Abstract
Dramatic improvements in the outcomes of patients with rectal cancer have occurred over the past 30 years. Advances in surgical pathology, refinements in surgical techniques and instrumentation, new imaging modalities, and the widespread use of neoadjuvant therapy have all contributed to these improvements. Several questions emerge as we learn of the benefits or lack thereof for components of the current multimodality treatment in subgroups of patients with nonmetastatic locally advanced rectal cancer (LARC). What is the optimal surgical technique for distal rectal cancers? Do all patients need postoperative chemotherapy? Do all patients need radiation? Do all patients need surgery, or is a nonoperative, organ-preserving approach warranted in selected patients? Answering these questions will lead to more precise treatment regimens, based on patient and tumor characteristics, that will improve outcomes while preserving quality of life. However, the idea of shifting the treatment paradigm (chemoradiotherapy, total mesorectal excision, and adjuvant therapy) currently applied to all patients with LARC to a more individually tailored approach is controversial. The paradigm shift toward organ preservation in highly selected patients whose tumors demonstrate clinical complete response to neoadjuvant treatment is also controversial. Herein, we highlight many of the advances and resultant controversies that are likely to dominate the research agenda for LARC in the modern era.
INTRODUCTION
In the past two decades, we have witnessed dramatic improvements in the outcomes of patients with rectal cancer. The rate of local recurrence (LR) has decreased, the probability of survival has increased, and quality of life has improved. Advances in surgical pathology, which have added to our understanding of the causes of locoregional recurrences, refinements in surgical techniques, new imaging modalities that help us select treatment and plan surgery, and the widespread use of neoadjuvant therapy have all contributed to these improvements.1 However, some of these advances have generated new controversies that are likely to dominate the research agenda in locally advanced rectal cancer (LARC) for years. In this article, we review some of the recent advances and controversies in the treatment of patients with nonmetastatic LARC.
EXTRALEVATOR ABDOMINOPERINEAL EXCISION
The introduction of the principles of total mesorectal excision (TME) using sharp dissection along the mesorectal fascia (MRF) has yielded high dividends in terms of decreased LR rates.2–6 TME is now considered the standard surgical technique in the treatment of most patients with rectal cancer. However, even when TME principles are applied, the LR rates are higher for tumors treated with an abdominoperineal excision (APE) compared with a sphincter-saving procedure (SSP).7 This disparity in outcome was attributed to differences in tumor biology and patterns of spread, but evidence from pathology audits of the surgical specimens suggests that inadequate surgery may also play a role.8,9
A LARC located in the vicinity of the anorectal ring likely involves the levator muscle or external anal sphincter. Extending the dissection along the MRF to the level of the anorectal ring separates the rectal wall from the levators and risks exposing the tumor. The surgical specimens resulting from this type of dissection typically have a “waist” where the tumor is closer to the circumferential resection margin (CRM; Figs 1A and 1B).9
Fig 1.
The anatomic considerations for extralevator abdominoperineal excision (ELAPE) are shown. (A-B) Blue arrows demonstrate a “waist” in the ELAPE specimen versus (C-D) a specimen with no “waist” (orange arrows). (D) A locally advanced rectal cancer specimen (patient underwent chemoradiotherapy) with an intact mesorectum (white arrow) and an acceptable ELAPE with no waist (orange arrow). (B) Adapted with permission.9
Pathologic and morphometric studies of APE specimens have found a smaller volume of tissue around the muscularis propria of the rectum and a higher rate of positive CRMs compared with specimens from SSPs.10 Patients treated with APE had higher LR rates (22.3% v 13.5%; P = .002) and lower 5-year cancer-specific survival (52.3% v 65.8%; P = .003) compared with patients treated with SSPs.10 The Dutch trial (Radiotherapy [RT] Plus Total Mesorectal Excision [TME] trial) pathology audit found higher positive CRMs (26.5% v 12.6%; P < .001) and more perforations (13.7% v 2.5%; P < .001) in specimens from patients with low rectal cancer (eg, ≤ 5 cm from the anal verge).8 Patients undergoing an APE had a higher risk of a positive CRM, independent of tumor height (30.4% v 10.7%; P = .002), as well as higher LR rates and lower rates of survival compared with patients who had SSPs.8 These studies indicate that differences in outcome between patients treated with APEs and SSPs could be attributed, at least in part, to inadequate surgical technique.
In recent years, there has been new emphasis on performing a more radical APE, in which the dissection along the MRF ends at the upper level of the levators, and the levator muscles are left in their natural position, attached to the distal rectum (Figs 1C and 1D). This procedure has been called cylindrical or extralevator APE (ELAPE), distinguishing it from the standard APE (SAPE) in which the levators are not removed with the specimen.9,11 The ELAPE procedure is not without controversy. The potential oncologic benefit of removing more tissue needs to be weighed against the increased morbidity potentially associated with a larger perineal defect, particularly in patients treated with neoadjuvant therapy.12 The results of a single-institution randomized trial13 and a recent meta-analysis of several case series indicate that ELAPE is associated with lower rates of intraoperative perforation, lower rates of positive CRM and LR, and similar complication rates compared with SAPE.14 ELAPE techniques were adopted earlier in Scandinavian and European countries, but their population-based tumor registry analyses have not demonstrated the benefit of ELAPE compared with SAPE.15,16 A prospective comparison of these techniques is unlikely, and therefore the controversy will persist. However, introduction of ELAPE principles has raised awareness about the importance of adequate preoperative imaging for surgical planning and precise surgical technique in patients with LARC who require APEs.
MINIMALLY INVASIVE TME
Laparoscopy has become the preferred approach to resection of colon cancer.17–20 Adoption of the laparoscopic approach to TME for rectal cancer has been slower because of the difficulty of working in the deep and narrow pelvic space using long, rigid, nonarticulated instruments. Three multi-institutional prospective randomized trials have compared open and laparoscopic TME for rectal cancer (Tables 1 and 2).21–23 A fourth prospective study, the American College of Surgeons Oncology Group ACOSOG-Z6051 (Laparoscopic-Assisted Resection or Open Resection in Treating Patients With Rectal Cancer),24 conducted in the United States has completed accrual but the results are pending. The combined experience of these trials indicates that laparoscopic TME results in longer operative time, less blood loss, faster bowel recovery, and shorter hospital stay compared with open TME. Operative mortality and intraoperative and postoperative complications were not different between groups. The proportion of patients having an SSP, a complete mesorectal excision, or a positive CRM was not different between groups, nor was the number of lymph nodes retrieved. However, conversion and positive CRM rates in the laparoscopic arms varied widely. These differences could be explained by trial design, inclusion criteria, randomization, and use of neoadjuvant therapy.19,21–23 The UK MRC-CLASICC trial (Conventional Surgery Compared With Laparoscopic-Assisted Surgery in Treating Patients With Colorectal Cancer)19 was conducted in the early years of the laparoscopic era, when many participating surgeons were still at the beginning of the learning curve, whereas the COREAN (Randomized Prospective Trial for Laparoscopic vs Open Resection for Rectal Cancer) trial22 was conducted recently by a limited number of surgeons with extensive laparoscopic experience. These data also suggest that with experience, some of the technical limitations of the laparoscopic approach can be overcome.
Table 1.
Multicentric, Prospective Randomized Trial Details
| Detail | Trial Name |
||
|---|---|---|---|
| CLASICC*† | COLOR II† | COREAN | |
| No. of patients | 38119, 21 | 1,10323 | 34022 |
| Recruitment | July 1996 to July 2002 | January 2004 to May 2010 | April 2006 to August 2009 |
| No. of participating institutions | 27 | 30 | 3 |
| Country | United Kingdom | 8 Countries | South Korea |
| Rectal segment, cm | All | 0-15 | 0-9 |
| Tumor stage | All | T1-3 (> 2 mm from MRF) | cT3N0-2 |
Abbreviations: CLASICC, Conventional Surgery Compared With Laparoscopic-Assisted Surgery in Treating Patients With Colorectal Cancer; COLOR II, Laparoscopic Versus Open Rectal Cancer Removal; COREAN, Randomized Prospective Trial for Laparoscopic vs Open Resection for Rectal Cancer; MRF, mesorectal fascia.
The CLASICC trial accrued a total of 794 patients (413 colon cancer; 381 rectal cancer).
Patients randomly assigned to open or laparoscopic surgery at a 1:2 ratio.
Table 2.
Multicentric, Prospective Randomized Trials Comparing Laparoscopic and Open TME for Rectal Adenocarcinoma
| Variable | Trial Name |
|||||
|---|---|---|---|---|---|---|
| CLASICC*† |
COLOR II† |
COREAN |
||||
| Laparascopic v Open | P | Laparascopic v Open | P | Laparascopic v Open | P | |
| Neoadjuvant RT and/or CRT | Stratified by RT | 58/59 | 100/100 | |||
| Operative time, minutes | 135/180‡ | 188/240 | < .001 | 197/224.9 | .001 | |
| Blood loss, mL | N.R. | 400/200 | < .001 | 217/200 | .006 | |
| Conversion rate, % | 34 | 17 | 1 | |||
| APE, % | 27/25 | N.S. | 23/29 | .120 | 14/11 | .708 |
| Complete TME, % | 67/79 | 92/88 | .250 | 88/92 | .55 | |
| Positive CRM, % | 14/16§ | .8 | 10/10 | .850 | 4/3 | .77 |
| No. of lymph nodes | 13.5/12 | 14/13 | .085 | 18/17 | .08 | |
| Time to first bowel movement, days | 6/5 | 3/2 | < .001 | 5.12/4.02 | < .001 | |
| Hospital stay, days | 13/11 | 9/8 | .036 | 9/8 | .056 | |
| 28-Day mortality, % | 5/4 | .57 | 2/1 | .41 | 0/0 | |
| Morbidity, % | 37/40 | 37/40 | .42 | 23.5/21.1 | .603 | |
| 3-Year oncologic outcomes | ||||||
| Local recurrence, % | 10.1/9.7 | .96 | N.A. | 4.9/2.6 | ||
| Distant metastasis, % | 16.4/18.6 | .68 | N.A. | 20/17 | ||
| DFS, % | N.A. | .87 | N.A. | 72.5/79.2 | ||
| OS, % | 67/73 | .12 | N.A. | 90.4/91.7 | ||
Abbreviations: APE, abdominoperineal excision; CLASICC, Conventional Surgery Compared With Laparoscopic-Assisted Surgery in Treating Patients With Colorectal Cancer; COLOR II, Laparoscopic Versus Open Rectal Cancer Removal; COREAN, Randomized Prospective Trial for Laparoscopic vs Open Resection for Rectal Cancer; CRM, circumferential resection margin; CRT, chemoradiotherapy; DFS, disease-free survival; N.A., not available; N.R., not reported; N.S., not significant; OS, overall survival; RT, radiotherapy; TME, total mesorectal excision.
The CLASICC trial accrued a total of 794 patients (413 colon cancer; 381 rectal cancer).
Patients randomly assigned to laparoscopic or open surgery at a 1:2 ratio.
Not discriminated colon and rectum.
Positive CRM in patients having sphincter-saving procedure was 12% for laparoscopic group and 6% for the open group.
