Abstract
The aim of the present study was to characterize the temporal patterns of sleep and wakefulness in a sample of the adult subjects from São Paulo city. All subjects filled the Morningness/Eveningness Questionnaire (MEQ) and wore an actigraph for at least three consecutive days. A total of 359 subjects were considered for the analyses. The mean age was 43±14 years, the mean body mass index was 26.7±5.7 kg/m2, and 60% were female. The mean MEQ score was 58.0±10.7. The sleep pattern evaluated by the actigraphic analyses showed that 92% had a monophasic sleep pattern, 7% biphasic, and 1% polyphasic sleep pattern. Cluster analysis, based on time to sleep onset, sleep efficiency, sleep latency, and total sleep time, was able to identify three different groups denominated: morning type, evening type, and undefined type. Morning type subjects were more frequent, older, and had higher MEQ scores than evening type subjects. Our results showed that the actigraph objectively assessed the sleep-wake cycle and was able to discriminate between morning and evening type individuals. These findings suggest that the actigraph could be a valuable tool for assessing temporal sleep patterns, including the circadian preferences.
Keywords: Temporal patterns, Actigraph, Circadian preferences, Sleep-wake pattern, Morningness, Eveningness
1. Introduction
Few epidemiological studies have evaluated the pattern of sleep-wake cycle in the general population. Questionnaires or sleep logs, in addition to the objective evaluation of sleep-wake patterns by actigraphs, are typically used in these studies [1–5]. It is important to note that the results of actigraphic recording as an instrument to investigate sleep pattern has been compared with polysomnography, which is considered the gold standard for objective evaluation of sleep, showing good correlation [6,7]. Previous studies have shown that duration and quality of sleep, which have consequences for health, are strongly associated with race, gender, and socioeconomic status [1]. It has also been demonstrated that the self-reported sleep duration is longer than the sleep duration objectively evaluated using an actigraph [2] and that morningness/eveningness preference is largely independent of ethnicity, gender, and socioeconomic status, indicating that this preference may be better explained by endogenous factors [3]. However, past studies with young adults showed female had significantly stronger tendency toward the morningness preference, and the authors considered the role of social-cultural factors in the existence of gender differences [4,5].
Depending on the evaluated population, the sleep-wake cycle is influenced by external cues that can greatly change the quality of life and cause significant consequences to the health of the individual and collective. Many cultural, environmental and social stimuli are related to an increase in light intensity to which the individual is subjected. Depending on time of the day that these stimuli occur, they can act on the circadian system and cause a phase delay or advance [8,9]. We should propose that the characterization of the sleep-wake cycle of a population is not trivial because exogenous and endogenous characteristics influence the habits of sleeping and waking. In this context, the aim of this study was to characterize the temporal pattern of sleep and wakefulness in a sample of the adult subjects from São Paulo city. The study was based on questionnaire and actigraphic data.
2. Materials and methods
2.1. Study participants
The study protocol was approved by the Ethics Committee for Research at the Universidade Federal de São Paulo (CEP 0593/06) and was registered with ClinicalTrials.gov (Identifier NCT00596713). All subjects read and signed an informed consent form.
The study was conducted with individuals who participated in the São Paulo Epidemiologic Sleep Study (EPISONO) and agreed to wear an actigraph for at least three consecutive days. The study design and methodology of EPISONO have been described in detail previously [10]. Briefly, EPISONO was a population-based survey developed to establish the epidemiological profile of sleep disorders in the adult population of São Paulo. A total sample of 1101 volunteers were enrolled as a representative population sample based on gender, age (20–80 years) and socioeconomic status, with 3% precision in the prevalence estimates [11]. A three-stage cluster sampling technique was used with unequal selection probability [11]. Pregnant and lactating women, subjects with physical or mental impairments that prevent self-care, and subjects who work every night were not included in the household drawing. Data collection was performed between July and December 2007.
2.2. Data collection
2.2.1. Morningness/Eveningness questionnaire (MEQ)
The Portuguese version [12] of the MEQ, developed by Horne and Ostberg [13], was used to score the circadian preferences of the evaluated population.
2.2.2. Actigraph
The subjects were instructed to wear the actigraph (Actiwach-64°, Respironics, Inc. Co. USA) for a minimum of three consecutive days, regardless of whether it was during the week or on the weekend. Data were analyzed using the Actiware 5.0 software, which evaluates the parameters of the activity/rest cycle.
