Abstract
Severe fever with thrombocytopenia syndrome (SFTS) is a tick-borne viral disease. The SFTS virus (SFTSV) has been detected in the Haemaphysalis longicornis, which acts as a transmission host between animals and humans. SFTSV was first confirmed in China in 2009 and has also been circulating in Japan and South Korea. However, it is not known if a genetic connection exists between the viruses in these regions and, if so, how SFTSV is transmitted across China, South Korea, and Japan. We therefore hypothesize that the SFTSV in South Korea share common phylogenetic origins with samples from China and Japan. Further, we postulate that migratory birds, well-known carriers of the tick H. longicornis, are a potential source of SFTSV transmission across countries. Our phylogenetic analysis results show that the SFTSV isolates in South Korea were similar to isolates from Japan and China. We connect this with previous work showing that SFTSV-infected H. longicornis were found in China, South Korea, and Japan. In addition, H. longicornis were found on migratory birds. The migratory bird routes and the distribution of H. longicornis are concurrent with the occurrence of SFTSV. Therefore, we suggest that migratory birds play an important role in dispersing H. longicornis-borne SFTSV.
Introduction
Severe fever with thrombocytopenia syndrome virus (SFTSV) is a tick-borne hemorrhagic fever virus of the genus Phlebovirus and family Bunyaviridae. SFTSV is an enveloped and tri-segmented (large [L], middle [M], and small [S]) negative-strand RNA virus. Phleboviruses have a broad geographic range and have been found in sand flies, mosquitoes, and ticks in the Americas, Asia, Africa, and Mediterranean region for many years.1,2 In 2012, another tick-borne Phlebovirus virus, the Heartland virus, was identified in northwestern Missouri in the United States.3 The major clinical symptoms of SFTS are acute and high fever (temperatures of 38°C or more), thrombocytopenia (platelet count < 100,000/mm3), leucopenia, elevated levels of serum hepatic enzymes, and multiorgan dysfunction.1,4,5
More recently, SFTSV has spread to eastern Asia. SFTSV was first reported in China in 2009 and became the first tick-borne Phlebovirus isolated in rural areas of Hubei and Henan provinces in central China (Figure 1 ).1 However, the etiology was not confirmed until 2011 following virus isolation.1 SFTS has been reported in at least 13 provinces in the central, eastern, and northeastern regions of China. Most patients are farmers living in wooded, hilly, or mountainous areas.1,4 In 2013, SFTS was also reported and confirmed in South Korea and in western regions of Japan.6–9
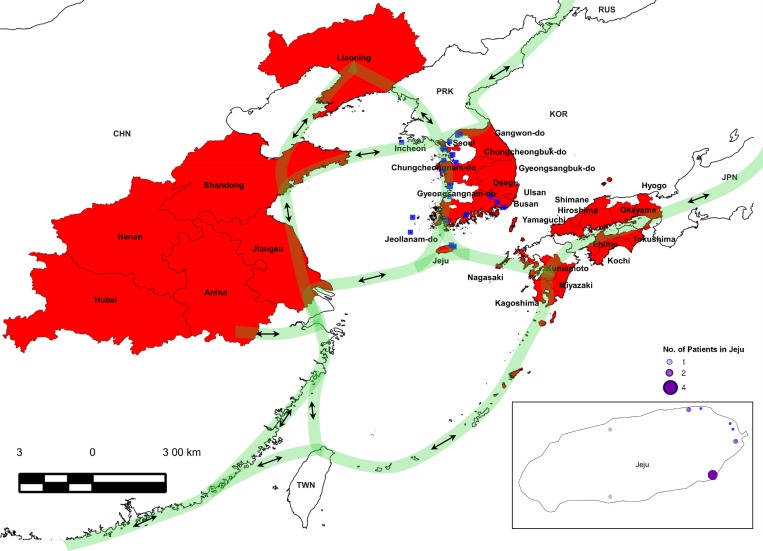
Figure 1.
Severe fever with thrombocytopenia syndrome (SFTS) patient distribution map and migration route of birds between China, South Korea, and Japan. The regions where SFTS patients were reported in China, South Korea, and Japan are shown in red, in Jeju are shown by purple circles, and the estimated migration route of birds are depicted by light green lines.1,6–12 Wetland habitats for birds in the Korean peninsula are indicated by blue squares.11
In China, SFTSV has an approximate case fatality rate of 12%. Retrospective analysis of cases in Japan show an even higher average case fatality rate, with four deaths out of eight confirmed cases.7,8 Additional suspected cases still need to be confirmed. In 2013, South Korea reported 36 SFTS cases with 17 fatalities, a 47.2% case fatality rate.9
Channels for SFTSV transition remains an important yet open question. SFTSV RNA has been detected in the ixodid tick Haemaphysalis longicornis, impacting both animals and human patients.1,4 As a consequence, H. longicornis has been identified as a vector of SFTSV.1,4–6
Migratory birds are regular hosts of H. longicornis and this tick species is widely distributed in the Asia–Pacific region. In addition, the tick distribution matches the bird migration flyways between China, South Korea, and Japan.1,4,10–16 This also coincides with the occurrence of SFTS, suggesting that migratory birds may play a role in the dissemination of SFTSV bearing H. longicornis.