At 3 years, LR, distant metastasis (DM), and survival in the CLASICC and COREAN trials were not different between groups.19,22 However, these results should be interpreted with caution, because the CLASICC trial was not specifically powered to detect differences between treatment groups in patients with rectal cancer, and the COREAN trial allowed a 15% difference as the noninferiority margin. Therefore, although these studies justify the use of laparoscopy in rectal cancer, it seems prudent to await final results of the larger COLOR II (COLOR II: Laparoscopic Versus Open Rectal Cancer Removal) and ACOSOG-Z6051 trials before endorsing laparoscopy as the preferred surgical approach to LARC.
The DaVinci platform25 was introduced to the surgical armamentarium to facilitate the minimally invasive approach for procedures such as prostatectomy and hysterectomy, which require optimal visualization and dexterity in the narrow pelvic space. The DaVinci platform is now also used to perform TME for LARC. The experience accumulated thus far, based on retrospective institutional case series, suggests that a TME performed with the DaVinci platform is equivalent to a laparoscopic TME in terms of completeness of the mesorectal excision, CRM positivity, and short-term oncologic outcomes.26–28 Conversion rates appear to be lower compared with laparoscopic TME, but hospital charges are higher. A prospective randomized study comparing laparoscopic and robotic TME29 was recently completed, but the results are pending. Therefore, because laparoscopy has not become standard for LARC, it is likely that the controversy regarding the benefit of robotic TME will continue for years.
MAGNETIC RESONANCE IMAGING
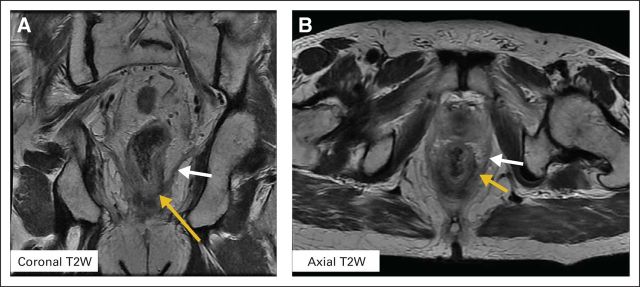
Magnetic resonance imaging (MRI) with a surface phased-array coil has become the preferred imaging modality for evaluating patients with LARC.30 T2-weighted MRI (T2W MRI) provides high tissue resolution and excellent anatomic depiction of the rectum, the mesorectum, the MRF, the levator muscles, and other pelvic structures relative to tumor location (Figs 2A and 2B). The addition of contrast and advanced functional sequences such as diffusion-weighted MRI (DW-MRI) and dynamic contrast-enhanced MRI (DCE-MRI) permit the quantification of tumor biologic processes such as microcirculation, vascular permeability, and tissue cellularity. These images are potentially useful for early assessment of rectal cancer response to neoadjuvant therapy.
Fig 2.
Representative T2-weighted (T2W) magnetic resonance images of a locally advanced rectal cancer tumor located at 1 cm above the dentate line. The yellow arrow indicates the tumor and the white arrow indicates its relationship to the levators. These images show high resolution and clear anatomic depictions of critical relationships involving the levator musculature, the tumor, and the mesorectum. (A) Coronal and (B) axial T2W magnetic resonance image.
Modern multidetector computed tomography scanners with their potential multiplanar reconstruction capabilities provide accurate images of the rectum, adjacent pelvic structures, and even the MRF; however, this imaging modality has lower resolution compared with MRI or endorectal ultrasound (ERUS).31 Therefore, multidetector computed tomography scanning is not considered an optimal modality for locoregional staging, but it is essential for excluding distant metastases. ERUS is the imaging modality that best depicts the different layers of the bowel wall, and it is especially useful for staging early rectal cancer.32 However, ERUS has a relatively short focal range and is not as accurate compared with MRI in assessing the relationship of LARC to important anatomic structures,33,34 in particular the MRF.
Tumor distance from the MRF—the CRM in TME surgery—has become one of the most important parameters in the preoperative evaluation of patients with rectal cancer. A recent systematic review and meta-analysis found that MRI had a sensitivity of 77% (95% CI, 57% to 90%) and specificity of 94% (95% CI, 88% to 97%) in predicting CRM involvement.35 The MERCURY trial (Magnetic Resonance Imaging and Rectal Cancer European Equivalence), a prospective observational study assessing the accuracy of MRI in predicting a curative resection in rectal cancer, reported 92% specificity in predicting a negative CRM.36 The 5-year follow-up report from that study noted a 67.2% disease-free survival (DFS) in patients with MRI-clear CRMs compared with 47.3% in patients with MRI-involved CRMs (hazard ratio, 1.65; 95% CI, 1.01 to 2.69).37 On multivariable analysis, MRI involvement of the CRM was the only preoperative staging parameter remaining significant for LR and survival.37 Other important tumor features reported by the MERCURY trial were extramural spread, extramural venous invasion, involvement of the peritoneal reflection, and distance of tumor from the levator muscle and sphincter complex. By using this information, the investigators were able to identify a group of patients with rectal cancer who had a good prognosis (eg, clear CRM, no evidence of extramural venous invasion, T2 or T3 < 5 mm and not involving the intersphincteric plane) with a 3% LR rate and an 85% 5-year DFS after treatment with surgery alone.38
There is uniform consensus about the value of MRI in the preoperative evaluation of patients with rectal cancer.30 The controversy lies in the use of the information provided by MRI images in different parts of the world. In the United States, decisions regarding the treatment of rectal cancer are based on clinical TNM staging.39,40 Patients with LARC (T3N0 or Tany, N1-2) are recommended to undergo combined-modality therapy with fluoropyrimidine and radiotherapy (RT), followed by TME, with consideration given to infusional fluorouracil, leucovorin, and oxaliplatin (FOLFOX) before chemoradiotherapy (CRT) as an acceptable option (Fig 3A). In Europe and Scandinavia, rectal cancers are stratified into different risk categories according to MRI features (Fig 3B). Patients within each risk category are recommended different treatment approaches.41–43 These MRI-based risk stratification schemas have been incorporated into clinical practice guidelines and clinical trial design (eg, the EXPERT-C trial [Randomized, Phase II European Multicenter Trial of Neoadjuvant Capecitabine Plus Oxaliplatin Chemotherapy (CAPOX) and Chemoradiation (CRT) With or Without Cetuximab Followed by Total Mesorectal Excision (TME) in Patients With MRI-Defined, High-Risk Rectal Cancer] and the RAPIDO trial [Rectal Cancer and Pre-Operative Induction Therapy Followed by Dedicated Operation: The RAPIDO trial]).44,45 However, the treatment approach guided by MRI risk categorization is based on results of prospective observational studies conducted in institutions with significant expertise in rectal cancer and has not been tested in prospective randomized trials.46
Fig 3.
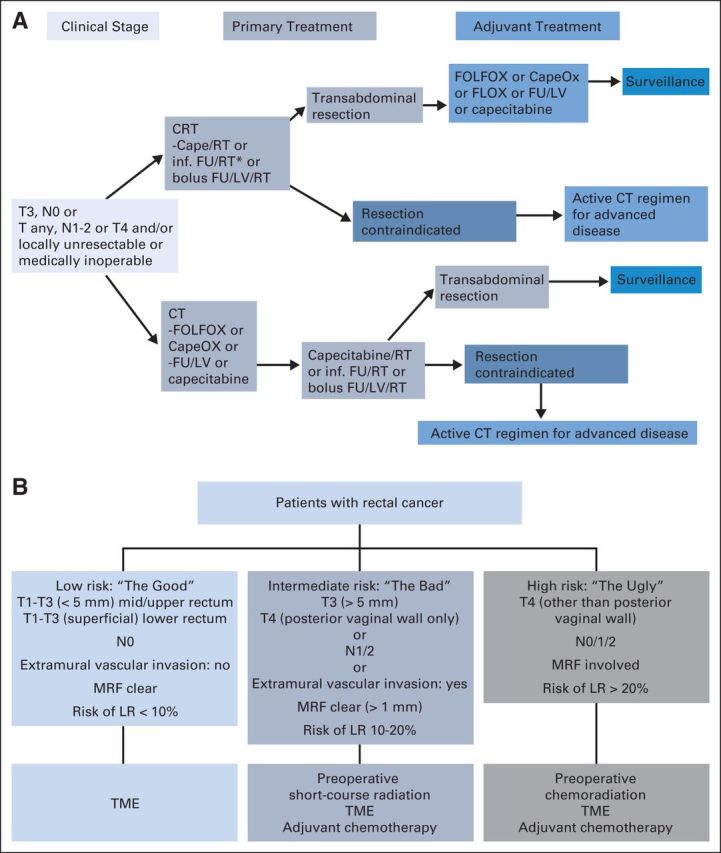
(A) The National Comprehensive Cancer Network guidelines, version 1.201540 for locally advanced rectal cancer. Adapted with permission.40 (B) The European/Scandinavian model of stratification for patients with locally advanced rectal cancer based on magnetic resonance imaging and then subsequent treatment decisions. Adapted with permission.42 Cape, capecitabine; CapeOx, capecitabine plus oxaliplatin; CRT, chemoradiotherapy; CT, chemotherapy; FLOX, fluorouracil, leucovorin, and oxaliplatin; FOLFOX, infusional fluorouracil, leucovorin, and oxaliplatin; FU, fluorouracil; inf., infusional; LR, local recurrence; LV, leucovorin; MRF, mesorectal fascia; RT, radiotherapy; TME, total mesorectal excision.
Most LARCs treated with neoadjuvant CRT demonstrate variable degrees of tumor response, including pathologic complete response (pCR) in up to 33% of patients.47,48 Whether tumor response justifies modifying the surgical approach is one of the most intriguing controversies in LARC today. Patients with tumors who have a CR to neoadjuvant therapy could potentially achieve excellent local tumor control and better quality of life with organ-sparing alternatives, such as local excision (LE) or even nonoperative management (NOM).49,50 However, without accurate restaging, surgeons have been reluctant to deviate from the initial surgical plan because of concerns about leaving viable cancer cells in the bowel wall or in the mesorectal nodes.51
Tumor necrosis in response to chemotherapy (CT) and/or RT leads to a local inflammatory reaction followed by degrees of fibrosis, challenging the detection of small remnants of viable tumor cells in the tumor bed and mesorectal lymph nodes. Clinical examination and conventional imaging can detect tumor shrinkage but cannot reliably identify patients with a pCR in the bowel wall or in the mesorectal lymph nodes.52–54
The value of MRI in restaging LARC after neoadjuvant therapy is controversial.55 Criteria for assessing tumor response in T2W MRI are based on the reduction of signal intensity relative to pretreatment images that occur when tumor is replaced by fibrosis. A T2W MRI–based tumor regression grade is associated with good tumor response and improved survival in patients with LARC treated with CRT.56 Other studies have reported that a T2W MRI–measured change in tumor volume greater than −70% after CRT is associated with tumor response.57,58 However, neither the visual assessment of morphologic features nor the objective measurements of tumor regression is a good predictor of pCR and therefore cannot be used reliably to modify treatment.
