Abstract
Background and Objectives:
Febrile neutropenia (FN) is considered a medical emergency. Patients with hematological malignancies (HM) commonly experience FN. Broad spectrum antibiotics have to be started empirically to prevent complications. This study depicts the clinical profile, microbiological profile, antibiotic sensitivity pattern, and outcome in high risk HM.
Materials and Methods:
In this prospective study, 72 patients with hematologic malignancies, diagnosed and treated for 108 high risk febrile neutropenic episodes from August 2011 to January 2013 at a Regional Cancer Center, in South India were analyzed. Cefoperazone-sulbactum was used as a first-line empiric antibiotic.
Results:
Majority of the patients with FN episodes had acute myeloid leukemia. Overall culture positivity was 29.62%. The most common organisms isolated were Gram-negative bacilli (63.64%), with Escherichia coli being the most frequent pathogen. All Gram-negative organisms were sensitive to imipenem, whereas sensitivity pattern to other antibiotics were as follows: 85.71%, 78.26%, 69.52%, 63.64%, 41.66% and 47.05% for pipercillin-tazoactum, meropenem, cefoperazone-sulbactum, amikacin, ceftazidime, ciprofloxacin respectively. Overall mortality was 13.5%. Most of the patients responded to empiric antibiotic cefoperazone-sulbactum.
Conclusions:
In the hematologic malignancies particularly in acute leukemia, there is high risk of developing FN. Empiric therapy with cefoperazone-sulbactum as a first line leads to satisfactory outcome in high risk FN and therapy should be tailored to the most appropriate antibiotics according to the bacterial culture results.
Keywords: Antibiotics, febrile neutropenia, Gram-negative organism, hematological malignancies, treatment
INTRODUCTION
Febrile neutropenia (FN) is a medical emergency. Fever in neutropenia is influenced by prolonged duration and severity of neutropenia.[1] It leads to prolonged hospital stay, increases the cost of treatment, morbidity and mortality. FN commonly affects gastrointestinal tract (GIT), where chemotherapy induced mucosal damage causes blood stream invasion by gut flora. Invasive devices like central venous catheter (CVC) also become a source for blood stream infections.[2] The other sites affected include respiratory system and skin infections. Inadvertently, FN leads to decrease in dose intensity and poor outcome of cancer.[3] With the use of empiric antibiotic, the mortality due to FN has gradually reduced in cancer patients from 75% to <10%.[4,5] More so, with the availability of broad spectrum antibiotics, highly aggressive chemotherapeutic regimens are being used. While there are innumerable studies from the Western countries with respect to FN and the infectious agent, choice of empiric antibiotic and outcome, data from India is relatively less. Hence, we thought it is worthwhile to evaluate the clinical profile, pathogenic organisms, pattern of antibiotic sensitivity and the outcome of treatment in high risk hematological malignancies (HM) with FN.
MATERIALS AND METHODS
In this prospective study, 72 patients with HM, diagnosed and treated for 108 high risk febrile neutropenic events from August 2011 to January 2013 at a Regional Cancer Center, in South India were evaluated. Consent was taken from all patients. All patients were older than 15 years. FN was diagnosed in the occurrence of a single oral temperature of ≥38.3°C (101°F) or 38.0°C (100.4°F) for more than 1 h along with an absolute neutrophil count (ANC) ≤500/µl or ≤1000/µl with predicted rapid decline during next 48 h.[2] All febrile patients were evaluated for relevant history, physical examination, complete hemogram, liver function test, renal function test, serum electrolytes and chest X-ray. Microbiological cultures of blood from peripheral vein, central line (if present) and urine were conducted routinely for all febrile episodes. Automated blood culture system was used for blood culture. Sputum, stool and/or pus cultures were done when clinically indicated. Blood culture (two sets of blood culture, 10 ml each, one from central line and the other from peripheral line) was repeated every 48 h if fever persisted. Organisms were identified according to standard bacteriological procedures. Antibiotic susceptibility testing was carried out in the first half of the study using the Kirby-Bauer disk diffusion method. Computed tomography (CT) scan of paranasal sinuses was done if patients had paranasal sinus tenderness or if fungal infection of the sinus was suspected. CT scan thorax was done in patients who had pleuritic chest pain; chest X-ray was suggestive of fungal mass or infiltrate or in clinical suspicion of fungal infection.