Given this information, we conducted a phylogenetic analysis of SFTSV isolated from human patients in South Korea. We compared the results with known samples from Japan and China to analyze the connection between SFTSV across northeastern Asia. We also conducted a meta-analysis of literature on the role of H. longicornis and migratory birds in the spread and geographic expansion of SFTSV. We found a clear phylogenetic connection between our SFTSV samples from South Korea and those from Japan and China. We also found strong suggestive evidence that migratory birds are a key connection in the spread of tick-containing SFTSV.
Materials and Methods
SFTS patients.
A total of 17 SFTS cases were laboratory confirmed in this study (Table 1): 14 of these were treated at Jeju National University Hospital on Jeju Island (the southern end of South Korea) from May 2013 to August 2014, one confirmed case was from Kangwon National University Hospital in Chuncheon (the northern end of South Korea) in 2013, one confirmed case was from Keimyung University Dongsan Medical Center in Daegu (the central part of South Korea) in 2013, and one confirmed case was from Patima Medical Center in Daegu (the southern end of South Korea) in 2013. All patients had no history of travel to China or Japan. Of the patients, 14 were farmers living in wooded, hilly areas and three made visits to similarly wooded, hilly, or mountainous areas before the onset of symptoms. This study was approved by institutional review board of Jeju National University Hospital and all subjects signed an informed consent form.
Table 1.
Basic characteristics of SFTS patients
| Patients | Age (years)/sex | Location | Occupation | Date of sampling | Temperature (°C) | ANC | Platelet (× 103/μL) | ALP (IU/L) | AST (IU/L) | ALT (IU/L) | Outcome |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Jeju-JP01-Korea-2013 | 58/M | Jeju | Farmer | July 2013 | 38.6 | 650 | 35 | 580 | 612 | 230 | Recovered |
| Jeju-JP02-Korea-2013 | 73/M | Jeju | Rancher | May 2013 | 38.3 | 530 | 23 | 281 | 5,858 | 165 | Death |
| Jeju-JP03-Korea-2013 | 82/F | Jeju | Farmer | May 2013 | 39.1 | 770 | 67 | 130 | 90 | 22 | Death |
| Jeju-JP04-Korea-2013 | 63/M | Jeju | Rancher | June 2013 | 37.3 | 7,080 | 75 | 652 | 184 | 77 | Death |
| Jeju-JP05-Korea-2013 | 61/F | Jeju | Farmer | June 2013 | 36.9 | 1,090 | 62 | 510 | 242 | 89 | Recovered |
| Jeju-JP06-Korea-2013 | 37/F | Jeju | Farmer | August 2013 | 37.7 | 1,640 | 72 | 197 | 82 | 40 | Recovered |
| Jeju-JP07-Korea-2013 | 70/M | Jeju | Rancher | October 2013 | 38.0 | 1,570 | 58 | 211 | 116 | 64 | Recovered |
| Jeju-JP01-Korea-2014 | 67/F | Jeju | Farmer | June 2014 | 39.9 | 170 | 68 | 132 | 39 | 14 | Recovered |
| Jeju-JP02-Korea-2014 | 78/F | Jeju | Unemployed | June 2014 | 38.8 | 960 | 107 | 204 | 90 | 25 | Recovered |
| Jeju-JP03-Korea-2014 | 57/M | Jeju | Farmer | June 2014 | 37.6 | 700 | 24 | 182 | 178 | 72 | Recovered |
| Jeju-JP04-Korea-2014 | 51/M | Jeju | Rancher | July 2014 | 38.6 | 200 | 115 | 196 | 241 | 109 | Recovered |
| Jeju-JP05-Korea-2014 | 65/M | Jeju | Rancher | July 2014 | 37.6 | 140 | 50 | 211 | 127 | 77 | Recovered |
| Jeju-JP06-Korea-2014 | 78/F | Jeju | Farmer | July 2014 | 38.1 | 1,680 | 87 | 268 | 40 | 17 | Recovered |
| Jeju-JP07-Korea-2014 | 69/M | Jeju | Farmer | August 2014 | 38.1 | 1,750 | 152 | 181 | 57 | 38 | Recovered |
| Jeju-AP01-Korea-2013 | 67/M | Andong | Businessman | October 2013 | 37.2 | 1,444 | 49 | 160 | 77 | 39 | Death |
| Jeju-CP01-Korea-2013 | 59/M | Chuncheon | Logger | August 2013 | 37.3 | 3,904 | 10 | 240 | 924 | 214 | Recovered |
| Jeju-DP01-Korea-2013 | 59/M | Daegu | Businessman | October 2013 | 37.3 | 2,188 | 49 | 46 | 188 | 88 | Recovered |
ALP = alkaline phosphatase; ALT = alanine aminotransferase; ANC = absolute neutrophil count; AST = aspartate aminotransferase; F = female; M = male; SFTS = severe fever with thrombocytopenia syndrome.