DCE-MRI depicts tumor microcirculation by measuring the pharmacokinetics of contrast agents. Semiquantitative perfusion parameters of DCE-MRI LARC imaging before and after neoadjuvant therapy have been correlated with tumor response,59 but results are conflicting.60 DW-MRI captures the dynamic cellular-level motion of water, providing images with higher signal-to-noise ratio compared with conventional morphologic sequences like T2W MRI. DW-MRI derives its image contrast from differences in the motion of water molecules in various tissues. Restriction of water molecule diffusion is considered a surrogate marker of tissue cellularity. Several studies note that DW-MRI improved the diagnostic performance in assessing rectal cancer response to CRT compared with T2W MRI.61–67 Although these results are promising, larger validation studies are necessary before functional MRI sequences can be incorporated into clinical practice and used to change surgical treatment of patients with LARC treated with neoadjuvant therapy.
NEOADJUVANT RT AND CRT
Preoperative short-course radiation therapy (SCRT; 5 Gy per day; total dose of 25 Gy) or conventionally fractionated radiation (1.8 to 2.0 Gy per day; total dose of 45 to 50 Gy) combined with sensitizing fluoropyrimidine-based CRT have been shown to improve local tumor control in patients with LARC treated with surgery, even when TME is performed.2,7,68–81 SCRT and surgery within 7 days have the potential benefits of shorter treatment duration, more efficient use of resources, and reduced cost compared with CRT and surgery within 6 to 8 weeks. However, higher dose per fraction increases delayed toxicity risk, and tumor regression is lower with SCRT.7,82 Two prospective randomized trials have compared SCRT with CRT.83,84 The Polish trial included patients with clinical T3-4 tumors within reach of the examining finger but not involving the anal sphincter.83 The study was powered to detect a difference in sphincter preservation of at least 15%. In all, 316 patients were accrued, of whom 312 were analyzable. The proportion of patients amenable to SSPs was similar in both groups (61% in the SCRT group v 58% in the CRT group). The pCR rate was lower in the SCRT group (1% v 16%), and the rate of CRM positivity was higher in the SCRT group (12.9% v 4.4%; P = .017), but LR, DM, overall survival (OS), and late toxicity were similar in both groups.83 The authors concluded that both regimens should be considered as alternatives in the treatment of rectal cancer; however, they favored SCRT on the basis of treatment compliance, safety profile, and cost. A more recent study from the Trans-Tasman Radiation Oncology Group randomly assigned 326 patients with ERUS- or MRI-staged T3N0-2M0 tumors located within 12 cm of the anal verge to SCRT and early surgery followed by 6 months of adjuvant CT or CRT (50.4 Gy of radiation; 1.8 Gy per fraction with continuous venous infusion of fluorouracil) and surgery in 4 to 6 weeks, followed by 4 months of adjuvant CT.84 All patients in the SCRT group received the full dose of radiation; in the CRT group, 93% received the full dose of radiation and 84% received CT within 10% of the prescribed dose. The cumulative LR rate was slightly higher in the SCRT group compared with the CRT group (7.5% v 4.4%; P = .24). Treatment groups were well balanced for most patient and tumor characteristics, but more patients in the SCRT group had distal tumors (< 5 cm from the anal verge) compared with the CRT group (30% v 19%), which may account for the higher LR rates in the SCRT group.84 The authors concluded that CRT may be more effective than SCRT in reducing the LR risk; this has been challenged, however, on the basis of an imbalance between patient groups.85,86 In summary, these studies have not solved the controversy about SCRT effectiveness compared with CRT, particularly for distal LARC.
Substitution of the oral prodrug capecitabine (converted to fluorouracil by intracellular thymidine phosphorylase) for continuous venous infusion (CVI) fluorouracil is attractive because it is convenient for patients.87 In a European trial, capecitabine was not inferior to CVI fluorouracil with respect to 5-year OS and was even superior to fluorouracil in 3-year DFS.88 In the National Surgical Adjuvant Breast and Bowel Project R04 (Radiation Therapy and Either Capecitabine or Fluorouracil With or Without Oxaliplatin, Before Surgery in Treating Patients With Resectable Rectal Cancer) trial, clinical stage II to III patients undergoing preoperative RT were randomly assigned to CVI fluorouracil with or without oxaliplatin, or capecitabine with or without oxaliplatin as radiosensitizers. In the 1,608 patients evaluated, grade 3 or 4 diarrhea was more frequent with use of oxaliplatin, but the surgical end points of pCR, SSPs, or conversion to SSPs were similar in all four groups.89 Although long-term oncologic outcomes are not yet available for that study, the data support the noninferiority of capecitabine versus CVI fluorouracil as a radiosensitizer for patients with LARC.88,89
The STAR-01 trial (Preoperative Fluorouracil [FU] -Based Chemoradiation With and Without Weekly Oxaliplatin in Locally Advanced Rectal Cancer),90 the ACCORD 12/0405-Prodige2 trial (Radiation Therapy and Capecitabine With or Without Oxaliplatin in Treating Patients Who Are Undergoing Surgery for Stage II or Stage III Rectal Cancer),91 and the aforementioned NSABP-R-04 trial89investigated adding oxaliplatin to a fluoropyrimidine as a radiosensitizer. They reported that addition of oxaliplatin once per week to various fluoropyrimidine-based CRT regimens in patients with LARC resulted in higher toxicity and worse therapeutic ratio. Conversely, the CAO/ARO/AIO-04 (Neoadjuvant Chemoradiotherapy and Adjuvant Chemotherapy With 5-Fluorouracil and Oxaliplatin Versus 5-Fluorouracil Alone in Rectal Cancer)92 trial found that the inclusion of oxaliplatin in a fluorouracil-based CRT regimen led to a higher pCR rate, with no increase in toxicity. However, the results of the CAO/ARO/AIO-04 trial should be interpreted with caution because the fluorouracil dosage and schedule were different in the control and oxaliplatin arms, which may have contributed to the different outcomes. These trials do not support adding oxaliplatin to fluoropyrimidines as a radiosensitizer in LARC.
It has been proposed that targeted therapy be incorporated into the multimodality treatment of patients with LARC. Both anti-vascular endothelial growth factor and anti-endothelial growth factor receptor agents have been tested in phase II trials as radiosensitizers; unfortunately, these studies have fallen short in meeting their primary end points (eg, pCR or progression-free survival [PFS]) and have not made it to phase III evaluation.44,93,94 Targeted therapies currently play no role in the neoadjuvant treatment of patients with potentially resectable LARC outside of clinical trials.
SYSTEMIC CT IN RELATION TO CRT AND TME
In the past, LR was the dominant problem in patients with LARC, but in the modern era, more patients develop distant relapse than LR. Consequently, patients with LARC treated with neoadjuvant RT and/or CT and TME are recommended postoperative adjuvant CT independent of the chemotherapeutic response.39 A meta-analysis of 21 randomized controlled trials concluded that postoperative fluorouracil-based CT is effective in patients with LARC.95 Therefore, the current recommendations for use of adjuvant CT in patients with LARC treated with CRT and TME is based in part on these data and extrapolation from colon cancer data.95–97 Similar to patients with colon cancer, patients with LARC treated with CRT and TME usually receive fluorouracil or capecitabine plus oxaliplatin-based adjuvant CT.39 Despite these recommendations, up to 27% of eligible patients with LARC never start adjuvant CT, and less than 50%98 receive the full prescribed treatment without interruptions or delays78,92 resulting from postoperative complications, slow recovery, interference with closure of their temporary ileostomy,99 or simply refusal of treatment.100 A systematic review of 10 studies that included more than 15,000 patients evaluated the effect of timing on the efficacy of postoperative adjuvant CT and demonstrated that each 4-week delay in treatment correlated with a 14% decrease in OS.101
Therefore, splitting adjuvant CT and delivering a limited number of cycles pre-CRT and then delivering the remaining cycles postsurgery has been proposed to increase tumor response in patients with LARC.102–106 An alternative is to deliver all of systemic CT before CRT and surgery. This neoadjuvant CT (NACT) has several potential advantages compared with the standard adjuvant CT. It treats occult micrometastases several months earlier and increases treatment compliance,107 potentially enhancing the efficacy of CT in preventing DMs and ultimately improving survival. Other benefits of NACT include increased response of the primary tumor, early identification of nonresponders, and earlier removal of the loop ileostomy.
A recent study at Memorial Sloan Kettering Cancer Center (MSKCC) investigated the safety and efficacy of FOLFOX before CRT, demonstrating excellent treatment compliance and no evidence of serious adverse effects requiring treatment delay.108 All patients undergoing TME had an R0 resection, and nearly half had a tumor response greater than 90%, including 30% who had either a pCR or a clinical complete response (cCR). CT can also be delivered as consolidation CT (after CRT completion and before surgery). The TIMING trial (NCT00335816; Timing of Rectal Cancer Response to Chemoradiotherapy Trial), which completed accrual in 2012, showed that delivering two, four, or six cycles of FOLFOX after CRT in patients with LARC increased the pCR rates up to 25%, 30%, and 38%, respectively, compared with CRT alone (18%), without any associated increase in adverse events or surgical complications.109 Eighty percent of patients received consolidation CT without interruption.109,110 These studies suggest that delivering systemic CT in the neoadjuvant setting, either before or after CRT, is well tolerated and has potential advantages for the patient. Although solid data from large prospective studies is still lacking, in the most recent edition of the National Comprehensive Cancer Network guidelines, NACT is contemplated as an option for the treatment of patients with LARC (Fig 3A).40
SHIFTING PARADIGMS: “LESS MAY BE MORE”
The results of the aforementioned clinical trials conducted over the last three decades have crystallized in the development of a multimodality treatment for patients with LARC that includes preoperative CRT, TME, and postoperative adjuvant CT.39 This approach has improved outcomes to the point that survival is now better for patients who have rectal cancer than for patients who have colon cancer.111 But this success has been achieved at the cost of significant morbidity and reduced quality of life. The challenge now is to identify treatment approaches that could maintain or even improve the oncologic outcomes while preserving quality of life.50
In the process of implementing an intense multimodality approach to LARC, we have identified patient subgroups with different tumor characteristics that have been associated with different risks of recurrence and survival probability. This risk stratification leads us to question whether all patients with LARC require all components of an intense multimodality approach.
Many patients with LARC experience various degrees of response to CRT, and tumor response is now one of the most important prognosticators in patients with LARC.112,113 The need for adjuvant CT in patients with a complete or near-complete response after CRT has been questioned.114,115 Recent work from a multi-institutional retrospective analysis of 3,133 patients shows that the benefit of adjuvant therapy differs between LARC subgroups. For example, patients with ypT1-2 or ypT3-4 tumors benefitted the most from adjuvant therapy compared with patients who had ypT0N0 tumors.116 Some centers now use postoperative CT selectively on the basis of tumor response to CRT. In the recently published phase II ADORE trial (Adjuvant Oxaliplatin in Rectal Cancer), which examined use of selective adjuvant CT, patients with LARC who had ypT3-4N0 or ypTanyN1-2 tumors after fluoropyrimidine-based CRT were randomly assigned to adjuvant CT with either four cycles of fluorouracil and leucovorin or eight cycles of FOLFOX. The administration of FOLFOX after surgery was associated with prolonged PFS in stage III patients but not in stage II patients. In addition, FOLFOX was associated with prolonged OS for stage II and stage III patients with rectal cancer.117 Identification of those patients most likely to derive benefit from adjuvant treatment will be better informed by carefully conducted correlative studies that more accurately delineate molecular, pathologic, and clinical markers of resistance.
