Abstract
The indicator used to measure progress toward the Millennium Development Goal (MDG) for water is access to an improved water supply. However, improved supplies are frequently fecally contaminated in developing countries. We examined factors associated with Escherichia coli contamination of improved water supplies in rural Pisco province, Peru. A random sample of 207 households with at least one child less than 5 years old was surveyed, and water samples from the source and storage container were tested for E. coli contamination. Although over 90% of households used an improved water source, 47% of source and 43% of stored water samples were contaminated with E. coli. Pouring or using a spigot to obtain water from the storage container instead of dipping a hand or object was associated with decreased risk of contamination of stored water (adjusted prevalence ratio [aPR] = 0.58, 95% confidence interval [CI] = 0.42, 0.80). Container cleanliness (aPR = 0.67, 95% CI = 0.45, 1.00) and correct handwashing technique (aPR = 0.62, 95% CI = 0.42, 0.90) were also associated with decreased contamination risk. These findings highlighted the limitations of improved water supplies as an indicator of safe water access. To ensure water safety in the home, household water treatment and improved hygiene, water handling, and storage practices should be promoted.
Introduction
Approximately 1.8 billion people use fecally contaminated water sources globally, with the majority living in low- and middle-income countries.1 Ingestion of pathogens in water contaminated with feces represents the greatest water-related health risk and is a major cause of diarrheal disease.2,3 The Millennium Development Goal (MDG) Target 7.C of halving the number of people without access to safe drinking water has already been reached, but it is widely recognized that this achievement overstates access because the indicator of progress—use of an “improved” source—is not an adequate proxy for safety.4–6 There is evidence of substantial between- and within-country variation in the fecal contamination of improved drinking water supplies,5,7,8 and it is important to accurately assess this risk to improve the precision of global estimates of the burden of disease attributable to unsafe drinking water, which currently assume that the use of an improved source implies no health risk.9
The World Health Organization/United Nations Children's Fund (WHO/UNICEF) Joint Monitoring Programme (JMP) for Water Supply and Sanitation, which monitors progress toward this MDG target, classifies a water source as “improved” if it has some measure of protection from outside fecal contamination such as a piped supply, boreholes, protected dug wells, protected springs, and rainwater.10 In contrast, an “unimproved” source includes unprotected dug wells, unprotected springs, water from tanker trucks or carts with small tanks or drums, bottled water, and surface water.10 In preparation for the end of the MDG monitoring period in 2015, the JMP has proposed new targets and indicators for measuring the expansion of access to safe drinking water.11 The proposed indicator of “basic” drinking water service is identical to the MDG indicator, except for the additional suggested requirement that the total collection time of the water source does not exceed 30 minutes.11 The proposed indicator of “intermediate” drinking water service requires access to an on-premises improved water supply that meets WHO guideline values for Escherichia coli, which implies that routine microbial testing will be added to all national household surveys.11
In Peru, as in other low- and middle-income countries, rural areas are at particularly high risk of having fecally contaminated drinking water, even when restricting to piped supplies.5,12 In coastal areas of Peru outside metropolitan Lima (the capital), an estimated 68% of households with children under 5 years of age use microbiologically contaminated water12 despite 81% access to a piped supply.13 National data indicate that the majority of piped water systems are at risk of contamination because of inadequate chlorination12,13 and intermittent service,14 which results in negative pressure in poorly maintained water pipes, permitting the entry of contaminants from the surrounding soil. The Peruvian coast is at particular risk of water infrastructure damage because of its high frequency of seismic activity. Intermittent supply also necessitates water storage in the home, where contamination can occur through unsafe storage and handling.15–17 To better understand household access to safe drinking water in a rural area on the southern coast of Peru, we evaluated source and stored water quality and examined factors associated with stored drinking water safety.
Materials and Methods
Study site.
In January 2014, we conducted a cross-sectional survey of households in Humay District of Pisco Province, which includes 33 communities and an estimated population of 5,800 people. The district is located in the agricultural Pisco River Valley, where the annual rainfall is approximately 2 mm. Of the population of rural areas of Ica Department (to which Pisco Province belongs), 16% are in the poorest national wealth index quintile, while almost half (49%) are in the second poorest quintile.13 The water in piped distribution systems comes either directly from the Pisco River, from irrigation channels (fed by the river) or, for one community, from a well. Water piped directly from the river runs through a sand or gravel filtration gallery before distribution; this is also the case for the water in some piped systems supplied by irrigation channels. Water piped from the well is unfiltered. The irrigation channels typically run through agricultural fields where animals are also pastured. In the two largest communities, chlorination of piped water is intermittent due to inconsistent treatment by the water authorities. The majority of water service is intermittent due to lack of supply, with daily service ranging from 2 to 20 hours. Typically, there are 1–2 standpipes per block in larger communities and at least one centrally located standpipe in smaller communities.