RNA extraction.
Viral RNA was extracted from the serum of 17 SFTS patients using QlAamp Viral RNA Mini kit (Qiagen Inc., Mainz, Germany) according to the manufacturer's instructions. The extracted RNA was preserved in the elution buffer at − 70°C until introduction into a reverse transcriptase polymerase chain reaction (RT-PCR) assay.
Real-time RT-PCR.
Real-time RT-PCR of partial S and L segments was performed for molecular diagnosis of SFTSV.17,18 The two sets of primers used were forward primer (5′-ACCTCTTTGACCCTGAGTTWGACA-3′) and reverse primer (5′-CTGAAGGAGACAGGTGGAGATGA-3′) for the partial S segments17 and forward primer (5′-AGTCTAGGTCATCTGATCCGTTYAG-3′) and reverse primer (5′-TGTAACTTCGCCCTTTGTCCAT-3′) for the partial L segments.18 The real-time RT-PCR mixture contained 8 μL one-step RT-PCR premix (Thermo Scientific, made in EU), 7 μL detection solution, and 5 μL RNA template in a total volume of 20 μL. PCR was performed under the following conditions: 30 minutes at 45°C, 10 minutes at 90°C, and 45 cycles of 15 seconds at 95°C and 30 seconds at 48°C.
Reverse transcriptase polymerase chain reaction.
RT-PCR of complete S segments, which is highly conserved in the Bunyaviridae, was performed for phylogenetic analysis. The set of primers used were forward primer (5′-ACACAAAGAACCCCCAAAAA-3′) and reverse primer (5′-CAGGCTCAAGCTGGTGAAGA-3′) for the complete S segments. The RT-PCR mixture contained 8 μL one-step RT-PCR premix, 7 μL detection solution, and 5 μL RNA template in a total volume of 20 μL. PCR was performed under the following conditions: 30 minutes at 45°C, 10 minutes at 90°C, and 45 cycles of 15 seconds at 95°C and 30 seconds at 48°C.
Sequencing of complete S segments of SFTSV.
For the complete S segments, RT-PCR products of the complete S segment (1,746 bp) were electrophoresed on a 1.2% agarose gel and purified using a QIAEX II gel extraction kit (Qiagen Inc.) according to the manufacturer's instructions. They were then sequenced using a BigDye Terminator Cycle Sequencing Kit (PerkinElmer Applied Biosystems, Warrington, United Kingdom).
Phylogenetic analysis of complete S segments of SFTSV.
The complete sequences of S segments were aligned using the multiple-alignment algorithm in the MegAlign program (Windows version 3.12e; DNASTAR, Madison, WI) and the ClustalX program.19 On the basis of the aligned sequences, phylogenetic analyses were conducted in MEGA6 and phylogenetic trees were constructed by the maximum likelihood method.20 The bootstrap consensus tree inferred from 1,000 replicates was used to represent the evolutionary history of the taxa analyzed.20
Results
Molecular diagnostic and phylogenetic analysis of SFTSV.
