Abstract
Purpose
To analyze the benefit and feasibility of this procedure compared with those of open method.
Methods
Abdominal procedure includes laparoscopic gastric mobilization, celiac axis lymph node dissection, formation of the gastric tube, and pyloromyotomy. The actual procedure performed during open surgery is the same as those of laparoscopic surgery except for the main incision. Minimally invasive esophagectomy (MIE) was performed on 54 patients with esophageal cancer. The short-term outcomes, including postoperative complications were analyzed and compared with 44 cases of open method.
Results
Although the total operative time was not different between 2 groups (349.8 minutes vs. 374.8 minutes, P = 0.153), the operation time of abdominal procedure was shorter in laparoscopic group (90.6 minutes vs. 162.1 minutes, P < 0.001). Operation related complications and hospital stay were not significantly different between the 2 groups. The number of transfused patients was significantly smaller in laparoscopic group (11.1% vs. 27.9%, P = 0.030).
Conclusion
Laparoscopic gastric tubing with pyloromyotomy is a feasible and safe treatment option for patients with esophageal cancer.
Keywords: Esophageal cancer, Minimally invasive surgical procedures, Laparoscopy, Feasibility studies
INTRODUCTION
Esophageal cancer is the eighth most common cause of cancer and the sixth most common cause of cancer-related death worldwide [1]. For resectable disease, esophagectomy for esophageal cancer has remained the primary treatment for patients with localized disease. In this approach, the stomach is the most frequently accessed conduit organ [2]. But the surgical damage associated with operation for esophageal cancer may be the greatest among all general surgical procedures [3]. Open esophagectomy (OE) is frequently associated with significant morbidity and mortality, even at highly rated treatment centers [4,5,6,7,8,9]. With the development of laparoscopic surgery, minimally invasive esophagectomy (MIE) has gradually taken place as a choice for esophageal cancer surgery. MIE, which is less traumatic, allows for earlier postoperative recovery, and incurs fewer complications, has, not surprisingly, attracted a great deal of interest from surgeons and researchers [10]. Furthermore, the enhanced visualization afforded can facilitate intraoperative procedures, alleviate blood loss, and reduce complications [11,12,13]. In general, it is reported that postoperative outcomes are better when abdominal surgeries are performed laparoscopically as compared with open surgery [14]. With this point of view, it can be thought that postoperative complication rates would be lower when the abdominal phase of esophagectomy is performed laparoscopically. In fact, as part of an effort to reduce the risks of esophagectomy, MIE has been adopted in many specialized centers. Luketich et al. [9] reported a method of MIE that yielded excellent outcomes.
The purpose of this study was to look at the safety and feasibility of laparoscopic gastric tubing with pyloromyotomy in patients who underwent esophagectomy for esophageal cancer and to compare with those who underwent open procedure.
METHODS
Patients
Between January 2009 and April 2013, 54 patients who underwent subtotal esophagectomy and laparoscopic gastric tubing were selected, and 44 patients who underwent open gastric tubing between 2006 and 2013 were selected. Before 2009, we had performed open gastric tubing and thereafter, laparoscopic gastric tubing has been standard procedure for esophageal cancer surgery. The primary inclusion criteria in patients with esophageal cancer included the presence of a resectable lesion after evaluation by endoscopic ultrasound and computed tomography. All of the 98 patients enrolled in the present study received a complete evaluation that included physical examinations, blood tests, chest and abdominal x-rays, upper gastrointestinal endoscopy, endoscopic ultrasound, bronchoscopy, chest and abdominal computed tomography, and PET-CT prior to neoadjuvant chemotherapy or surgery.
The operative procedure entailed laparoscopic gastric tubing with lymph node dissection, followed by thoracoscopic esophageal mobilization and esophagogastric anastomosis in the thorax or neck. Chart reviews of the patients yielded clinical data on age, gender, body mass index (BMI), operation time, length of postoperative hospital stay, and postoperative complications. Approval for this study was obtained from our Institutional Review Broad (IRB).
