Abstract
Purpose
We evaluated the feasibility of administering carboplatin as a radiosensitizer during craniospinal radiation therapy (CSRT) to patients with high-risk medulloblastomas (MBs) and supratentorial primitive neuroectodermal tumors, and we report the outcome in the subset with metastatic (M+) MB.
Patients and Methods
After surgery, patients received 36 Gy CSRT with boosts to sites of disease. During radiation, patients received 15 to 30 doses of carboplatin (30-45 mg/m2/dose), along with vincristine (VCR) once per week for 6 weeks. Patients on regimen A received 6 months of maintenance chemotherapy (MC) with cyclophosphamide and VCR. Once the recommended phase II dose (RP2D) of carboplatin was determined, cisplatin was added to the MC (regimen B).
Results
In all, 161 eligible patients (median age, 8.7 years; range, 3.1 to 21.6 years) were enrolled. Myelosuppression was dose limiting and 35 mg/m2/dose × 30 was determined to be the RP2D of carboplatin. Twenty-nine (36%) of 81 patients with M+ MB had diffuse anaplasia. Four patients were taken off study within 11 months of completing radiotherapy for presumed metastatic progression and are long-term survivors following palliative chemotherapy. Excluding these four patients, 5-year overall survival ± SE and progression-free survival ± SE for M+ patients treated at the RP2D on regimen A was 82% ± 9% and 71% ± 11% versus 68% ± 10% and 59% ± 10% on regimen B (P = .36). There was no difference in survival by M stage. Anaplasia was a negative predictor of outcome.
Conclusion
The use of carboplatin as a radiosensitizer is a promising strategy for patients with M+ MB. Early progression should be confirmed by biopsy.
INTRODUCTION
Medulloblastomas (MBs) account for 20% of pediatric brain tumors, with approximately one third of patients having metastatic spread (M+) at diagnosis.1,2 Whereas 80% of children with localized MB will be cured following treatment with reduced dose craniospinal radiation therapy (CSRT) and chemotherapy,3 the success rate for patients with disseminated disease, as well as for those with supratentorial primitive neuroectodermal tumors (PNETs), has historically been poor despite full-dose CSRT and chemotherapy.1,4–6
The Goldie-Coldman model of therapeutic resistance states that the prompt elimination of tumor through the use of multiple drugs given concurrently reduces the likelihood of the emergence of resistant clones.7 CSRT is the most effective treatment modality against MB and can be modeled as a non–cross-resistant chemotherapeutic agent. Chemoradiotherapy therefore has the potential to achieve maximal tumor-cell kill in the shortest period of time. Carboplatin not only has significant activity against PNETs8,9 but is also a potent radiosensitizer, possibly by enhancing the production and persistence of DNA single- and double-strand breaks.10,11 RT may also enhance the cellular uptake and DNA-binding of carboplatin.12 Radiopotentiating concentrations of platinum can be found in intracerebral tumors and surrounding edematous brain following administration of low systemic doses of cisplatin (CDDP), with lower concentrations of platinum found in brain distant to the tumor and in the cerebrospinal fluid (CSF).13 Carboplatin binds more slowly to plasma proteins than CDDP does, allowing a greater proportion of free platinum to cross the blood-brain barrier.14 We sought to identify a feasible dose and duration of carboplatin given daily with craniospinal and boost radiotherapy (RT) to children with high-risk MB and PNET and, herein, report on the results of this approach in patients with M+ MB.
PATIENTS AND METHODS
Patients and Eligibility
Patients between the ages of 3 and 21 years with newly diagnosed high-risk PNET were eligible for enrollment. High-risk PNET was defined as those with M+ MB, more than 1.5 cm3 postoperative residual tumor, or supratentorial PNET, regardless of M stage or resection. Patients were classified as M1 when they had positive CSF cytology without other evidence of metastatic disease, as M2 when they had supratentorial without spinal metastases, and M3 when they had spinal metastases with (M3b) or without (M3a) supratentorial disease. Patients underwent a complete staging evaluation, including pre- and postoperative brain magnetic resonance imaging scans, spinal magnetic resonance imaging scans, and CSF cytology. Patients with extraneural metastases were excluded. All patients began therapy within 31 days of definitive surgery. Eligibility criteria also included normal renal function (creatinine < 1.5 × normal for age), bone marrow function (absolute neutrophil count [ANC] > 1,500/μL, platelet count > 100,000/μL, hemoglobin > 10 g/dL), and liver function (bilirubin < 1.5 mg/dL, ALT < 2.5 × normal).
