Abstract
Background:
Alarming rise of vancomycin-resistant enterococci (VRE) is a global cause of concern. Several factors have been held responsible for such rise, of which antibiotic usage is a prominent one.
Objective:
This study was undertaken to determine the intestinal VRE colonization rate amongst hospitalized patients in relation to use of various antibiotics in the Intensive Care Unit (ICU) of a tertiary care university hospital, India.
Materials and Methods:
Stool samples were collected weekly from all the patients in the adult ICU for a period of 6 months and processed for isolation and phenotypic and genotypic characterization of VRE isolates. Patient and treatment details were noted and cases (those with VRE in stool) and controls (those without VRE in stool) were compared statistically. Further, a multivariate analysis was done to identify those antibiotics as independent risk factors for VRE colonization.
Results:
VRE colonization was found in 34.56% (28/81) of the patients studied, with the majority 75% (21/28) carrying the vanA gene. The cases had significantly more (P < 0.05) duration of hospital stay and antibiotic exposure. Intake of metronidazole, vancomycin, and piperacillin-tazobactam were identified as significant risk factors both in univariate and multivariate analysis.
Conclusion:
A potential reservoir of VRE was thus revealed even in low VRE prevalence setting. Based on this high colonization status, restriction of empirical antibiotic use, reviewing of the ongoing antibiotic policy, and active VRE surveillance as an integral part of infection control strategy were suggested.
Keywords: Antibiotics, colonization, hospitalized, surveillance, vancomycin-resistant enterococci
INTRODUCTION
During the last few years, there has been an alarming rise of vancomycin-resistant enterococci (VRE), especially in the hospital environment of developed countries like Europe and USA.[1] Of the several causes responsible for such rise, excessive antibiotics use in hospitals leading to increased VRE transmission is a prominent one.[2] Situations might be similar in developing nations too where factors responsible for such increase are very much present but scarce data to document. Certain classes of antibiotics have been associated with establishment and persistence of VRE gut colonization in the hospitalized patients though few studies have documented it. Prevalence of VRE in clinical infections have been studied in India, but there is no substantial data of the many reservoirs of VRE whether within the hospital environment or outside. Moreover, even in countries where VRE infections have not been remarkable, potential intestinal VRE reservoirs have been reported.[3] To meet this end, the following pilot study was undertaken to determine the intestinal VRE colonization rate in the hospitalized patients of the adult Intensive Care Unit (ICU) in relation to the use of various antibiotics.
MATERIALS AND METHODS
Study setting
The study was carried out prospectively in the 25 bedded adult ICU of a 1200 bedded tertiary care center catering to the need of approximately 30 crore population in the northern part of India for a period of 6 months. Active VRE surveillance was not a part of hospital infection control policy in our hospital during the conduct of this study.
Microbiological study
Stool samples were collected as early as within 2 days of admission in the ICU irrespective of their previous hospitalization status and then weekly until discharge from all the patients and transported to the microbiology laboratory. Samples were then plated directly on bile esculin azide agar (BEAA) with and without 6 μg/ml vancomycin (HiMedia, Mumbai) and also following enrichment in BEA broth with 6 μg/ml vancomycin for 24 h at 37°C. All the plates were incubated aerobically at 37°C for 48 h. Four to five colonies with dark brown halo morphologically resembling enterococcal colonies were selected and further tested by Gram's stain, PYRase test and growth in 6.5% NaCl. Speciation of the isolates was done based on standardized biochemical classification.[4] Enterococcus fecalis ATCC 29212 and previously characterized vanA E. faecium strains were taken as controls. Minimum inhibitory concentration (MIC) for vancomycin and teicoplanin were determined by agar dilution method.[5] Detection of the resistant determinants were done by polymerase chain reaction amplification of vanA, vanB, and vanC genes.[6]
Study design and data collection
A case-control study was performed considering the VRE culture positive patients as cases and VRE screening culture negative patients as controls. Clinical data were collected from the available patient treatment records based on age, sex, underlying diseases, clinical diagnosis, severity of illness, invasive procedures, length of hospital stay including days in ICU, antibiotics used for more than 3 days, and duration of exposure to antibiotics.