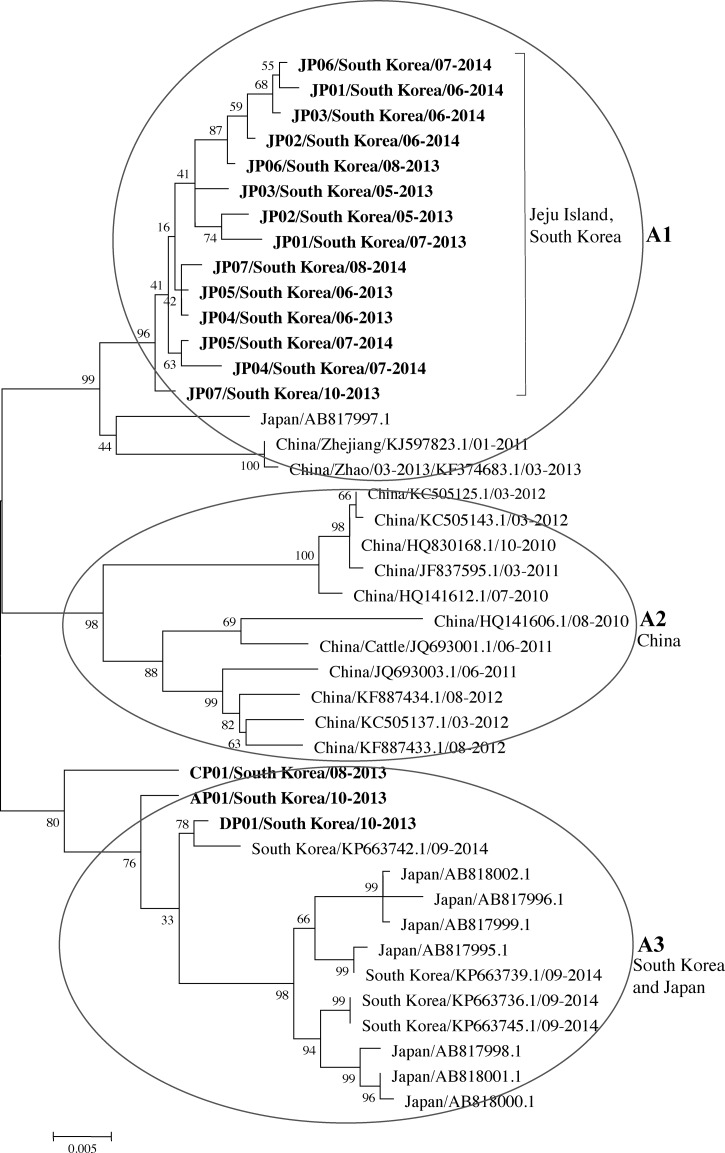
The partial S and L segments and complete S segment of SFTSV were confirmed from 17 SFTS patients by real-time RT-PCR and RT-PCR, respectively.17,18 Following dideoxy sequencing of these fragments, we undertook a phylogenetic analysis of the complete S sequences (1,746 bp), which is highly conserved in the Bunyaviridae. The resulting phylogenetic tree of the S segment shows that the isolates were similar to isolates from China and Japan and were separated into three groups based on the root (the A1 group included Jeju Island, South Korea, an isolate from Japan, and two isolates from China; the A2 group included China; and the A3 group included South Korea and Japan) (Figure 2 ).6
Figure 2.
Phylogenetic tree constructed based on the complete S segments. The tree was constructed using the maximum likelihood method with MEGA6.20 S sequence of 17 severe fever with thrombocytopenia syndrome (SFTS) cases in this study are shown in bold. JP01/South Korea/07-2013 to JP07/South Korea/08-2014 were amplified from SFTS patients from Jeju Island (the southern end of South Korea) from May 2013 to August 2014, CP01/South Korea/08-2013 was amplified from an SFTS patient from Chuncheon (the northern end of South Korea), AP01/South Korea/10-2013 was amplified from an SFTS patient from Andong (the central part of South Korea), and DP01/South Korea/10-2013 was amplified from an SFTS patient from Daegu (the southern end of South Korea) (GenBank accession numbers KR612072 to KR612088, respectively). The S sequence data of the viruses identified in China, South Korea, and Japan were obtained from the National Center for Biotechnology Information (NCBI)/Basic Local Alignment Search Tool (BLAST).
The relationship between H. longicornis and migratory birds.
We conducted a meta-analysis of literature on the role of H. longicornis and migratory birds in the spread and geographic expansion of SFTSV. Previous work has found the ixodid tick H. longicornis, a vector of SFTSV, were found on four wild fowl: Zoothera aurea, Turdus hortulorum, Halcyon coromanda, and Pitta nympha.15 These migratory birds are known to breed and migrate between China, South Korea, and Japan (Figures 1 and 3 ).8,10,12,14,15 Another previous study detected tick-borne encephalitis virus (TBEV) in the H. longicornis collected from various habitats on Jeju Island (including wetland habitats for migratory birds) before SFTSV was first reported in Korea in 2013.14
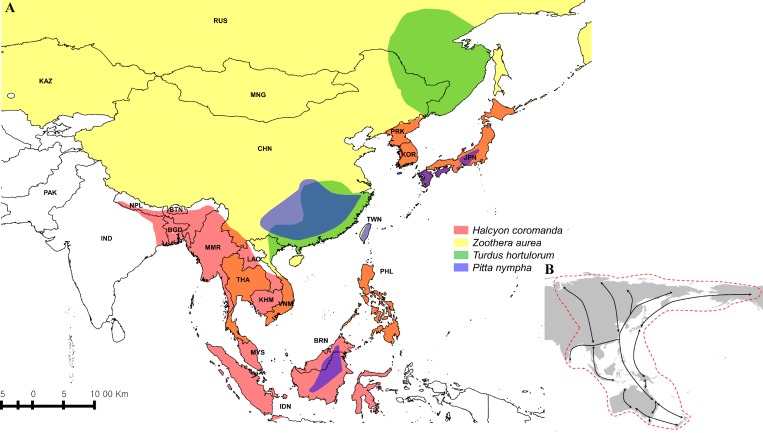
Figure 3.