Study Design
This phase I/II study included a dose-escalation phase followed by a comparison of maintenance chemotherapy (MC) with or without CDDP. The craniospinal axis received 36 Gy in 1.8 Gy fractions and was treated before boosts. A boost of 19.8 Gy was administered to the entire posterior fossa for patients with MB or to the supratentorial primary tumor site. Focal spinal cord metastases were boosted to 45 Gy if they were above the termination of the cord and to 50.4 Gy if they were below it. RT films and doses were centrally reviewed. Intravenous vincristine (VCR) 1.5 mg/m2 was administered weekly × 6 during RT. Patients received carboplatin at the assigned dose level over 15 to 20 minutes, 1 to 4 hours before each fraction of radiation. The dose and duration of carboplatin was assigned at study entry by using a phase I dose-escalation design, starting with 35 mg/m2/dose × 15 doses. Subsequent dose levels increased the number of doses of carboplatin to 20, 25, and 30, and thereafter the dose of carboplatin was increased in increments of 5 mg/m2/dose up to 50 mg/m2/dose. A minimum of six evaluable patients were treated at each dose level. If two or fewer of the first six patients treated at any dose level experienced dose-limiting toxicity (DLT) during the 12-week evaluation period, up to six additional patients were treated at that dose level. Up to 24 additional patients could be enrolled on the highest safe dose level during evaluation of a higher dose. The maximum-tolerated dose was defined as the dose level immediately below that at which at least three patients in a cohort of six, or at least four patients in a cohort of 12, experienced a DLT.
Toxicities were graded on the basis of the National Cancer Institute Common Toxicity Criteria, version 1.0. DLT was defined as death related to toxicity; more than a 2-week delay in completion of RT; inability to start MC within 7 weeks of completing RT; grade 4 nonhematologic toxicity, with the exception of weight loss, infection, or electrolyte abnormalities; grade 3 nonhematologic toxicity, with the exception of weight loss, dysphagia-esophagitis related to RT lasting ≤ 7 days, skin toxicity, infection, electrolyte abnormalities, nausea and vomiting, and liver function abnormalities that returned to grade ≤ 2 within 7 days; and VCR-related toxicities. RT was not withheld for myelosuppression alone and was held only for a “severe medical condition precluding radiation therapy,” not including fever and neutropenia as long as the patient was clinically stable. If a radiation treatment was not given, carboplatin was also held.
Granulocyte colony-stimulating factor (G-CSF) was administered on the weekend if the ANC fell ≤ 1,500/μL on Friday; if the ANC dropped to less than 1,000/μL on any Monday or Wednesday, G-CSF was administered on that day and the following day. Patients were transfused with platelets to maintain a count of more than 30,000/μL and RBCs to maintain a hematocrit ≥ 30% during RT. No dose modifications of carboplatin were made for myelosuppression. VCR was held for grade 3 to 4 foot drop, severe paresis, disabling paresthesias or ileus and was resumed at 1 mg/m2 once symptoms improved.
During the dose-finding phase, all patients were nonrandomly assigned to receive MC with cyclophosphamide (CPM) and VCR. Once the optimal dose and duration of carboplatin was determined, patients were treated at the recommended phase II dose (RP2D) and received MC with VCR, CPM, and CDDP. Six cycles were given 4 weeks apart beginning 6 weeks after RT was completed or when the ANC was more than 1,000/μL and platelet count was more than 100,000/μL. Regimen A consisted of CPM 1,000 mg/m2 on days 0 and 1 of each course and VCR 1.5 mg/m2 on days 0 and 7. G-CSF was administered until the postnadir ANC exceeded 1,500/μL. Regimen B consisted of the same chemotherapy with the addition of 75 mg/m2 CDDP on day 0. Subsequent courses of chemotherapy were administered once the ANC was more than 750/μL and the platelet count was more than 75,000/μL. The CPM dose was reduced by 25% if the counts had not recovered by the time the next course was due. Audiograms were obtained before each course of CDDP with dose reductions dependent on the grade of the toxicity.
