Abstract
Purpose
Children with Hodgkin's lymphoma (HL) routinely undergo surveillance computed tomography (CT) imaging for up to 5 years after therapy, resulting in cost and radiation exposure, without clear benefit. The objective of this study was to determine the contribution of surveillance CT, as compared with clinical findings, to detection of disease recurrence.
Patients and Methods
Two hundred sixteen patients, age ≤ 21 years old, were treated on the multicenter Pediatric Oncology Group 9425 trial. Data for patients who experienced relapse were retrospectively reviewed to determine whether imaging or clinical events prompted suspicion of disease recurrence. Correlation was made to disease stage, time to recurrence, relapse site, and overall survival (OS).
Results
With a median follow-up time of 7.4 years, 25 (11.6%) of 216 patients had experienced a relapse, of whom 23 experienced local relapse. Median time to relapse was 7.6 months (range, 0.2 to 48.9 months). Nineteen relapses (76%) were detected based on symptoms, laboratory or physical examination findings, and two relapses (8%) were detected by imaging within the first year after therapy. Only four patients (16%) had their recurrence detected exclusively by surveillance imaging after the first year. Six deaths occurred, all in patients who experienced relapse within the first year after therapy. No patient with a recurrence after 1 year off treatment has died, regardless of how the recurrence was detected.
Conclusion
The majority of pediatric HL relapses occurred within the first year after therapy or were detected based on change in clinical status. Detecting late relapse, whether by imaging or clinical change, did not affect OS. These findings indicate that CT is overused for routine surveillance of patients with HL.
INTRODUCTION
Children with Hodgkin's lymphoma (HL) have excellent overall survival (OS) rates, exceeding 90%.1–4 Patients frequently undergo routine surveillance computed tomography (CT) imaging for up to 5 years after completing their chemotherapy. Most patients with HL who experience relapse do so within the first 2 years after completing therapy.5,6 Most relapses are local, occurring at original sites of disease and within radiation fields. There is little debate about monitoring for recurrence during the early post-treatment period when patients are at greatest risk, particularly for patients with advanced-stage or unfavorable disease. However, it is unclear what role surveillance imaging (ie, routine imaging that is not prompted by symptoms or change in clinical status) plays in detecting disease relapse. Studies in adult patients with HL have suggested that most relapses are symptomatic and that routine CT, in addition to being expensive, has poor specificity and provides minimal OS benefit.7–10 There have been no studies in pediatric HL investigating the role of routine CT surveillance imaging for detection of relapse.
There is increasing concern that children who undergo CT scanning, even with age- and weight-adjusted optimal-dose CT scanning, may have an increased risk of developing cancers later in life that could be attributable to the CT scans.11 Recent retrospective studies of diagnostic imaging examinations in pediatric patients with cancer showed the highest cumulative effective radiation doses in patients with neuroblastoma and lymphoma, primarily from CT and nuclear medicine examinations.6,12 Because of the high curability of HL with current treatment, renewed emphasis has been placed on decreasing long-term treatment-associated morbidity,2 including that related to radiation from diagnostic imaging.6,12
It was our hypothesis that children with HL undergo a large number of surveillance CT scans that may not contribute significantly to detection of relapse or impact OS, resulting in unnecessary cost and additional radiation exposure. We further hypothesized that the majority of patients would either have their relapse occur within the first year or would have their disease suspected or detected based on clinical and physical examination findings, and not solely based on surveillance imaging. Therefore, the purpose of our study was to determine the contribution of surveillance CT imaging to detecting disease relapse compared with clinical symptoms and laboratory or physical examination findings and to determine whether detecting late relapses had any impact on OS.
PATIENTS AND METHODS
Data were reviewed from 216 patients enrolled onto the Children's Oncology Group (COG) multi-institutional Pediatric Oncology Group 9425 trial conducted at Pediatric Oncology Group institutions from 1997 to 2001. Written informed consent for treatment was obtained according to institutional guidelines and in accordance with the Declaration of Helsinki. All studies were approved by the US Department of Health and Human Services. Clinical results of this trial have recently been published.13
Patients
Patients were ≤ 21 years old and had either intermediate-risk (stage IB, IIA/IIIA1 with large mediastinal adenopathy, or IIIA2) or high-risk (stage IIB, IIIB, or IV) disease. All patients had biopsy-proven classic HL; patients with lymphocyte-predominant HL were excluded. Patients were treated with doxorubicin, bleomycin, vincristine, etoposide, prednisone, and cyclophosphamide (ABVE-PC) chemotherapy, either three or five cycles depending on their early response, followed by radiation therapy, as described.13 Rapid early response (RER) was defined as ≥ 50% reduction in the sum of the products of the perpendicular diameters of measurable lesions and negative gallium scan after three chemotherapy cycles. Slow early response (SER) was failure to achieve RER. RER patients received involved-field radiation; SER patients received two additional cycles of ABVE-PC (five total cycles) followed by radiation.