The following parameters were evaluated: total sleep time (TST) for each sleep episode (“main episode TST”) and over each 24-hour period (including naps) (“24 h TST”), sleep latency, sleep efficiency, wake after sleep onset (WASO), and time to sleep onset.
The study protocol allowed the volunteers to choose the most appropriate dates to wear the actigraph. A subsample of 92 volunteers wore the actigraph for at least three consecutive days during the weekdays and also during the weekend. Those data were analyzed and no significant differences (p>0.05) were found in the main characteristics of sleep during the weekdays compared to the weekends (TST, sleep latency, sleep efficiency, WASO) (Table 1). Thus, data from both weekdays and weekends were included in the same test sample.
Table 1.
Analyses of the sleep characteristics evaluated by actigraph wore during weekdays and weekend in a sample of 92 volunteers. Data presented on average±standard deviation.
| Weekdays | Weekends | p | |
|---|---|---|---|
| Total Sleep Time (min) | 352.2±48.4 | 364.4±73.5 | 0.31 |
| Sleep Efficiency (%) | 81.2±4.7 | 80.4±5.3 | 0.54 |
| Sleep Latency (min) | 12.2±11.2 | 12.8±12.6 | 0.91 |
| WASO (min) | 57.1±17.8 | 62.3±22.8 | 0.23 |
WASO=wake after sleep onset.
The number of episodes of sleep over each 24-hour period was classified as monophasic (predominantly a single episode of sleep), biphasic (presence of a second sleep episode during more than 50% of the days in the time series), and polyphasic (presence of more than two sleep episodes during more than 50% of the days in the time series).
2.3. Statistical analysis
For comparisons between groups as a function of demographics and actigraphic variables, chi-square or ANOVA with Tukey post-hoc test were used, depending of the level of measurement. For variables that were not normally distributed, the results were checked by the nonparametric Mann–Whitney and Kruskal–Wallis tests, and their results were presented only if they differed from the ANOVA results. One-way ANOVA tests were used to compare the data from subjects who used the actigraph both during the weekdays and on the weekend.
A two-step cluster analysis with two different sets of variables was performed. The first set included time to sleep onset, sleep efficiency, sleep latency, and TST. In the second set, the sleep efficiency was replaced by WASO. In both data sets, the first two components were sufficient to understand the relationships between the variables explaining 70% and 65% of the total proportion of the variability. The clusters were used as independent variables in comparison with the MEQ and a one-way ANOVA of this comparison was performed.
All results were considered significant when p<0.05. The STATA 10.0 package was used to perform the analyses.
3. Results
Of the evaluated sample (N=1101), 533 subjects agreed to wear the actigraph. A total of 174 subjects were excluded due to problems such as incorrect device use, insufficient time of usage and technical problems. A final sample of 359 subjects was considered for the analyses. No differences in age (p=0.83) or in the proportion of males and females (p=0.08) were found among the sample of subjects who did not wear the actigraph (N=568), those who wore but were excluded (N=174), and the final sample (N=359). A significantly higher proportion of subjects from the upper socioeconomic class agreed to wear the actigraph (excluded and final sample) compared to the overall sample and the subjects who had not agreed (p=0.001).
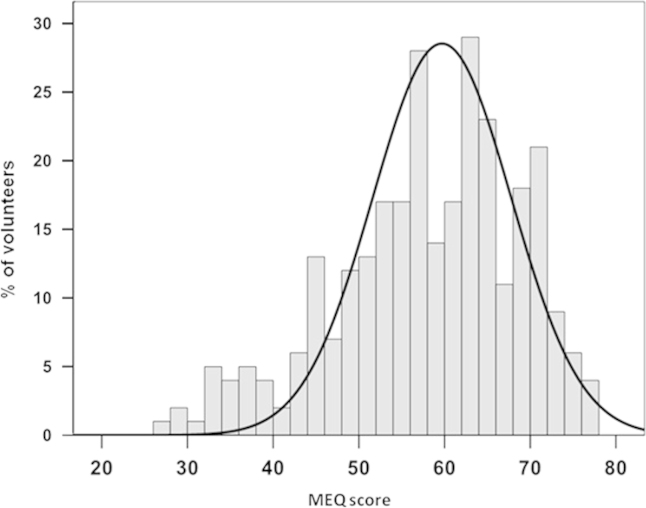
Of the 359 subjects, the age (mean±standard deviation) was 43±14 years, body mass index (BMI) was 26.7±5.7 kg/m2, and 60% were female. The assessment of circadian preferences by MEQ showed a score of 58.0±10.7. The frequency of the subjects based on MEQ score is presented in Fig. 1 and shows a tendency toward morningness.