Follow-up imaging was performed 4 to 6 weeks after the completion of RT, at 3-month intervals during MC, and at 4- to 12-month intervals thereafter. Progressive disease was defined as an increase of more than 25% in area of residual disease compared with the best response at that site or the reappearance or new appearance of tumor. Overall survival (OS) and progression-free survival (PFS) for patients treated at the maximum-tolerated dose was estimated by using the product limit (Kaplan-Meier) method, with SE via the Peto-Pike formula.10 Survival distributions among subgroups were compared by using the log-rank test. Association of survival distributions with continuous covariates was investigated by using Cox proportional hazards regression models. Pathology slides from patients with M+ MBs were centrally reviewed to confirm the diagnosis and assess for anaplasia by a reviewer blinded to clinical factors and outcome (P.C.B.). Anaplastic MBs were defined as those with marked nuclear pleomorphism, nuclear molding, cell-cell wrapping, and high mitotic activity. Large-cell MBs had large vesicular nuclei, prominent nucleoli, and scant eosinophilic cytoplasm. Large-cell areas were usually topographically distinct areas in lesions that elsewhere were anaplastic. A tumor was coded as anaplastic if at least half the tissue contained anaplastic or large cells. With high-grade and/or large-cell features, a third of the tissue was considered sufficient.
RESULTS
Between March 1998 and November 2004, 168 patients were enrolled. Seven patients were considered ineligible. Four patients were considered unevaluable for toxicity. For the 161 eligible patients, the median age at study entry was 8.7 years (range, 3.1 to 21.6 years); 92 (58%) were male, and 33 patients were treated on regimen B. Median follow-up was 8.5 and 6.4 years for patients on regimens A and B, respectively. One patient was not included in the MB outcome analysis because the diagnosis could not be confirmed on central review, and one patient was not included in the anaplasia analysis because of crush artifact.
Study Toxicity
There were no treatment-related deaths (Tables 1 and 2). Delayed myelosuppression occurring 2 to 3 weeks after the completion of RT and occasionally causing a delay in the initiation of MC was seen starting at dose level 7 (35 mg/m2 × 6 weeks). Specific radiation-related toxicities were not common, occurring in less than 10% of patients at each dose level except for dose level 10 (50 mg/m2 × 6 weeks) in which two of eight patients developed grade 3 skin breakdown and severe esophagitis. Sixteen patients (10%) had interruptions in RT for medical reasons (median, 2 days; range, 1 to 6 days).
Table 1.
DLTs by Dose Level
| Dose Level | Carboplatin Dose | No. of Patients Evaluable for DLT* | No. of DLTs | DLT Type | No. of Patients With DLT |
|---|---|---|---|---|---|
| 3 | 30 mg/m2 × 3 weeks | 11 | 2 | Seizures | 1 |
| Somnolence | 1 | ||||
| 4 | 35 mg/m2 × 3 weeks | 11 | 1 | Grade 3 esophagitis | |
| 5 | 35 mg/m2 × 4 weeks | 12 | 1 | Grade 3 esophagitis | |
| 6 | 35 mg/m2 × 5 weeks | 12* | 1 | Kidney stone | |
| 7 | 35 mg/m2 × 6 weeks | 70 | 11 | Inability to start maintenance therapy | 7 |
| Esophagitis/inability to start maintenance therapy | 1 | ||||
| Grade 4 nausea/vomiting | 2 | ||||
| Grade 3 esophagitis/dermatitis | 1 | ||||
| 8 | 40 mg/m2 × 6 weeks | 24 | 4 | Inability to start maintenance therapy | 3 |
| Grade 3 esophagitis | 1 | ||||
| 9 | 45 mg/m2 × 6 weeks | 10 | 1 | Grade 4 dermatitis | |
| 10 | 50 mg/m2 × 6 weeks | 7 | 2 | Grade 3 esophagitis | 1 |
| Dermatitis | 1 |
Abbreviation: DLT, dose-limiting toxicity.
One patient (no DLT) was inadvertently treated with an additional week of carboplatin (ie, dose level 7).
Table 2.