The risk of LR in LARC depends on tumor stage and also on the distance of the tumor from the anal verge and the proximity of the tumor to the MRF.7,38 Upper rectal tumors away from the MRF have a low risk of LR when treated with TME. The added benefit of RT in these patients has been questioned because RT is associated with significant toxicity, including bowel obstruction, hip fractures, sexual and urinary dysfunction, and proctitis.82,118,119 A growing body of evidence suggests that RT could be safely avoided in patients with intermediate-risk rectal cancer (eg, rectal cancers located between 5 and 12 cm from the anal verge that do not threaten the MRF) on MRI.120,121 In a pilot phase II trial conducted at MSKCC, 32 patients with resectable, clinical stage II to III rectal cancer were treated with preoperative FOLFOX and/or anti- vascular endothelial growth factor and selective CRT on the basis of tumor response.122 The 30 patients who completed preoperative CT had tumor regression and underwent TME without preoperative CRT. No local recurrences were noted at 4 years, and an 84% DFS was achieved.122 These data were used as proof of concept for the design of the phase II/III PROSPECT trial (Chemotherapy Alone or Chemotherapy Plus Radiation Therapy in Treating Patients With Locally Advanced Rectal Cancer Undergoing Surgery), which is now accruing worldwide.123 The overarching goal of this randomized trial is to determine whether pelvic RT can be used selectively in patients with LARC.121 The trial uses selective rather than reflexive CRT to individualize treatment based on patient response to neoadjuvant FOLFOX (the study schema is shown in Fig 4A). The hope is that a trial that tailors therapy more precisely, based on clinical subgroups and tumor response to treatment, will help eliminate the over- or undertreatment noted in previous trials.120
Fig 4.
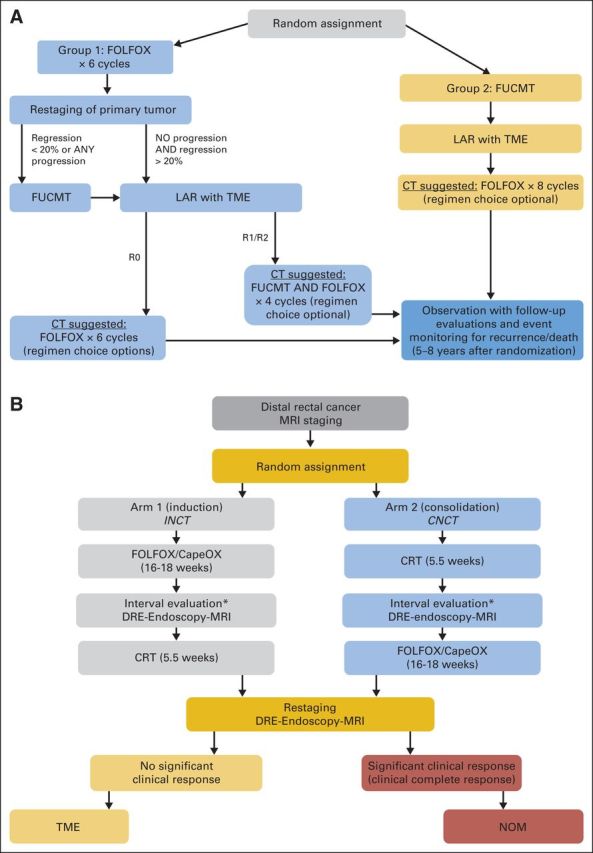
(A) The PROSPECT trial (Chemotherapy Alone or Chemotherapy Plus Radiation Therapy in Treating Patients With Locally Advanced Rectal Cancer Undergoing Surgery) study schema is shown.121,123 This is a phase II/III randomized trial to evaluate the impact of selective use of radiotherapy compared with nonselective use of chemoradiotherapy (CRT) for all patients with locally advanced rectal cancer. Cycle length, 14 days; fluorouracil or capecitabine plus radiotherapy (FUCMT) duration, 5.5 weeks. Regression is estimated by tumor imaging and clinical tumor response on endoscopy. If there is evidence of progressive disease at restaging of the primary tumor after six cycles of infusional fluorouracil, leucovorin, and oxaliplatin (FOLFOX), group 1 patients would proceed to FUCMT instead of event monitoring. (B) Memorial Sloan Kettering Cancer Center phase II trial schema that is underway to test the feasibility of incorporating a nonoperative management (NOM) approach to the multimodality treatment of rectal cancer in a multi-institutional setting. This study will evaluate 3-year disease-free survival in patients with locally advanced rectal cancer treated with chemotherapy (CT) plus induction (INCT) or chemotherapy plus consolidation (CNCT) and total mesorectal excision (TME) or NOM.124 CapeOx, capecitabine plus oxaliplatin; DRE, digital rectal examination; LAR, low anterior resection; MRI, magnetic resonance imaging. (*) Patients with tumor progression at the interval evaluation will be treated according to standard of care.
TME is the cornerstone of the treatment algorithm for patients with LARC. However, up to 33% of patients with LARC treated with neoadjuvant CRT exhibit a pCR at the time of surgical resection.47,48 Patients with a pCR have improved oncologic outcomes, with LR rates of less than 1% and a 5-year survival rate of more than 95%,112,113 leading us to question the added benefit of TME for these patients. The potential gains of avoiding TME—reduced morbidity, improvement in quality of life, and potential reduction of health care expenses—could be significant. The current challenge lies in accurately identifying which patients have achieved a pCR and could safely avoid TME.52 Although cCR does not always correlate with pCR, and current imaging modalities cannot distinguish tumor remnants from tissue fibrosis with certainty,52–54 several institutions have reported their experience with the selective use of an organ-preserving or NOM approach in patients with a cCR after CRT.125–129 The largest experience with the NOM approach to rectal cancer comes from Habr-Gama's group in Sao Paulo, Brazil.125–128 Patients with persistent tumor underwent TME; those with a cCR underwent monthly clinical evaluations. Patients with evidence of tumor relapse were directed to surgery, whereas patients with a sustained cCR after 1 year continued surveillance every 3 months for an additional year and every 6 months thereafter. Twenty-seven percent of patients with rectal cancer treated according to this protocol had a sustained cCR and were spared TME. LR during follow-up developed in 10% of patients entered in the NOM protocol, but all had curative TME. The oncologic results in this NOM group were equivalent to those of patients who had a pCR after TME. A group from Maastricht University in the Netherlands reported their NOM experience in 21 patients with cCR as determined on clinical examination, MRI, and endoscopic biopsy, among 192 patients treated with CRT between 2004 and 2010.128 After a mean follow-up of 25 ± 19 months, one patient developed LR but was able to undergo curative salvage surgery. The other 20 patients are alive without disease. Outcomes in patients with cCR treated according to the NOM protocol were similar to outcomes of patients with a pCR after TME. At MSKCC, patients with rectal cancer with a cCR have been managed under a NOM strategy since 2006. Of the 32 patients starting treatment before 2010 who were followed for a median of 23 months, six patients (21%) developed relapse, and all underwent curative salvage surgery; three of these patients also developed DMs.129 The combined experience of these series suggests that NOM may be an alternative approach to TME in highly selected patients with distal LARC who achieve a cCR to neoadjuvant therapy (Table 3). However, the safety and efficacy of the NOM approach outside of centers specializing in the treatment of rectal cancer is controversial. It is now clear that even with strict cCR definitions, some patients will later develop LR, emphasizing the importance of close surveillance because the success of this approach relies on the early diagnosis of recurrences and timely salvage therapy. In addition, the risk of DM in patients with an apparent cCR who develop local tumor regrowth, along with subsequent outcomes, is unknown.
Table 3.
Summary of Outcomes With Nonoperative Versus Operative Management of LARC After CRT in High-Volume Centers
| Reference | No. of cCRs | % | Mean Interval to LR (months) | No. of Patients | OS |
DFS |
||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| NOM |
Operative Arm |
NOM |
Operative Arm |
|||||||||
| Survival | % | Survival | % | Survival | % | Survival | % | |||||
| Habr-Gama et al125 | 71 | 27 | 60 | 2 | 5-year | 100 | 5-year | 88 | 5-year | 92 | 5-year | 83 |
| Habr-Gama et al126 | 90 | 49 | 17 | 28 | 5-year | 91 | N.A. | 5-year | 68 | N.A. | ||
| Maas et al127 | 21 | 11 | 22 | 1 | 2-year | 100 | 2-year | 91 | 2-year | 89 | 2-year | 93 |
| Smith et al128 | 32 | N.A. | 11 | 6 | 2-year | 97 | 2-year | 88 | 2-year | 100 | 2-year | 98 |
Abbreviations: cCR, clinical complete response; CRT, chemoradiotherapy; DFS, disease-free survival; LARC, locally advanced rectal cancer; LR, local recurrence; N.A., not available; NOM, nonoperative management; OS, overall survival; RT, radiotherapy;
LE with or without RT has been used for patients with stage I rectal cancer.130–132 In recent years, LE has been used as an alternative to TME in patients with LARC who were downstaged by CRT to avoid the sequelae of removing the rectum. Perez et al133 have reported a 15% LR rate after LE at 15 months in 27 patients with clinical stage II or III disease who experienced a partial clinical response after CRT. Because the median time to LR in patients with LARC treated by TME after CRT is 3 years, and because almost one third of recurrences are diagnosed after 5 years,134 it can be expected that the recurrence rate will continue to increase as follow-up matures. In addition, recent work by Park et al48 noted that patients with an intermediate (ypT1-2N0) or poor (ypT3-4 or N+) response experienced significantly worse 5-year PFS, LR, and distant recurrence compared with patients with a pCR, suggesting more aggressive biologic behavior. These data indicate that LE may be insufficient surgical treatment in patients with LARC who have a partial response to CRT. Conversely, LE may be unnecessary in patients with a cCR because it can interfere with appropriate follow-up studies, and with a proper TME option should the tumor regrow. These observations indicate that there is not a safe role for LE after CRT in patients with LARC with a partial or cCR to CRT.135
The design of large, prospective randomized trials investigating the efficacy of the NOM approach in selected patients is challenging, given the relatively small proportion of patients with a cCR to standard neoadjuvant CRT and the disparity of the treatment arms—observation versus TME. However, several prospective observational studies136–138 and phase II trials, including our own (Fig 4B) are underway to test the feasibility of incorporating a NOM approach into the multimodality treatment of rectal cancer in a multi-institutional setting.124,139
SUMMARY
Decades of clinical research have resulted in a multimodality treatment paradigm for patients with LARC that provides unprecedented local tumor control and patient survival. This intense multimodality treatment is associated with significant morbidity and long-term sequelae that permanently impair quality of life. Identifying patients at different risk levels for tumor recurrence and survival based on baseline tumor characteristics and tumor response to therapy makes it possible to customize this multimodality approach to individual patients. This patient-specific approach, along with efforts aimed at early diagnosis, should further improve surgical outcomes while preserving quality of life. Finding predictors of tumor response and ways to identify response early in the treatment course should help improve the treatment of patients with LARC.