Abdominal procedure: total laparoscopy
Laparoscopic gastric mobilization
The patient was positioned in a supine split leg position with the surgeon operating on the right, the camera assistant in the middle, and the first assistant on the left. Under general anesthesia, pneumoperitoneum was established at the umbilicus via the open technique, and an intra-abdominal pressure of 12-15 mmHg was obtained. Four ports were placed under direct laparoscopic visualization. Access to the abdominal cavity was obtained using two 5-mm laparoscopic trocars and two 12-mm trocars (Fig. 1A). The liver was retracted upward using a "V-shaped liver retraction" method described previously [15].
Fig. 1. (A) Trocar placement. (B) Lymph node dissection around celiac axis. (C) Gastric tube formation using stapler. (D) Pyloromyotomy using monopolar cautery. LGA, left gastric artery; CHA, common hepatic artery; SA, splenic artery.
Subsequently, first, the greater omentum was divided proximally about 4 to 5 cm from the mid portion of the right gastroepiploic arcade toward the junction of the left- and right-side gastroepiploic arcades, using an ultrasonic scalpel (Ethicon Endo-Surgery Inc., Cincinnati, OH, USA). The left gastroepiploic and short gastric vessels were divided in order to mobilize the fundus of the stomach. We then moved backward to the roots of the right-side gastroepiploic vessels. Second, the hepatogastric ligament was divided beyond the right gastric vessels to enable access to the lesser sac and to expose the right crus of the esophageal hiatus.
Celiac axis lymph node dissection
The lymph nodes with fatty tissue along the anterosuperior aspect of the common hepatic artery, the celiac axis, and the proximal splenic artery [16] were dissected along each artery using an ultrasonic dissector and a hook-type monopolar bovie (Fig. 1B). The left gastric vein was divided, and the root of the left gastric artery was exposed and divided by double clipping, thereby allowing dissection of the left gastric artery lymph nodes. The perigastric lymph nodes were dissected along the upper lesser curvature up to the esophagogastric junction. The esophageal hiatus opening of the diaphragm was enlarged to facilitate exposure and smooth passage of the gastric tube.
Formation of gastric tube
First, the mid portion of the lesser curvature was dissected in preparation for stapling. The first linear 45-mm endostapler (Covidien, Norwalk, CT, USA) was fired just above the angle of the lesser curvature. Then, one or two additional staplings were performed in a curvilinear fashion (Fig. 1C). To facilitate both delivery to the chest and insertion of the circular stapler body into the tubed stomach, the stomach was not completely divided.
Pyloromyotomy
In this final step, pyloromyotomy in place of pyloroplasty was performed. The pyloric ring was identified, and a 1-cm-long pyloromyotomy was performed using monopolar cautery (Fig. 1D). There have been some suggestions that the incidence of symptoms such as regurgitation, diarrhea, bile reflux, and dumping syndrome was not different between pyloroplasty and pyloromyotomy [17].
Open surgery
The actual procedure performed during open surgery is the same as those of laparoscopic surgery except for the main incision. We used upper midline incision and most of the procedure is similar to laparoscopic surgery. In all cases of open procedure, we performed pyloroplasty.
Thoracic procedure (video-assited thoracoscopic surgery)
Patient position was changed to left lateral decubitus and right side chest was entered. We made a 6-cm utility incision with another three or four port incisions. The operator was usually located on the patient's left side. A 10.5-mm camera port was placed in the 7th or 8th intercostal space at midaxillary line. We used 30-degree telescope. A utility incision was made along the anterior axillary line at the 5th or 6th intercostal space without division of the rib or utilization of rib retractor. A thin plastic wound protector (Applied Medical, Rancho Santa Margarita, CA, USA) was applied for easy instrumentation and for avoiding tumor contamination during specimen removal. A 5-mm port was placed below the scapular tip for the assistant. The third 10.5-mm port was inserted at the anterior axillary line of the 3rd intercostal space. Shortly after, a fourth port was inserted a little to the anterior side of the posterior axillary line at the 5th intercostal space for assistance. After selective single lung ventilation, the azygos vein was divided using the endostapler (TriStapler, Covidien, Norwalk, CT, USA) or 10-mm-sized Hemolok. The mediastinal pleura was opened over the esophagus entirely. Esophageal dissection was performed using a Harmonic scalpel (Ethicon Endo-Surgery Inc.). During esophageal dissection, regional lymph nodes, and soft tissue were removed from the level of thoracic inlet to the diaphragm including recurrent laryngeal lymph nodes and subcarinal lymph nodes. The thoracic duct was routinely clipped at the lowest possible level. After the entire intrathoracic esophagus was mobilized, a single muscular purse-string suture was placed at the uppermost portion of the esophagus using 2-0 Prolene.