Fig. 1.
Distribution of the Morningness/Eveningness Questionnaire (MEQ) score in the evaluated subjects (n=359).
The average number of days wearing the actigraph was 5.8±2.1 days (range 3–22.6 days). Actigraphic analyses showed that the “main episode TST” was 365.4±57.4 min and the “24 h TST” was 371.6±58.1 min, the sleep efficiency was 80.6±6.7%, the sleep latency was 12.5±11.0 min, and WASO was 53.9±21.2 min.
The assessment of the sleep pattern showed that 92% had a monophasic pattern, 7% had a biphasic pattern, and only 1% had a polyphasic pattern.
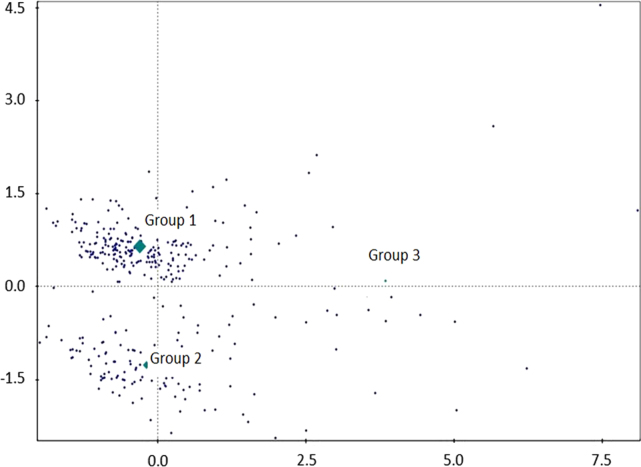
Fig. 2 shows the dispersion of the subjects in the cluster analysis based on the actigraphic variables of time to sleep onset, sleep efficiency, sleep latency, and TST; these data characterized the distribution of the temporal sleep pattern in the subjects. A clear division along the horizontal axis was observed and was able to divide the subjects according to time to sleep onset: Group 1=morning type; Group 2=evening type; and Group 3=undefined type. The undefined type group appears to be representative of subjects with reduced sleep efficiency, almost independent of time to sleep onset. However, the highest score is below the reference line nearest the evening type group. Table 2 shows the demographics and actigraphic variables description of the study groups. The subjects in the evening type group (40.3±13.5 years) were younger than those in the morning type group (44.4±14.4 years) and the undefined type group (43.6±17.2 years). As expected, actigraphic variables were significantly different among the groups.
Fig. 2.
Dispersion of subjects according to the cluster analysis of the actigraphic variables (time to sleep onset, sleep efficiency, sleep latency, and Total Sleep Time). Groups were divided based on the temporal pattern of sleep: Group 1=morning type; Group 2=evening type; and Group 3=undefined type.
Table 2.
Description of the groups based on the temporal pattern of sleep defined by the cluster analysis of the actigraphic variables (mean values±standard deviation) of time to sleep onset, sleep efficiency, sleep latency, and total sleep time (TST).
| Group 1 (morning type) | Group 2 (evening type) | Group 3 (undefined type) | P | |
| N(%) | 220 (61) | 116 (32) | 23 (7) | 0.001 |
| Age (years) | 44.4±14.4 | 40.3±13.5* | 43.6±17.2 | 0.04 |
| Gender (%) | ||||
| Male | 36.8 | 44 | 56.5 | 0.41 |
| Female | 63.2 | 56 | 43.5 | |
| BMI (kg/m2) | 26.7±5.7 | 26.5±5.2 | 27.8±7.1 | 0.58 |
| Occupation (%) | ||||
| Employed | 74.5 | 82 | 65.2 | 0.14 |
| Housewife | 8.2 | 4.3 | 8.7 | |
| Retired | 14.1 | 6.9 | 17.4 | |
| Unemployed | 3.2 | 2.6 | 8.7 | |
| Actigraphic variables | ||||
| Time of sleep onset (h:min) | 22:13‡ | 01:54 | Variable† | 0.001 |
| Sleep latency (min) | 11.9±9.4 | 11.1±9.9 | 25.2±20.1† | <0.001 |
| Sleep efficiency (%) | 81.0±6.4 | 81.5±6.5 | 72.3±5.5† | <0.001 |
| “24 h TST” (min) | 379.8±52.0‡ | 354.8±67.6 | 377.1±44.9 | 0.001 |
| “Main episode TST” (min) | 374.4±52.4‡ | 349.0±65.7 | 362.4±57.7 | 0.001 |
significant difference between the evening type group and the morning type and undefined type groups (ANOVA with Tukey post-hoc test).