Acknowledgment
We thank Jenifer Levin for her editorial review of the manuscript.
Footnotes
Supported by Grant No. 1R01CA182551-01 from the National Cancer Institute.
Authors' disclosures of potential conflicts of interest are found in the article online at www.jco.org. Author contributions are found at the end of this article.
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Disclosures provided by the authors are available with this article at www.jco.org.
AUTHOR CONTRIBUTIONS
Manuscript writing: All authors
Final approval of manuscript: All authors
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Advances and Challenges in Treatment of Locally Advanced Rectal Cancer
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to www.asco.org/rwc or jco.ascopubs.org/site/ifc.
J. Joshua Smith
No relationship to disclose
Julio Garcia-Aguilar
No relationship to disclose
REFERENCES
- 1.Minsky BD. Chemoradiation for rectal cancer: Rationale, approaches, and controversies. Surg Oncol Clin N Am. 2010;19:803–818. doi: 10.1016/j.soc.2010.06.001. [DOI] [PubMed] [Google Scholar]
- 2.Heald RJ, Ryall RD. Recurrence and survival after total mesorectal excision for rectal cancer. Lancet. 1986;1:1479–1482. doi: 10.1016/s0140-6736(86)91510-2. [DOI] [PubMed] [Google Scholar]
- 3.Arbman G, Nilsson E, Hallböök O, et al. Local recurrence following total mesorectal excision for rectal cancer. Br J Surg. 1996;83:375–379. doi: 10.1002/bjs.1800830326. [DOI] [PubMed] [Google Scholar]
- 4.Wibe A, Møller B, Norstein J, et al. A national strategic change in treatment policy for rectal cancer: Implementation of total mesorectal excision as routine treatment in Norway—A national audit. Dis Colon Rectum. 2002;45:857–866. doi: 10.1007/s10350-004-6317-7. [DOI] [PubMed] [Google Scholar]
- 5.Kapiteijn E, Putter H, van de Velde CJ. Impact of the introduction and training of total mesorectal excision on recurrence and survival in rectal cancer in The Netherlands. Br J Surg. 2002;89:1142–1149. doi: 10.1046/j.1365-2168.2002.02196.x. [DOI] [PubMed] [Google Scholar]
- 6.Martling A, Holm T, Rutqvist LE, et al. Impact of a surgical training programme on rectal cancer outcomes in Stockholm. Br J Surg. 2005;92:225–229. doi: 10.1002/bjs.4834. [DOI] [PubMed] [Google Scholar]
- 7.Kapiteijn E, Marijnen CA, Nagtegaal ID, et al. Preoperative radiotherapy combined with total mesorectal excision for resectable rectal cancer. N Engl J Med. 2001;345:638–646. doi: 10.1056/NEJMoa010580. [DOI] [PubMed] [Google Scholar]
- 8.Nagtegaal ID, van de Velde CJ, Marijnen CA, et al. Low rectal cancer: A call for a change of approach in abdominoperineal resection. J Clin Oncol. 2005;23:9257–9264. doi: 10.1200/JCO.2005.02.9231. [DOI] [PubMed] [Google Scholar]
- 9.West NP, Finan PJ, Anderin C, et al. Evidence of the oncologic superiority of cylindrical abdominoperineal excision for low rectal cancer. J Clin Oncol. 2008;26:3517–3522. doi: 10.1200/JCO.2007.14.5961. [DOI] [PubMed] [Google Scholar]
- 10.Marr R, Birbeck K, Garvican J, et al. The modern abdominoperineal excision: The next challenge after total mesorectal excision. Ann Surg. 2005;242:74–82. doi: 10.1097/01.sla.0000167926.60908.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.West NP, Anderin C, Smith KJ, et al. Multicentre experience with extralevator abdominoperineal excision for low rectal cancer. Br J Surg. 2010;97:588–599. doi: 10.1002/bjs.6916. [DOI] [PubMed] [Google Scholar]
- 12.Musters GD, Buskens CJ, Bemelman WA, et al. Perineal wound healing after abdominoperineal resection for rectal cancer: A systematic review and meta-analysis. Dis Colon Rectum. 2014;57:1129–1139. doi: 10.1097/DCR.0000000000000182. [DOI] [PubMed] [Google Scholar]
- 13.Han JG, Wang ZJ, Wei GH, et al. Randomized clinical trial of conventional versus cylindrical abdominoperineal resection for locally advanced lower rectal cancer. Am J Surg. 2012;204:274–282. doi: 10.1016/j.amjsurg.2012.05.001. [DOI] [PubMed] [Google Scholar]
- 14.Yu HC, Peng H, He XS, et al. Comparison of short- and long-term outcomes after extralevator abdominoperineal excision and standard abdominoperineal excision for rectal cancer: A systematic review and meta-analysis. Int J Colorectal Dis. 2014;29:183–191. doi: 10.1007/s00384-013-1793-7. [DOI] [PubMed] [Google Scholar]
- 15.Prytz M, Angenete E, Ekelund J, et al. Extralevator abdominoperineal excision (ELAPE) for rectal cancer: Short-term results from the Swedish Colorectal Cancer Registry—Selective use of ELAPE warranted. Int J Colorectal Dis. 2014;29:981–987. doi: 10.1007/s00384-014-1932-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Klein M, Fischer A, Rosenberg J, et al. Extralevatory abdominoperineal excision (ELAPE) does not result in reduced rate of tumor perforation or rate of positive circumferential resection margin: A nationwide database study. Ann Surg. doi: 10.1097/SLA.0000000000000910. [epub ahead of print on September 10, 2014] [DOI] [PubMed] [Google Scholar]
- 17.Lacy AM, García-Valdecasas JC, Delgado S, et al. Laparoscopy-assisted colectomy versus open colectomy for treatment of non-metastatic colon cancer: A randomised trial. Lancet. 2002;359:2224–2229. doi: 10.1016/S0140-6736(02)09290-5. [DOI] [PubMed] [Google Scholar]
- 18.Clinical Outcomes of Surgical Therapy Study Group. A comparison of laparoscopically assisted and open colectomy for colon cancer. N Engl J Med. 2004;350:2050–2059. doi: 10.1056/NEJMoa032651. [DOI] [PubMed] [Google Scholar]
- 19.Jayne DG, Guillou PJ, Thorpe H, et al. Randomized trial of laparoscopic-assisted resection of colorectal carcinoma: 3-year results of the UK MRC CLASICC Trial Group. J Clin Oncol. 2007;25:3061–3068. doi: 10.1200/JCO.2006.09.7758. [DOI] [PubMed] [Google Scholar]
- 20.Colon Cancer Laparoscopic or Open Resection Study Group. Buunen M, Veldkamp R, et al. Survival after laparoscopic surgery versus open surgery for colon cancer: Long-term outcome of a randomised clinical trial. Lancet Oncol. 2009;10:44–52. doi: 10.1016/S1470-2045(08)70310-3. [DOI] [PubMed] [Google Scholar]
- 21.Jayne DG, Thorpe HC, Copeland J, et al. Five-year follow-up of the Medical Research Council CLASICC trial of laparoscopically assisted versus open surgery for colorectal cancer. Br J Surg. 2010;97:1638–1645. doi: 10.1002/bjs.7160. [DOI] [PubMed] [Google Scholar]
- 22.Kang SB, Park JW, Jeong SY, et al. Open versus laparoscopic surgery for mid or low rectal cancer after neoadjuvant chemoradiotherapy (COREAN trial): Short-term outcomes of an open-label randomised controlled trial. Lancet Oncol. 2010;11:637–645. doi: 10.1016/S1470-2045(10)70131-5. [DOI] [PubMed] [Google Scholar]
- 23.Van der Pas MH, Haglind E, Cuesta MA, et al. Laparoscopic versus open surgery for rectal cancer (COLOR II): Short-term outcomes of a randomised, phase 3 trial. Lancet Oncol. 2013;14:210–218. doi: 10.1016/S1470-2045(13)70016-0. [DOI] [PubMed] [Google Scholar]
- 24.Alliance for Clinical Trials in Oncology. Laparoscopic-Assisted Resection or Open Resection in Treating Patients With Rectal Cancer. American College of Surgeons Oncology Group (ACOSOG) Z6051. https://clinicaltrials.gov/ct2/show/NCT00726622. [Google Scholar]
- 25.Intuitive Surgical. Home page. www.intuitivesurgical.com. [Google Scholar]
- 26.Baek JH, McKenzie S, Garcia-Aguilar J, et al. Oncologic outcomes of robotic-assisted total mesorectal excision for the treatment of rectal cancer. Ann Surg. 2010;251:882–886. doi: 10.1097/SLA.0b013e3181c79114. [DOI] [PubMed] [Google Scholar]
- 27.Pigazzi A, Luca F, Patriti A, et al. Multicentric study on robotic tumor-specific mesorectal excision for the treatment of rectal cancer. Ann Surg Oncol. 2010;17:1614–1620. doi: 10.1245/s10434-010-0909-3. [DOI] [PubMed] [Google Scholar]
- 28.Memon S, Heriot AG, Murphy DG, et al. Robotic versus laparoscopic proctectomy for rectal cancer: A meta-analysis. Ann Surg Oncol. 2012;19:2095–2101. doi: 10.1245/s10434-012-2270-1. [DOI] [PubMed] [Google Scholar]
- 29.Pigazzi A. RObotic Versus LAparoscopic Resection for Rectal Cancer (ROLARR) https://clinicaltrials.gov/ct2/show/NCT01736072. [Google Scholar]
- 30.Costa-Silva L, Brown G. Magnetic resonance imaging of rectal cancer. Magn Reson Imaging Clin N Am. 2013;21:385–408. doi: 10.1016/j.mric.2013.01.006. [DOI] [PubMed] [Google Scholar]
- 31.Kwok H, Bissett IP, Hill GL. Preoperative staging of rectal cancer. Int J Colorectal Dis. 2000;15:9–20. doi: 10.1007/s003840050002. [DOI] [PubMed] [Google Scholar]
- 32.Bipat S, Glas AS, Slors FJ, et al. Rectal cancer: Local staging and assessment of lymph node involvement with endoluminal US, CT, and MR imaging[em]A meta-analysis. Radiology. 2004;232:773–783. doi: 10.1148/radiol.2323031368. [DOI] [PubMed] [Google Scholar]
- 33.Brown G, Davies S, Williams GT, et al. Effectiveness of preoperative staging in rectal cancer: Digital rectal examination, endoluminal ultrasound or magnetic resonance imaging? Br J Cancer. 2004;91:23–29. doi: 10.1038/sj.bjc.6601871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Beets-Tan RG, Beets GL. Local staging of rectal cancer: A review of imaging. J Magn Reson Imaging. 2011;33:1012–1019. doi: 10.1002/jmri.22475. [DOI] [PubMed] [Google Scholar]
- 35.Al-Sukhni E, Milot L, Fruitman M, et al. Diagnostic accuracy of MRI for assessment of T category, lymph node metastases, and circumferential resection margin involvement in patients with rectal cancer: A systematic review and meta-analysis. Ann Surg Oncol. 2012;19:2212–2223. doi: 10.1245/s10434-011-2210-5. [DOI] [PubMed] [Google Scholar]