Statistical analyses
Patient characteristics and treatment history of the VRE positive patients as cases and VRE negative patients as controls were statistically analyzed (odd's ratio were calculated and compared with Fischer's exact test using SPSS version 15, SPSS Inc, Chicago) and studied. Continuous variables were compared by Wilcoxon sum test. Significance was set at P < 0.05. A multivariate analysis was performed for VRE carriage and prior antibiotic use. VRE carriage was the dependent variable in logistic regression. Use of metronidazole, vancomycin, and piperacillin-tazobactam were included as variables in the model.
RESULTS
A total of 252 stool samples were collected from 81 patients during the study period. VRE was isolated from 28 patients (34.56%) with decreased susceptibility to vancomycin and teicoplanin as determined by their MIC values. In these, 20 isolates were identified as E. faecium containing vanA gene (MIC for vancomycin 128 µg/ml, teicoplanin 32 µg/ml), 1 E. fecalis with vanA gene (MIC for vancomycin 64 µg/ml, teicoplanin 16 µg/ml), and 7 E. gallinarum containing vanC gene (4 isolates with MIC for vancomycin 12 µg/ml, 3 isolates with MIC for vancomycin 8 µg/ml).
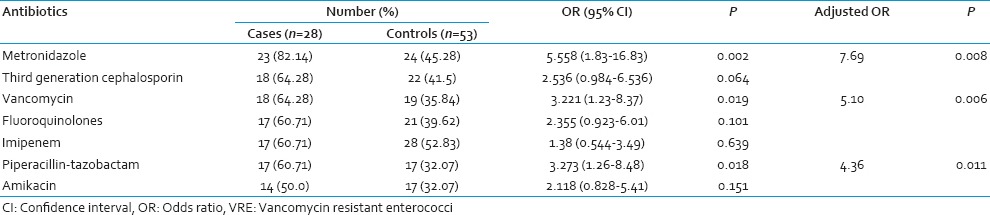
All the initial VRE culture positive cases remained so throughout their stay in the ICU. However, 3 patients with initial VRE culture negative status became positive on subsequent screening. All the 3 isolates were identified as vanA containing E. faecium. The demographic and epidemiological characteristics of the cases and controls have been summarized in Table 1. Sex and age were comparable in both the groups but mortality was significantly more (P < 0.05) in VRE carriers. Mean duration of antibiotic intake prior to the first detection of VRE and hospital stay were significantly more amongst the cases than controls. Among the controls, 7 patients did not receive any antibiotic throughout the study period. VRE carriers were significantly associated with intake of antibiotics like metronidazole, vancomycin, and piperacillin-tazobactam, both in the univariate and multivariate analysis [Table 2].
Table 1.
Risk factors associated with VRE carriage in hospitalized patients
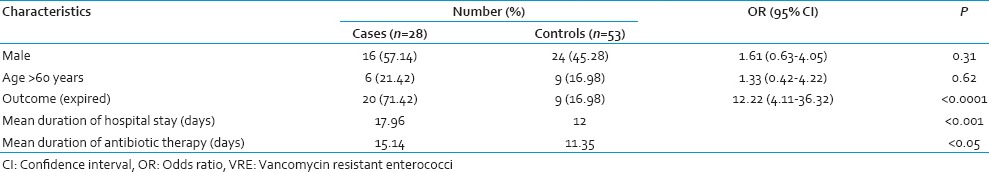
Table 2.
Association of VRE carriage with antibiotics in hospitalized patients
DISCUSSION
It is a known fact that “drug selection pressure” contributes immensely in the evolution of multidrug resistant organisms.[7] Emergence of VRE has been regarded to provide an “illustrative example” of various associations of antibiotic usage and level of resistance in both hospital and community environments.[8] In this study, it was found that a potential nosocomial reservoir of VRE in the ICU exists despite the lack of any major outbreak of VRE yet reported.