Sample size.
On the basis of previous water monitoring data from this area, we estimated that 88% of stored water samples would be contaminated with E. coli. A sample size of 210 was chosen to measure the prevalence of E. coli contamination with a margin of error less than 4%, assuming a 95% confidence level and a population size of 450 eligible households estimated from census data. This margin of error was chosen to meet the convention of measuring prevalences > 10% or < 90% to a precision of at least ±5% points18; the sample size was calculated using a finite population correction, which is appropriate when the sample size is large relative to the size of the total study population.19
Enrollment.
A district-wide census was conducted before the study start to identify households meeting the eligibility criteria for study participation, which included a child under the age of 5 years, an adult female member > 18 years, and ability to heat water in the home. A randomly ordered list of eligible households was created using a pseudorandom number generator in STATA 13.1 (StataCorp, College Station, TX). Eligible houses from the list were approached in order and enrolled until a total of 210 participants were reached. If the female head of household was unavailable at the first visit to the home, one additional attempt was made to enroll the household.
Data collection.
After obtaining informed consent, study workers administered a survey to ascertain participant demographic information, socioeconomic status, water supply, behaviors related to water boiling and other household water treatment methods, sanitation and hygiene behaviors, presence of a handwashing station (defined as a designated place for handwashing with a water source and soap present), and ability to demonstrate correct handwashing technique (lathering all surfaces of the hands with soap). Household source and stored drinking water were tested for the presence of total chlorine using an orthotolidine (OTO) pool test kit (Pentair, Minneapolis, MN). Samples of source and household stored drinking water were collected in sterile 100-mL bottles and transported on ice to the study center, where they were tested for E. coli using the Compartment Bag Test (CBT; Aquagenx, Chapel Hill, NC). Samples were processed within 6 hours of collection and incubated for at least 20 hours at 35–44.5°C.20 Positive and negative controls were processed and incubated on each day of water sample collection. The CBT is a validated measure of the most probable number (MPN) of E. coli ranging from undetectable to > 100 E. coli/100 mL.21
Data analysis.
All data were entered into a Microsoft Access 2010 (Microsoft, Redmond, WA) database and analyzed using STATA 13.1. We considered drinking water to be safe if < 1 MPN E. coli was detected in a 100-mL sample, per the WHO standard.3 Descriptive statistics were generated using cross-tabulations. χ2 or Fisher's exact tests were used to determine associations between stored household water quality and demographic and socioeconomic characteristics, and water, sanitation, and hygiene knowledge and practices. For continuous variables with non-normal distributions, equality-of-medians tests were used to compare median values by stored drinking water contamination. The distribution of source and stored drinking water samples were stratified by the WHO E. coli risk categories: low risk/safe (< 1 E. coli/100 mL), intermediate to high risk (1–100 E. coli/100 mL), and very high risk (> 100 E. coli/100 mL) for human consumption.3 The WHO intermediate (1–10 E. coli/100 mL) and high (11–100 E. coli/100 mL) risk categories were combined in this analysis to reduce the probability of misclassification, given the lack of precision of individual MPN values.20 For participants who reported boiling, a McNemar's test was used to compare the prevalence of E. coli contamination between source and stored water samples to evaluate the degree to which this practice was associated with an improvement in water quality.
We used log-binomial models to estimate the prevalence ratios (PRs) for the association of household characteristics with the contamination of stored household drinking water. The WHO/UNICEF JMP for Water and Sanitation definitions were used to classify water sources as “piped on premises” (piped into the dwelling, plot, or yard), “other improved” (i.e., public tap), and “unimproved” (i.e., uncovered well or irrigation channel), while sanitation infrastructure was categorized as household ownership of a toilet or latrine or other (shared facilities/open defecation).10 Principal component analysis of household assets, building materials, and home ownership (by self or a family member) was used to calculate a socioeconomic index for each household.22 The first principal component of each variable was used to generate the index23 and natural breakpoints in the data were used to group households into terciles for analysis. The question “Do you do anything to treat the water you use for drinking?” was administered to assess household drinking water treatment. Households were considered to have treated their stored drinking water if they responded affirmatively to this question and reported using an adequate method to treat the drinking water currently stored in the home. Treatment methods were considered as adequate or inadequate per the JMP definitions.24 Water source, treatment, storage and handling variables, demographic variables, and socioeconomic status were considered as potential confounders and were included in adjusted models if they altered the prevalence ratio by 10% or more, and cell sizes allowed for adequate adjustment. A forward stepwise approach was used for the inclusion of confounders in adjusted models, and a significance level of 0.05 was used for all hypothesis testing. To assess the sensitivity of results to using a different level of drinking water safety, we additionally estimated the PRs for the association of household characteristics with a very high risk stored water (containing > 100 E. coli/100 mL).