Response assessments included chest x-ray, CT scan, and gallium scan (until negative) and were obtained at the end of three cycles of chemotherapy, after completion of chemotherapy, and after radiation therapy. Surveillance CT scans were required per protocol at 0, 6, 12, 24, and 30 months after completing all chemotherapy and radiation therapy. When relapses occurred, relapse data forms and imaging studies were submitted to the COG study center. Imaging studies from the time of relapse were available for 22 patients and were centrally reviewed to confirm site(s) and pattern of relapse.14
Relapse data forms were retrospectively reviewed to determine symptoms, physical examination findings, and laboratory findings reported at the time of relapse. If no symptoms or changes in clinical status were reported, sites were asked to affirm that the patients were indeed asymptomatic at the time of relapse. Only patients who experienced disease recurrence after showing an objective response to therapy were considered to have experienced relapse and are included in the study population evaluated here. Patients who were either nonresponsive or who experienced disease progression on therapy were not considered to have experienced relapse for the purposes of this study.
Statistical Methods
OS was defined as time from treatment start to death from any cause. Postrelapse survival among patients who experienced a relapse was defined as time from disease relapse to death from any cause. The Kaplan-Meier method was used to compute the survival estimates. The log-rank test was used to investigate differences in survival curves. The Wilcoxon rank sum test was used to compare the distribution of time to relapse between RER patients with relapse and SER patients with relapse. Differences with P ≤ .05 were considered statistically significant. Analysis for this report was performed based on the data available in the COG Statistics and Data Center as of October 2007.
RESULTS
Of 219 patients initially enrolled, 216 were eligible, with 53 patients (25%) having intermediate-risk disease and 163 patients (75%) having high-risk disease. With a median follow-up of 7.4 years, events have been reported in 35 patients (16%). Ten of these patients experienced events that were not related to disease relapse and were excluded from further analysis, including two patients who had nonresponsive or progressive disease on study, five patients with second malignancies, and two patient deaths (one occurring on study; the other unrelated, occurring years off study). One additional patient was taken off study for toxicity. The remaining 25 patients experienced relapse after showing an objective response to therapy and constitute the study population evaluated here (Table 1).
Table 1.
Patient Characteristics
| Patient No. | Stage | Histology | Response | Time to Relapse (months) | Detection of Relapse | Method of Detection | Type of Symptom(s) | Relapse Site | Local Relapse | Biopsy Proven |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | IIA | NS | RER | 43.0 | Sx | CT/Ga | Chest/shoulder pain | Mediastinum | Yes | Yes |
| 2 | IVB | MC | RER | 33.8 | Sx | Ga | Adenopathy | Axilla | Yes | Yes |
| 3 | IIIA | NS | RER | 12.4 | Sx | CT | Adenopathy | Axilla | Yes | Yes |
| 4 | IVB | NS | SER | 1.2 | Imaging | Ga | NA | Cervical/supraclavicular | Yes | Yes |
| 5 | IIIB | LP | RER | 10.6 | Sx | CT/Ga | Adenopathy | Axilla/supraclavicular | Yes | Yes |
| 6 | IIA | NS | RER | 48.9 | Imaging | CT | NA | Mediastinum | Yes | Yes |
| 7 | IVB | NS | RER | 5.4 | Sx | Ga | Adenopathy | Axilla | Yes | Yes |
| 8 | IVB | NS | RER | 1.6 | Sx | Ga | “B” symptoms/adenopathy | Cervical | Yes | Yes |
| 9 | IVB | NS | RER | 4.6 | Sx | CT | “B” symptoms/adenopathy | Paratracheal/axilla/liver/spleen | Yes | Yes |
| 10 | IVB | NS | RER | 20.6 | Imaging | CT | NA | Periaortic | Yes | Yes |
| 11 | IVB | NS | RER | 4.8 | Sx | None | Adenopathy | Cervical | Yes | Yes |