Worldwide distribution of four wild fowl: Zoothera aurea, Turdus hortulorum, Halcyon coromanda, and Pitta nympha (A) and East Asia/Australasia Flyway (B). Distribution of Z. aurea is shown in light yellow, T. hortulorum in light green, Halcyon coromanda in light red, and P. nympha in light blue. Areas of overlapping distribution are indicated in mixed colors (A).10 East Asia/Australasia is depicted by the red dotted line (B).12
Discussion
Comparing our results with an SFTSV phylogenetic tree, we found that SFTSV from South Korea is genetically similar to observations from China and Japan.6 Since we found evidence that SFTSV strains have been circulating between China, South Korea, and Japan, the question remains as to how ticks carrying SFTSV can move overseas, especially across water bodies including the East China Sea, Yellow Sea, and Sea of Korea (Figure 2).6
We now review and connect existing evidence surrounding virus transmission to ticks and migratory birds. There is strong evidence that SFTSV is transmitted, at least in part, by the H. longicornis tick and moved over long distances through migratory birds, and we support this hypothesis through a meta-analysis of the literature.8,10,12,14,15
H. longicornis is a vector of SFTSV, and regular hosts of H. longicornis include mammals such as goats, cattle, sheep, yak, donkeys, pigs, deer, cats, rats, mice, hedgehogs, weasels, brushtail possums, chickens, and humans, along with some birds. This tick is widely distributed in the Asia–Pacific region, including China, Korea, Japan, Australia, the Pacific Islands, and New Zealand.1,4 SFTSV-infected H. longicornis have been found in China, South Korea, and Japan.1,4,7,9
It is known that birds can act as long-distance carriers for ticks and are important in disseminating disease.21–24 Migratory bird routes cross China, South Korea, and Japan via the East Asia/Australasia Flyway, which extends from Arctic Russia and North America to the southern limits of Australia and New Zealand and encompasses large parts of east Asia (Figure 3B).12 Bird species using the East Asia/Australasia Flyway route include Z. aurea, T. hortulorum, Halcyon coromanda, and P. nympha (Figure 3A).10 Therefore, these migratory birds, which breed in and migrate through China, Korea, and Japan, have the potential to disperse H. longicornis-borne SFTSV in these countries (Figure 3A).10,12
Previous work has found H. longicornis ticks on the Z. aurea, T. hortulorum, Halcyon coromanda, and P. nympha bird species in South Korea.15 On Jeju Island, Choi and others collected and examined migratory birds for ticks and showed that the larva of H. longicornis were found on Z. aurea, T. hortulorum, Halcyon coromanda, and P. nympha.15 H. longicornis is also the most dominant and widely distributed tick species on Jeju Island, and it has also been reported to harbor the SFTSV. In addition, TBEV was detected in the H. longicornis before SFTSV was first reported in Korea 2013.6,14,15 Therefore, we infer that SFTSV can also exist in H. longicornis collected from migratory birds based on these previous works.14,15
Jeju Island lies at the center of the East Asia/Australasia Flyway in east Asia and is home to one of the largest populations of migratory birds on the Korean Peninsula (Figures 1 and 3A, B).11,12,14,15 The H. longicornis distribution matches with bird migration patterns between China, South Korea, and Japan (Figures 1 and 3A).10–12 In South Korea in 2013, 36 cases of SFTS were reported including 17 fatalities, with 10 of the 36 cases on Jeju Island, which is a high-prevalence region (Figure 1).9 On the basis of these concurrent geographic ranges, we suggest that migratory birds potentially disseminate SFTSV-carrying H. longicornis between China, South Korea, and Japan. (Figures 1 and 3A, B).14,15,21–24