These patients were categorized as high risk based on Infectious Diseases Society of America guidelines. It includes patients with profound neutropenia ≤100 cells/µl, neutropenia anticipated to extend beyond 7 days, significant comorbid conditions, hemodynamic instability, pneumonia, new onset of abdominal pain or neurologic change.[6] All patients received standard chemotherapy. No patient received high dose chemotherapy.
All FN patients received cefoperazone/sulbactum empirically. Once microbiological culture report was available, antibiotics were modified accordingly. However, in culture negative patients same antibiotic was continued. Vancomycin was administered additionally to the patients, who had persistent fever, hypotension, redness or tenderness at central line insertion site and pneumonia. Amphotericin B was initiated empirically in patients in whom fever persisted despite antibiotics on day 4 or 5, in sinusitis with suspected fungal infection, pleuritic chest pain or chest X-ray suggested presence of fungal ball. In culture negative and stable patients intravenous antibiotics were continued for 5–7 days or until ANC recovered to >500/µl. Subsequently, the patients were started on oral antibiotics which included amoxicillin/clavulanate, ciprofloxacin or levofloxacin on an outpatient basis. Bacterial pathogens in all samples yielding culture positivity were identified and their antibiogram was recorded. Diagnosis, clinical features, chest X-ray findings, type of chemotherapy, duration of neutropenia, use of growth factor, culture positivity, antibiotic use and outcome of FN were recorded for all patients. These outcomes were evaluated for all SPSS 19 - SPSS Inc., IL, USA. It's a software package used for statistical analysis. SPSS is the Statistical Package for the Social Sciences.
RESULTS
A total of 72 patients with HM were diagnosed and treated for 108 FN events. Totally 22 patients had CVC, 21 had recurrent FN (episodes 14 patients had two episodes, 2 patients had three episodes, 2 had four episodes and 3 patients had five episodes of FN). Acute myeloid leukemia (55 episodes) was the most common etiology in our study followed by non Hodgkins lymphoma (31 episodes) and acute lymphoblastic leukemia (16 episodes). Only in 25% episodes growth factor has been used. Majority of the patients with FN had neutropenia for more than 7 days (72/108 episodes, 76.67%). Each of these 108 FN events were analyzed for associated clinical symptoms as shown in Table 1. Most common site of infection was the GIT (18.52%).
Table 1.
Clinical profile
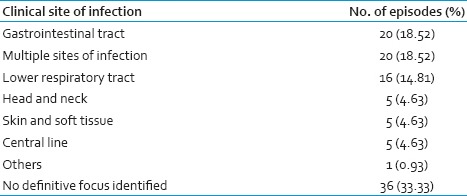
Overall the culture positivity was identified in 32 of 108 episodes (29.62%): (Blood culture 19.44%, sputum 7.41% and pus 2.77%). Poly-microbial infection was noted in 4.62% of cases. Gram-negative bacilli (GNB) (63.64%) were isolated more commonly followed by Gram-positive cocci (GPC) (36.36%). The details of which are given in Table 2. Among Gram-negative infections, Escherichia coli was the most common isolate (32.14%) followed by Klebsiella pneumoniae, Acinetobacter baumannii and Pseudomonas aeruginosa (25%, 21.43%, and 17.86%) respectively. Staphylococcus aureus was the most common isolate amongst Gram-positive infections (three were methicillin-resistant S. aureus [MRSA]). Blood culture was positive was in 21/108 (65.62% of all culture positive) episodes. In blood culture, 12 and 9 isolates were GNB and GPC respectively. GPC accounted for 42.86% of blood stream infection with S. aureus being the most common isolate. Sputum culture was positive in 7/108 (7.41%) of FN episodes. The most common isolate in sputum was P. aeruginosa and E. coli followed by K. pneumoniae, A. baumannii and S. aureus. Fungi were isolated in four sputum samples with two samples yielding Aspergillus species and two Candida species. No bacterial pathogens were isolated from urine.
Table 2.
Bacteriological profile
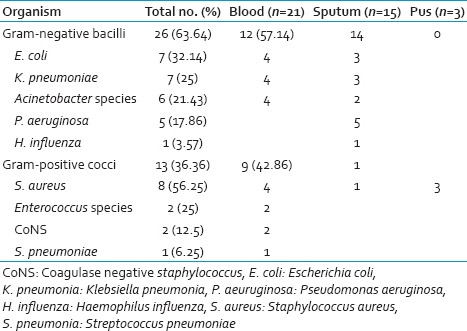
The antibiotic sensitivity among GNB was highest for imipenem (100%), followed by pipercillin-tazoactum (86.95%). Most of the E. coli isolates were resistant to cefoperazone-sulbactum, piperacillin-tazobactum. Gram-positive isolates including MRSA were uniformly sensitive to vancomycin, teicoplanin, linezolid and levofloxacin. With first line antibiotic (cefoperazone-sulbactum), 68.5% FN episodes showed an improvement, 26% episodes improved with second line (piperacillin-tazobactum with amikacin), another 5.5% required third line antibiotics (imipenem/cilastin or meropenem). Additional vancomycin was required in 14.5% FN episodes, while 17% episodes required addition of antifungal agent on day 5 due to unresponsive fever.
