Abstract
Background/Aims
We aimed to investigate how neuropsychological test measures at presentation might differentiate frontotemporal degenerations (FTD) from Alzheimer disease (AD).
Methods
We compared autopsy-confirmed frontotemporal lobar degeneration (FTLD) and definite AD with Clinical Dementia Rating ≤1. Factor scores and t-values of each neuropsychological test measure were compared between FTLD and AD patients. Logistic regression analyses were applied to identify independent predictors within test measures for differentiation of FTLD from AD.
Results
Factor analyses showed that memory domain was more severely impaired in AD than in FTLD, whereas language and attention domain were more severely impaired in FTLD than in AD. Multiple logistic regression analysis showed that Letter Fluency, Boston Naming Test, and delayed memory recall remained as independent predictors of FTLD compared to AD. However, test measures did not discriminate between FTLD-tau and FTLD-ubiqutin (FTLD-U).
Conclusion
We confirm that memory and language function tests discriminate between FTLD and AD.
Keywords: Frontotemporal lobar degeneration, Frontotemporal dementia, Alzheimer disease, Mild cognitive impairment, Neuropsychological assessment
Introduction
The clinical diagnosis of frontotemporal degeneration (FTD) in early stages remains challenging. Although biomarkers from neuroimaging studies such as magnetic resonance imaging (MRI) [1] and positron emission tomography (PET) [2], or cerebrospinal fluid such as tau and β amyloid [3] may help distinguish FTD patients from patients with Alzheimer disease (AD), often neuropsychological testing is used for diagnosis in the clinical setting. However, it is less clear whether neuropsychological testing can discriminate FTD from AD, especially in the early stages of dementia [4]. A meta-analytic review has suggested that the overlap in the neuropsychological performance of FTD and AD patients can cause difficulties in differentiation of FTD from AD [5]. And while many authors have reported that memory function of FTD patients is relatively preserved compared with AD, detailed profiles of memory function have been less consistent [6,7]. Finally, it is also not established as to how much cognitive testing can help discriminate between the different pathologies underlying FTD, which principally include frontotemporal lobar degeneration (FTLD)-tau and FTLD-TAR DNA-binding protein of 43kDa (FTLD-TDP-43) [8–11].
In the present study, we investigated the neuropsychological testing features at presentation to our center of FTD patients who had Clinical Dementia Rating [12] (CDR) ≤1, and then ultimately had autopsy-confirmed FTLD. The objectives of this study were (1) to assess the differences of cognitive profiles between FTD and AD at the early stage, (2) to investigate how to improve the diagnostic accuracy for differentiation of FTD from AD using neuropsychological measures, and (3) to delineate the neuropsychological features of different pathological bases of FTD.
Materials and Methods
Case selection
Cases from the autopsy cohort of the Alzheimer Disease Research Center (ADRC) at Columbia University were selected for this study if they met primary neuropathological diagnosis of FTLD-tau, FTLD-ubiquitine/TDP-43 positive inclusions (FTLD-U), or definite AD, and presented with CDR ≤1, ie. the very mild stages of dementia. All ADRC participants are informed of the opportunity to participate in the brain bank, and as of 2010, this research clinic referral-based brain bank consisted of 607 brains from autopsies performed over 21 years. Twelve patients had FTLD-tau, 13 patients had FTLD-U, and 89 patients were pathologically diagnosed as definite AD. FTLD-tau patients included five with Pick disease, six with progressive supranuclear palsy (PSP), and one with corticobasal degeneration (CBD) according to the nosologic criteria for FTLD [13,14]. The ADRC research protocols are approved by the Institutional Review Boards of the New York State Psychiatric Institute and Columbia University.
Clinical assessment
At the initial visit to our center, all cases were evaluated with medical history, physical and neurologic examination, and neuropsychological test battery. A modified form of the Unified Parkinson Disease Rating Scale (mUPDRS) [15] was used to rate extrapyramidal motor signs. Patients with at least 1 motor sign rated mild-to-moderate were considered to have extrapyramidal tract signs. Visual hallucinations, depression, and other behavioral symptoms were assessed using the Columbia University Scale for Psychopathology in Alzheimer’s Disease [16]. CDR [12] was evaluated to rate the overall severity of dementia.
Neuropsychological assessment
The neuropsychological test battery performed at the ADRC included the Mini-Mental State Examination (MMSE) [17], the Selective Reminding Test (SRT) [18], the Boston Naming Test (BNT) (short 15-item version) [19], verbal fluency tests of initial letter and category [20], the Rosen Drawing Test (five item version) [21], and the Digit Span subtest from the Wechsler Adult Intelligence Scale Revised [22]. Orientation was assessed by ten items about time and place from the MMSE. Selective Reminding Test sub-measures included short-term recall (SRT-STR), long-term retrieval (SRT-LTR), delayed free recall (SRT-DR), and delayed recognition (SRT-Rcg). An SRT retention score (= delayed recall number /correct retrieval number at last trial of encoding phase) was also calculated. Controlled Oral Word Association test included letter verbal fluency test using C, F, and L for English speakers, and A, B, and S for Spanish speakers (7 AD cases) [20], and semantic category fluency task was using animal naming. T-scores of all neuropsychological tests were applied based on established age- and education-adjusted norms [23,24].
Neuropathological evaluation
Neuropathological assessment was performed using a protocol described by Vonsattel et al [25,26]. Neuritic plaques and neurofibrillary tangles were detected using hematoxylin-eosin and the modified Bielschowsky silver method. Immunohistochemistry was performed for beta-amyloid, phosphorylated tau, ubiquitin (p62), TDP-43, and glial fibrillary acidic protein. Autopsy-confirmed AD patients have been previously described [27]. Briefly, all of the AD cases met the criteria of Braak stage ≥4 neurofibrillary pathological findings, and also met CERAD neuropathological criteria for definite AD. The neuropathological diagnosis of FTLD-tau was based on the presence of tau-positive inclusions: Pick body (Pick disease), tau-positive tufted astrocytes (PSP), and ballooned achromatic neurons and astrocytic plaques (CBD). The neuropathological diagnosis of FTLD-U was based on the presence of typical TDP-43-type cytoplasmic ubiquinated inclusions. Cases with FTLD did not demonstrate significant AD pathology (Braak stage ≤3, and NIA-Reagan criteria low likelihood).