| 12 | IIIB | NS | SER | 0.2 | Sx | None | Adenopathy | Inguinal | Yes | Yes |
| 13 | IVA | NS | SER | 2.2 | Sx | CT/Ga | Adenopathy | Cervical | Yes | Yes |
| 14 | IIB | NS | RER | 20.2 | Imaging | CT | NA | Mediastinum | Yes | NA |
| 15 | IVB | NS | SER | 39.6 | Imaging | Ga/MRI/PET | NA | Axilla/mediastinum/bone | Yes | Yes |
| 16 | IIA | MC | RER | 20.4 | Sx | CT | Chest pain/ESR | Supraclavicular | Yes | Yes |
| 17 | IVB | NS | SER | 0.6 | Sx | CT/Ga | Adenopathy | Preauricular/cervical | Yes | Yes |
| 18 | IVB | NS | SER | 7.4 | Sx | CT | ESR/weight loss | Mediastinum | Yes | Yes |
| 19 | IVA | NS | RER | 7.6 | Sx | CT | ESR | Lung | No (lung nodule) | Yes |
| 20 | IVB | NS | RER | 26.6 | Sx | CT | Pneumonia | Axilla | No (RT axilla) | Yes |
| 21 | IIA | NS | SER | 6.0 | Imaging | CT/Ga | NA | Mediastinum/cervical | Yes | No |
| 22 | IIB | NS | RER | 4.1 | Sx | CT/Ga | ESR | Mediastinum | Yes | Yes |
| 23 | IVB | NOS | SER | 1.9 | Sx | CT/PET/Ga | ESR | Mediastinum/lung | Yes | No |
| 24 | IIA | NS | RER | 7.8 | Sx | None, PE | ESR, adenopathy, chest pain | Cervical | Yes | Yes |
| 25 | IVB | NS | SER | 12.1 | Sx | CT | Pain, adenopathy, alkaline phosphatase | Bilateral axilla, mediastinum, effusion | Yes | NA |
Abbreviations: CT, computed tomography; ESR, erythrocyte sedimentation rate; Ga, gallium scan; LP, lymphocyte predominant; MC, mixed cellularity; MRI, magnetic resonance imaging; NA, not applicable; NOS, not otherwise specified; NS, nodular sclerosis; PE, physical examination; PET, positron emission tomography; RER, rapid early response; RT, right; SER, slow early response; Sx, symptoms.
All 25 patients who experienced relapse had complete data sheets submitted. Nineteen patients had recurrence suspected based on reported change in clinical symptoms, laboratory values, or physical examination findings (Table 2). Symptoms/clinical findings included eight patients with palpable adenopathy, two with recurrent “B” symptoms and adenopathy, and eight with combinations of pain, weight loss, adenopathy, and/or abnormal laboratory values. Six patients were asymptomatic at the time of relapse and had their disease detected based on routine imaging. Two of these relapses occurred within the first year after completion of therapy, the time when patients are at greatest risk of disease recurrence. Only four patients who experienced relapse after the first year and who did not have new clinical symptoms, laboratory findings, or physical examination findings had their relapses detected solely based on routine surveillance imaging. Asymptomatic late relapses occurred in both intermediate-stage (IIA, n = 1) and advanced-stage disease (IIB, n = 1; IVB, n = 2). One additional patient had disease recurrence detected on a CT performed for unrelated symptoms (question of pneumonia). Relapses were detected by both CT and gallium scintigraphy (Table 1). Twenty-one of 25 relapses were biopsy confirmed; two patients had positive correlative gallium imaging. For the remaining two patients, disease recurrence was treated presumptively.
Table 2.
Symptoms of Patients at the Time of Relapse
| Symptom | No. of Patients (N = 25) |
|---|---|
| Clinical symptoms/laboratory findings at time of relapse | 19 |
| Palpable lymph node enlargement | 8 |
| Recurrent “B” symptoms and lymph node enlargement | 2 |
| Pain, elevated ESR or alkaline phosphatase, lymph node enlargement | 2 |
| Chest pain, shoulder pain | 1 |
| Chest pain, elevated ESR | 1 |
| Weight loss, elevated ESR | 1 |
| Elevated ESR | 3 |
| Unrelated symptoms (pneumonia) | 1 |
| Clinically asymptomatic at time of relapse | 6 |
| Time to relapse < 1 year | 2* |
| Time to relapse > 1 year | 4† |
Abbreviation: ESR, erythrocyte sedimentation rate.
One patient with stage IIA disease and one patient with stage IVB disease.
One patient with stage IIA disease, one patient with stage IIB disease, and two patients with stage IVB disease.