The esophagus was opened longitudinally 4 to 5 centimeters below the purse-string suture. An additional full-length esophageal stitch was placed and tied just above the proximal end of the esophagotomy to prevent further upward laceration during anvil placement. A 28-mm anvil was placed in the proximal esophagus and secured with a purse-string suture. The esophagus was divided just below the tied purse-sting. The stomach was pulled into the thorax through esophageal hiatus. The esophagus and stomach were pulled out through the utility incision and takedown of specimen and final gastric tubing procedure was done extracorporeally using a linear stapler leaving a 4-cm opening for EEA body insertion (DST EEA 28, Tyco Healthcare, Norwalk, CT, USA). The stomach graft was then returned to the thoracic cavity. A 28-mm size EEA body was inserted into the thoracic cavity through the utility incision. Then the EEA head portion was gently introduced into the stomach graft through the gastric opening and a spike pierced the posterior upper part of the stomach. Careful coaptation of the anvil and body is needed; gentle and tensionless approximation of the stomach and esophagus without any interference by adjacent soft tissue. Firm and sustained squeezing of the handle can make a good intrathoracic anastomosis. An anastomotic stapled line can be observed internally by thoracoscopy. The remaining stomach opening was closed with a linear stapler. The anastomosis was also examined outside and a few clips were applied alongside the anastomosis for later identification by x-ray. The gastric tube was positioned deep into the mediastinum and the incised pleura was sutured or sutured to the stomach wall for prevention of gastric bulging or elongation into the pleural cavity.
Statistical analysis
Continuous data were expressed as the mean ± standard deviation. Data analysis was performed using SPSS ver. 12.0 (SPSS Inc., Chicago, IL, USA).
RESULTS
There were no significant differences in clinicopathological characteristics between the 2 groups (Table 1). As indicated in Table 1, most of the patients (96.3% vs. 93.2%, P = 0.690) had squamous cell carcinoma (SCC). The perioperative data are presented in Table 2. The total operation time was not different between the 2 groups (349.8±77.4 minutes vs. 374.8±94.0 minutes, P = 0.153), but abdominal operation time was significantly shorter in the laparoscopic group (90.6±27.6 minutes vs. 162.1±97.3 minutes, P < 0.001). There were no cases of conversion to open laparotomy. The mean estimated operative blood loss was not different between the two groups (460.0±355.5 mL vs. 465±323.3 mL, P = 0.945), whereas the number of transfused patients was smaller in laparoscopic than open group (11.1% vs. 27.9%, P = 0.030). With respect to the postoperative complication rate, there were no significant differences between the two groups (24.1% vs. 27.9%, P = 0.817). In all 25 patients who had complications, 1 suffered from anastomosis bleeding, 5 from anastomosis leakage, 9 from pneumonia, and 1 from intra-abdominal bleeding. No significant differences were found in terms of mortality rate (5.1% vs. 4.5%, P > 0.999). Among the 5 patients, 1 patient died from pneumonia and 4 from anastomosis leakage. Postoperative hospital stay was not significantly different between the 2 groups (16.7±12.8 days vs. 19.1±13.4 days, P = 0.370).
Table 1. Clinicopathological characteristics of patients.