This transmission mechanism is not unique to SFTSV. Migratory birds have long acted as long-distance carriers of ticks containing various human pathogens.21–24 Several studies have shown that migratory birds play a major role in the dispersal of Crimean-Congo hemorrhagic fever virus (CCHFV).21–24 CCHFV-carrying ticks belonging to the Hyalomma marginatum complex, also known as the Mediterranean Hyalomma, are common in large parts of the African and Eurasian continents.21–24 Hyalomma marginatum is found widely across northern Africa and Middle East where there are reports of CCHFV from Algeria, Armenia, Azerbaijan, Egypt, Ethiopia, Georgia, Iran, Iraq, Israel, Morocco, Sudan, Syria, Tunisia, and Turkey.24 CCHFV is also present in ticks in southern and eastern Europe, including Albania, Bosnia and Herzegovina, Bulgaria, Croatia, Cyprus, France, Greece, Italy, Kosovo, Macedonia, Moldova, Montenegro, Portugal, Romania, Russia, Serbia, Spain, and the Ukraine.24
Recently, Tian and others showed that movement of the highly pathogenic avian influenza virus H5N1 is closely associated with known bird migration routes in Asia.25 Thus, there is a very good evidence that migratory birds play an important role in the dissemination of the main tick vector of CCHFV and spread of H5N1.21–25
Evidence that migratory birds may play a role in the distribution of CCHFV in Europe and spread of H5N1 in Asia point to the expectation that SFTSV will have a similarly wide range. Adults, nymphs, and larvae of H. longicornis were found on Z. aurea, T. hortulorum, Halcyon coromanda, and P. nympha in South Korea. These birds are known to breed in and migrate between China, Korea, and Japan (Figures 1 and 3A). SFTSV also has been detected in these ticks in South Korea.8,10–16
In conclusion, SFTSV samples from humans in South Korea show a clear phylogenetic link with SFTSV samples in Japan and China. We suggest that SFTSV-carrying H. longicornis ticks are likely transported across eastern Asia through bird migration. Alternative pathways for example, land-bound mammals and infected humans traveling internationally are implausible because land-bound mammals cannot move overseas and 17 patients had no history of travel to China or Japan before the onset. We suspect that the wild fowl Z. aurea, T. hortulorum, Halcyon coromanda, and P. nympha play a major part in this dispersal (Figures 1 and 3A, B).10–12,14
We conducted a meta-analysis of literature on the role of H. longicornis and migratory birds in the spread and geographic expansion of SFTSV.8,10–12,14,15 Further research such as detection and isolation of SFTSV in H. longicornis collected from migratory birds and serological surveillance for SFTSV in migratory birds is needed to elucidate the interaction between migratory birds and ticks to better understand the ecological transmission dynamics and geographic distribution of SFTSV.1,4
ACKNOWLEDGMENTS
We thank L. Bakkensen for the comments on this paper.
Footnotes
Financial support: This work was supported by the research grant of the Jeju National University in 2012.
Authors' addresses: Yeojun Yun, Ewha Medical Research Institute, Ewha Womans University, Seoul, South Korea, E-mail: yyun@ewha.ac.kr. Sang Taek Heo, Hyemin Kim, Dahee Park, and Keun Hwa Lee, Jeju National University School of Medicine, Jeju, South Korea, E-mails: neosangtaek@naver.com, hyemin36@hanmail.net, archons1004@naver.com, and yomust7@jejunu.ac.kr. Gwanghun Kim and Nam-Hyuk Cho, Department of Microbiology and Immunology, Department of Biomedical Science, Seoul National University College of Medicine, Seoul, South Korea, E-mails: kimgh@snu.ac.kr and chonh@snu.ac.kr. Roger Hewson, Virology and Pathogenesis Group, WHO Collaborating Centre for Virus Reference and Research, Public Health England, Porton Down, Salisbury Wiltshire, United Kingdom, E-mail: roger.hewson@phe.gov.uk. Won Sup Oh, Department of Internal Medicine, Kangwon National University School of Medicine, Chuncheon, South Korea, E-mail: onesbi@gmail.com. Seong Yeol Ryu, Department of Internal Medicine, Keimyung University Dongsan Medical Center, Daegu, South Korea, E-mail: 121rsy@hanmail.net. Ki Tae Kwon, Division of Infectious Diseases, Daegu Fatima Hospital, Daegu, South Korea, E-mail: idktkwon@gmail.com. Jolyon M. Medlock, Medical Entomology and Zoonoses Ecology, Microbial Risk Assessment, Emergency Response Department, Public Health England, Porton Down, Wiltshire, United Kingdom, E-mail: jolyon.medlock@phe.gov.uk.