The median time to relapse from end of treatment was 7.6 months (range, 0.2 to 48.9 months). Sixteen (64%) of 25 patients experienced relapse within the first year after therapy. Most patients who experienced a relapse were successfully treated with salvage therapy, with 5-year event-free survival of 84% and 5-year OS of 95% reported for the entire patient population.13 The majority of the relapses were identified at previously involved sites of disease or at both new and previous sites (Table 3). Only two (8%) of the 25 relapses were exclusively outside the original sites of disease.
Table 3.
Comparison of Patients With Relapse With Total Patient Cohort
| Characteristic | All Patients (N = 216) |
Patients With Relapse (n = 25) |
||
|---|---|---|---|---|
| No. | % | No. | % | |
| Site of relapse | ||||
| Local | 22 | 88 | ||
| Distant | 2 | 8 | ||
| Both | 1 | 4 | ||
| Risk category of patients at diagnosis | ||||
| Intermediate stage | 53 | 25 | 6 | 24 |
| IB | 1 | 1 | 0 | 0 |
| IIA | 34 | 16 | 5 | 20 |
| IIIA | 18 | 8 | 1 | 4 |
| Advanced stage | 163 | 75 | 19 | 76 |
| IIB | 49 | 22 | 2 | 8 |
| IIIB | 37 | 17 | 2 | 8 |
| IVA | 26 | 12 | 2 | 8 |
| IVB | 51 | 24 | 13 | 52 |
| Bulky disease | ||||
| Yes | 125 | 58 | 17 | 68 |
| No | 79 | 42 | 8 | 32 |
| Early response group | ||||
| SER | 77 | 39 | 9 | 36 |
| RER | 132 | 61 | 16 | 64 |
| Histology | ||||
| NS | 185 | 86 | 21 | 84 |
| LP | 3 | 1 | 1 | 4 |
| MC | 19 | 9 | 2 | 8 |
| NOS | 9 | 4 | 1 | 4 |
Abbreviations: LP, lymphocyte predominant; MC, mixed cellularity; NOS, not otherwise specified; NS, nodular sclerosis; RER, rapid early response; SER, slow early response.
Relapses occurred at approximately the same rate (11% to 12%) in patients with intermediate-stage (six of 53 patients) and advanced-stage disease (19 of 163 patients) and among SER patients (nine of 77 patients) and RER patients (16 of 132 patients). The distribution of relapses reflected the frequency with which these disease stages and response groups were represented in the study population (Table 3). Similarly, relapses occurred across all of the HL histologies, with the same frequency represented by the respective histologic subtype in the study population. The presence of bulk disease did not significantly distinguish the population of patients who experienced relapse. There was a significant difference (P = .044) in time to relapse between RER patients and SER patients; the median time to relapse was 11.5 months (range, 1.6 to 48.9 months) for RER patients and 2.2 months (range, 0.2 to 39.5 months) for SER patients.
To examine the contribution of surveillance imaging to OS, we separated the population of patients who experienced relapse into the following three groups: relapse within 12 months after therapy, detected either by imaging or clinical change; relapse after 12 months, detected by clinical change; and relapse after 12 months, detected only by imaging. As shown in Figure 1A, six patients died, all of whom had experienced relapse within the first 12 months after therapy. There were no deaths in patients who experienced relapse after 12 months, regardless how their relapse was detected. Although the number of patients is relatively small, the difference in survival rate between patients who experienced relapse early (ie, within the first year after completing therapy) and those who experienced relapse later was significant (P = .04).
Fig 1.
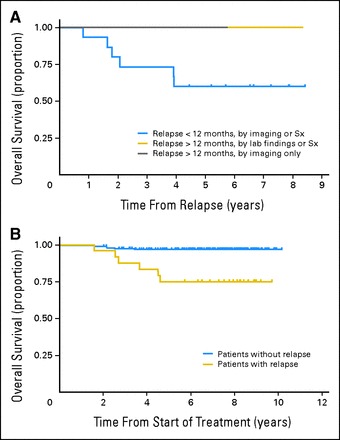
(A) Overall survival (OS) for patients with a reported relapse (n = 25). Patients were grouped as follows based on method of relapse detection: group 1 (relapse within the first 12 months, detected either by imaging, symptoms [Sx], or clinical findings); group 2 (relapse after 12 months, detected by Sx or clinical findings); and group 3 (relapse after 12 months, detected by imaging only, no Sx or clinical findings). Six patients died, all of them from group 1. The differences in postrelapse OS curves between group 1 and group 2 and between group 1 and group 3 are not statistically significant (P = .12 and P = .16, respectively) because of the small sample size. Combining groups 2 and 3, the difference between group 1 (relapse < 12 months) and groups 2 and 3 (relapse > 12 months) resulted in P = .04. (B) OS for patients with a relapse (n = 25) versus the remaining study cohort (n = 191). Six deaths were reported among the 25 patients with a relapse, and six deaths were reported among the remaining patients. The difference is statistically significant (P < .001).