Selected Grade 3 and 4 Toxicities During Maintenance Courses for Regimen A (dose level 7) and Regimen B
| Toxicity | Course 1 |
Course 2 |
Course 3 |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Regimen A |
Regimen B |
Regimen A |
Regimen B |
Regimen A |
Regimen B |
|||||||
| No. | % | No. | % | No. | % | No. | % | No. | % | No. | % | |
| ANC | 32 | 89 | 30 | 97 | 28 | 85 | 27 | 96 | 23 | 72 | 26 | 93 |
| Platelet count | 19 | 53 | 26 | 84 | 15 | 45 | 26 | 93 | 17 | 53 | 27 | 96 |
| Hearing | 0 | 0 | 0 | 0 | 0 | 0 | 2 | 7 | 0 | 0 | 4 | 14 |
| Infection | 9 | 25 | 15 | 48 | 5 | 16 | 9 | 32 | 5 | 16 | 8 | 29 |
Abbreviation: ANC, absolute neutrophil count.
The majority of patients required G-CSF and/or transfusion support toward the end of RT, particularly at the higher dose levels. As enrollment proceeded, there was concern that the incidence of myelosuppression and infection at the higher dose levels was becoming unacceptable. A logistic regression dose-response analysis of the incidence of platelet transfusion and infection showed a clear increase in platelet transfusions as the total dose of carboplatin increased, and dose level 7 (35 mg/m2 × 6 weeks) was chosen as the RP2D.
Table 2 shows the major grade 3 and 4 toxicities during MC for regimens A and B at dose level 7. Regimen B was uniformly more toxic, with the differences most apparent in the rates of thrombocytopenia and infection. As expected, ototoxicity was also more severe on regimen B, with 14 (42%) of 33 patients requiring CDDP dose reductions. No unexpected long-term toxicities have been reported to date.
Outcome for Patients With M+ MB
By using a Cox proportional hazard model, neither OS (P = .50) nor PFS (P = .42) was associated with the total dose of carboplatin received. There were 59 patients with centrally reviewed M+ MB and five M0 patients. Combining all dose levels, 5-year OS ± SE and PFS ± SE for centrally reviewed M+ patients on regimen A was 80% ± 5% and 66% ± 6%. Investigation into the unexpected discrepancy between OS and PFS revealed four patients with reported progression within 11 months of completing RT (6 weeks, and 4, 9, and 11 months). All recurrences involved equivocal worsening of leptomeningeal disease, none of whom were biopsied. All four are long-term survivors (9, 8, 7, and 7 years) following treatment with palliative chemotherapy alone. Excluding these four patients, 5-year OS ± SE and PFS ± SE for M+ patients was 78% ± 6% and 71% ± 6% (Fig 1).
Fig 1.
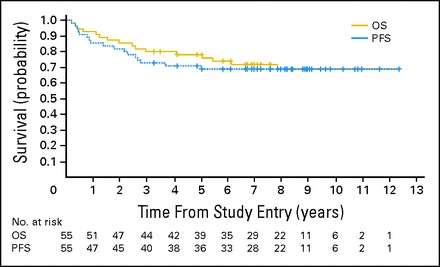
Kaplan-Meier curves showing the overall survival (OS) and progression-free survival (PFS) of patients with centrally reviewed metastatic medulloblastoma treated on regimen A, excluding four patients who were felt to have had pseudoprogression. The numbers below the survival curves reflect the number of patients at risk at any given time point.
Comparison of M+ Patients Enrolled at the RP2D: Regimen A Versus Regimen B
There was no significant difference in distribution of M stage, demographics, or percentage of anaplasia between patients enrolled at the RP2D on regimen A versus regimen B. Figure 2 shows the outcome for centrally reviewed patients with M+ MB who received dose level 7 of regimen A (n = 19) or regimen B (n = 22). There was no significant difference in outcome between the two regimens. The 5-year OS ± SE was 82% ± 9% for regimen A and 68% ± 10% for regimen B (log-rank P = .68); the 5-year PFS ± SE was 71% ± 11% for regimen A and 59% ± 10% for regimen B (log-rank P = .36).
Fig 2.
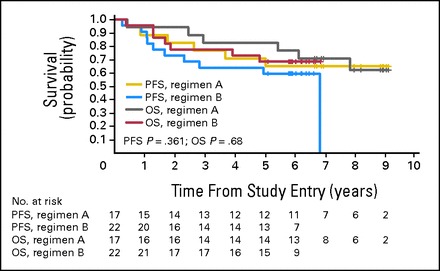
Kaplan-Meier curves showing the overall survival (OS) and progression-free survival (PFS) of patients with centrally reviewed metastatic medulloblastoma treated at the recommended phase II dose of carboplatin on regimen A and regimen B. The numbers below the survival curves reflect the number of patients at risk at any given time point.