Fifteen (13.5%) patients died due to septicemia (8 acute myeloid leukemia, 3 diffuse large B-cell lymphoma, 2 peripheral T-cell lymphoma, 1 acute lymphoblastic leukemia and 1 acute promyelocytic leukemia). Six patients died of pneumonia (5-bacterial infection and 1-fungal pneumonia). Four patients succumbed to blood stream infections. In five patients, the source of infection could not be identified. One patient developed multiorgan dysfunction and one had septicemic shock. In eight patients who have died, a bacterial pathogen was isolated either from blood or sputum (6-GNB, 2-GPC). Most of the acute myeloid leukemia deaths (7/8) occurred during induction chemotherapy.
DISCUSSION
This study was undertaken to review the clinical profile, antibiotic sensitivity pattern, severity of infection and outcome of treatment in FN with high risk HM patients. We found FN to be most common in acute myeloid leukemia patients on chemotherapy. Similar incidence was also seen in other studies.[7,8] This high incidence of FN in acute myeloid leukemia could be attributed to the use of intensive chemotherapy leading to prolonged and profound neutropenia, which increases the risk of infection. Similar to other studies FN more commonly occurred during induction chemotherapy, than during consolidation and maintenance chemotherapy.[9,10] In this study, majority of the patients had prolonged neutropenia (>7 days) and had more episodes of FN (both bacterial and fungal) with associated morbidity and mortality, which was in concordance to other studies.[11] Bacterial culture yielded pathogens in 29.62% of the FN episodes. Culture positive episodes have been identified in 15–38% patients in other studies.[7,8,9,10,12] In the western countries, while infections with GNB predominated during 1970 and 80, a predominant shift to GPC infections has been reported in later years.[13,14,15,16] This is usually attributed to frequent use of indwelling CVCs and the use of fluoroquinolone prophylaxis which suppresses the aerobic GNB colonizing the GIT but fails to suppress the microaerophilic GPC.[17,18] In our study only three cases with MRSA were identified, all of which were vancomycin susceptible. In contrast to the Western studies, studies from India including the present one still find GNB as the predominant infection in HM.[12,19,20,21] Overall E. coli was found to be the most common isolate including all cultures and S. aureus was the most common isolate from blood. Similar results were also reported from other studies.[9] The reason for low incidence of S. aureus infection in our study is due to infrequent use of CVCs. Increased incidence of S. aureus isolates from the blood in our study was due to use of CVCs in all acute leukemia patients. Also Gram-negative isolates from only two cases produced extended spectrum beta lactamases. We found that GNB were highly sensitive to imipenem, piperacillin/tazobactum and meropenem. They were also moderately sensitive to amikacin and cefoperazone/sulbactum. Most of the GNB showed high resistance to third generation cephalosporins. Similar antibiotic sensitivity pattern of high sensitivity of GNB to carbapenems, piperacillin/tazobactum, cefoperazone/sulbactum and resistance to third generation cephalosporins has been reported in studies.[22,23] Even though, in vitro sensitivity to cefoperazone/sulbactum was low, most patients responded to it clinically, when administered empirically. Sensitivity to fluoroquinolones is low among GNB with higher resistance seen in E. coli. All S.aureus including MRSA were highly sensitive to amikacin, linezolid, vancomycin, teicoplanin, and levofloxacin. Overall mortality in this study was 13.50%. All the deaths were attributed to infections. Similar results have been found in other studies both from India and abroad.[4,7] Cancer patients on chemotherapy are significantly predisposed to FN due to immune deficiency mediated either by the underlying malignancy and/or anticancer chemotherapy and mucosal breach due to chemotherapy.[6] This is reflected by the fact that nearly 80% of these infections are due to endogenous flora.[24] With the present menace of antibiotic resistant “superbugs,” judicious use of available antibiotics is the need of an hour. The strength of our study is that it is a large single institutional study which has taken into account the FN in HM. Though observational, it can go a long way in transforming the health policies and antibiotic policies in our tertiary care oncology center. Furthermore, the importance of clinical judgment and not chasing laboratory reports is well emphasized through this study. For instance though cefoperazone-sulbactum was only 70% sensitive according to the culture sensitivity, 70% of episodes did respond to the first line antibiotic suggesting an in vivo and in vitro discordance, which has been proven before. Since sporadic data exist from this part of the world, this study can be a useful guide for oncologists managing FN in India and building institutional antibiotic policy. The weakness of this study is that it is a nonrandomsied observational study.