Recurrence of disease was not the only cause of death. Six deaths were reported among the remaining study cohort of 191 patients who did not experience relapse and were the result of treatment toxicity, second malignancy, or other unspecified causes. However, when compared with the six deaths that occurred in the 25 patients with a relapse, the difference is statistically significant (P < .001) and indicates that early disease recurrence, independent of the method of detection, was still the most important predictor of OS (Fig 1B).
DISCUSSION
Children treated for HL have excellent long-term survival. As a result, there has been a shift in treatment paradigm, with emphasis on reducing treatment-related toxicity and late effects. Concurrently, there has been increasing concern that use of CT for routine post-treatment surveillance imaging exposes a large number of patients to potentially unnecessary radiation to detect a small number of relapses, with little effect on OS. In the study reported here, the majority of the relapses were detected within 12 months after completing therapy, in agreement with other studies indicating that most relapses occur within 2 years after completing therapy.5,15 Routine surveillance imaging performed beyond 1 year after completing therapy detected relapses in only four patients who did not have concurrent clinical findings to prompt suspicion for relapse. All deaths as a result of recurrent disease occurred in patients who experienced relapse with the first year after completion of therapy; detecting recurrence beyond 1 year had no effect on OS. On the basis of these findings, it is our conclusion that CT is overutilized for the routine surveillance of patients with HL, with little impact on overall outcome in patients who do not experience an early relapse.
The current protocol of 216 patients required five off-therapy CT scans. Therefore, approximately 1,080 CT scans were obtained to detect four asymptomatic late relapses. Extrapolating to the recently completed COG intermediate-risk HL study AHOD0031, in which more than 1,700 patients were treated, approximately 10,000 surveillance CT scans are being performed after 1 year after completion of therapy to detect what we anticipate will be a small number of late relapses. This approach bears reconsideration, because most patients who experience relapse can be successfully treated with salvage therapy, with more than 90% OS. Furthermore, as was shown here, the use of surveillance imaging to detect asymptomatic late relapses did not impact OS.
The risk of surveillance imaging in terms of radiation exposure can be estimated.6 For example, the 1,700 patients enrolled onto AHOD0031 are required to undergo six surveillance CTs. If we assume, conservatively, CTs limited only to the thorax, there is an additional cumulative exposure of 30 mSv (5 mSv/chest CT) attributable to the surveillance imaging. Using Biological Effects of Ionizing Radiation VII estimates, assuming a median age of 15 years and equal numbers of males and females,16 this added exposure results in an additional lifetime attributable risk of cancer incidence equal to 0.48%, indicating that at least eight new cancers can be expected to occur within the population of 1,700 patients as a result of surveillance imaging. Undoubtedly these risk estimates must be interpreted with caution. Biological Effects of Ionizing Radiation VII assumes normal patient populations, with no other exposures contributing to baseline lifetime cancer risk,16 and does not account for the additional attributable risk imposed by chemotherapy, radiation therapy, and extensive diagnostic staging and early response assessments. Although we expect populations of patients such as ours to have higher baseline rates of cancer occurrence,17 along with increased incidences of second malignancies and other late effects,18,19 any additional risk attributable to surveillance imaging should be minimized.
Significant financial cost is also incurred by surveillance imaging, with only a modest projected gain in quality-adjusted life-years compared with routine clinical follow-up.10 On the basis of the earlier AHOD0031 example, a conservative estimate of the cost for six additional surveillance chest CT scans, using a 2011 Centers for Medicare and Medicaid Services reimbursement rate of $341.13 per chest CT, is an additional $2,046 per patient. Even with this conservative price estimate (private third-party payers may reimburse at rates substantially higher than the Medicare rate), the aggregate cost of these scans to the health care system is at least $3.48 million for the 1,700 patients enrolled.