Statistical analyses
Baseline characteristics were compared among the groups using Pearson χ2 tests or Fisher’s exact test for nominal data. For continuous variables, Mann-Whitney test and Kruskal Wallis tests were used to assess differences between AD and FTLD (FTLD-tau + FTLD-U), and differences among AD, FTLD-tau, and FTLD-U, respectively. To identify the underlying factor structure, a factor analysis was performed on 11 neuropsychological variables using generalized least squares method with Promax rotation. Factor extraction was based on the Kaiser-Guttman rule of retaining components with eigenvalues >1. Factor loading of each test >0.35 was considered as significant contributor to the factor. Mann-Whitney test and Kruskal Wallis test were applied to the each factor score and the age- and education-adjusted t-values of each neuropsychological test to examine the differences between AD and FTLD, and the differences among AD, FTLD-tau, and FTLD-U, respectively.
To assess the relative impairments in letter fluency compared to category fluency in FTLD, paired t-tests after repeated measures ANOVA were applied with test condition (letter fluency or category fluency). An unpaired t-test was used to compare the result of each fluency test between AD and FTLD. The “semantic index score” (SI=semantic fluency/(semantic fluency + letter fluency)) was calculated as suggested by Rascovsky et al. [28].
The contribution of each neuropsychological variable to the distinction between autopsy-confirmed AD and FTLD was examined by selecting age- and education-adjusted t-values of 11 neuropsychological variables with P <0.05 in the univariate analysis and included them simultaneously in multiple logistic regression analysis using a forward selection method. To identify independent predictors for differentiation of FTLD-U from AD, for differentiation of FTLD-tau from AD, and for differentiation of FTLD-U from FTLD-tau, separate logistic regression analyses were performed. Analyses were conducted using SPSS Version 19 (IBM Corporation, New York, NY, USA) and statistical significance was defined as p = 0.05.
Results
Comparison of clinical features
Demographic data is shown in table 1. The FTLD group was younger than the AD group at age of onset, age at initial visit, and age at death. Subgroup analysis revealed that both the FTLD-tau and FTLD-U group showed younger age of onset compared with the AD group. Duration between onset of symptoms and death was shorter in the patients with FTLD-U relative to patients in the AD patients. The FTLD group showed increased frequency of extrapyramidal tract signs at initial presentation compared with the AD group (Fisher’s exact test; p < 0.001; table 1). Subgroup analysis showed higher rate of extrapyramidal tract signs in both the FTLD-tau and FTLD-U group compared with the AD patients (Fisher’s exact test; p = 0.001, post-hoc analysis; p = 0.011 and p = 0.001, respectively). There were no group differences with regard to other symptoms, including depression.
Table 1.
Demographics of Patient Groups
| Pathological group | ||||||
|---|---|---|---|---|---|---|
| AD (n=89) |
FTLD (n=25) |
FTLD subtype | p - value | |||
| FTLD-tau (n=12) |
FTLD-U (n=13) |
AD FTLD |
AD FTLD-tau FTLD-U |
|||
| Demographic data | ||||||
| Age of onset | 68.3 (9.8) | 58.1 (9.4) | 60.0 (10.4) | 56.5 (8.7) | <0.001‡a | <0.001§b,c |
| Age at initial visit | 71.6 (9.6) | 63.6 (10.5) | 67.2 (11.5) | 60.2 (8.6) | 0.001‡a | 0.001§c |
| Age at death | 78.6 (10.4) | 68.8 (11.2) | 74.0 (10.2) | 63.9 (10.2) | <0.001‡a | <0.001§c,d |
| Disease duration (years) | 10.3 (4.1) | 9.8 (7.4) | 12.6 (7.5) | 7.4 (6.7) | 0.10‡ (ns) | 0.018§c |
| Education (years) | 14.7 (4.3) | 15.7 (2.9) | 14.3 (2.6) | 17.0 (2.7) | 0.55‡ (ns) | 0.071§ (ns) |
| Gender (male %) | 47.2 | 68.0 | 66.7 | 69.2 | 0.066* (ns) | 0.18† (ns) |
| Race (White/Black/Hispanic/Other) | 73/6/9/1 | 24/1/0/0 | 12/0/0/0 | 12/1/0/0 | 0.40† (ns) | 0.78† (ns) |
| Blessed Functional Activity Scale | 2.8 (1.7) | 3.0 (2.6) | 4.1 (2.9) | 2.0 (1.8) | 0.69‡ (ns) | 0.099§ (ns) |
| MMSE | 21.2 (4.8) | 23.7 (4.0) | 23.0 (4.7) | 24.3 (3.3) | 0.021‡a | 0.060§ (ns) |
| CDR | 0.74 (0.28) | 0.64 (0.34) | 0.58 (0.36) | 0.69 (0.33) | 0.16‡ (ns) | 0.27§ (ns) |
| Clinical Symptoms (%) | ||||||
| Extrapyramidal sign | 9.9% (n=81) |
50.0% (n=20) |
41.7% (n=12) |
62.5% (n=8) |
<0.001†a | <0.001†b,c |
| Visual hallucination | 4.2% (n=71) |
0% (n=19) |
0% (n=9) |
0% (n=10) |
1.00† (ns) | 1.00† (ns) |
| Illusion | 4.3% (n=70) |
0% (n=19) |
0% (n=9) |
0% (n=10) |
1.00† (ns) | 1.00† (ns) |
| Delusion | 7.7% (n=56) |
5.3% (n=19) |
11.1% (n=9) |
0% (n=10) |
1.00† (ns) | 0.57†(ns) |
| Agitation | 9.8% (n=51) |
11.1% (n=18) |
12.5% (n=8) |
10.0% (n=10) |
1.00† (ns) | 1.00† (ns) |
| Depression | 7.8% (n=51) |
11.1% (n=18) |
12.5% (n=8) |
10.0% (n=10) |
0.65† (ns) | 0.80† (ns) |
Continuous variables are presented as mean (SD).