Several studies have revealed the association of different antibiotics with VRE colonization. Of these, the most significant were extended-spectrum cephalosporins and agents with potent antianaerobic activities. A previous meta-analysis considering a good number of studies have already shown a significant association between administration of antianaerobic agents like metronidazole with VRE colonization.[9] Similarly, in this study metronidazole was significantly associated with VRE carriage. In India, metronidazole is the third most commonly prescribed antibiotic in the community,[10] along with its irrational use in almost all cases of diarrhea as an antiprotozoal in fixed dose combinations.[11] In our hospital too, metronidazole is prescribed prophylactically to provide potent antianaerobic activity. Therefore, its association with VRE is nothing unexpected but indicative of its widespread use.
The above-mentioned analysis also showed a significant association between broad-spectrum cephalosporins administration and VRE colonization. However, we did not find any significant association with third generation cephalosporin despite the notable increase in the purchase of cephalosporin in the country.[7] In this context, similar findings of a “nonsignificant trend” in the association between the purchase of third generation cephalosporin and VRE and no association of the extended spectrum like cefepime have been studied.[12] Mixed results with ceftriaxone have been reported.
Piperacillin-tazobactam, which has been rarely considered as a risk factor for VRE carriage showed a positive association in our study. A statistically significant association between the purchase of ticarcillin-clavulanate combination and VRE carriage along with the nonsignificant association with piperacillin-tazobactam purchase has been reported.[13] Instead piperacillin-tazobactam was associated with the persistence of high-level VRE colonization along with vancomycin and metronidazole as in our study. The protective effect of piperacillin-tazobactam on VRE colonization was not reflected in our study. This could be explained by the fact that the apparent protection provided by this antibiotic is appreciated in a moderately colonized gut and not in a heavily colonized one as in our case. Contrasting our findings, imipenem possessing both antianaerobic, and antienterococcal activities was not associated with VRE carriage, as against other reports. Amikacin, on the other hand, being a commonly used aminoglycoside was not associated as per most of the studies.
Vancomycin-resistant enterococci colonized patients clearly received antibiotics for longer duration along with increased hospital stay in the ICU as compared to controls, which is the most frequently identified risk factor in its epidemiology. The increased mortality in this group can relate to the use of more antibiotics owing to the seriousness of their illness.
Besides, a small sample size with skewed number of cases and controls, one of the major limitation of our study was that we could not perform VRE screening test prior to admission in ICU and consequently could not comment on whether the studied antibiotics led to the persistence of a previously VRE colonized gut or promoted the establishment of gut colonization. The exact VRE density was not measured. However, the majority of the isolates (21 of 28, 75%) were detected directly even when unenriched. This might be due to higher VRE load in the stool due to constant antibiotic exposure in the ICU. This requires attention as it has been shown that the VRE load and ease of transmission are directly related.[8]
Even though no major VRE outbreak is a cause of concern in our hospital, the study is of importance as often patient's gastrointestinal tract has been seen to be a possible reservoir for VRE even in hospitals where VRE infections have not yet been reported.[14] In the study period, we did not find any VRE from hand samples of the ICU staff or environmental samples in the ICU, which could explain the nonoccurrence of any outbreak. Despite this potential reservoir in the hospital environment, not only alerts for proper infection control practices, but also indirectly hints towards optimization of antibiotic usage. In addition, it should be emphasized that excessive antibiotic use in the ICU is a modifiable risk factor that can be controlled by a judicious and prudent approach. We recommend a reconsideration of the empirical antibiotic prescribing practice in the ICU based on the high VRE colonization status.
CONCLUSION
Because antibiotic usage causes several changes in the gut ecology, the effects of antibiotics on VRE colonization are not simple. However, owing to the fact that since last few years a steady and bothering increase in VRE has been seen among clinical and commensal isolates and previous studies in the same ward of this hospital had reported vanA containing vancomycin-intermediate Staphylococcus aureus isolates in patients with VRE in their stool,[15] active VRE surveillance should be included as an integral part of hospital infection control policy especially with developing countries with notable consumption of antibiotics.
ACKNOWLEDGMENT
We thank the entire staff of the ICU for helping us in conducting the study.
Footnotes
Source of Support: Nil.
Conflict of Interest: None declared.