significant difference between the undefined type group and the morning type and evening type groups (ANOVA with Tukey post-hoc test).
significant difference between the morning type group and the evening type group (ANOVA with Tukey post-hoc test).
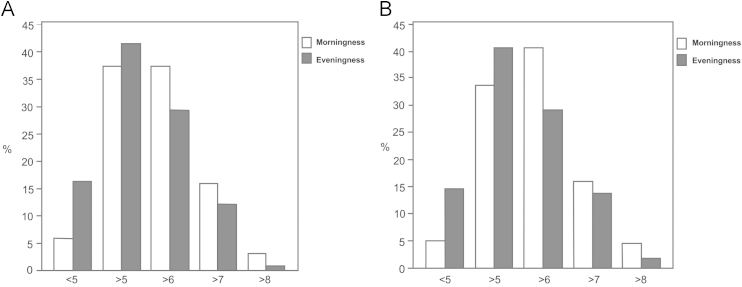
Fig. 3 shows the distribution of the “main episode TST” and “24 h TST” according to morning and evening types, both as defined by the actigraph variables. There was a trend of higher frequency of evening type among the subjects who had less than six hours of sleep compared to the morning type.
Fig. 3.
Distribution of the hours of sleep among the morning and evening type groups, both evaluated by actigraph (n=359).A: main total sleep time; B: total sleep time over each 24-hour.
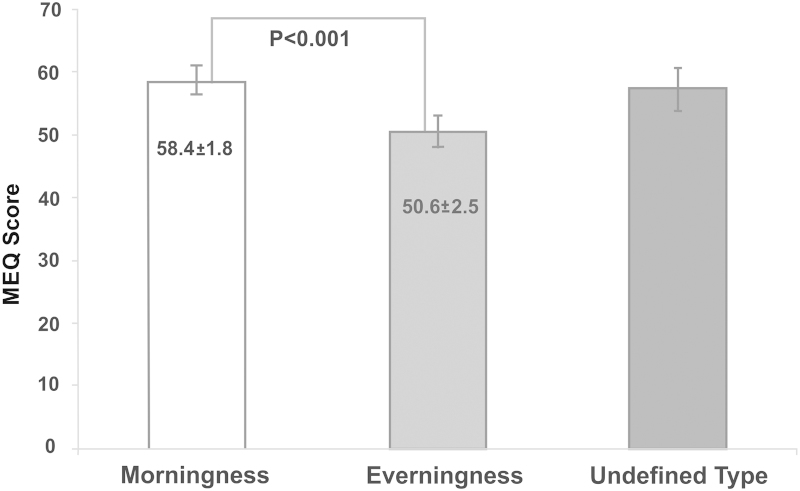
The evaluation of the MEQ score according to the temporal pattern defined by the actigraph showed that the morning type group had higher scores than the evening type group (Fig. 4).
Fig. 4.
Morningness/Eveningness Questionnaire (MEQ) score according the temporal pattern defined by the cluster analysis based on the actigraph data (n=359).
4. Discussion
The evaluated sample of the São Paulo city population assessed by actigraph showed average sleep duration of six hours, sleep efficiency of 81%, temporal pattern of sleep that was predominantly monophasic and a trend toward morningness.