Impact of M Stage
No significant differences in OS and PFS were found between the centrally reviewed patients with M1 (n = 18), M2 (n = 10), or M3 (n = 49) MB (log-rank P = .4 and P = .34, respectively; Fig 3). The 5-year OS ± SE was 83% ± 9% for M1, 70% ± 16% for M2, and 73% ± 6% for M3; the 5-year PFS ± SE was 77% ± 10% for M1, 50% ± 16% for M2, and 67% ± 7% for M3.
Fig 3.
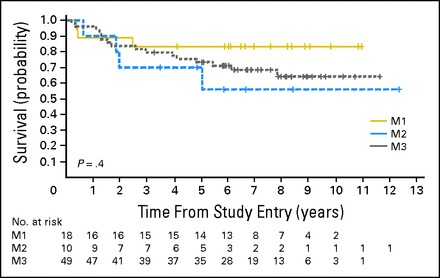
Kaplan-Meier curves showing no significant difference in the overall survival of patients with centrally reviewed metastatic medulloblastoma based on M stage. The numbers below the survival curves reflect the number of patients at risk at any given time point.
Impact of Anaplasia
Figure 4 shows the OS and PFS plots comparing 29 patients with anaplasia and 48 patients without anaplasia. There was no statistical difference in the distribution of anaplasia across the two regimens (Fisher's exact test P = .80). There was no difference in age, ratio of males to females, or distribution of M stage between those with or without anaplasia. The 5-year PFS ± SE was 75% ± 6% for patients without anaplasia and 55% ± 9% for those with anaplasia (log-rank P = .019); the 5-year OS ± SE rate was 83% ± 5% for patients without anaplasia and 62% ± 9% for those with anaplasia (log-rank P = .064).
Fig 4.
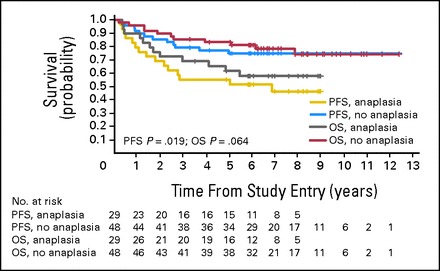
Kaplan-Meier curves showing the overall survival (OS) and progression-free survival (PFS) of patients with centrally reviewed metastatic medulloblastoma with and without anaplasia. The numbers below the survival curves reflect the number of patients at risk at any given time point.
Impact of Surgical Resection
There was no difference in OS or PFS for the patients with M2/3 MB who had at least a radical resection of the primary tumor versus those with less aggressive resection (log-rank P = .62 and P = .91 for OS and PFS, respectively).
Incidence of Second Malignant Neoplasms (SMNs)
Four of 161 patients (2.5%; 95% exact CI, 0.7% to 6.2%), all with MB, developed a SMN by year 8: one developed a malignant melanoma in the radiation field 2 years after diagnosis; one was diagnosed with acute myeloid leukemia 4 years after receiving high-dose chemotherapy with thiotepa, etoposide (VP-16), and carboplatin, with stem-cell rescue as treatment for recurrence; one developed myelodysplastic syndrome 3 years after diagnosis; and one developed a glioblastoma 7 years after diagnosis. There was no difference between the incidence of SMNs in the entire study group or the subset of patients with MB compared with that of a historical cohort of standard-risk patients treated with lower doses of CSRT15 (Fisher's exact P = .6 and P = .76, respectively).
DISCUSSION
We have shown that a regimen administering daily carboplatin concurrently with RT for patients with high-risk MB is feasible, and with appropriate supportive care, radiosensitizing and cytotoxic doses of carboplatin can be administered. At the RP2D, a cumulative dose of more than 1 g/m2 carboplatin was administered over 6 weeks. The substantial doses of carboplatin administered early on in treatment may account for why the addition of CDDP to MC provided no increase in survival. The incidence of radiation-related adverse effects was lower than expected and was not what determined the RP2D.