- 36.MERCURY Study Group. Diagnostic accuracy of preoperative magnetic resonance imaging in predicting curative resection of rectal cancer: Prospective observational study. BMJ. 2006;333:779. doi: 10.1136/bmj.38937.646400.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Taylor FG, Quirke P, Heald RJ, et al. Preoperative magnetic resonance imaging assessment of circumferential resection margin predicts disease-free survival and local recurrence: 5-year follow-up results of the MERCURY study. J Clin Oncol. 2014;32:34–43. doi: 10.1200/JCO.2012.45.3258. [DOI] [PubMed] [Google Scholar]
- 38.Taylor FG, Quirke P, Heald RJ, et al. Preoperative high-resolution magnetic resonance imaging can identify good prognosis stage I, II, and III rectal cancer best managed by surgery alone: A prospective, multicenter, European study. Ann Surg. 2011;253:711–719. doi: 10.1097/SLA.0b013e31820b8d52. [DOI] [PubMed] [Google Scholar]
- 39.Benson AB, 3rd, Bekaii-Saab T, Chan E, et al. Rectal cancer. J Natl Compr Canc Netw. 2012;10:1528–1564. doi: 10.6004/jnccn.2012.0158. [DOI] [PubMed] [Google Scholar]
- 40.Benson AB, Venook AP, Saltz L. NCCN Guidelines version 2.2015 (rectal cancer), 2015. www.nccn.org. [Google Scholar]
- 41.Smith N, Brown G. Preoperative staging of rectal cancer. Acta Oncol. 2008;47:20–31. doi: 10.1080/02841860701697720. [DOI] [PubMed] [Google Scholar]
- 42.Blomqvist L, Glimelius B. The ‘good’, the ‘bad’, and the ‘ugly’ rectal cancers. Acta Oncol. 2008;47:5–8. doi: 10.1080/02841860701802585. [DOI] [PubMed] [Google Scholar]
- 43.Glynne-Jones R, Tan D, Goh V. Pelvic MRI for guiding treatment decisions in rectal cancer. Oncology (Williston Park) 2014;28:667–677. [PubMed] [Google Scholar]
- 44.Dewdney A, Cunningham D, Tabernero J, et al. Multicenter randomized phase II clinical trial comparing neoadjuvant oxaliplatin, capecitabine, and preoperative radiotherapy with or without cetuximab followed by total mesorectal excision in patients with high-risk rectal cancer (EXPERT-C) J Clin Oncol. 2012;30:1620–1627. doi: 10.1200/JCO.2011.39.6036. [DOI] [PubMed] [Google Scholar]
- 45.Nilsson PJ, van Etten B, Hospers GA, et al. Short-course radiotherapy followed by neo-adjuvant chemotherapy in locally advanced rectal cancer: The RAPIDO trial. BMC Cancer. 2013;13:279. doi: 10.1186/1471-2407-13-279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sautter-Bihl ML, Hohenberger W, Fietkau R, et al. MRI-based treatment of rectal cancer: Is prognostication of the recurrence risk solid enough to render radiation redundant? Ann Surg Oncol. 2014;21:197–204. doi: 10.1245/s10434-013-3236-7. [DOI] [PubMed] [Google Scholar]
- 47.Francois Y, Nemoz CJ, Baulieux J, et al. Influence of the interval between preoperative radiation therapy and surgery on downstaging and on the rate of sphincter-sparing surgery for rectal cancer: The Lyon R90-01 randomized trial. J Clin Oncol. 1999;17:2396. doi: 10.1200/JCO.1999.17.8.2396. [DOI] [PubMed] [Google Scholar]
- 48.Park IJ, You YN, Agarwal A, et al. Neoadjuvant treatment response as an early response indicator for patients with rectal cancer. J Clin Oncol. 2012;30:1770–1776. doi: 10.1200/JCO.2011.39.7901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Glynne-Jones R. Rectal cancer: The times they are a-changing. Lancet Oncol. 2012;13:651–653. doi: 10.1016/S1470-2045(12)70214-0. [DOI] [PubMed] [Google Scholar]
- 50.Glynne-Jones R, Harrison M, Hughes R. Challenges in the neoadjuvant treatment of rectal cancer: Balancing the risk of recurrence and quality of life. Cancer Radiother. 2013;17:675–685. doi: 10.1016/j.canrad.2013.06.043. [DOI] [PubMed] [Google Scholar]
- 51.Tepper JE, O'Neil BH. Minimizing therapy and maximizing outcomes in rectal cancer. J Clin Oncol. 2011;29:4604–4606. doi: 10.1200/JCO.2011.38.1335. [DOI] [PubMed] [Google Scholar]
- 52.Guillem JG, Chessin DB, Shia J, et al. Clinical examination following preoperative chemoradiation for rectal cancer is not a reliable surrogate end point. J Clin Oncol. 2005;23:3475–3479. doi: 10.1200/JCO.2005.06.114. [DOI] [PubMed] [Google Scholar]
- 53.Pastor C, Subtil JC, Sola J, et al. Accuracy of endoscopic ultrasound to assess tumor response after neoadjuvant treatment in rectal cancer: Can we trust the findings? Dis Colon Rectum. 2011;54:1141–1146. doi: 10.1097/DCR.0b013e31821c4a60. [DOI] [PubMed] [Google Scholar]
- 54.Guillem JG, Ruby JA, Leibold T, et al. Neither FDG-PET nor CT can distinguish between a pathological complete response and an incomplete response after neoadjuvant chemoradiation in locally advanced rectal cancer: A prospective study. Ann Surg. 2013;258:289–295. doi: 10.1097/SLA.0b013e318277b625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Van der Paardt MP, Zagers MB, Beets-Tan RG, et al. Patients who undergo preoperative chemoradiotherapy for locally advanced rectal cancer restaged by using diagnostic MR imaging: A systematic review and meta-analysis. Radiology. 2013;269:101–112. doi: 10.1148/radiol.13122833. [DOI] [PubMed] [Google Scholar]
- 56.Patel UB, Taylor F, Blomqvist L, et al. Magnetic resonance imaging-detected tumor response for locally advanced rectal cancer predicts survival outcomes: MERCURY experience. J Clin Oncol. 2011;29:3753–3760. doi: 10.1200/JCO.2011.34.9068. [DOI] [PubMed] [Google Scholar]
- 57.Dresen RC, Beets GL, Rutten HJ, et al. Locally advanced rectal cancer: MR imaging for restaging after neoadjuvant radiation therapy with concomitant chemotherapy: Part I. Are we able to predict tumor confined to the rectal wall? Radiology. 2009;252:71–80. doi: 10.1148/radiol.2521081200. [DOI] [PubMed] [Google Scholar]
- 58.Nougaret S, Rouanet P, Molinari N, et al. MR volumetric measurement of low rectal cancer helps predict tumor response and outcome after combined chemotherapy and radiation therapy. Radiology. 2012;263:409–418. doi: 10.1148/radiol.12111263. [DOI] [PubMed] [Google Scholar]
- 59.Gollub MJ, Gultekin DH, Akin O, et al. Dynamic contrast enhanced-MRI for the detection of pathological complete response to neoadjuvant chemotherapy for locally advanced rectal cancer. Eur Radiol. 2012;22:821–831. doi: 10.1007/s00330-011-2321-1. [DOI] [PubMed] [Google Scholar]
- 60.Lim JS, Kim D, Baek SE, et al. Perfusion MRI for the prediction of treatment response after preoperative chemoradiotherapy in locally advanced rectal cancer. Eur Radiol. 2012;22:1693–1700. doi: 10.1007/s00330-012-2416-3. [DOI] [PubMed] [Google Scholar]
- 61.Lambregts DM, Vandecaveye V, Barbaro B, et al. Diffusion-weighted MRI for selection of complete responders after chemoradiation for locally advanced rectal cancer: A multicenter study. Ann Surg Oncol. 2011;18:2224–2231. doi: 10.1245/s10434-011-1607-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Dzik-Jurasz A, Domenig C, George M, et al. Diffusion MRI for prediction of response of rectal cancer to chemoradiation. Lancet. 2002;360:307–308. doi: 10.1016/S0140-6736(02)09520-X. [DOI] [PubMed] [Google Scholar]
- 63.Kim SH, Lee JM, Hong SH, et al. Locally advanced rectal cancer: Added value of diffusion-weighted MR imaging in the evaluation of tumor response to neoadjuvant chemo- and radiation therapy. Radiology. 2009;253:116–125. doi: 10.1148/radiol.2532090027. [DOI] [PubMed] [Google Scholar]
- 64.Kim YC, Lim JS, Keum KC, et al. Comparison of diffusion-weighted MRI and MR volumetry in the evaluation of early treatment outcomes after preoperative chemoradiotherapy for locally advanced rectal cancer. J Magn Reson Imaging. 2011;34:570–576. doi: 10.1002/jmri.22696. [DOI] [PubMed] [Google Scholar]
- 65.Barbaro B, Vitale R, Valentini V, et al. Diffusion-weighted magnetic resonance imaging in monitoring rectal cancer response to neoadjuvant chemoradiotherapy. Int J Radiat Oncol Biol Phys. 2012;83:594–599. doi: 10.1016/j.ijrobp.2011.07.017. [DOI] [PubMed] [Google Scholar]
- 66.Kim SH, Lee JY, Lee JM, et al. Apparent diffusion coefficient for evaluating tumour response to neoadjuvant chemoradiation therapy for locally advanced rectal cancer. Eur Radiol. 2011;21:987–995. doi: 10.1007/s00330-010-1989-y. [DOI] [PubMed] [Google Scholar]
- 67.Lambrecht M, Vandecaveye V, De Keyzer F, et al. Value of diffusion-weighted magnetic resonance imaging for prediction and early assessment of response to neoadjuvant radiochemotherapy in rectal cancer: Preliminary results. Int J Radiat Oncol Biol Phys. 2012;82:863–870. doi: 10.1016/j.ijrobp.2010.12.063. [DOI] [PubMed] [Google Scholar]
- 68.Fisher B, Wolmark N, Rockette H, et al. Postoperative adjuvant chemotherapy or radiation therapy for rectal cancer: Results from NSABP protocol R-01. J Natl Cancer Inst. 1988;80:21–29. doi: 10.1093/jnci/80.1.21. [DOI] [PubMed] [Google Scholar]
- 69.[No authors listed] Randomised trial of surgery alone versus radiotherapy followed by surgery for potentially operable locally advanced rectal cancer: Medical Research Council Rectal Cancer Working Party. Lancet. 1996;348:1605–1610. [PubMed] [Google Scholar]
- 70.[No authors listed] Prolongation of the disease-free interval in surgically treated rectal carcinoma: Gastrointestinal Tumor Study Group. N Engl J Med. 1985;312:1465–1472. doi: 10.1056/NEJM198506063122301. [DOI] [PubMed] [Google Scholar]
- 71.Cedermark B, Johansson H, Rutqvist LE, et al. The Stockholm I trial of preoperative short term radiotherapy in operable rectal carcinoma: A prospective randomized trial—Stockholm Colorectal Cancer Study Group. Cancer. 1995;75:2269–2275. doi: 10.1002/1097-0142(19950501)75:9<2269::aid-cncr2820750913>3.0.co;2-i. [DOI] [PubMed] [Google Scholar]