Categorical variables are reported as % or number of patients.
; Pearson χ2,
; Fisher’s Exact Test,
; U of Mann-Whitney,
; χ2 of Kruskal Wallis
; AD vs. FTLD,
; AD vs. FTLD-tau,
; AD vs. FTLD-U,
; FTD-tau vs. FTLD-U
AD = Alzheimer disease; CDR = Clinical Dementia Rating; FTLD = frontotemporal lobar degeneration; FTLD-U = FTLD with ubiquitin-positive inclusions; MMSE = Mini-Mental State Examination.
The retrospective review of clinical data of all FTLD patients showed that all of the Pick patients presented with behavioral or personality changes, whereas CBD, PSP, and FTLD-U patients presented with heterogeneous cognitive symptoms such as language dysfunction, memory disturbance, depression, as well as in some cases motor symptoms (see supplementary table 1). Abnormalities in expressive language function were present in 5/11 (45.5%) FTLD-tau and 6/13 (46.2%) FTLD-U patients. No patients presented with clinical features of semantic dementia. Motor neuron disease findings were ultimately present in 12/13 (92.3%) of FTLD-U patients, but only three patients initially presented with such findings.
Neuropsychological testing domains using factor analysis
The explanatory factor analysis solutions with the t-values of all 11 neuropsychological tests revealed a factor structure consisting of three distinctive factors. The loadings from the rotated solution are shown in table 2a (rotated eigenvalues = 2.69, 2.35, and 1.89, respectively). The three factors were representative of memory, language, and attention domain, according to the neuropsychological subscores from which high factor loadings were extracted. The correlation between memory factor and language factor was 0.300 and the correlation between language factor and attention factor was 0.436. However, the correlation between memory factor and attention factor was not significant (table 2b).
Table 2.
Factor Analysis of Neuropsychological tests
| 2a | |||
|---|---|---|---|
| Factor 1 Memory | Factor 2 Language | Factor 3 Attention | |
| SRT-DR | 0.911 | −0.133 | 0.014 |
| SRT-LTR | 0.867 | 0.057 | −0.031 |
| SRT-DRcg | 0.702 | 0.022 | 0.153 |
| Orientation | 0.412 | 0.293 | −0.070 |
| Category Fluency | 0.182 | 0.814 | −0.030 |
| Letter Fluency | −0.010 | 0.667 | 0.185 |
| RDT-5 | −0.058 | 0.429 | −0.104 |
| BNT-15 | 0.245 | 0.365 | −0.126 |
| SRT-STR | −0.290 | 0.356 | 0.364 |
| Digit Forward | 0.078 | −0.250 | 0.882 |
| Digit Backward | 0.072 | 0.118 | 0.755 |
| eigenvalue | 2.69 | 2.35 | 1.89 |
| 2b | |||
|---|---|---|---|
| Factor 1 | Factor 2 | Factor 3 | |
| Factor 1 | 1.000 | 0.300 | 0.011 |
| Factor 2 | 1.000 | 0.436 | |
| Factor 3 | 1.000 | ||
Factor loadings ≥0.35 are in bold.
BNT = Boston naming test; RDT = Rosen Drawing Test; SRT = Selective Reminding Test; SRT-DR = SRT delayed free recall; SRT-DRcg = SRT delayed recognition; SRT-LTR = SRT long-term retrieval; SRT-STR = SRT short term recall.
Table 3a shows that the memory factor score in the AD group was lower than in the FTLD group (p = 0.024), whereas the language factor and attention factor scores in the FTLD group was lower than in the AD group (p = 0.002, p = 0.015, respectively). Subgroup analysis demonstrated that language and attention factor scores in the FTLD-U group were significantly lower than in the AD (p = 0.006, p = 0.005, respectively).
Table 3.
Results of Neuropsychological tests
| 3a | ||||||
|---|---|---|---|---|---|---|
| Pathological group | ||||||
| AD (n=89) |
FTLD (n=25) |
FTLD subtype | p - value | |||
| FTLD-tau (n=12) |
FTLD-U (n=13) |
AD FTLD |
AD FTLD-tau FTLD-U |
|||
| Memory Factor | −0.13 (0.80) | 0.46 (1.27) | 0.39 (1.39) | 0.52 (1.21) | p = 0.024a | p = 0.071 |
| Language Factor | 0.14 (0.92) | −0.49 (0.71) | −0.44 (0.79) | −0.53 (0.67) | p = 0.002a | p = 0.006c |
| Attention Factor | 0.088 (0.93) | −0.31 (0.72) | −0.097(0.86) | −0.51 (0.52) | p = 0.015a | p = 0.016c |
| 3b | |||||||
|---|---|---|---|---|---|---|---|
| Pathological group | |||||||
| AD (n=89) |
FTLD (n=25) |
FTLD subtype | p - value | ||||
| FTLD-tau (n=12) |
FTLD-U (n=13) |
AD FTLD |
AD FTLD-tau FTLD-U |
||||
| Attention | |||||||
| Digit Span Forward | 5.7 (1.2) | 5.7 (0.68) | 5.7 (0.78) | 5.8 (0.60) | p = 0.63 | p = 0.72 | |
| Digit Span Backward | 3.5 (1.2) | 3.4 (1.1) | 3.4 (1.4) | 3.3 (0.63) | p = 0.031a | p = 0.027c | |
| SRT-STR | 15.8 (5.9) | 12.1 (6.2) | 14.8 (6.3) | 9.6 (5.2) | p = 0.067 | p = 0.021c | |
| Memory | |||||||
| SRT-LTR | 8.4 (8.2) | 15.5 (14.6) | 13.3(14.7) | 17.5 (14.9) | p = 0.38 | p = 0.60 | |
| SRT-DR | 1.2 (1.9) | 4.1 (3.4) | 3.8 (3.5) | 4.3(3.4) | p = 0.002a | p = 0.008c | |
| SRT-DRcg | 7.6 (3.0) | 9.3 (2.8) | 9.3(3.2) | 9.3 (2.5) | p = 0.098 | p = 0.24 | |
| Language | |||||||
| Category Fluency | 10.2 (6.2) | 8.9 (5.1) | 8.4 (4.9) | 9.3 (5.5) | p = 0.12 | p = 0.30 | |
| Letter Fluency | 10.5 (5.6) | 5.7 (4.1) | 6.2 (4.5) | 5.2(3.7) | p <0.001a | p <0.001b,c | |
| Orientation | 6.9 (2.5) | 8.4 (1.9) | 7.8 (2.0) | 8.9 (1.8) | p = 0.013a | p = 0.018c | |
| BNT-15 | 12.8 (2.0) | 12.2 (4.6) | 12.3 (4.5) | 12.0 (4.8) | p = 0.72 | p = 0.68 | |
| Visuospatial | |||||||
| RDT-5 | 3.1 (1.1) | 2.7 (1.4) | 2.1 (1.4) | 3.2 (1.2) | p = 0.036a | p = 0.033b | |
Continuous variables are reported as means (SD)
Statistics was performed using age and education adjusted t-score.