CONCLUSION
This study attempts to highlight the common infectious agents, their antibiotic sensitivity pattern and outcome of high risk FN with cefoperazone-sulbactum. This study showed that FN was more common in acute leukemia during induction chemotherapy. Prolonged neutropenia was associated with increased risk of FN. Around 30% patients had culture proven infections with predominance of GNB. Empiric therapy with cefoperazone-sulbactum leads to satisfactory clinical outcome in high risk FN and indiscriminate use of higher antibiotics must be restricted even in this medical emergency and therapy should be tailored to the most appropriate antibiotic according to the bacterial culture.
Footnotes
Source of Support: Nil.
Conflict of Interest: None declared.
REFERENCES
- 1.Freifeld AG, Walsh TJ, Pizzo PA. Infectious complications in the pediatric cancer patient. In: Pizzo PA, Poplack DG, editors. Principles and Practice of Pediatric Oncology. Philadelphia: Lippincott-Raven; 1997. pp. 1069–114. [Google Scholar]
- 2.Hughes WT, Armstrong D, Bodey GP, Bow EJ, Brown AE, Calandra T, et al. 2002 guidelines for the use of antimicrobial agents in neutropenic patients with cancer. Clin Infect Dis. 2002;34:730–51. doi: 10.1086/339215. [DOI] [PubMed] [Google Scholar]
- 3.Lyman GH, Dale DC, Friedberg J, Crawford J, Fisher RI, et al. Incidence and predictors of low chemotherapy dose-intensity in aggressive non-Hodgkin's lymphoma: A nationwide study. J Clin Oncol. 2004;22:4302–11. doi: 10.1200/JCO.2004.03.213. [DOI] [PubMed] [Google Scholar]
- 4.Kang CI, Kim SH, Kim HB, Park SW, Choe YJ, Oh MD, et al. Pseudomonas aeruginosa bacteremia: Risk factors for mortality and influence of delayed receipt of effective antimicrobial therapy on clinical outcome. Clin Infect Dis. 2003;37:745–51. doi: 10.1086/377200. [DOI] [PubMed] [Google Scholar]
- 5.Kuderer NM, Dale DC, Crawford J, Cosler LE, Lyman GH. Mortality, morbidity, and cost associated with febrile neutropenia in adult cancer patients. Cancer. 2006;106:2258–66. doi: 10.1002/cncr.21847. [DOI] [PubMed] [Google Scholar]
- 6.Freifeld AG, Bow EJ, Sepkowitz KA, Boeckh MJ, Ito JI, Mullen CA, et al. Clinical practice guideline for the use of antimicrobial agents in neutropenic patients with cancer: 2010 update by the infectious diseases society of america. Clin Infect Dis. 2011;52:e56–93. doi: 10.1093/cid/cir073. [DOI] [PubMed] [Google Scholar]
- 7.Ghosh I, Raina V, Kumar L, Sharma A, Bakhshi S, Thulkar S, et al. Profile of infections and outcome in high-risk febrile neutropenia: Experience from a tertiary care cancer center in India. Med Oncol. 2012;29:1354–60. doi: 10.1007/s12032-011-9858-3. [DOI] [PubMed] [Google Scholar]
- 8.Cordonnier C, Herbrecht R, Pico JL, Gardembas M, Delmer A, Delain M, et al. Cefepime/amikacin versus ceftazidime/amikacin as empirical therapy for febrile episodes in neutropenic patients: A comparative study. The French Cefepime Study Group. Clin Infect Dis. 1997;24:41–51. doi: 10.1093/clinids/24.1.41. [DOI] [PubMed] [Google Scholar]
- 9.Advani SH, Kochupillai V, Lalitha N, Shanta V, Maitreyan V, Nair R, et al. Infections in the immunocompromised host: A prospective multicenter survey in patients receiving chemotherapy for acute leukemia. J Assoc Physicians India. 1996;44:769–73. [PubMed] [Google Scholar]
- 10.Jagarlamudi R, Kumar L, Kochupillai V, Kapil A, Banerjee U, Thulkar S. Infections in acute leukemia: An analysis of 240 febrile episodes. Med Oncol. 2000;17:111–6. doi: 10.1007/BF02796205. [DOI] [PubMed] [Google Scholar]