Other studies that evaluated a population-based sample [2] and a population of young adults [1] also showed that the average sleep duration and sleep efficiency were similar to our results (~6 h and ~80%, respectively). It is important to note that habitual sleep duration among adults shows considerable variance within and between individuals [14], with a reported daily average of sleep of 6.8 hours [15]. However, some studies suggested that sleep duration as reported by North American adults decreased over the past 10 years [16]. Sleep efficiency was also decreased in our study, compared with the normal values found by polysomnograph (>85%). Considering that actigraph may systematically overestimated sleep efficiency when compared to polysomnograph [6] probably the sleep efficiency found in this study could be lower than 81%. There is considerable debate whether sleep duration decrease is resulting in higher rates of chronic sleep restriction or sleep debt and effects of sleep deprivation to the general population, including neurobehavioral and physiological (endocrine, immune and cardiovascular) consequences [16–18].
As expected for the general adult population [19], our study showed that 92% of the subjects had a monophasic pattern. The monophasic sleep pattern remains during adulthood, except in some societies that have the habit of napping after lunch [20]. Several factors could determine the ideal total sleep duration, if it can be achieved with a single sleep episode (monophasic pattern) or with more than one (biphasic or polyphasic). First, there is an individual propensity that determines the ability of a subject to sleep at different times [21,22]. Moreover, social factors could determine the need to perform various sleep episodes [23–26]. Biphasic and polyphasic sleep patterns, represented by 8% of the evaluated population, may be associated with the ability of humans to fall asleep several times a day. This ability to fragment sleep within a 24-hour period may be an adaptive strategy in individuals who cannot have the desired amount of sleep [27].
Our results showed that although the mean MEQ score characterized the circadian preferences of the subjects as indifferent (58±11), there was a rightward shift of the Gaussian curve of the MEQ scores, i.e., showing a tendency toward morningness. Furthermore, using the MEQ to compare groups determined by the cluster analysis, both groups, morning and evening types based on the cluster analysis, were classified as intermediate by the MEQ. One possible explanation for this result is the difference between the preference of the subjects in relation to the habits of sleeping, waking up, and performing their daily activities and what actually occurs in their daily routine as revealed by actigraph. However, the group defined by actigraph as morning type presented MEQ scores that were significantly higher than those of the evening type. This finding seems to strengthen the role of the actigraph as a tool for assessing the circadian preferences that are closer to the real situation regarding sleep in population studies.
Our data also showed that most of the subjects were defined by the actigraph as morning type. Other researchers observed an increase in morning type subjects in the general population. An epidemiological study conducted in the adult population of New Zealand, using MEQ scores, showed that 49.8% of the population was characterized as morning type [3]. Another study that evaluated 566 adult subjects, without sleep complaints, found that 62.1% were morning type based on the MEQ score [28]. One can speculate that light exposure would be an environmental factor that is associated with the genetic predisposition to the phenotypic expression of morningness and eveningness [8]. In São Paulo, the difference between the shortest and the longest day throughout the year is approximately two hours, whereas in London this difference is eight hours. However, in a study performed in France [28], similar proportions of morningness were observed. Thus, other factors seem to influence this increased frequency of morningness in São Paulo city.
We found that the morning type group had higher TST (“main episode” and “24 h”) than the other groups and were also more common among those who slept more than six hours per day. These data corroborate the study by Taillard and colleagues [29], which showed that evening type subjects had shorter time in bed and increased need for sleep. We suggest that evening type individuals could experience consequences associated with chronic sleep deprivation.
Our study showed that the mean age of the evening type subjects, identified by the cluster analysis, was lower than that in the other groups, which is consistent with other studies that showed a trend for increased morningness with age [30], even after correcting for socioeconomic and demographic factors [3,31].
The main limitation of our study was the difficulty of sampling the general population of São Paulo with the use of the actigraph. Our sample of subjects who wore an actigraph was comparable to the general population in terms of the male/female proportion and age, but significant differences were found for the socioeconomic status. Furthermore, from the sample who agreed to wear the actigraph, many subjects were excluded from the analysis, mainly due to failure in the consecutive days of use. This may represent the difficulty of compliance in wearing the actigraph despite the time spent training the subjects. As expected in a population-based survey [11], the evaluated sample of subjects is heterogeneous and the health, lifestyle or occupational issues were not considered in this study. Moreover, MEQ was applied by trained interviewers. This could have some bias since the MEQ is a self-reported questionnaire.
We conclude that the actigraph was valuable for establishing the profile of the temporal sleep pattern of the evaluated population, including circadian preferences, based on the variables of sleep efficiency, TST, bedtime, and sleep latency. These data demonstrated that the population was mostly classified as morning type mainly due to the primary phenotype and/or the adaptation imposed on the inhabitants by the social habits of a large metropolis. We suggest that the actigraph, which has the capacity to record long periods of the wake-sleep cycle, is also reliable and valid to detect circadian preferences in an adult population.