Radiation dose and cost considerations notwithstanding, certain experimental treatment protocols may justify more intensive post-treatment monitoring if higher rates of relapse are anticipated. Although some have hypothesized that the majority of the unnecessary surveillance imaging is driven by treatment protocols, a recent study reported that only 34% of 690 imaging procedures performed on patients with lymphoma were protocol required.20 The remainder of the scans were discretionary investigations; approximately 40% of these surveillance studies were obtained when recurrence risk was low and without an indication in the medical record, emphasizing the need for more thoughtful approach to imaging in these patients.
One naturally assumes that close imaging surveillance will lead to earlier detection of relapse and improved OS. However, earlier studies in adults suggested that clinical history and physical examination are more reliable in detecting relapse than routine imaging surveillance,7,8 with no difference in outcome between patients with early versus late detection of relapse.9 Our results are in agreement with these adult studies and consistent with reports investigating salvage therapy in children and adolescents, in which relapses occurring more than 1 year after completion of therapy did not predict poor OS, as opposed to refractory or progressive disease during initial therapy.21,22
In many pediatric cancers, and in HL in particular, new response-based treatment paradigms emphasize early response to chemotherapy as a means of identifying patients with chemotherapy-sensitive disease, in whom treatment intensity could be reduced. Extending this paradigm to imaging surveillance, patients who have an RER to chemotherapy, and a lower likelihood of relapse, may require much less frequent radiologic follow-up. Slow responders to therapy, in contrast, may benefit from more intensive surveillance early on, with reduction in imaging as the risk of relapse decreases over time. Our results support this, showing no benefit from long-term surveillance imaging on OS.
There is currently no established role for the use of fluorodeoxyglucose (FDG) positron emission tomography (PET) or PET CT in routine surveillance.23,24 FDG-PET imaging is primarily used for staging and early response assessment and for confirmation of sites of suspected relapse, although use of early PET response data may help direct surveillance imaging to patients at greatest risk.25,26
There were limitations to our study. Although this was a retrospective review, relapse imaging studies were centrally reviewed in the majority of patients. Relapse data sheets were complete for all of our patients, and the majority of the relapses were biopsy confirmed. There are other factors contributing to cumulative radiation exposure in the cohort of patients studied here. Because of the retrospective and multi-institutional nature of this work, it was not possible to determine the total number of imaging studies (CT, nuclear medicine) each patient received while undergoing treatment. Estimates of such cumulative exposures have been reported elsewhere and are likely to be similar.6 Although we provided a rough estimate of cost incurred by performing tests that may not contribute meaningfully to patient outcome (surveillance chest CT), a more rigorous assessment of such costs, both in absolute dollars and in terms of risk-adjusted life-years, has been recently reported.10
This study, examining the role of surveillance imaging in a large cohort of patients with intermediate- and advanced-stage HL treated on a multi-institutional study, identifies an opportunity to reduce both unnecessary medical expense and radiation exposure by decreasing the number of imaging studies being routinely performed on patients with HL. Given these findings, we recommend reducing the routine use of CT surveillance imaging to the initial 12 months after therapy. Consideration could also be given to further reductions for patients with low-stage/low-risk disease and for patients who have RER to therapy based on sensitive functional imaging techniques, such as FDG-PET,23–26 although such response-based reductions in surveillance should probably be performed in the context of a clinical trial. This approach (Fig 2) could include limiting scanning to original site(s) of disease, where relapse is most likely, incorporating chest x-ray, physical examination, and other routine laboratory tests into the disease surveillance regimen and reserving CT scans for new clinical concerns. In all cases, CT dose should be reduced to the lowest achievable dose using the As Low As Reasonably Achievable (ALARA) principle. In many institutions, faster magnetic resonance imaging sequences and parallel imaging techniques have allowed magnetic resonance imaging to effectively replace CT.27 Finally, a comprehensive outcome analysis is needed to determine whether surveillance imaging of any kind is a cost-effective means of monitoring a disease for which late, clinically occult relapses are unusual and for which OS rates remain high.
Fig 2.
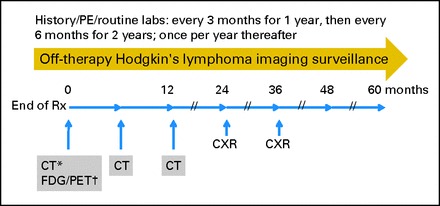
Proposed surveillance scheme for routine post-therapy monitoring in Hodgkin's lymphoma. (*) CT or MRI, provided the treating institution has demonstrated feasibility and efficacy of MRI at detecting known sites of disease at diagnosis or on interim early response assessment. Off-therapy computed tomography (CT) or magnetic resonance imaging (MRI) should be limited to initial sites of involvement (ie, stage IIA patients with disease limited to the mediastinum would have routine surveillance imaging limited to the thorax). (†) Fluorodeoxyglucose (FDG) positron emission tomography (PET) or PET/CT should only be obtained off therapy if still positive during on-therapy monitoring. Patients with persistent FDG-avid disease after completion of therapy should be considered for retrieval therapy rather than further routine surveillance. CXR, chest x-ray; Rx, therapy.