; AD vs. FTLD,
; AD vs. FTLD-tau,
; AD vs. FTLD-U
AD = Alzheimer disease; BNT = Boston naming test; FTLD = frontotemporal lobar degeneration; FTLD-U = FTLD with ubiquitin-positive inclusions; RDT = Rosen Drawing Test; SRT = Selective Reminding Test; SRT-DR = SRT delayed free recall; SRT-DRcg = SRT delayed recognition; SRT-LTR = SRT long-term retrieval; SRT-STR = SRT short term recall.
Comparison of neuropsychological testing
As shown in table 3b, the FTLD group showed, in comparison to AD, significantly lower scores on Digit Span Backwards (p = 0.031), Letter Fluency (p <0.001), and RDT-5 (p = 0.036) tests, but higher scores on SRT-DR (p = 0.002) and Orientation (p = 0.013). In the subgroup analysis, the FTLD-U group showed higher score in SRT-DR (p = 0.011) and orientation (p = 0.005), and lower score in Digit Span Backwards (p = 0.007), SRT-STR (p = 0.006), and Letter Fluency (p <0.001) compared with AD patients. The FTLD-tau group’s scores on RDT-5 and Letter Fluency were lower than those of AD (p = 0.010, p = 0.006, respectively).
The memory retention score (= delayed recall number /correct retrieval number at last trial of encoding phase) was lower in AD patients (M = 0.20, SD = 0.29) than in FTLD patients (M = 0.60, SD = 0.48); Mann-Whitney U = 524.5, p <0.001. Kruskal Wallis test and post hoc separate analyses of FTLD-tau and FTLD-U revealed that retention scores in AD patients (M = 0.20, SD = 0.29) were lower for both FTLD-tau (M = 0.63, SD = 0.52, p = 0.003) and FTLD-U patients (M = 0.57, SD = 0.44, p = 0.005).
Verbal fluency tests
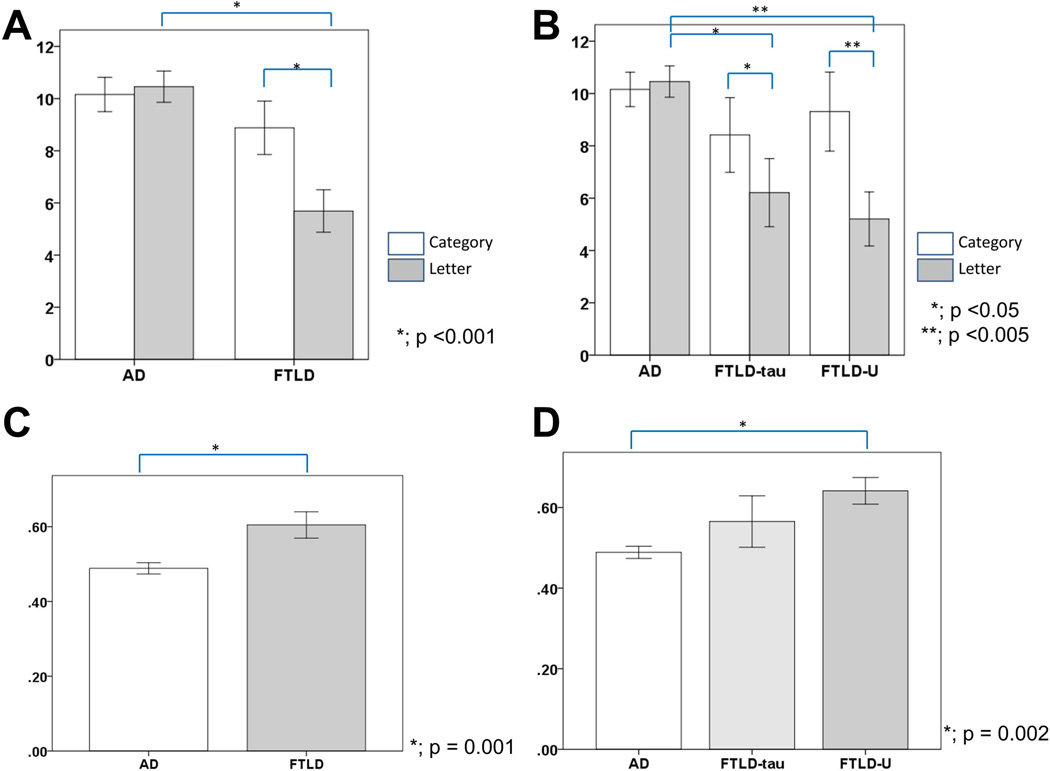
Letter and category fluency task performance differed in FTLD and AD patients, with repeated-measures analysis of covariance’s for fluency test revealing a significant 2-way interaction of Diagnosis by Fluency, F(1, 112) = 9.27, p = 0.003. In Letter Fluency, FTLD was impaired compared to AD (t (112) = 3.93, p <0.001), whereas in Category Fluency, there was not a significant difference between FTLD and AD (t (112) = 0.942, p = 0.35) (fig. 1A). As shown in fig. 1B, in subgroup analysis, Letter Fluencies in FTLD-tau and FTLD-U were impaired more than those in AD (F (2,111) = 7.78, p = 0.001, post-hoc test; p = 0.035, p = 0.004, respectively). The semantic index (SI) was significantly lower in AD patients (M = 0.49, SD = 0.14) compared with FTLD patients (M = 0.60, SD = 0.18), t (112) = 3.41, p = 0.001 (fig. 1C). Subgroup analysis showed that SI in AD (M = 0.49, SD = 0.14) was significantly lower than FTLD-U (M = 0.64, SD = 0.12) (p = 0.002) (fig. 1D).