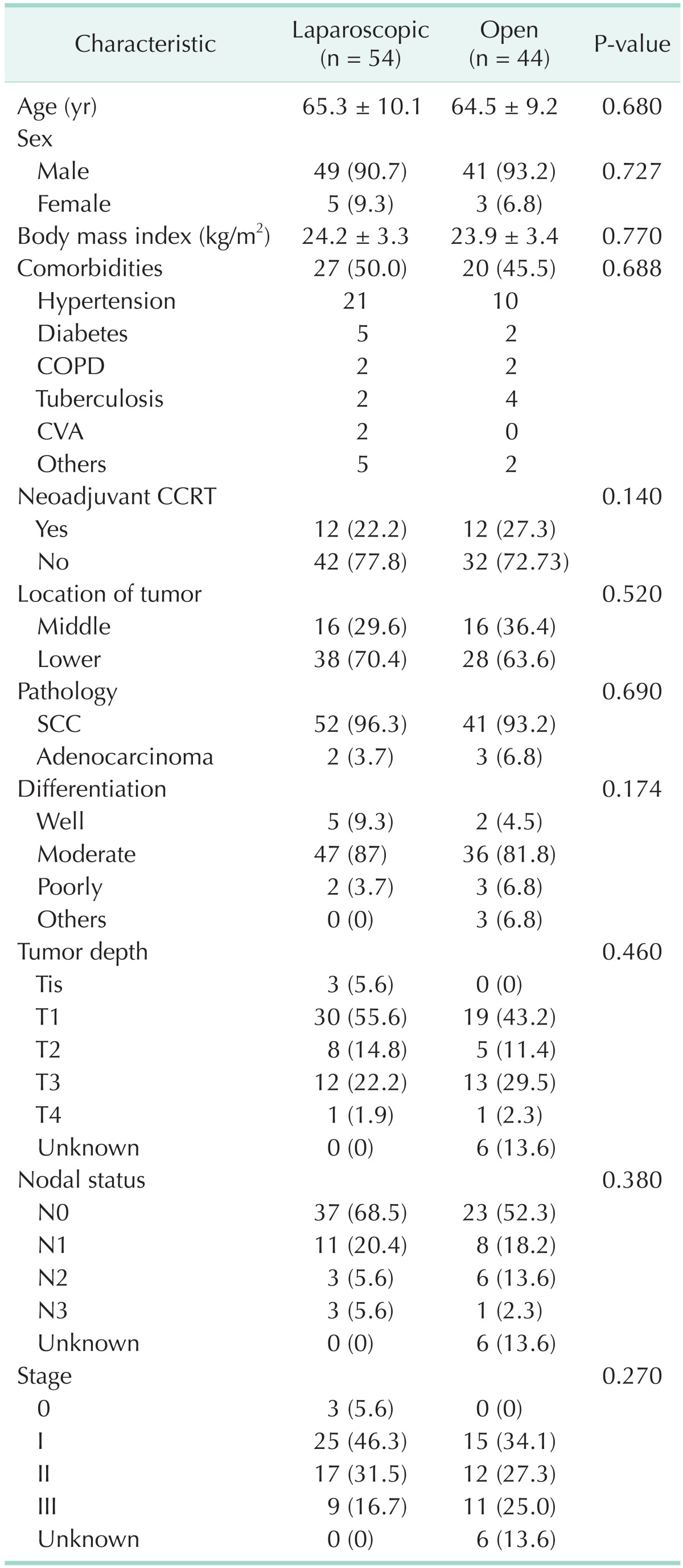
Values are presented as mean ± standard deviation or number (%).
COPD, chronic obstructive pulmonary disease; CVA, Cerebrovascular accident; CCRT, concomitant chemoradiotherapy; SCC, squamous cell carcinoma.
Table 2. Surgical and early postoperative outcomes.