References
- 1.Yu XJ, Liang MF, Zhang SY, Liu Y, Li JD, Sun YL, Zhang L, Zhang QF, Popov VL, Li C, Qu J, Li Q, Zhang YP, Hai R, Wu W, Wang Q, Zhan FX, Wang XJ, Kan B, Wang SW, Wan KL, Jing HQ, Lu JX, Yin WW, Zhou H, Guan XH, Liu JF, Bi ZQ, Liu GH, Ren J, Wang H, Zhao Z, Song JD, He JR, Wan T, Zhang JS, Fu XP, Sun LN, Dong XP, Feng ZJ, Yang WZ, Hong T, Zhang Y, Walker DH, Wang Y, Li DX. Fever with thrombocytopenia associated with a novel bunyavirus in China. N Engl J Med. 2011;364:1523–1532. doi: 10.1056/NEJMoa1010095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bouloy M. Molecular biology of phleboviruses. In: Plyusnin A, Elliot RM, editors. Bunyaviridae, Molecular and Cellular Biology. Norfolk, United Kingdom: Caister Academic Press; 2011. pp. 95–128. [Google Scholar]
- 3.McMullan LK, Folk SM, Kelly AJ, MacNeil A, Goldsmith CS, Metcalfe MG, Batten BC, Albariño CG, Zaki SR, Rollin PE, Nicholson WL, Nichol ST. A new phlebovirus associated with severe febrile illness in Missouri. N Engl J Med. 2012;367:834–841. doi: 10.1056/NEJMoa1203378. [DOI] [PubMed] [Google Scholar]
- 4.Niu G, Li J, Liang M, Jiang X, Jiang M, Yin H, Wang Z, Li C, Zhang Q, Jin C, Wang X, Ding S, Xing Z, Wang S, Bi Z, Li D. Severe fever with thrombocytopenia syndrome virus among domesticated animals, China. Emerg Infect Dis. 2013;19:756–763. doi: 10.3201/eid1905.120245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Deng B, Zhang S, Geng Y, Zhang Y, Wang Y, Yao W, Wen Y, Cui W, Zhou Y, Gu Q, Wang W, Wang Y, Shao Z, Wang Y, Li C, Wang D, Zhao Y, Liu P. Cytokine and chemokine levels in patients with severe fever with thrombocytopenia syndrome virus. PLoS One. 2012;7:e41365. doi: 10.1371/journal.pone.0041365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kim KH, Yi J, Kim G, Choi SJ, Jun KI, Kim NH, Choe PG, Kim NJ, Lee JK, Oh MD. Severe fever with thrombocytopenia syndrome, South Korea, 2012. Emerg Infect Dis. 2013;19:1892–1894. doi: 10.3201/eid1911.130792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Takahashi T, Maeda K, Suzuki T, Ishido A, Shigeoka T, Tominaga T, Kamei T, Honda M, Ninomiya D, Sakai T, Senba T, Kaneyuki S, Sakaguchi S, Satoh A, Hosokawa T, Kawabe Y, Kurihara S, Izumikawa K, Kohno S, Azuma T, Suemori K, Yasukawa M, Mizutani T, Omatsu T, Katayama Y, Miyahara M, Ijuin M, Doi K, Okuda M, Umeki K, Saito T, Fukushima K, Nakajima K, Yoshikawa T, Tani H, Fukushi S, Fukuma A, Ogata M, Shimojima M, Nakajima N, Nagata N, Katano H, Fukumoto H, Sato Y, Hasegawa H, Yamagishi T, Oishi K, Kurane I, Morikawa S, Saijo M. The first identification and retrospective study of severe fever with thrombocytopenia syndrome in Japan. J Infect Dis. 2014;209:816–827. doi: 10.1093/infdis/jit603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.National Institutes of Infectious Diseases, Japan http://www.nih.go.jp/niid/ja/sfts/3143-sfts.html Available at.
- 9.The Korea Centers for Disease Control and Prevention Prevention of Severe Fever with Thrombocytopenia Syndrome. http://is.cdc.go.kr/nstat/index.jsp Available at.
- 10.International Union for Conservation of Nature Zoothera aurea, Turdus hortulorum, Halcyon coromanda and Pitta nympha, IUCN Red List of Threatened Species. 2013. http://www.iucnredlist.org IUCN 2013 Version 2013.2. Available at.
- 11.Birds Korea Status of Birds, 2014. 2014. http://www.birdskorea.org Available at.
- 12.East Asia/Australasia Flyway-Birdlife International http://www.birdlife.org/datazone/userfiles/file/sowb/flyways/8_EastAsia_Australasia_NEW.pdf Available at.