Many strategies have been used over the last several years to improve the outcome of patients with M+ MB. The initial encouraging results of the Packer regimen16 in 15 patients with M+ MB showing a 66% ± 15% 5-year PFS could not be reproduced in either German or US cooperative group trials1,5 in which the identical regimen resulted in a 30% to 40% 5-year PFS. Studies that used preirradiation chemotherapy have shown similar results, although a delay in administration of RT has been suggested to have a negative impact on outcome.1 In the International Society of Pediatric Oncology/United Kingdom Children's Cancer Study GroupPNET 3 (SIOP/UKCCSG/PNET-3) trial,4 two courses of etoposide, carboplatin, cytoxan, and VCR were administered before RT. Patients with M2/3 disease subsequently received 35 Gy CSRT with a boost to the poster fossa. Five-year event-free survival and OS were 34.7% and 43.9%. Allen6 evaluated five courses of pre-RT chemotherapy followed by hyperfractionated RT. The 5-year PFS and OS for patients with M+ MB were 46% ± 10% and 48% ± 10%. A recent limited-institution trial reported more encouraging results with the use of four courses of dose-intense CPM-based chemotherapy requiring peripheral blood stem-cell rescue following CSRT.17 M1 patients received 36 Gy and M2/3 patients received 39.6 Gy CSRT with boosts to bulk disease. The 5-year event-free survival for M+ patients was 66% ± 18%. Our 5-year PFS of 71% ± 6% compares favorably, despite the use of lower CSRT doses and less intensive chemotherapy.
There were four M+ patients with early progression of leptomeningeal disease who are long-term survivors following palliative chemotherapy alone. In retrospect, these patients are not felt to have had true progressive disease. This highlights the need for great caution in determining progression, particularly when it occurs early in treatment. The use of chemotherapy during RT has been associated with an increased risk of pseudoprogression in patients with high-grade gliomas,18 and this risk may be higher when using a potent radiosensitizer. Independent of the risk of pseudoprogression, the ability to determine true worsening of leptomeningeal disease is fraught with uncertainty, given the technical limitations precluding the ability to accurately compare the size and presence of small areas of tumor deposits.
Attempts have been made to define a histopathologic grading system for MB that can identify patients with either a better or worse prognosis so that treatment can be tailored appropriately. The degree of anaplasia has been associated with more aggressive behavior.19,20 In a study evaluating prognostic variables in 207 patients with MB, metastatic disease and large-cell/anaplastic phenotype were significantly associated with poor PFS.21 Packer3 found that anaplasia was also a negative prognostic factor for OS in average-risk MB. In our study, anaplasia was seen in approximately one third of patients with M+ MB and was a significant negative prognostic factor.
In conclusion, our results using chemoradiotherapy followed by a well-tolerated, short duration, nonintensive MC regimen are at least as good as, if not better than, other recent trials using higher doses of CSRT and/or higher-intensity alkylator-based chemotherapy. The incidence of second malignancies in patients treated for MB is substantial and appears to be increasing with increased use of alkylator-based therapy.15 With improving survival, the impact of long-term treatment-related morbidity and mortality increases dramatically, making it imperative to keep alkylator and RT doses as low as possible without sacrificing efficacy. It is also important for neurosurgeons to be judicious about the need for aggressive surgical resection in the setting of metastatic disease, because there was no apparent benefit seen in this study. An ongoing Children's Oncology Group study randomizing between carboplatin during radiation and RT alone in patients with high-risk PNET will definitively answer the question regarding the impact of carboplatin on outcome, as well as formally evaluate long-term neurocognitive outcome.
Acknowledgment
We thank Shawn Lesh for his assistance with the statistics.
Footnotes
Supported by Grant No. U10 CA 98543 from the Children's Oncology Group.
Presented at the 43rd Annual Meeting of the American Society of Clinical Oncology, June 1-5, 2007, and the 12th International Symposium on Pediatric Neuro-Oncology, Nara, Japan, June 6-9, 2006.
Authors' disclosures of potential conflicts of interest and author contributions are found at the end of this article.
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Although all authors completed the disclosure declaration, the following author(s) indicated a financial or other interest that is relevant to the subject matter under consideration in this article. Certain relationships marked with a “U” are those for which no compensation was received; those relationships marked with a “C” were compensated. For a detailed description of the disclosure categories, or for more information about ASCO's conflict of interest policy, please refer to the Author Disclosure Declaration and the Disclosures of Potential Conflicts of Interest section in Information for Contributors.