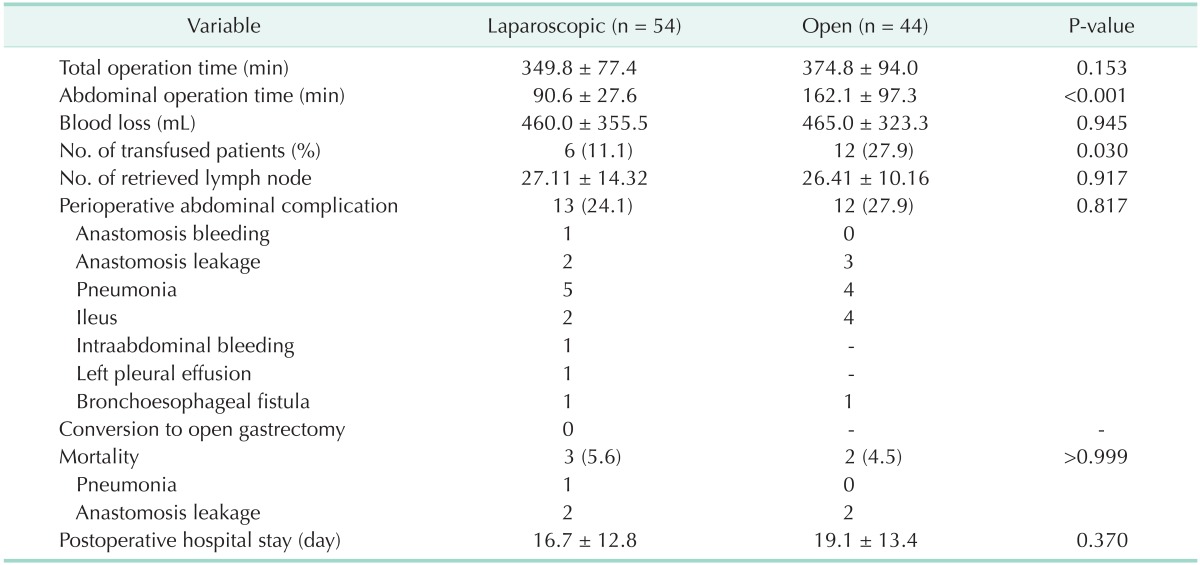
Values are presented as mean ± standard deviation or number (%).
Mortality cases include.
DISCUSSION
Esophagectomy for esophageal cancer is a complex procedure associated with significant postoperative morbidity. Esophageal resection with gastric tube reconstruction includes an abdominal operation for gastric mobilization and celiac lymphadenectomy. Thoracotomy is very hazardous and additional laparotomy can cause significant disturbance of pulmonary function and increase postoperative complications. To solve these problems, MIE was proposed as an alternative choice for esophageal cancer surgery.
It has been reported that the laparoscopic approach could improve the clinical course after abdominal surgery. Since laparoscopic cholecystectomy was first performed in 1989, laparoscopic surgery has been widely applied in patients with various gastrointestinal diseases [7]. Laparoscopic surgery has several advantages over conventional open surgery, including less invasiveness, less pain, earlier recovery, and better cosmesis [18]. This minimally invasive surgical technique has been refined in conjunction with the development of advanced surgical instrumentation, and has been applied to more complicated organ systems and disease processes. The indications of laparoscopic surgery have been extended to malignant diseases such as those involving the colon, stomach, liver, and other organs and tissues [19].
Esophageal surgeons also have increasingly incorporated minimally invasive surgery into their practices; several clinical series, in fact, have demonstrated the benefits of minimally invasive surgery in the treatment of gastroesophageal reflux disease [20], achalasia [21], and other benign esophageal diseases.