- 13.Kang JG, Kim HC, Choi CY, Nam HY, Chae HY, Chong ST, Klein TA, Ko S, Chae JS. Molecular detection of Anaplasma, Bartonella, and Borrelia species in ticks collected from migratory birds from Hong-do Island, Republic of Korea. Vector Borne Zoonotic Dis. 2013;13:215–225. doi: 10.1089/vbz.2012.1149. [DOI] [PubMed] [Google Scholar]
- 14.Ko S, Kang JG, Kim SY, Kim HC, Klein TA, Chong ST, Sames WJ, Yun SM, Ju YR, Chae JS. Prevalence of tick-borne encephalitis virus in ticks from southern Korea. J Vet Sci. 2010;11:197–203. doi: 10.4142/jvs.2010.11.3.197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Choi CY, Kang CW, Kim EM, Lee S, Moon KH, Oh MR, Yamauchi T, Yun YM. Ticks collected from migratory birds, including a new record of Haemaphysalis formosensis, on Jeju Island, Korea. Exp Appl Acarol. 2014;62:557–566. doi: 10.1007/s10493-013-9748-9. [DOI] [PubMed] [Google Scholar]
- 16.Chong ST, Kim HC, Lee IY, Kollars TM, Jr, Sancho AR, Sames WJ, Chae JS, Klein TA. Seasonal distribution of ticks in four habitats near the demilitarized zone, Gyeonggi-do (Province), Republic of Korea. Korean J Parasitol. 2013;51:319–325. doi: 10.3347/kjp.2013.51.3.319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zhang YZ, He YW, Dai YA, Xiong Y, Zheng H, Zhou DJ, Li J, Sun Q, Luo XL, Cheng YL, Qin XC, Tian JH, Chen XP, Yu B, Jin D, Guo WP, Li W, Wang W, Peng JS, Zhang GB, Zhang S, Chen XM, Wang Y, Li MH, Li Z, Lu S, Ye C, de Jong MD, Xu J. Hemorrhagic fever caused by a novel Bunyavirus in China: pathogenesis and correlates of fatal outcome. Clin Infect Dis. 2012;54:527–533. doi: 10.1093/cid/cir804. [DOI] [PubMed] [Google Scholar]
- 18.Sun Y, Liang M, Qu J, Jin C, Zhang Q, Li J, Jiang X, Wang Q, Lu J, Gu W, Zhang S, Li C, Wang X, Zhan F, Yao W, Bi Z, Wang S, Li D. Early diagnosis of novel SFTS bunyavirus infection by quantitative real-time RT-PCR assay. J Clin Virol. 2012;53:48–53. doi: 10.1016/j.jcv.2011.09.031. [DOI] [PubMed] [Google Scholar]
- 19.Thompson JD, Gibson TJ, Plewniak F, Jeanmougin F, Higgins DG. The Clustal X windows interface: flexible strategies for multiple sequence alignment aided by quality analysis tools. Nucleic Acids Res. 1997;25:4876–4882. doi: 10.1093/nar/25.24.4876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tamura K, Stecher G, Peterson D, Filipski A, Kumar S. MEGA6: Molecular Evolutionary Genetics Analysis version 6.0. Mol Biol Evol. 2013;30:2725–2729. doi: 10.1093/molbev/mst197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lindeborg M, Barboutis C, Ehrenborg C, Fransson T, Jaenson TG, Lindgren PE, Lundkvist A, Nyström F, Salaneck E, Waldenström J, Olsen B. Migratory birds, ticks, and Crimean-Congo hemorrhagic fever virus. Emerg Infect Dis. 2012;18:2095–2097. doi: 10.3201/eid1812.120718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Waldenström J, Lundkvist A, Falk KI, Garpmo U, Bergström S, Lindegren G, Sjöstedt A, Mejlon H, Fransson T, Haemig PD, Olsen B. Migrating birds and tickborne encephalitis virus. Emerg Infect Dis. 2007;13:1215–1218. doi: 10.3201/eid1308.061416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Palomar AM, Portillo A, Santibáñez P, Mazuelas D, Arizaga J, Crespo A, Gutiérrez Ó, Cuadrado JF, Oteo JA. Crimean-Congo hemorrhagic fever virus in ticks from migratory birds, Morocco. Emerg Infect Dis. 2013;19:260–263. doi: 10.3201/eid1902.121193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lee KH, Medlock JM, Heo ST. Severe fever with thrombocytopenia syndrome virus, Crimean-Congo haemorrhagic fever virus, and migratory birds. J Bacteriol Virol. 2013;43:235–243. [Google Scholar]
- 25.Tian H, Zhou S, Dong L, Van Boeckel TP, Cui Y, Wu Y, Cazelles B, Huang S, Yang R, Grenfell BT, Xu B. Avian influenza H5N1 viral and bird migration networks in Asia. Proc Natl Acad Sci USA. 2015;112:172–177. doi: 10.1073/pnas.1405216112. [DOI] [PMC free article] [PubMed] [Google Scholar]