Acknowledgments
The authors thank the staff and participants of the study, in particular Dr. Altay Alves Lino de Souza, PhD, for their important contributions.
This study was supported by grants from the Associaçao Fundo de Incentivo a Pesquisa (AFIP) and Fundação de Amparo à Pesquisa do Estado de São Paulo (FAPESP) (#07/50525-1 to RS-S and #98/14303-3 to ST). ST and LB received fellowships from Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq). LM received fellowships from Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Footnotes
Peer review under responsibility of Brazilian Association of Sleep.
References
- 1.Lauderdale DS, Knutson KL, Yan LL, Rathouz PJ, Hulley SB, Sidney S. Objectively measured sleep characteristics among early-middle-aged adults: the CARDIA study. Am J Epidemiol. 2006;164(1):5–16. doi: 10.1093/aje/kwj199. [DOI] [PubMed] [Google Scholar]
- 2.Jean-Louis G, Kripke DF, Ancoli-Israel S, Klauber MR, Sepulveda RS. Sleep duration, illumination, and activity patterns in a population sample: effects of gender and ethnicity. Biol Psychiat. 2000;47:921–927. doi: 10.1016/s0006-3223(99)00169-9. [DOI] [PubMed] [Google Scholar]
- 3.Paine SJ, Gander PH, Travier N. The epidemiology of morningness/eveningness: influence of age, gender, ethnicity, and socioeconomic factors in adults (30-49 years) J Biol Rhythm. 2006;21(1):68–76. doi: 10.1177/0748730405283154. [DOI] [PubMed] [Google Scholar]
- 4.Chelminski I, Ferraro FR, Petros T, Plaud JJ. Horne and Ostberg questionnaire: a score distribution in a large sample of young adults. Pers Indiv Differ. 1997;23:647–652. [Google Scholar]
- 5.Adan A, Natale V. Gender differences in morningness-eveningness preference. Chronobiol Int. 2002;19(4):709–720. doi: 10.1081/cbi-120005390. [DOI] [PubMed] [Google Scholar]
- 6.Souza L, Benedito-Silva AA, Pires ML, Poyares D, Tufik S, Calil HM. Further validation of actigraphy for sleep studies. Sleep. 2003;26:81–85. doi: 10.1093/sleep/26.1.81. [DOI] [PubMed] [Google Scholar]
- 7.Morgenthaler T, Alessi C, Friedman L, Owens J, Kapur V, Boehlecke B. Practice parameters for the use of actigraphy in the assessment of sleep and sleep disorders: an update for 2007. Standards of practice committee; american academy of sleep medicine. Sleep. 2007;30(4):519–529. doi: 10.1093/sleep/30.4.519. [DOI] [PubMed] [Google Scholar]
- 8.Pereira DS, Tufik S, Pedrazzoli M. Moléculas que marcam o tempo: implicações para os fenóticos circadianos. Rev Bras Psiquiatr. 2009;31(1):63–71. doi: 10.1590/s1516-44462009000100015. [DOI] [PubMed] [Google Scholar]
- 9.Peixoto CA, Silva AG, Carskadon MA, Louzada FM. Adolescents living in homes without electric lighting have earlier sleep times. Behav Sleep Med. 2009;7(2):73–80. doi: 10.1080/15402000902762311. [DOI] [PubMed] [Google Scholar]
- 10.Santos-Silva R, Tufik S, Conway SG, Taddei JA, Bittencourt LRA. São Paulo epidemiologic sleep study: rationale, design, sampling, and procedures. Sleep Med. 2009;10:679–685. doi: 10.1016/j.sleep.2008.11.001. [DOI] [PubMed] [Google Scholar]
- 11.Korn EL, Graubard BI. John Wiley & Sons Inc; New York: 1999. Analyses of health surveys. [Google Scholar]
- 12.Benedito-Silva AA, Menna-Barreto L, Marques N, Tenreiro SA. A self-assessment questionnaire for the determination of morningness-eveningness types in Brazil. Prog Clin Biol Res. 1990;341B:89–98. [PubMed] [Google Scholar]
- 13.Horne JA, Ostberg O. A self-assessment questionnaire to determine morningness-eveningness in human circadian rhythms. Int J Chronobiol. 1976;4(2):97–110. [PubMed] [Google Scholar]
- 14.Van Dongen HP, Vitellaro KM, Dinges DF. Review individual differences in adult human sleep and wakefulness: leitmotif for a research agenda. Sleep. 2005;28(4):479–496. doi: 10.1093/sleep/28.4.479. [DOI] [PubMed] [Google Scholar]
- 15.National Sleep Foundation. Washington, DC: National sleep foundation. 2006. “Sleep in America” poll.