REFERENCES
- 1.Harbarth S, Cosgrove S, Carmeli Y. Effects of antibiotics on nosocomial epidemiology of vancomycin-resistant enterococci. Antimicrob Agents Chemother. 2002;46:1619–28. doi: 10.1128/AAC.46.6.1619-1628.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Murray BE. Vancomycin-resistant enterococcal infections. N Engl J Med. 2000;342:710–21. doi: 10.1056/NEJM200003093421007. [DOI] [PubMed] [Google Scholar]
- 3.Gordts B, Van Landuyt H, Ieven M, Vandamme P, Goossens H. Vancomycin-resistant enterococci colonizing the intestinal tracts of hospitalized patients. J Clin Microbiol. 1995;33:2842–6. doi: 10.1128/jcm.33.11.2842-2846.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Facklam RR, Collins MD. Identification of Enterococcus species isolated from human infections by a conventional test scheme. J Clin Microbiol. 1989;27:731–4. doi: 10.1128/jcm.27.4.731-734.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Vol. 31. Wayne, PA: Clinical Laboratory and Standards Institute; 2011. Clinical Laboratory and Standards Institute. Performance Standards for Antimicrobial Susceptibility Testing; 21st Informational Supplement, M100-S21; pp. 84–87. [Google Scholar]
- 6.Arbour N, Weirich A, Cornejo-Palma D, Prevost S, Ramotar K, Harder CJ. Real-time PCR Detection of VRE. Spartan Bioscience. 2008:1–3. [Google Scholar]
- 7.Ganguly NK, Arora NK, Chandy SJ, Fairoze MN, Gill JP, Gupta U, et al. Rationalizing antibiotic use to limit antibiotic resistance in India. Indian J Med Res. 2011;134:281–94. [PMC free article] [PubMed] [Google Scholar]
- 8.Barbosa TM, Levy SB. The impact of antibiotic use on resistance development and persistence. Drug Resist Updat. 2000;3:303–11. doi: 10.1054/drup.2000.0167. [DOI] [PubMed] [Google Scholar]
- 9.Cosgrove S, Harbarth S, Carmeli Y. Abstr. 12th Annual Scientific SHEA Meeting abstr. 72. 2002 [Google Scholar]
- 10.Lalan BK, Hiray RS, Ghongane BB. Drug prescription pattern of outpatients in a tertiary care teaching hospital in Maharashtra. Int J Pharm Bio Sci. 2012;3:225–9. [Google Scholar]
- 11.Chakrabarti A. Prescription of fixed dose combination drugs for diarrhoea. Indian J Med Ethics. 2007;4:165–7. doi: 10.20529/IJME.2007.065. [DOI] [PubMed] [Google Scholar]
- 12.Rice LB. Antimicrobial resistance in gram-positive bacteria. Am J Med. 2006;119:S11–9. doi: 10.1016/j.amjmed.2006.03.012. [DOI] [PubMed] [Google Scholar]
- 13.Donskey CJ, Schreiber JR, Jacobs MR, Shekar R, Salata RA, Gordon S, et al. A polyclonal outbreak of predominantly VanB vancomycin-resistant enterococci in northeast Ohio. Northeast Ohio Vancomycin-Resistant Enterococcus Surveillance Program. Clin Infect Dis. 1999;29:573–9. doi: 10.1086/598636. [DOI] [PubMed] [Google Scholar]
- 14.Gambarotto K, Ploy MC, Turlure P, Grélaud C, Martin C, Bordessoule D, et al. Prevalence of vancomycin-resistant enterococci in fecal samples from hospitalized patients and nonhospitalized controls in a cattle-rearing area of France. J Clin Microbiol. 2000;38:620–4. doi: 10.1128/jcm.38.2.620-624.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Banerjee T, Anupurba S. Colonization with vancomycin-intermediate Staphylococcus aureus strains containing the VanA resistance gene in a tertiary-care center in North India. J Clin Microbiol. 2012;50:1730–2. doi: 10.1128/JCM.06208-11. [DOI] [PMC free article] [PubMed] [Google Scholar]