- 72.[No authors listed] Improved survival with preoperative radiotherapy in resectable rectal cancer: Swedish Rectal Cancer Trial. N Engl J Med. 1997;336:980–987. doi: 10.1056/NEJM199704033361402. [DOI] [PubMed] [Google Scholar]
- 73.Krook JE, Moertel CG, Gunderson LL, et al. Effective surgical adjuvant therapy for high-risk rectal carcinoma. N Engl J Med. 1991;324:709–715. doi: 10.1056/NEJM199103143241101. [DOI] [PubMed] [Google Scholar]
- 74.O'Connell MJ, Martenson JA, Wieand HS, et al. Improving adjuvant therapy for rectal cancer by combining protracted-infusion fluorouracil with radiation therapy after curative surgery. N Engl J Med. 1994;331:502–507. doi: 10.1056/NEJM199408253310803. [DOI] [PubMed] [Google Scholar]
- 75.Smalley SR, Benedetti JK, Williamson SK, et al. Phase III trial of fluorouracil-based chemotherapy regimens plus radiotherapy in postoperative adjuvant rectal cancer: GI INT 0144. J Clin Oncol. 2006;24:3542–3547. doi: 10.1200/JCO.2005.04.9544. [DOI] [PubMed] [Google Scholar]
- 76.[No authors listed] NIH consensus conference: Adjuvant therapy for patients with colon and rectal cancer. JAMA. 1990;264:1444–1450. [PubMed] [Google Scholar]
- 77.Bosset JF, Calais G, Mineur L, et al. Enhanced tumorocidal effect of chemotherapy with preoperative radiotherapy for rectal cancer: Preliminary results—EORTC 22921. J Clin Oncol. 2005;23:5620–5627. doi: 10.1200/JCO.2005.02.113. [DOI] [PubMed] [Google Scholar]
- 78.Bosset JF, Collette L, Calais G, et al. Chemotherapy with preoperative radiotherapy in rectal cancer. N Engl J Med. 2006;355:1114–1123. doi: 10.1056/NEJMoa060829. [DOI] [PubMed] [Google Scholar]
- 79.Gérard JP, Conroy T, Bonnetain F, et al. Preoperative radiotherapy with or without concurrent fluorouracil and leucovorin in T3-4 rectal cancers: Results of FFCD 9203. J Clin Oncol. 2006;24:4620–4625. doi: 10.1200/JCO.2006.06.7629. [DOI] [PubMed] [Google Scholar]
- 80.Sauer R, Becker H, Hohenberger W, et al. Preoperative versus postoperative chemoradiotherapy for rectal cancer. N Engl J Med. 2004;351:1731–1740. doi: 10.1056/NEJMoa040694. [DOI] [PubMed] [Google Scholar]
- 81.Roh MS, Colangelo LH, O'Connell MJ, et al. Preoperative multimodality therapy improves disease-free survival in patients with carcinoma of the rectum: NSABP R-03. J Clin Oncol. 2009;27:5124–5130. doi: 10.1200/JCO.2009.22.0467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Peeters KC, van de Velde CJ, Leer JW, et al. Late side effects of short-course preoperative radiotherapy combined with total mesorectal excision for rectal cancer: Increased bowel dysfunction in irradiated patients—A Dutch Colorectal Cancer Group study. J Clin Oncol. 2005;23:6199–6206. doi: 10.1200/JCO.2005.14.779. [DOI] [PubMed] [Google Scholar]
- 83.Bujko K, Nowacki MP, Nasierowska-Guttmejer A, et al. Long-term results of a randomized trial comparing preoperative short-course radiotherapy with preoperative conventionally fractionated chemoradiation for rectal cancer. Br J Surg. 2006;93:1215–1223. doi: 10.1002/bjs.5506. [DOI] [PubMed] [Google Scholar]
- 84.Ngan SY, Burmeister B, Fisher RJ, et al. Randomized trial of short-course radiotherapy versus long-course chemoradiation comparing rates of local recurrence in patients with T3 rectal cancer: Trans-Tasman Radiation Oncology Group trial 01.04. J Clin Oncol. 2012;30:3827–3833. doi: 10.1200/JCO.2012.42.9597. [DOI] [PubMed] [Google Scholar]
- 85.Tan D, Glynne-Jones R. But some neoadjuvant schedules are more equal than others. J Clin Oncol. 2013;31:1799–1800. doi: 10.1200/JCO.2012.47.7844. [DOI] [PubMed] [Google Scholar]
- 86.Bujko K. Short-course preoperative radiotherapy for low rectal cancer. J Clin Oncol. 2013;31:1799. doi: 10.1200/JCO.2012.47.3769. [DOI] [PubMed] [Google Scholar]
- 87.Fernández-Martos C, Nogué M, Cejas P, et al. The role of capecitabine in locally advanced rectal cancer treatment: An update. Drugs. 2012;72:1057–1073. doi: 10.2165/11633870-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 88.Hofheinz RD, Wenz F, Post S, et al. Chemoradiotherapy with capecitabine versus fluorouracil for locally advanced rectal cancer: A randomised, multicentre, non-inferiority, phase 3 trial. Lancet Oncol. 2012;13:579–588. doi: 10.1016/S1470-2045(12)70116-X. [DOI] [PubMed] [Google Scholar]
- 89.O'Connell MJ, Colangelo LH, Beart RW, et al. Capecitabine and oxaliplatin in the preoperative multimodality treatment of rectal cancer: Surgical end points from National Surgical Adjuvant Breast and Bowel Project trial R-04. J Clin Oncol. 2014;32:1927–1934. doi: 10.1200/JCO.2013.53.7753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Aschele C, Cionini L, Lonardi S, et al. Primary tumor response to preoperative chemoradiation with or without oxaliplatin in locally advanced rectal cancer: Pathologic results of the STAR-01 randomized phase III trial. J Clin Oncol. 2011;29:2773–2780. doi: 10.1200/JCO.2010.34.4911. [DOI] [PubMed] [Google Scholar]
- 91.Gérard JP, Azria D, Gourgou-Bourgade S, et al. Comparison of two neoadjuvant chemoradiotherapy regimens for locally advanced rectal cancer: Results of the phase III trial ACCORD 12/0405-Prodige 2. J Clin Oncol. 2010;28:1638–1644. doi: 10.1200/JCO.2009.25.8376. [DOI] [PubMed] [Google Scholar]
- 92.Rödel C, Liersch T, Becker H, et al. Preoperative chemoradiotherapy and postoperative chemotherapy with fluorouracil and oxaliplatin versus fluorouracil alone in locally advanced rectal cancer: Initial results of the German CAO/ARO/AIO-04 randomised phase 3 trial. Lancet Oncol. 2012;13:679–687. doi: 10.1016/S1470-2045(12)70187-0. [DOI] [PubMed] [Google Scholar]
- 93.Nogué M, Salud A, Vicente P, et al. Addition of bevacizumab to XELOX induction therapy plus concomitant capecitabine-based chemoradiotherapy in magnetic resonance imaging-defined poor-prognosis locally advanced rectal cancer: The AVACROSS study. Oncologist. 2011;16:614–620. doi: 10.1634/theoncologist.2010-0285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Dipetrillo T, Pricolo V, Lagares-Garcia J, et al. Neoadjuvant bevacizumab, oxaliplatin, 5-fluorouracil, and radiation for rectal cancer. Int J Radiat Oncol Biol Phys. 2012;82:124–129. doi: 10.1016/j.ijrobp.2010.08.005. [DOI] [PubMed] [Google Scholar]
- 95.Petersen SH, Harling H, Kirkeby LT, et al. Postoperative adjuvant chemotherapy in rectal cancer operated for cure. Cochrane Database Syst Rev. 2012;3:CD004078. doi: 10.1002/14651858.CD004078.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Quasar Collaborative Group. Gray R, Barnwell J, et al. Adjuvant chemotherapy versus observation in patients with colorectal cancer: A randomised study. Lancet. 2007;370:2020–2029. doi: 10.1016/S0140-6736(07)61866-2. [DOI] [PubMed] [Google Scholar]
- 97.André T, Boni C, Navarro M, et al. Improved overall survival with oxaliplatin, fluorouracil, and leucovorin as adjuvant treatment in stage II or III colon cancer in the MOSAIC trial. J Clin Oncol. 2009;27:3109–3116. doi: 10.1200/JCO.2008.20.6771. [DOI] [PubMed] [Google Scholar]
- 98.Bosset JF, Calais G, Mineur L, et al. Fluorouracil-based adjuvant chemotherapy after preoperative chemoradiotherapy in rectal cancer: Long-term results of the EORTC 22921 randomised study. Lancet Oncol. 2014;15:184–190. doi: 10.1016/S1470-2045(13)70599-0. [DOI] [PubMed] [Google Scholar]
- 99.Hayden DM, Pinzon MC, Francescatti AB, et al. Hospital readmission for fluid and electrolyte abnormalities following ileostomy construction: Preventable or unpredictable? J Gastrointest Surg. 2013;17:298–303. doi: 10.1007/s11605-012-2073-5. [DOI] [PubMed] [Google Scholar]
- 100.Khrizman P, Niland JC, ter Veer A, et al. Postoperative adjuvant chemotherapy use in patients with stage II/III rectal cancer treated with neoadjuvant therapy: A National Comprehensive Cancer Network analysis. J Clin Oncol. 2013;31:30–38. doi: 10.1200/JCO.2011.40.3188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Biagi JJ, Raphael MJ, Mackillop WJ, et al. Association between time to initiation of adjuvant chemotherapy and survival in colorectal cancer: A systematic review and meta-analysis. JAMA. 2011;305:2335–2342. doi: 10.1001/jama.2011.749. [DOI] [PubMed] [Google Scholar]
- 102.Calvo FA, Serrano FJ, Diaz-González JA, et al. Improved incidence of pT0 downstaged surgical specimens in locally advanced rectal cancer (LARC) treated with induction oxaliplatin plus 5-fluorouracil and preoperative chemoradiation. Ann Oncol. 2006;17:1103–1110. doi: 10.1093/annonc/mdl085. [DOI] [PubMed] [Google Scholar]
- 103.Chau I, Brown G, Cunningham D, et al. Neoadjuvant capecitabine and oxaliplatin followed by synchronous chemoradiation and total mesorectal excision in magnetic resonance imaging-defined poor-risk rectal cancer. J Clin Oncol. 2006;24:668–674. doi: 10.1200/JCO.2005.04.4875. [DOI] [PubMed] [Google Scholar]
- 104.Fernández-Martos C, Pericay C, Aparicio J, et al. Phase II, randomized study of concomitant chemoradiotherapy followed by surgery and adjuvant capecitabine plus oxaliplatin (CAPOX) compared with induction CAPOX followed by concomitant chemoradiotherapy and surgery in magnetic resonance imaging-defined, locally advanced rectal cancer: Grupo cancer de recto 3 study. J Clin Oncol. 2010;28:859–865. doi: 10.1200/JCO.2009.25.8541. [DOI] [PubMed] [Google Scholar]