- 16.Banks S, Dinges DF. Behavioral and physiological consequences of sleep restriction. J Clin Sleep Med. 2007;3(5):519–528. [PMC free article] [PubMed] [Google Scholar]
- 17.Dinges DF. Sleep debt and scientific evidence. Sleep. 2004;27(6):1050–1052. [PubMed] [Google Scholar]
- 18.Horne J. Is there a sleep debt? Sleep. 2004;27(6):1047–1049. [PubMed] [Google Scholar]
- 19.Lack LC, Wright HR. Chronobiology of sleep in humans. Cell Mol Life Sci. 2007;64(10):1205–1215. doi: 10.1007/s00018-007-6531-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Milner CE, Cote KA. Benefits of napping in healthy adults: impact of nap length, time of day, age, and experience with napping. J Sleep Res. 2009;18(2):272–281. doi: 10.1111/j.1365-2869.2008.00718.x. [DOI] [PubMed] [Google Scholar]
- 21.Adams J, Folkard S, Young M. Coping strategies used by nurses on night duty. Ergonomics. 1986;29:185–196. doi: 10.1080/00140138608968259. [DOI] [PubMed] [Google Scholar]
- 22.Allebrandt KV, Roenneberg T. The search for circadian clock components in humans: new perspectives for association studies. Braz J Med Biol Res. 2008;41(8):716–721. doi: 10.1590/s0100-879x2008000800013. [DOI] [PubMed] [Google Scholar]
- 23.Knauth P, Rutenfranz J. Duration of sleep related to the type of shift work. In: Reinberg A, Vieux N, Andlauer P.,, editors. Night and shiftwork: biological and social aspects. Pergamon Press; Oxford: 1981. pp. 187–196. [Google Scholar]
- 24.Akerstedt T. Is there an optimal sleep-wake pattern in shift work? Scand J Work Environ Health. 1998;24(3):18–27. [PubMed] [Google Scholar]
- 25.Rotenberg L, Portela LF, Marcondes WB, Moreno C, Nascimento CP. Gender and night work: sleep, daily life and the experiences of night shift workers. Cad Saude Publica. 2001;17(3):639–649. doi: 10.1590/s0102-311x2001000300018. [DOI] [PubMed] [Google Scholar]
- 26.Ribeiro-Silva F, Rotenberg L, Soares RE, Pessanha J, Ferreira FL, Oliveira P. Sleep on the job partially compensates for sleep loss in night-shift nurses. Chronobiol Int. 2006;23(6):1389–1399. doi: 10.1080/07420520601091931. [DOI] [PubMed] [Google Scholar]
- 27.Stampi C. Birkhauser; Boston: 1992. Why we nap: evolution, chronobiology and functions of polyphasic and ultrashort sleep. [Google Scholar]
- 28.Taillard J, Philip P, Chastang JF, Bioulac B. Validation of Horne and Ostberg Morningness-Eveningness Questionnaire in a middle-aged population of French workers. J Biol Rhythm. 2004;19:76–86. doi: 10.1177/0748730403259849. [DOI] [PubMed] [Google Scholar]
- 29.Taillard J, Philip P, Bioulac B. Morningness-eveningness and the need for sleep. J Sleep Res. 1999;8(4):291–295. doi: 10.1046/j.1365-2869.1999.00176.x. [DOI] [PubMed] [Google Scholar]
- 30.Roepke SE, Duffy JF. Differential impact of chronotype on weekday and weekend sleep timing and duration. Nat Sci Sleep. 2010;2:213–220. doi: 10.2147/NSS.S12572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Waterhouse JM, Minors DS. Circadian rhythms in the neonate and in old age: what do they tell us about the development and decay of the body clock in humans? Braz J Med Biol Res. 1996;29(1):87–94. [PubMed] [Google Scholar]