Interest recently, however, has been growing: a minimally invasive approach to the treatment of esophageal cancer is theoretically attractive, as it is less traumatic, allows for faster and easier postoperative recovery, and incurs fewer incision- or cardiopulmonary-related complications. Enhanced visualization, moreover, can facilitate intraoperative procedures, minimize blood loss, and reduce complications. In this study, we found that abdominal operation time was significantly shorter in the laparoscopic group compared with the open group. Also, we found that there was no significant difference between the two groups in terms of postoperative complications and mortality. There was no significant difference between the two groups regarding intraoperative blood loss, but this estimated blood loss could have been subjective and inaccurate, so we analyzed the number of transfused patients, which was significantly smaller in patients treated with laparoscopic procedure than those with open procedure. In 1998, Luketich et al. [9] reported eight cases of the minimally invasive approach to esophagectomy, including one case of total thoracoscopic and laparoscopic esophagectomy with cervical anastomosis.
In the latter, combined approach, thoracoscopy was performed to mobilize the thoracic esophagus, followed by laparoscopy to prepare the gastric conduit, and finally by esophagogastrostomy performed on the neck. In 2003, Luketich et al. [8] published promising results for 222 consecutive patients who had undergone this treatment. The procedure was successfully completed in 93% of the patients. Thereafter, a number of studies reported that MIE has some advantage related to the postoperative short-term outcomes and oncologic outcomes [11,12,22], and some studies demonstrated that the incidences of complications were significantly lower with MIE than with open procedure [23,24]. Furthermore, Mu et al. [13] concluded in their retrospective study that MIE and OE was equivalent with regard to early oncological outcomes. However, there was a trend that hospital length of stay and hospital expense were reduced in the MIE group than OE group.
There were some suggestions that hand-assisted laparoscopic transhiatal approach has several advantages over the thoracoscopic approach in patients with middle and lower esophageal cancer. Shiozaki et al. [25] demonstrated that thoracic trauma was minimized and duration of one-lung ventilation was reduced with this procedure. Also, it enables easy access to the posterior mediastinal lymph nodes, including the para-aortic and left pulmonary ligament lymph nodes. They insisted that resection of the left pulmonary ligament lymph nodes via a right thoracic approach carries an increased risk of serious complication such as respiratory morbidity.
Our institution developed and standardized operative techniques based on the multidisciplinary approach. The abdominal component is performed by a gastrointestinal surgeon (K.Y.S.) with an abundance of experience in laparoscopic gastrectomy. Laparoscopic surgery for treatment of gastric cancer, along with an effective screening system, has been well developed in Korea due to the high incidence of that malignancy among Koreans. As is well known, the Ivor Lewis operation should include two-field or three-field lymph node dissection in cases where radical surgery is required, to which lymph node dissection around the celiac axis is critical. In this respect, extensive node dissection experience can enhance the efficacy of the abdominal component. Also, reconstruction after esophagectomy is difficult and many problems may be encountered. The organ of first choice for reconstruction is the stomach followed by the colon [26]. And Zhang et al. [27] demonstrated that patients who underwent gastric tube reconstruction developed less postoperative complications, and have a quicker recovery and a better quality of life during the follow-up period than those who underwent whole stomach reconstruction.
We applied the principles and empirical records of laparoscopic gastrectomy to esophageal cancer surgery in several aspects including trocar placement, liver retraction, gastric mobilization, celiac node dissection, and stapling. We did not perform pyloroplasty but rather, pyloromyotomy. No patient experienced gastric stasis or passage disturbance, even though all had undergone total vagotomy.
Overall, we consider that this novel method of total laparoscopic gastric tube formation and pyloromyotomy can greatly facilitate MIE. Also, we found that the postoperative outcomes of this procedure were comparable to those of open procedure for patients with esophageal cancer.
In conclusion, laparoscopic gastric tubing with pyloromyotomy is a feasible and safe treatment option for patients with esophageal cancer.
Footnotes
CONFLICTS OF INTEREST: No potential conflict of interest relevant to this article was reported.