Fig.1.
FTLD scores compared with AD.
Letter and category fluency scores are shown in panel A and B. Semantic index (semantic fluency/(semantic fluency + letter fluency)) scores are shown in panels C and D.
Brackets show the significant differences between scores.
Error bars represent standard error of the mean.
AD = Alzheimer disease; FTLD = frontotemporal lobar degeneration; FTLD-U = FTLD with ubiquitin-positive inclusions;
Predictive value of neuropsychological tests
Logistic regression analysis using a stepwise forward selection method showed that SRT-DR (p <0.001, OR = 1.179, 95% CI = 1.095 – 1.269), the BNT (p = 0.020, OR = 0.966, 95% CI = 0.939 – 0.995), and letter fluency (p <0.001, OR = 0.881, 95% CI = 0.823 – 0.943) remained as independent predictors of FTLD compared to AD in the final model. With this model, the diagnostic sensitivity for FTLD was 64.0%, while specificity was 95.5%, and accuracy was 88.6%. Separate logistic regression analyses showed that SRT-DR and Letter Fluency remained as independent predictors of FTLD-U compared to AD (sensitivity 58.3%, specificity 98.9%, accuracy 94.1%), and also showed that RDT-5, SRT-STR, SRT-DR, and Letter Fluency remained as independent predictors of FTLD-tau compare to AD (sensitivity 46.2%, specificity 98.9%, accuracy 92.2%). Although RDT-5 and SRT-STR remained as independent predictors of FTLD-U compared to FTLD-tau in the final model, they did not reach statistical significance (p = 0.063 and p = 0.054; table 4).
Table 4.
Multivariate logistic model comparisons
| partial regression coefficient |
p-value | OR | 95% CI | ||
|---|---|---|---|---|---|
| Lower | Upper | ||||
| FTLD-U versus AD | |||||
| SRT-DR | 0.145 | 0.001 | 1.156 | 1.062 | 1.257 |
| Letter Fluency | −0.171 | 0.003 | 0.843 | 0.752 | 0.944 |
| Constant | 0.100 | 0.964 | |||
| FTLD-tau versus AD | |||||
| RDT-5 | −0.085 | 0.019 | 0.919 | 0.856 | 0.986 |
| SRT-STR | 0.210 | 0.049 | 1.233 | 1.001 | 1.520 |
| SRT-DR | 0.159 | 0.001 | 1.173 | 1.067 | 1.289 |
| Letter fluency | −0.123 | 0.004 | 0.884 | 0.812 | 0.962 |
| Constant | −9.386 | 0.095 | |||
| FTLD-U versus FTLD-tau | |||||
| RDT-5 | 0.095 | 0.063 | 1.099 | 0.995 | 1.215 |
| SRT-STR | −0.256 | 0.054 | 0.774 | 0.597 | 1.004 |
| Constant | 8.637 | 0.146 | |||
AD = Alzheimer disease; FTLD = frontotemporal lobar degeneration; FTLD-U= FTLD with ubiquitin-positive inclusions; RDT = Rosen Drawing Test; SRT = selective reminding test; SRT-DR = SRT- delayed free recall; SRT-STR = SRT-short term recall.
Discussion
We compared neuropsychological features at initial presentation in patients with early stages of dementia (CDR ≤1) in patients eventually confirmed by autopsy as FTLD or AD. Some prior studies have indicated that neuropsychological characteristics of FTLD include early changes in verbal fluency, planning, working memory, and executive function, in comparison to the more significant deficits in episodic memory, visuospatial function, and praxis skills are characteristic in AD [6,29–31]. But other studies have failed to find such differences between AD and FTLD [5,32–34]. These discrepancies may relate to different neuropsychological measures used, small sample sizes, difficulties with clinical classification, and heterogeneity of FTD. To elucidate the validity of neuropsychological testing in differentiating FTLD from AD, we restricted our analysis to subjects with early stages of disease (CDR ≤1), and examined the results using factor analysis of neuropsychological test scores, as well as examining components of memory testing.
Factor analysis showed three latent factors: memory, language, and attention. Comparison of the factor scores showed dissociative patterns with the FTLD patients performing better on the memory factor and worse on the language and attention factors compared with the AD patients. Factor analysis allows us to reduce large amount of complicated data of neuropsychological scores, to evaluate the construct validity of a test battery adopted, and to reveal the cognitive background of the diseases [35,36]. Therefore, data reduction using factor analysis and investigating affected cognitive domains would be useful for discriminating between diseases whose neuropsychological manifestation mimic to each other.