Footnotes
See accompanying editorial on page 2579
Authors' disclosures of potential conflicts of interest and author contributions are found at the end of this article.
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
The author(s) indicated no potential conflicts of interest.
AUTHOR CONTRIBUTIONS
Conception and design: Stephan D. Voss, Allen Chauvenet, Sue C. Kaste, Thomas Slovis, Cindy L. Schwartz
Administrative support: Stephan D. Voss
Collection and assembly of data: Stephan D. Voss, Cindy L. Schwartz
Data analysis and interpretation: Stephan D. Voss, Lu Chen, Louis S. Constine, Allen Chauvenet, Thomas J. Fitzgerald, Sue C. Kaste,Cindy L. Schwartz
Manuscript writing: All authors
Final approval of manuscript: All authors
REFERENCES
- 1.Schwartz CL. Special issues in pediatric Hodgkin's disease. Eur J Haematol Suppl. 2005;66:55–62. doi: 10.1111/j.1600-0609.2005.00456.x. [DOI] [PubMed] [Google Scholar]
- 2.Hodgson DC, Hudson MM, Constine LS. Pediatric Hodgkin lymphoma: Maximizing efficacy and minimizing toxicity. Semin Radiat Oncol. 2007;17:230–242. doi: 10.1016/j.semradonc.2007.02.009. [DOI] [PubMed] [Google Scholar]
- 3.Punnett A, Tsang RW, Hodgson DC. Hodgkin lymphoma across the age spectrum: Epidemiology, therapy, and late effects. Semin Radiat Oncol. 2010;20:30–44. doi: 10.1016/j.semradonc.2009.09.006. [DOI] [PubMed] [Google Scholar]
- 4.Freed J, Kelly KM. Current approaches to the management of pediatric Hodgkin lymphoma. Paediatr Drugs. 2010;12:85–98. doi: 10.2165/11316170-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 5.Dhakal S, Biswas T, Liesveld JL, et al. Patterns and timing of initial relapse in patients subsequently undergoing transplantation for Hodgkin's lymphoma. Int J Radiat Oncol Biol Phys. 2009;75:188–192. doi: 10.1016/j.ijrobp.2008.10.048. [DOI] [PubMed] [Google Scholar]
- 6.Ahmed BA, Connolly BL, Shroff P, et al. Cumulative effective doses from radiologic procedures for pediatric oncology patients. Pediatrics. 2010;126:e851–e858. doi: 10.1542/peds.2009-2675. [DOI] [PubMed] [Google Scholar]
- 7.Dryver ET, Jernstrom H, Tompkins K, et al. Follow-up of patients with Hodgkin's disease following curative treatment: The routine CT scan is of little value. Br J Cancer. 2003;89:482–486. doi: 10.1038/sj.bjc.6601052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Radford JA, Eardley A, Woodman C, et al. Follow up policy after treatment for Hodgkin's disease: Too many clinic visits and routine tests? A review of hospital records. BMJ. 1997;314:343–346. doi: 10.1136/bmj.314.7077.343a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Torrey MJ, Poen JC, Hoppe RT. Detection of relapse in early-stage Hodgkin's disease: Role of routine follow-up studies. J Clin Oncol. 1997;15:1123–1130. doi: 10.1200/JCO.1997.15.3.1123. [DOI] [PubMed] [Google Scholar]
- 10.Guadagnolo BA, Punglia RS, Kuntz KM, et al. Cost-effectiveness analysis of computerized tomography in the routine follow-up of patients after primary treatment for Hodgkin's disease. J Clin Oncol. 2006;24:4116–4122. doi: 10.1200/JCO.2006.07.0409. [DOI] [PubMed] [Google Scholar]
- 11.Brenner DJ, Hall EJ. Computed tomography: An increasing source of radiation exposure. N Engl J Med. 2007;357:2277–2284. doi: 10.1056/NEJMra072149. [DOI] [PubMed] [Google Scholar]
- 12.Robbins E. Radiation risks from imaging studies in children with cancer. Pediatr Blood Cancer. 2008;51:453–457. doi: 10.1002/pbc.21599. [DOI] [PubMed] [Google Scholar]