References
- 1.Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D. Global cancer statistics. CA Cancer J Clin. 2011;61:69–90. doi: 10.3322/caac.20107. [DOI] [PubMed] [Google Scholar]
- 2.Pennathur A, Luketich JD. Resection for esophageal cancer: strategies for optimal management. Ann Thorac Surg. 2008;85:S751–S756. doi: 10.1016/j.athoracsur.2007.11.078. [DOI] [PubMed] [Google Scholar]
- 3.Wu W, Zhu Q, Chen L, Liu J. Technical and early outcomes of Ivor Lewis minimally invasive oesophagectomy for gastric tube construction in the thoracic cavity. Interact Cardiovasc Thorac Surg. 2014;18:86–91. doi: 10.1093/icvts/ivt448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Takeuchi H, Kawakubo H, Kitagawa Y. Current status of minimally invasive esophagectomy for patients with esophageal cancer. Gen Thorac Cardiovasc Surg. 2013;61:513–521. doi: 10.1007/s11748-013-0258-9. [DOI] [PubMed] [Google Scholar]
- 5.Uttley L, Campbell F, Rhodes M, Cantrell A, Stegenga H, Lloyd-Jones M. Minimally invasive esophagectomy versus open surgery: is there an advantage? Surg Endosc. 2013;27:4401–4402. doi: 10.1007/s00464-013-3068-3. [DOI] [PubMed] [Google Scholar]
- 6.Shiozaki A, Fujiwara H, Murayama Y, Komatsu S, Kuriu Y, Ikoma H, et al. Perioperative outcomes of esophagectomy preceded by the laparoscopic transhiatal approach for esophageal cancer. Dis Esophagus. 2014;27:470–478. doi: 10.1111/j.1442-2050.2012.01439.x. [DOI] [PubMed] [Google Scholar]
- 7.Vierra M. Minimally invasive surgery. Annu Rev Med. 1995;46:147–158. doi: 10.1146/annurev.med.46.1.147. [DOI] [PubMed] [Google Scholar]
- 8.Luketich JD, Alvelo-Rivera M, Buenaventura PO, Christie NA, McCaughan JS, Litle VR, et al. Minimally invasive esophagectomy: outcomes in 222 patients. Ann Surg. 2003;238:486–494. doi: 10.1097/01.sla.0000089858.40725.68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Luketich JD, Nguyen NT, Weigel T, Ferson P, Keenan R, Schauer P. Minimally invasive approach to esophagectomy. JSLS. 1998;2:243–247. [PMC free article] [PubMed] [Google Scholar]
- 10.Smithers BM, Gotley DC, Martin I, Thomas JM. Comparison of the outcomes between open and minimally invasive esophagectomy. Ann Surg. 2007;245:232–240. doi: 10.1097/01.sla.0000225093.58071.c6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cash JC, Zehetner J, Hedayati B, Bildzukewicz NA, Katkhouda N, Mason RJ, et al. Outcomes following laparoscopic transhiatal esophagectomy for esophageal cancer. Surg Endosc. 2014;28:492–499. doi: 10.1007/s00464-013-3230-y. [DOI] [PubMed] [Google Scholar]
- 12.Xie MR, Liu CQ, Guo MF, Mei XY, Sun XH, Xu MQ. Short-term outcomes of minimally invasive Ivor-Lewis esophagectomy for esophageal cancer. Ann Thorac Surg. 2014;97:1721–1727. doi: 10.1016/j.athoracsur.2014.01.054. [DOI] [PubMed] [Google Scholar]
- 13.Mu J, Yuan Z, Zhang B, Li N, Lyu F, Mao Y, et al. Comparative study of minimally invasive versus open esophagectomy for esophageal cancer in a single cancer center. Chin Med J (Engl) 2014;127:747–752. [PubMed] [Google Scholar]
- 14.Cense HA, Busch OR, Bemelman WA, Obertop H, van Lanschot JJ. Results of the combination of open transthoracic esophagectomy with laparoscopic gastric tube formation for esophageal cancer. Dig Surg. 2006;23:164–168. doi: 10.1159/000094350. [DOI] [PubMed] [Google Scholar]