- 105.Maréchal R, Vos B, Polus M, et al. Short course chemotherapy followed by concomitant chemoradiotherapy and surgery in locally advanced rectal cancer: A randomized multicentric phase II study. Ann Oncol. 2012;23:1525–1530. doi: 10.1093/annonc/mdr473. [DOI] [PubMed] [Google Scholar]
- 106.Schou JV, Larsen FO, Rasch L, et al. Induction chemotherapy with capecitabine and oxaliplatin followed by chemoradiotherapy before total mesorectal excision in patients with locally advanced rectal cancer. Ann Oncol. 2012;23:2627–2633. doi: 10.1093/annonc/mds056. [DOI] [PubMed] [Google Scholar]
- 107.Glynne-Jones R, Grainger J, Harrison M, et al. Neoadjuvant chemotherapy prior to preoperative chemoradiation or radiation in rectal cancer: Should we be more cautious? Br J Cancer. 2006;94:363–371. doi: 10.1038/sj.bjc.6602960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Cercek A, Goodman KA, Hajj C, et al. Neoadjuvant chemotherapy first, followed by chemoradiation and then surgery, in the management of locally advanced rectal cancer. J Natl Compr Canc Netw. 2014;12:513–519. doi: 10.6004/jnccn.2014.0056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Garcia-Aguilar J, Smith DD, Avila K, et al. Optimal timing of surgery after chemoradiation for advanced rectal cancer: Preliminary results of a multicenter, nonrandomized phase II prospective trial. Ann Surg. 2011;254:97–102. doi: 10.1097/SLA.0b013e3182196e1f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Garcia-Aguilar J, Marcet J, Coutsoftides T, et al. Impact of neoadjuvant chemotherapy following chemoradiation on tumor response, adverse events, and surgical complications in patients with advanced rectal cancer treated with TME. J Clin Oncol. 2011;29(suppl):224s. abstr 3514. [Google Scholar]
- 111.Renouf DJ, Woods R, Speers C, et al. Improvements in 5-year outcomes of stage II/III rectal cancer relative to colon cancer. Am J Clin Oncol. 2013;36:558–564. doi: 10.1097/COC.0b013e318256f5dc. [DOI] [PubMed] [Google Scholar]
- 112.Maas M, Nelemans PJ, Valentini V, et al. Long-term outcome in patients with a pathological complete response after chemoradiation for rectal cancer: A pooled analysis of individual patient data. Lancet Oncol. 2010;11:835–844. doi: 10.1016/S1470-2045(10)70172-8. [DOI] [PubMed] [Google Scholar]
- 113.Park IJ, Eng C, You YN, et al. Exploratory analysis of adjuvant chemotherapy benefits after preoperative chemoradiotherapy and radical resection for rectal cancer. J Clin Oncol. 2012;30(suppl):21s. abstr 557. [Google Scholar]
- 114.Fietkau R, Barten M, Klautke G, et al. Postoperative chemotherapy may not be necessary for patients with ypN0-category after neoadjuvant chemoradiotherapy of rectal cancer. Dis Colon Rectum. 2006;49:1284–1292. doi: 10.1007/s10350-006-0570-x. [DOI] [PubMed] [Google Scholar]
- 115.Nelson VM, Benson AB., 3rd Pathological complete response after neoadjuvant therapy for rectal cancer and the role of adjuvant therapy. Curr Oncol Rep. 2013;15:152–161. doi: 10.1007/s11912-013-0297-5. [DOI] [PubMed] [Google Scholar]
- 116.Maas M, Nelemans PJ, Valentini V, et al. Adjuvant chemotherapy in rectal cancer: Defining subgroups who may benefit after neoadjuvant chemoradiation and resection—A pooled analysis of 3,313 patients. Int J Cancer. doi: 10.1002/ijc.29355. [epub ahead of print on November 22, 2014] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Hong YS, Nam BH, Kim KP, et al. Oxaliplatin, fluorouracil, and leucovorin versus fluorouracil and leucovorin as adjuvant chemotherapy for locally advanced rectal cancer after preoperative chemoradiotherapy (ADORE): An open-label, multicentre, phase 2, randomised controlled trial. Lancet Oncol. 2014;15:1245–1253. doi: 10.1016/S1470-2045(14)70377-8. [DOI] [PubMed] [Google Scholar]
- 118.Birgisson H, Påhlman L, Gunnarsson U, et al. Adverse effects of preoperative radiation therapy for rectal cancer: Long-term follow-up of the Swedish Rectal Cancer Trial. J Clin Oncol. 2005;23:8697–8705. doi: 10.1200/JCO.2005.02.9017. [DOI] [PubMed] [Google Scholar]
- 119.Joye I, Haustermans K. Early and late toxicity of radiotherapy for rectal cancer. Recent Results Cancer Res. 2014;203:189–201. doi: 10.1007/978-3-319-08060-4_13. [DOI] [PubMed] [Google Scholar]
- 120.Gunderson LL, Sargent DJ, Tepper JE, et al. Impact of T and N stage and treatment on survival and relapse in adjuvant rectal cancer: A pooled analysis. J Clin Oncol. 2004;22:1785–1796. doi: 10.1200/JCO.2004.08.173. [DOI] [PubMed] [Google Scholar]
- 121.Schrag D. Evolving role of neoadjuvant therapy in rectal cancer. Curr Treat Options Oncol. 2013;14:350–364. doi: 10.1007/s11864-013-0242-8. [DOI] [PubMed] [Google Scholar]
- 122.Schrag D, Weiser MR, Goodman KA, et al. Neoadjuvant chemotherapy without routine use of radiation therapy for patients with locally advanced rectal cancer: A pilot trial. J Clin Oncol. 2014;32:513–518. doi: 10.1200/JCO.2013.51.7904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Alliance for Clinical Trials in Oncology. Chemotherapy Alone or Chemotherapy Plus Radiation Therapy in Treating Patients With Locally Advanced Rectal Cancer Undergoing Surgery (The PROSPECT Trial) https://clinicaltrials.gov/ct2/show/NCT01515787. [Google Scholar]
- 124.Memorial Sloan-Kettering Cancer Center. Trial Evaluating 3-year Disease Free Survival in Patients With Locally Advanced Rectal Cancer Treated With Chemoradiation Plus Induction or Consolidation Chemotherapy and Total Mesorectal Excision or Non-operative Management. doi: 10.1186/s12885-015-1632-z. https://clinicaltrials.gov/ct2/show/NCT02008656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Habr-Gama A, Perez RO, Nadalin W, et al. Operative versus nonoperative treatment for stage 0 distal rectal cancer following chemoradiation therapy: Long-term results. Ann Surg. 2004;240:711–717. doi: 10.1097/01.sla.0000141194.27992.32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Habr-Gama A, Perez RO, Proscurshim I, et al. Patterns of failure and survival for nonoperative treatment of stage c0 distal rectal cancer following neoadjuvant chemoradiation therapy. J Gastrointest Surg. 2006;10:1319–1328. doi: 10.1016/j.gassur.2006.09.005. [DOI] [PubMed] [Google Scholar]
- 127.Habr-Gama A, Gama-Rodrigues J, São Julião GP, et al. Local recurrence after complete clinical response and watch and wait in rectal cancer after neoadjuvant chemoradiation: Impact of salvage therapy on local disease control. Int J Radiat Oncol Biol Phys. 2014;88:822–828. doi: 10.1016/j.ijrobp.2013.12.012. [DOI] [PubMed] [Google Scholar]
- 128.Maas M, Beets-Tan RG, Lambregts DM, et al. Wait-and-see policy for clinical complete responders after chemoradiation for rectal cancer. J Clin Oncol. 2011;29:4633–4640. doi: 10.1200/JCO.2011.37.7176. [DOI] [PubMed] [Google Scholar]
- 129.Smith JD, Ruby JA, Goodman KA, et al. Nonoperative management of rectal cancer with complete clinical response after neoadjuvant therapy. Ann Surg. 2012;256:965–972. doi: 10.1097/SLA.0b013e3182759f1c. [DOI] [PubMed] [Google Scholar]
- 130.You YN, Baxter NN, Stewart A, et al. Is the increasing rate of local excision for stage I rectal cancer in the United States justified?: A nationwide cohort study from the National Cancer Database. Ann Surg. 2007;245:726–733. doi: 10.1097/01.sla.0000252590.95116.4f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Greenberg JA, Shibata D, Herndon JE, 2nd, et al. Local excision of distal rectal cancer: An update of Cancer and Leukemia Group B 8984. Dis Colon Rectum. 2008;51:1185–1191. doi: 10.1007/s10350-008-9231-6. [DOI] [PubMed] [Google Scholar]
- 132.Stitzenberg KB, Sanoff HK, Penn DC, et al. Practice patterns and long-term survival for early-stage rectal cancer. J Clin Oncol. 2013;31:4276–4282. doi: 10.1200/JCO.2013.49.1860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Perez RO, Habr-Gama A, Lynn PB, et al. Transanal endoscopic microsurgery for residual rectal cancer (ypT0-2) following neoadjuvant chemoradiation therapy: Another word of caution. Dis Colon Rectum. 2013;56:6–13. doi: 10.1097/DCR.0b013e318273f56f. [DOI] [PubMed] [Google Scholar]
- 134.Sauer R, Liersch T, Merkel S, et al. Preoperative versus postoperative chemoradiotherapy for locally advanced rectal cancer: Results of the German CAO/ARO/AIO-94 randomized phase III trial after a median follow-up of 11 years. J Clin Oncol. 2012;30:1926–1933. doi: 10.1200/JCO.2011.40.1836. [DOI] [PubMed] [Google Scholar]
- 135.Garcia-Aguilar J. Transanal endoscopic microsurgery following neoadjuvant chemoradiation therapy in rectal cancer: A word of caution about patient selection? Dis Colon Rectum. 2013;56:1–3. doi: 10.1097/DCR.0b013e318273f58c. [DOI] [PubMed] [Google Scholar]
- 136.Vejle Hospital. Watchful Waiting. An Observational Study of Patients with Rectal Cancer After Concomitant Radiation and Chemotherapy. https://clinicaltrials.gov/ct2/show/NCT00952926. [Google Scholar]
- 137.Maria Sklodowska-Curie Memorial Cancer Center, Institute of Oncology. Organ Preservation in Elderly Patients with Rectal Cancer. https://clinicaltrials.gov/ct2/show/NCT01863862. [Google Scholar]
- 138.Instituto do Cancer do Estado de São Paulo. Observation Versus Surgical Resection in Patients with Rectal Cancer Who Achieved Complete Clinical Response After Neoadjuvant Chemoradiotherapy. https://clinicaltrials.gov/ct2/show/NCT02052921. [Google Scholar]
- 139.Cancer Research UK-Pelican Cancer Foundation. Timing and Deferral of Rectal Surgery Following a Continued Response to Pre-operative Chemoradiotherapy. http://public.ukcrn.org.uk/search/StudyDetail.aspx?StudyID=8565. [Google Scholar]