- 13.Schwartz CL, Constine LS, Villaluna D, et al. A risk-adapted, response-based approach using ABVE-PC for children and adolescents with intermediate- and high-risk Hodgkin lymphoma: The results of P9425. Blood. 2009;114:2051–2059. doi: 10.1182/blood-2008-10-184143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Constine L, Marcus R, Chauvenet A, et al. Patterns of failure after response-based, dose-dense therapy for intermediate/high risk pediatric Hodgkin's disease (POG 9425) Int J Radiat Oncol Biol Phys. 2005;63(suppl):S21–S22. [Google Scholar]
- 15.Das P, Ng A, Constine LS, et al. ACR appropriateness criteria on Hodgkin's lymphoma: Favorable prognosis stage I and II. J Am Coll Radiol. 2008;5:1054–1066. doi: 10.1016/j.jacr.2008.06.016. [DOI] [PubMed] [Google Scholar]
- 16.Alessio AM, Phillips GS. A pediatric CT dose and risk estimator. Pediatr Radiol. 2010;40:1816–1821. doi: 10.1007/s00247-010-1761-0. [DOI] [PubMed] [Google Scholar]
- 17.O'Brien MM, Donaldson SS, Balise RR, et al. Second malignant neoplasms in survivors of pediatric Hodgkin's lymphoma treated with low-dose radiation and chemotherapy. J Clin Oncol. 2010;28:1232–1239. doi: 10.1200/JCO.2009.24.8062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bhatia S, Constine LS. Late morbidity after successful treatment of children with cancer. Cancer J. 2009;15:174–180. doi: 10.1097/PPO.0b013e3181a58f46. [DOI] [PubMed] [Google Scholar]
- 19.Schwartz CL. Long-term survivors of childhood cancer: The late effects of therapy. Oncologist. 1999;4:45–54. [PubMed] [Google Scholar]
- 20.Chong AL, Grant RM, Ahmed BA, et al. Imaging in pediatric patients: Time to think again about surveillance. Pediatr Blood Cancer. 2010;55:407–413. doi: 10.1002/pbc.22575. [DOI] [PubMed] [Google Scholar]
- 21.Schellong G, Dorffel W, Claviez A, et al. Salvage therapy of progressive and recurrent Hodgkin's disease: Results from a multicenter study of the pediatric DAL/GPOH-HD study group. J Clin Oncol. 2005;23:6181–6189. doi: 10.1200/JCO.2005.07.930. [DOI] [PubMed] [Google Scholar]
- 22.Metzger ML, Hudson MM, Krasin MJ, et al. Initial response to salvage therapy determines prognosis in relapsed pediatric Hodgkin lymphoma patients. Cancer. 2010;116:4376–4384. doi: 10.1002/cncr.25225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Crocchiolo R, Fallanca F, Giovacchini G, et al. Role of 18FDG-PET/CT in detecting relapse during follow-up of patients with Hodgkin's lymphoma. Ann Hematol. 2009;88:1229–1236. doi: 10.1007/s00277-009-0752-4. [DOI] [PubMed] [Google Scholar]
- 24.Meany HJ, Gidvani VK, Minniti CP. Utility of PET scans to predict disease relapse in pediatric patients with Hodgkin lymphoma. Pediatr Blood Cancer. 2007;48:399–402. doi: 10.1002/pbc.20797. [DOI] [PubMed] [Google Scholar]
- 25.Gallamini A, Fiore F, Sorasio R, et al. Interim positron emission tomography scan in Hodgkin lymphoma: Definitions, interpretation rules, and clinical validation. Leuk Lymphoma. 2009;50:1761–1764. doi: 10.3109/10428190903308072. [DOI] [PubMed] [Google Scholar]
- 26.Furth C, Steffen IG, Amthauer H, et al. Early and late therapy response assessment with [18F]fluorodeoxyglucose positron emission tomography in pediatric Hodgkin's lymphoma: Analysis of a prospective multicenter trial. J Clin Oncol. 2009;27:4385–4391. doi: 10.1200/JCO.2008.19.7814. [DOI] [PubMed] [Google Scholar]
- 27.Kwee TC, van Ufford HM, Beek FJ, et al. Whole-body MRI, including diffusion-weighted imaging, for the initial staging of malignant lymphoma: Comparison to computed tomography. Invest Radiol. 2009;44:683–690. doi: 10.1097/RLI.0b013e3181afbb36. [DOI] [PubMed] [Google Scholar]