Ethical considerations.
The study protocol was reviewed and approved by the Institutional Review Boards of the University of Washington, the U.S. Naval Medical Research Unit No. 6, and the Ica regional Ministry of Health. Written informed consent was obtained from all study participants. Personal identifying information was irreversibly removed from databases at the end of the study.
Results
Demographic characteristics of the study population.
Of 210 women enrolled, three did not meet the study eligibility criteria and were excluded from analysis. The median age of the 207 remaining participants was 31 years (range = 18–64) and the median number of household members was 4 (range = 2–15; Table 1). Of the participants, 57% had completed secondary school or more education.
Table 1.
Participant characteristics by Escherichia coli contamination of stored household drinking water in Pisco, Peru, January 2014*
| Uncontaminated (N = 115) | Contaminated (N = 85) | P | |||
|---|---|---|---|---|---|
| n (%) | n (%) | ||||
| Median age (range) | 30 (18–62) | 35 (19–64) | 0.02 | ||
| Median household size (range) | 4 (2–15) | 5 (2–14) | 0.01 | ||
| Education | |||||
| Less than complete secondary | 35 | (40.7) | 51 | (59.3) | < 0.001 |
| Secondary complete or above | 80 | (70.2) | 34 | (29.8) | |
| Socioeconomic index† | |||||
| Poorest tercile | 32 | (51.6) | 30 | (48.4) | 0.34 |
| Middle tercile | 39 | (60.9) | 25 | (39.1) | |
| Wealthiest tercile | 39 | (60.0) | 26 | (40.0) | |
| Primary water source | |||||
| Unimproved source | 6 | (42.9) | 8 | (57.1) | 0.01 |
| Other improved source | 31 | (44.9) | 38 | (55.1) | |
| Piped source on premises | 77 | (67.5) | 37 | (32.5) | |
| Storage container type | |||||
| Teapot | 17 | (77.3) | 5 | (22.7) | 0.054 |
| Wide-mouthed container | 97 | (55.8) | 77 | (44.3) | |
| Covered storage container‡ | |||||
| Yes | 111 | (59.4) | 76 | (40.6) | 0.13 |
| No | 4 | (33.3) | 8 | (66.7) | |
| Clean storage container‡ | |||||
| Yes | 112 | (59.6) | 76 | (40.4) | 0.06 |
| No | 3 | (27.3) | 8 | (73.7) | |
| Method of water extraction | |||||
| Poured/dispensed with a spigot | 88 | (65.7) | 46 | (34.3) | 0.002 |
| Dipped with a cup, other utensil, or hands | 23 | (41.1) | 33 | (58.9) | |
| Boiled currently stored water‡ | |||||
| Yes | 112 | (59.6) | 76 | (40.4) | 0.02 |
| No | 2 | (20.0) | 8 | (80.0) | |
| Toilet/latrine | |||||
| Yes | 93 | (58.1) | 67 | (41.9) | 0.85 |
| No | 22 | (56.4) | 17 | (43.6) | |
| Presence of a hand washing station | |||||
| Yes | 80 | (63.0) | 47 | (37.0) | 0.048 |
| No | 35 | (48.6) | 37 | (51.4) | |
| Correct hand washing‡ | |||||
| Yes | 101 | (62.4) | 61 | (37.7) | 0.02 |
| No | 4 | (28.6) | 10 | (71.4) | |
Seven subjects did not have stored water for collection at the time of the survey and were therefore excluded from this analysis. Numbers in the table may not sum to total because of missing values.
P value for trend; ‡P value for Fisher's exact test.
Water sources, treatment, and storage.
The primary drinking water source of 93% of participants was improved, including piped water inside the home (59%), piped water outside the home (33%), and a covered well (1%). Unimproved primary water sources reported by participants included surface water (5%) and an uncovered well (1%). Household piped supplies were fed directly by the river (86%), by irrigation channels (9%), or by a well (5%). Forty percent of participants believed their water was safe to drink.