Employment or Leadership Position: Joel Goldwein, Elekta (C); Minesh Mehta, Pharmacyclics (C) Consultant or Advisory Role: Minesh Mehta, Bristol-Myers Squibb (C), Elekta (C), TomoTherapy (C), Vertex Pharmaceuticals (C) Stock Ownership: Joel Goldwein, Elekta; Minesh Mehta, Accuray, Colby Pharmaceutical, Pharmacyclics, ProCertus BioPharm, Honoraria: Minesh Mehta, Medical Communications Media, Medscape, Merck, Novartis, prIME Oncology, Strategic Edge Communications, Vindico Medical Education, WebMD Research Funding: None Expert Testimony: None Other Remuneration: None
AUTHOR CONTRIBUTIONS
Conception and design: Regina I. Jakacki, Joel Goldwein,Roger J. Packer
Provision of study materials or patients: Roger J. Packer, Regina Jakacki, Nancy Tarbell, Joanne Hilden
Collection and assembly of data: Peter C. Burger, Tianni Zhou, Roger J. Packer, Charles Fitz, Gilbert Vezina
Data analysis and interpretation: Regina I. Jakacki, Peter C. Burger, Tianni Zhou, Emiko J. Holmes, Mehmet Kocak, Arzu Onar, Minesh Mehta, Roger J. Packer, Nancy Tarbell, Charles Fitz, Joanne Hilden, Ian F. Pollack
Manuscript writing: All authors
Final approval of manuscript: All authors
REFERENCES
- 1.Kortmann RD, Kühl J, Timmermann B, et al. Postoperative neoadjuvant chemotherapy before radiotherapy as compared to immediate radiotherapy followed by maintenance chemotherapy in the treatment of medulloblastoma in childhood: Results of the German prospective randomized trial HIT '91. Int J Radiat Oncol Biol Phys. 2000;46:269–279. doi: 10.1016/s0360-3016(99)00369-7. [DOI] [PubMed] [Google Scholar]
- 2.Bouffet E, Gentet JC, Doz F, et al. Metastatic medulloblastoma: The experience of the French Cooperative M7 Group. Eur J Cancer. 1994;30A:1478–1483. doi: 10.1016/0959-8049(94)00256-5. [DOI] [PubMed] [Google Scholar]
- 3.Packer RJ, Gajjar A, Vezina G, et al. Phase III study of craniospinal radiation therapy followed by adjuvant chemotherapy for newly diagnosed average-risk medulloblastoma. J Clin Oncol. 2006;24:4202–4208. doi: 10.1200/JCO.2006.06.4980. [DOI] [PubMed] [Google Scholar]
- 4.Taylor RE, Bailey CC, Robinson KJ, et al. Outcome for patients with metastatic (M2-3) medulloblastoma treated with SIOP/UKCCSG PNET-3 chemotherapy. Eur J Cancer. 2005;41:727–734. doi: 10.1016/j.ejca.2004.12.017. [DOI] [PubMed] [Google Scholar]
- 5.Zeltzer PM, Boyett JM, Finlay JL, et al. Metastasis stage, adjuvant treatment, and residual tumor are prognostic factors for medulloblastoma in children: Conclusions from the Children's Cancer Group 921 randomized phase III study. J Clin Oncol. 1999;17:832–845. doi: 10.1200/JCO.1999.17.3.832. [DOI] [PubMed] [Google Scholar]
- 6.Allen J, Donahue B, Mehta M, et al. A phase II study of preradiotherapy chemotherapy followed by hyperfractionated radiotherapy for newly diagnosed high-risk medulloblastoma/primitive neuroectodermal tumor: A report from the Children's Oncology Group (CCG 9931) Int J Radiat Oncol Biol Phys. 2009;74:1006–1011. doi: 10.1016/j.ijrobp.2008.09.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Goldie JH, Coldman AJ. A mathematic model for relating the drug sensitivity of tumors to their spontaneous mutation rate. Cancer Treat Rep. 1979;63:1727–1733. [PubMed] [Google Scholar]