- 11.Talcott JA, Siegel RD, Finberg R, Goldman L. Risk assessment in cancer patients with fever and neutropenia: A prospective, two-center validation of a prediction rule. J Clin Oncol. 1992;10:316–22. doi: 10.1200/JCO.1992.10.2.316. [DOI] [PubMed] [Google Scholar]
- 12.Srivastava VM, Krishnaswami H, Srivastava A, Dennison D, Chandy M. Infections in haematological malignancies: An autopsy study of 72 cases. Trans R Soc Trop Med Hyg. 1996;90:406–8. doi: 10.1016/s0035-9203(96)90524-6. [DOI] [PubMed] [Google Scholar]
- 13.Yadegarynia D, Tarrand J, Raad I, Rolston K. Current spectrum of bacterial infections in patients with cancer. Clin Infect Dis. 2003;37:1144–5. doi: 10.1086/378305. [DOI] [PubMed] [Google Scholar]
- 14.Zinner SH. Changing epidemiology of infections in patients with neutropenia and cancer: Emphasis on gram-positive and resistant bacteria. Clin Infect Dis. 1999;29:490–4. doi: 10.1086/598620. [DOI] [PubMed] [Google Scholar]
- 15.Wisplinghoff H, Seifert H, Wenzel RP, Edmond MB. Current trends in the epidemiology of nosocomial bloodstream infections in patients with hematological malignancies and solid neoplasms in hospitals in the United States. Clin Infect Dis. 2003;36:1103–10. doi: 10.1086/374339. [DOI] [PubMed] [Google Scholar]
- 16.Viscoli C, Varnier O, Machetti M. Infections in patients with febrile neutropenia: Epidemiology, microbiology, and risk stratification. Clin Infect Dis. 2005;40 Suppl 4:S240–5. doi: 10.1086/427329. [DOI] [PubMed] [Google Scholar]
- 17.Edmond MB, Wallace SE, McClish DK, Pfaller MA, Jones RN, Wenzel RP. Nosocomial bloodstream infections in United States hospitals: A three-year analysis. Clin Infect Dis. 1999;29:239–44. doi: 10.1086/520192. [DOI] [PubMed] [Google Scholar]
- 18.Rubio M, Palau L, Vivas JR, del Potro E, Diaz-Mediavilla J, Alvarez A, et al. Predominance of gram-positive microorganisms as a cause of septicemia in patients with hematological malignancies. Infect Control Hosp Epidemiol. 1994;15:101–4. doi: 10.1086/646869. [DOI] [PubMed] [Google Scholar]
- 19.Madiajagane R. A retrospective study of infections in neutropenic patients. J Indian Med Assoc. 1998;96:239–40, 244. [PubMed] [Google Scholar]
- 20.Kumar L, Kochupillai V, Bhujwala RA. Infections in acute myeloid leukemia. Study of 184 febrile episodes. J Assoc Physicians India. 1992;40:18–20. [PubMed] [Google Scholar]
- 21.Das PK, Chhotaray MK, Rath RN, Kar S, Das B, Parida B. Infection in haematological malignancies. J Indian Med Assoc. 1994;92:328–30. [PubMed] [Google Scholar]
- 22.Swati M, Gita N, Sujata B, Farah J, Preeti M. Microbial etiology of febrile neutropenia. Indian J Hematol Blood Transfus. 2010;26:49–55. doi: 10.1007/s12288-010-0029-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kumar P, Medhekar A, Ghadyalpatil NS, Noronha V, Biswas S, Kurkure P, et al. The effect of age on the bacteria isolated and the antibiotic-sensitivity pattern in infections among cancer patients. Indian J Cancer. 2010;47:391–6. doi: 10.4103/0019-509X.73574. [DOI] [PubMed] [Google Scholar]
- 24.Schimpff SC, Young VM, Greene WH, Vermeulen GD, Moody MR, Wiernik PH. Origin of infection in acute nonlymphocytic leukemia. Significance of hospital acquisition of potential pathogens. Ann Intern Med. 1972;77:707–14. doi: 10.7326/0003-4819-77-5-707. [DOI] [PubMed] [Google Scholar]