Memory test sub-measures were useful in discriminating FTLD from AD. FTLD patients showed preserved memory retention compared with AD patients, although the other sub-measures of SRT such as long term retrieval (LTR) and delayed recognition in FTLD patients were comparable to those in AD patients. Examining FTLD-U and FTLD-tau separately, short term recall (STR) only in FTLD-U was significantly lower than AD. Considering three aspects of episodic long term memory including encoding phase, storage, and retrieval, SRT sub-measures allow assessment of working memory by STR, memory encoding by LTR, and memory retrieval by Delayed Recall [18]. Our results suggest that FTLD patients show preserved abilities in memory storage and retrieval phases, compared with AD patients, whereas FTLD patients, especially FTLD-U patients, showed more deficits in working memory. Prior studies have been inconsistent in this regard, with some reporting that the memory disturbance of FTLD is similar to that of AD [37], while others reporting differences between FTLD and AD for retention, but no differences for encoding in word list memory tests [38]. Memory disturbance in FTLD may result from frontal-executive impairments such as inefficient memory strategies and working memory rather than storage or recall deficits themselves [39] or impaired access to semantic representations [40]. This contrasts with memory impairment in AD which likely relates principally to encoding impairment due to entorhinal cortex and hippocampus dysfunction present at the earliest stages of the disease [41]. These pathological differences are likely responsible for the difference in memory sub-measures between the groups, which may allow better differentiation of FTLD from AD.
Verbal fluency tests also allowed some distinction between FTLD and AD. Our results showed that both types of verbal fluency in FTLD were more affected than AD. However, letter fluency was more impaired than category fluency in FTLD compared with AD. Prior studies have also showed that letter fluency may be more impaired than category fluency in FTLD compared with AD [42,43]. Rascovsky et al. [28] have shown the disparate phonemic letter fluency and semantic category fluency deficits in autopsy-confirmed FTLD. Semantic fluency likely depends upon integrity of semantic memory which demands temporal-lobe-mediated semantic system, whereas phonemic fluency may be more sensitive to executive dysfunction which may be caused by frontal lobe damage. [44]. Thus the differences in performance on these tasks are consistent with differing principal pathological involvement in AD, with early temporal involvement, and FTLD with early frontal involvement.
Discriminant analysis for FTLD versus AD using logistic regression showed that Letter Fluency, Boston Naming Test, and SRT delayed recall were independent predictors of FTLD pathology. Although cut-off points in our study did not allow sufficient discrimination of the two groups, it is possible that combinations of certain tests measuring specific cognitive domain such as language function and memory retrieval might be useful to discriminate FTLD from AD. Similarly, while we were able to discriminate between FTLD-U and AD, and between FTLD-tau and AD, we were unable to successfully discriminate between FTLD-U and FTLD-tau using logistic regression analysis. This may relate to the tests used, or to overlap between the impairments in these subgroups. Prior work has included some reporting differences between FTLD-tau and FTLD-U/TDP [8,10], some failing to find such neuropsychological differences [11]. It may be necessary to incorporate measures of behavioral change, neuropsychiatric symptoms, concomitant neurological signs, and neuroimaging data to make such distinction between different FTLD pathologies.
There are several limitations of the present study. One limitation is the selection of neuropsychological battery. More detailed evaluation of specific areas of function may better characterize the differences between patients with AD and FTLD. Frontal lobe functions such as executive function, judgment, and personality have been particularly implicated in FTLD [6], and our battery had only limited tests in these functions. Secondly, this sample is not population-based but clinic-based, and the sample size of FTLD group is small, therefore the results may not be generalizable. Thirdly, not every case had TDP-43 staining, so heterogeneity of the FTLD-U cases is possible. Finally, we do not have sufficient longitudinal data to determine if rate of change measures might be more useful [31].
Conclusions
Our study suggests that: (1) FTLD patients at early symptomatic stages show lower test scores in attentional and language domains and higher test scores in memory domains than do AD patients; (2) FTLD patients showed a pattern of memory subtest findings suggesting working memory deficits, whereas AD patients showed delayed recall deficits; (3) FTLD patients showed more impaired letter fluency than category fluency, unlike AD patients; (4) FTLD can possibly be discriminated from AD using combined test scores of Letter Fluency, Boston naming test, and delayed recall; (5) FTLD-U and FTLD-tau patients were not distinguishable by their neuropsychological test performances.
Acknowledgements
This study was supported by the NIH grants P50AG008702 (PI M. Shelanski) and UL1RR024156 (PI H. Ginsberg), the Alzheimer’s Association, the Alzheimer’s Drug Discovery Foundation, Henry P. Panasci Fund, and the Taub Institute for Research on Alzheimer’s Disease and the Aging Brain.
Footnotes
Conflict of interest: There is no conflict of interest.
References