- 8.Mastrangelo R, Lasorella A, Riccardi R, et al. Carboplatin in childhood medulloblastoma/PNET: Feasibility of an in vivo sensitivity test in an “up-front” study. Med Pediatr Oncol. 1995;24:188–196. doi: 10.1002/mpo.2950240309. [DOI] [PubMed] [Google Scholar]
- 9.Gaynon PS, Ettinger LJ, Baum ES, et al. Carboplatin in childhood brain tumors: A Children's Cancer Study Group Phase II trial. Cancer. 1990;66:2465–2469. doi: 10.1002/1097-0142(19901215)66:12<2465::aid-cncr2820661204>3.0.co;2-n. [DOI] [PubMed] [Google Scholar]
- 10.Yang L, Douple EB, O'Hara JA, et al. Enhanced radiation-induced cell killing by carboplatin in cells of repair-proficient and repair-deficient cell lines. Radiat Res. 1995;144:230–236. [PubMed] [Google Scholar]
- 11.Yang LX, Douple EB, O'Hara JA, et al. Carboplatin enhances the production and persistence of radiation-induced DNA single-strand breaks. Radiat Res. 1995;143:302–308. [PubMed] [Google Scholar]
- 12.Yang LX, Douple EB, Wang HJ. Irradiation enhances cellular uptake of carboplatin. Int J Radiat Oncol Biol Phys. 1995;33:641–646. doi: 10.1016/0360-3016(95)00202-A. [DOI] [PubMed] [Google Scholar]
- 13.Stewart DJ, Leavens M, Maor M, et al. Human central nervous system distribution of cis-diamminedichloroplatinum and use as a radiosensitizer in malignant brain tumors. Cancer Res. 1982;42:2474–2479. [PubMed] [Google Scholar]
- 14.Jacobs SS, Fox E, Dennie C, et al. Plasma and cerebrospinal fluid pharmacokinetics of intravenous oxaliplatin, cisplatin, and carboplatin in non-human primates. Clin Cancer Res. 2005;11:1669–1674. doi: 10.1158/1078-0432.CCR-04-1807. [DOI] [PubMed] [Google Scholar]
- 15.Packer RJ, Holmes E, Zhou T, et al. Secondary malignant neoplasms (SMNS) following “successful” treatment of non-disseminated medulloblastoma (MB): A 10 year follow-up of patients treated on a COG study. Neuro-Oncology. 2010:ii8. [Google Scholar]
- 16.Packer RJ, Sutton LN, Elterman R, et al. Outcome for children with medulloblastoma treated with radiation and cisplatin, CCNU, and vincristine chemotherapy. J Neurosurg. 1994;81:690–698. doi: 10.3171/jns.1994.81.5.0690. [DOI] [PubMed] [Google Scholar]
- 17.Gajjar A, Chintagumpala M, Ashley D, et al. Risk-adapted craniospinal radiotherapy followed by high-dose chemotherapy and stem-cell rescue in children with newly diagnosed medulloblastoma (St Jude Medulloblastoma-96): Long-term results from a prospective, multicentre trial. Lancet Oncol. 2006;7:813–820. doi: 10.1016/S1470-2045(06)70867-1. [DOI] [PubMed] [Google Scholar]
- 18.Brandsma D, Stalpers L, Taal W, et al. Clinical features, mechanisms, and management of pseudoprogression in malignant gliomas. Lancet Oncol. 2008;9:453–461. doi: 10.1016/S1470-2045(08)70125-6. [DOI] [PubMed] [Google Scholar]
- 19.Brown HG, Kepner JL, Perlman EJ, et al. “Large cell/anaplastic” medulloblastomas: A Pediatric Oncology Group Study. J Neuropathol Exp Neurol. 2000;59:857–865. doi: 10.1093/jnen/59.10.857. [DOI] [PubMed] [Google Scholar]
- 20.Eberhart CG, Kepner JL, Goldthwaite PT, et al. Histopathologic grading of medulloblastomas: A Pediatric Oncology Group study. Cancer. 2002;94:552–560. doi: 10.1002/cncr.10189. [DOI] [PubMed] [Google Scholar]
- 21.Ellison DW, Kocak M, Dalton J, et al. Definition of disease-risk stratification groups in childhood medulloblastoma using combined clinical, pathologic, and molecular variables. J Clin Oncol. 2011;29:1400–1407. doi: 10.1200/JCO.2010.30.2810. [DOI] [PMC free article] [PubMed] [Google Scholar]


