in old age: an economic perspective. Disabil Rehabil. 2005;27:1129–1141. doi: 10.1080/09638280500055529. [DOI] [PubMed] [Google Scholar]
- 38.Stevens JA, Corso PS, Finkelstein EA, Miller TR. The costs of fatal and non-fatal falls among older adults. Inj Prev. 2006;12:290–295. doi: 10.1136/ip.2005.011015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hogan P, Dall T, Nikolov P. Economic costs of diabetes in the US in 2002. Diabetes Care. 2003;26:917–932. doi: 10.2337/diacare.26.3.917. [DOI] [PubMed] [Google Scholar]
- 40.Thom T, Haase N, Rosamond W, et al. Heart disease and stroke statistics--2006 update: a report from the American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Circulation. 2006;113:e85–151. doi: 10.1161/CIRCULATIONAHA.105.171600. [DOI] [PubMed] [Google Scholar]
- 41.Summary of the FY 2007 President’s Budget. Vol. 2006. National Institutes of Health; 2006. [Google Scholar]
- 42.Edelstein DM, Aharonoff GB, Karp A, Capla EL, Zuckerman JD, Koval KJ. Effect of postoperative delirium on outcome after hip fracture. Clin Orthop. 2004:195–200. doi: 10.1097/01.blo.0000128649.59959.0c. [DOI] [PubMed] [Google Scholar]
- 43.Franco K, Litaker D, Locala J, Bronson D. The cost of delirium in the surgical patient. Psychosomatics. 2001;42:68–73. doi: 10.1176/appi.psy.42.1.68. [DOI] [PubMed] [Google Scholar]
- 44.Milbrandt EB, Deppen S, Harrison PL, et al. Costs associated with delirium in mechanically ventilated patients. Crit Care Med. 2004;32:955–962. doi: 10.1097/01.ccm.0000119429.16055.92. [DOI] [PubMed] [Google Scholar]