- 1.Whitwell JL, Jack CR, Przybelski SA, Parisi JE, Senjem ML, Boeve BF, Knopman DS, Petersen RC, Dickson DW, Josephs KA. Temporoparietal atrophy: A marker of AD pathology independent of clinical diagnosis. Neurobiol Aging. 2011;32:1531–1541. doi: 10.1016/j.neurobiolaging.2009.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rabinovici G, Furst A, O'Neil J, Racine C, Mormino E, Baker S, Chetty S, Patel P, Pagliaro T, Klunk W, Mathis C, Rosen H, Miller B, Jagust W. 11C-PIB PET imaging in Alzheimer disease and frontotemporal lobar degeneration. Neurology. 2007;68:1205–1212. doi: 10.1212/01.wnl.0000259035.98480.ed. [DOI] [PubMed] [Google Scholar]
- 3.Bian H, Van Swieten JC, Leight S, Massimo L, Wood E, Forman M, Moore P, de Koning I, Clark CM, Rosso S, Trojanowski J, Lee VM, Grossman M. CSF biomarkers in frontotemporal lobar degeneration with known pathology. Neurology. 2008;70:1827–1835. doi: 10.1212/01.wnl.0000311445.21321.fc. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mendez MF, Shapira JS, McMurtray A, Licht E, Miller BL. Accuracy of the clinical evaluation for frontotemporal dementia. Arch Neurol. 2007;64:830–835. doi: 10.1001/archneur.64.6.830. [DOI] [PubMed] [Google Scholar]
- 5.Hutchinson AD, Mathias JL. Neuropsychological deficits in frontotemporal dementia and Alzheimer's disease: A meta-analytic review. J Neurol Neurosurg Psychiatry. 2007;78:917–928. doi: 10.1136/jnnp.2006.100669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Seelaar H, Rohrer JD, Pijnenburg YA, Fox NC, van Swieten JC. Clinical, genetic and pathological heterogeneity of frontotemporal dementia: A review. J Neurol Neurosurg Psychiatry. 2011;82:476–486. doi: 10.1136/jnnp.2010.212225. [DOI] [PubMed] [Google Scholar]
- 7.Thomas-Antérion C, Jacquin K, Laurent B. Differential mechanisms of impairment of remote memory in Alzheimer's and frontotemporal dementia. Dement Geriatr Cogn Disord. 2000;11:100–106. doi: 10.1159/000017221. [DOI] [PubMed] [Google Scholar]
- 8.Yokota O, Tsuchiya K, Arai T, Yagishita S, Matsubara O, Mochizuki A, Tamaoka A, Kawamura M, Yoshida H, Terada S, Ishizu H, Kuroda S, Akiyama H. Clinicopathological characterization of Pick's disease versus frontotemporal lobar degeneration with ubiquitin/TDP-43-positive inclusions. Acta Neuropathol. 2009;117:429–444. doi: 10.1007/s00401-009-0493-4. [DOI] [PubMed] [Google Scholar]
- 9.Josephs KA, Hodges JR, Snowden JS, Mackenzie IR, Neumann M, Mann DM, Dickson DW. Neuropathological background of phenotypical variability in frontotemporal dementia. Acta Neuropathol. 2011;122:137–153. doi: 10.1007/s00401-011-0839-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Forman MS, Farmer J, Johnson JK, Clark CM, Arnold SE, Coslett HB, Chatterjee A, Hurtig HI, Karlawish JH, Rosen HJ, Van Deerlin V, Lee VM, Miller BL, Trojanowski JQ, Grossman M. Frontotemporal dementia: Clinicopathological correlations. Ann Neurol. 2006;59:952–962. doi: 10.1002/ana.20873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mendez MF, Joshi A, Tassniyom K, Teng E, Shapira JS. Clinicopathologic differences among patients with behavioral variant frontotemporal dementia. Neurology. 2013;80:561–568. doi: 10.1212/WNL.0b013e3182815547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Morris JC. The clinical dementia rating (CDR): Current version and scoring rules. Neurology. 1993;43:2412–2414. doi: 10.1212/wnl.43.11.2412-a. [DOI] [PubMed] [Google Scholar]
- 13.Cairns NJ, Bigio EH, Mackenzie IR, Neumann M, Lee VM, Hatanpaa KJ, White CL, Schneider JA, Grinberg LT, Halliday G, Duyckaerts C, Lowe JS, Holm IE, Tolnay M, Okamoto K, Yokoo H, Murayama S, Woulfe J, Munoz DG, Dickson DW, Ince PG, Trojanowski JQ, Mann DM. Neuropathologic diagnostic and nosologic criteria for frontotemporal lobar degeneration: Consensus of the consortium for frontotemporal lobar degeneration. Acta Neuropathol. 2007;114:5–22. doi: 10.1007/s00401-007-0237-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mackenzie IR, Neumann M, Bigio EH, Cairns NJ, Alafuzoff I, Kril J, Kovacs GG, Ghetti B, Halliday G, Holm IE, Ince PG, Kamphorst W, Revesz T, Rozemuller AJ, Kumar-Singh S, Akiyama H, Baborie A, Spina S, Dickson DW, Trojanowski JQ, Mann DM. Nomenclature for neuropathologic subtypes of frontotemporal lobar degeneration: Consensus recommendations. Acta Neuropathol. 2009;117:15–18. doi: 10.1007/s00401-008-0460-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Richards M, Marder K, Bell K, Dooneief G, Mayeux R, Stern Y. Interrater reliability of extrapyramidal signs in a group assessed for dementia. Arch Neurol. 1991;48:1147–1149. doi: 10.1001/archneur.1991.00530230055021. [DOI] [PubMed] [Google Scholar]
- 16.Devanand DP, Miller L, Richards M, Marder K, Bell K, Mayeux R, Stern Y. The Columbia university scale for psychopathology in Alzheimer's disease. Arch Neurol. 1992;49:371–376. doi: 10.1001/archneur.1992.00530280051022. [DOI] [PubMed] [Google Scholar]
- 17.Folstein M, Folstein S, McHugh P. "Mini-mental state". A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12:189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- 18.Buschke H, Fuld PA. Evaluating storage, retention, and retrieval in disordered memory and learning. Neurology. 1974;24:1019–1025. doi: 10.1212/wnl.24.11.1019. [DOI] [PubMed] [Google Scholar]
- 19.Kaplan E, Goodglass H, Weintraub S. Boston naming test. Philadelphia PA: Lea & Febiger; 1983. [Google Scholar]
- 20.Benton AL, Hamsher K. Multilingual aphasia examination. Iowa City IA: University of Iowa; 1976. [Google Scholar]
- 21.Rosen W. The Rosen drawing test. Bronx NY: Veterans Administration Medical Center; 1981. [Google Scholar]
- 22.Wechsler D. Wechsler memory scale-revised manual. San Antonio TX: The Psychological Corporation; 1987. [Google Scholar]