- 15.Oh DK, Hur H, Kim JY, Han SU, Cho YK. V-shaped liver retraction during a laparoscopic gastrectomy for gastric cancer. J Gastric Cancer. 2010;10:133–136. [Google Scholar]
- 16.Song KY, Kim SN, Park CH. Laparoscopy-assisted distal gastrectomy with D2 lymph node dissection for gastric cancer: technical and oncologic aspects. Surg Endosc. 2008;22:655–659. doi: 10.1007/s00464-007-9431-5. [DOI] [PubMed] [Google Scholar]
- 17.Law S, Cheung MC, Fok M, Chu KM, Wong J. Pyloroplasty and pyloromyotomy in gastric replacement of the esophagus after esophagectomy: a randomized controlled trial. J Am Coll Surg. 1997;184:630–636. [PubMed] [Google Scholar]
- 18.Kim HH, Han SU, Kim MC, Hyung WJ, Kim W, Lee HJ, et al. ERRATUM: Correction for the number of the recruited patients and the participating institutions. Prospective randomized controlled trial (phase III) to comparing laparoscopic distal gastrectomy with open distal gastrectomy for gastric adenocarcinoma (KLAS 01) Ann Surg Treat Res. 2014;87:51–52. [Google Scholar]
- 19.Kim W, Song KY, Lee HJ, Han SU, Hyung WJ, Cho GS. The impact of comorbidity on surgical outcomes in laparoscopy-assisted distal gastrectomy: a retrospective analysis of multicenter results. Ann Surg. 2008;248:793–799. doi: 10.1097/SLA.0b013e3181887516. [DOI] [PubMed] [Google Scholar]
- 20.Ackroyd R, Watson DI, Majeed AW, Troy G, Treacy PJ, Stoddard CJ. Randomized clinical trial of laparoscopic versus open fundoplication for gastro-oesophageal reflux disease. Br J Surg. 2004;91:975–982. doi: 10.1002/bjs.4574. [DOI] [PubMed] [Google Scholar]
- 21.Patti MG, Pellegrini CA, Horgan S, Arcerito M, Omelanczuk P, Tamburini A, et al. Minimally invasive surgery for achalasia: an 8-year experience with 168 patients. Ann Surg. 1999;230:587–593. doi: 10.1097/00000658-199910000-00014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Levy RM, Wizorek J, Shende M, Luketich JD. Laparoscopic and thoracoscopic esophagectomy. Adv Surg. 2010;44:101–116. doi: 10.1016/j.yasu.2010.05.002. [DOI] [PubMed] [Google Scholar]
- 23.Nagpal K, Ahmed K, Vats A, Yakoub D, James D, Ashrafian H, et al. Is minimally invasive surgery beneficial in the management of esophageal cancer? A meta-analysis. Surg Endosc. 2010;24:1621–1629. doi: 10.1007/s00464-009-0822-7. [DOI] [PubMed] [Google Scholar]
- 24.Ben-David K, Sarosi GA, Cendan JC, Howard D, Rossidis G, Hochwald SN. Decreasing morbidity and mortality in 100 consecutive minimally invasive esophagectomies. Surg Endosc. 2012;26:162–167. doi: 10.1007/s00464-011-1846-3. [DOI] [PubMed] [Google Scholar]
- 25.Shiozaki A, Fujiwara H, Konishi H, Morimura R, Komatsu S, Murayama Y, et al. Middle and lower esophagectomy preceded by hand-assisted laparoscopic transhiatal approach for distal esophageal cancer. Mol Clin Oncol. 2014;2:31–37. doi: 10.3892/mco.2013.201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Makuuchi H. Reconstruction after thoracic esophagectomy. Nihon Geka Gakkai Zasshi. 2008;109:256–260. [PubMed] [Google Scholar]
- 27.Zhang C, Wu QC, Hou PY, Zhang M, Li Q, Jiang YJ, et al. Impact of the method of reconstruction after oncologic oesophagectomy on quality of life: a prospective, randomised study. Eur J Cardiothorac Surg. 2011;39:109–114. doi: 10.1016/j.ejcts.2010.04.032. [DOI] [PubMed] [Google Scholar]