- 23.Stricks L, Pittman J, Jacobs DM, Sano M, Stern Y. Normative data for a brief neuropsychological battery administered to English- and Spanish-speaking community-dwelling elders. J Int Neuropsychol Soc. 1998;4:311–318. [PubMed] [Google Scholar]
- 24.Weintraub S, Salmon D, Mercaldo N, Ferris S, Graff-Radford NR, Chui H, Cummings J, DeCarli C, Foster NL, Galasko D, Peskind E, Dietrich W, Beekly DL, Kukull WA, Morris JC. The alzheimer's disease centers' uniform data set (UDS): The neuropsychologic test battery. Alzheimer Dis Assoc Disord. 2009;23:91–101. doi: 10.1097/WAD.0b013e318191c7dd. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Vonsattel JP, Aizawa H, Ge P, DiFiglia M, McKee AC, MacDonald M, Gusella JF, Landwehrmeyer GB, Bird ED, Richardson EP. An improved approach to prepare human brains for research. J Neuropathol Exp Neurol. 1995;54:42–56. doi: 10.1097/00005072-199501000-00006. [DOI] [PubMed] [Google Scholar]
- 26.Vonsattel JP, Del Amaya MP, Keller CE. Twenty-first century brain banking. Processing brains for research: The Columbia university methods. Acta Neuropathol. 2008;115:509–532. doi: 10.1007/s00401-007-0311-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yoshizawa H, Vonsattel JP, Honig LS. Early neuropsychological discriminants for Lewy body disease: An autopsy series. J Neurol Neurosurg Psychiatry. doi: 10.1136/jnnp-2012-304381. in press. [DOI] [PubMed] [Google Scholar]
- 28.Rascovsky K, Salmon DP, Hansen LA, Thal LJ, Galasko D. Disparate letter and semantic category fluency deficits in autopsy-confirmed frontotemporal dementia and Alzheimer's disease. Neuropsychology. 2007;21:20–30. doi: 10.1037/0894-4105.21.1.20. [DOI] [PubMed] [Google Scholar]
- 29.Harciarek M, Jodzio K. Neuropsychological differences between frontotemporal dementia and Alzheimer's disease: A review. Neuropsychol Rev. 2005;15:131–145. doi: 10.1007/s11065-005-7093-4. [DOI] [PubMed] [Google Scholar]
- 30.Piguet O, Hornberger M, Mioshi E, Hodges JR. Behavioural-variant frontotemporal dementia: Diagnosis, clinical staging, and management. Lancet Neurol. 2011;10:162–172. doi: 10.1016/S1474-4422(10)70299-4. [DOI] [PubMed] [Google Scholar]
- 31.Binetti G, Locascio JJ, Corkin S, Vonsattel JP, Growdon JH. Differences between Pick disease and Alzheimer disease in clinical appearance and rate of cognitive decline. Arch Neurol. 2000;57:225–232. doi: 10.1001/archneur.57.2.225. [DOI] [PubMed] [Google Scholar]
- 32.Collette F, Amieva H, Adam S, Hogge M, Van der Linden M, Fabrigoule C, Salmon E. Comparison of inhibitory functioning in mild Alzheimer's disease and frontotemporal dementia. Cortex. 2007;43:866–874. doi: 10.1016/s0010-9452(08)70686-5. [DOI] [PubMed] [Google Scholar]
- 33.Giovagnoli AR, Erbetta A, Reati F, Bugiani O. Differential neuropsychological patterns of frontal variant frontotemporal dementia and Alzheimer's disease in a study of diagnostic concordance. Neuropsychologia. 2008;46:1495–1504. doi: 10.1016/j.neuropsychologia.2007.12.023. [DOI] [PubMed] [Google Scholar]
- 34.Castiglioni S, Pelati O, Zuffi M, Somalvico F, Marino L, Tentorio T, Franceschi M. The frontal assessment battery does not differentiate frontotemporal dementia from Alzheimer's disease. Dement Geriatr Cogn Disord. 2006;22:125–131. doi: 10.1159/000093665. [DOI] [PubMed] [Google Scholar]
- 35.Johnson DK, Morris JC, Galvin JE. Verbal and visuospatial deficits in dementia with Lewy bodies. Neurology. 2005;65:1232–1238. doi: 10.1212/01.wnl.0000180964.60708.c2. [DOI] [PubMed] [Google Scholar]
- 36.Gustafson L, Erikson C, Warkentin S, Brun A, Englund E, Passant U. A factor analytic approach to symptom patterns in dementia. Int J Alzheimers Dis. 2010;2011:632604. doi: 10.4061/2011/632604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hornberger M, Piguet O, Graham AJ, Nestor PJ, Hodges JR. How preserved is episodic memory in behavioral variant frontotemporal dementia? Neurology. 2010;74:472–479. doi: 10.1212/WNL.0b013e3181cef85d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wicklund AH, Johnson N, Rademaker A, Weitner BB, Weintraub S. Word list versus story memory in Alzheimer disease and frontotemporal dementia. Alzheimer Dis Assoc Disord. 2006;20:86–92. doi: 10.1097/01.wad.0000213811.97305.49. [DOI] [PubMed] [Google Scholar]
- 39.Pasquier F, Grymonprez L, Lebert F, Van der Linden M. Memory impairment differs in frontotemporal dementia and Alzheimer's disease. Neurocase. 2001;7:161–171. doi: 10.1093/neucas/7.2.161. [DOI] [PubMed] [Google Scholar]
- 40.Hodges JR, Davies RR, Xuereb JH, Casey B, Broe M, Bak TH, Kril JJ, Halliday GM. Clinicopathological correlates in frontotemporal dementia. Ann Neurol. 2004;56:399–406. doi: 10.1002/ana.20203. [DOI] [PubMed] [Google Scholar]
- 41.Braak H, Braak E. Neuropathological stageing of Alzheimer-related changes. Acta Neuropathol. 1991;82:239–259. doi: 10.1007/BF00308809. [DOI] [PubMed] [Google Scholar]
- 42.Rogers TT, Ivanoiu A, Patterson K, Hodges JR. Semantic memory in Alzheimer's disease and the frontotemporal dementias: A longitudinal study of 236 patients. Neuropsychology. 2006;20:319–335. doi: 10.1037/0894-4105.20.3.319. [DOI] [PubMed] [Google Scholar]
- 43.Laisney M, Matuszewski V, Mezenge F, Belliard S, de la Sayette V, Eustache F, Desgranges B. The underlying mechanisms of verbal fluency deficit in frontotemporal dementia and semantic dementia. J Neurol. 2009;256:1083–1094. doi: 10.1007/s00415-009-5073-y. [DOI] [PubMed] [Google Scholar]
- 44.Salmon DP, Heindel WC, Lange KL. Differential decline in word generation from phonemic and semantic categories during the course of Alzheimer's disease: Implications for the integrity of semantic memory. J Int Neuropsychol Soc. 1999;5:692–703. doi: 10.1017/s1355617799577126. [DOI] [PubMed] [Google Scholar]