Of 207 participants, 203 (98%) reported treating their drinking water, 201 by boiling and 2 by chlorinating and boiling. Although 97% of those who reported water treatment also reported having treated water currently stored in their home, 32% admitted to sometimes not treating their drinking water. Definitions of boiling provided by 194 participants included bubbles rising from the bottom to the top of the container (82%), steam rising from the surface of the water (13%), the teapot whistling (4%), and bubbles breaking on the surface (1%). Water treatment was reported to be expensive by 46% and easy by 92% of respondents. When asked why they boiled their drinking water, 49% cited health, 18% said to make it clean, 13% to kill microbes or parasites, and 11% to make the water safe for drinking. The two most common reasons for nontreatment were that it took too much time (45%) and a lack of fuel (20%). Among 203 participants who reported treating their water, 77% primarily used a gas stove for cooking, 9% an open fire, 7% a “plancha” stove (which has a griddle and combustion chamber), and 7% used a gas stove and a wood-burning stove with equal frequency.
Water storage containers reported by participants included plastic beverage container (51%), cooking pot (17%), teapot (11%), barrel (9%), and bucket (9%). At the time of the visit, drinking water was observed to be stored in a covered container in 94% of households; 95% appeared to be clean (free of dirt, debris, garbage, fecal matter, etc.). Chlorine was detected in 9 (5%) source water samples and no stored water samples.
Sanitation and hygiene.
Of 207 participating households, 81% used a household toilet or latrine, 14% practiced open defecation, and 5% used the bathroom of a neighbor or relative. A handwashing station was observed in 64% of participant homes; 91% of participants were able to demonstrate correct handwashing technique.
Microbiological water quality and boiling.
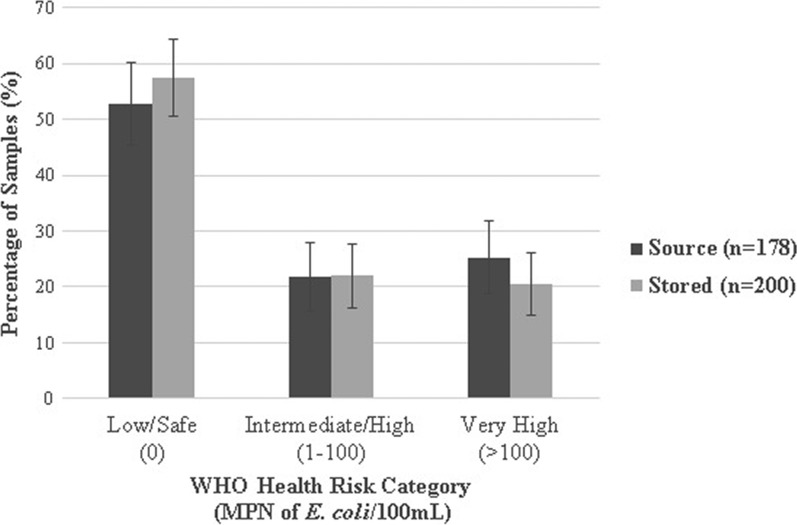
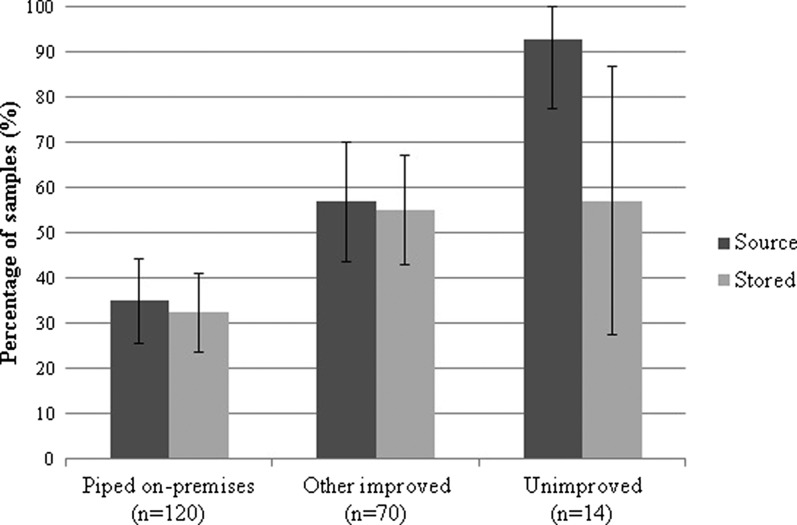
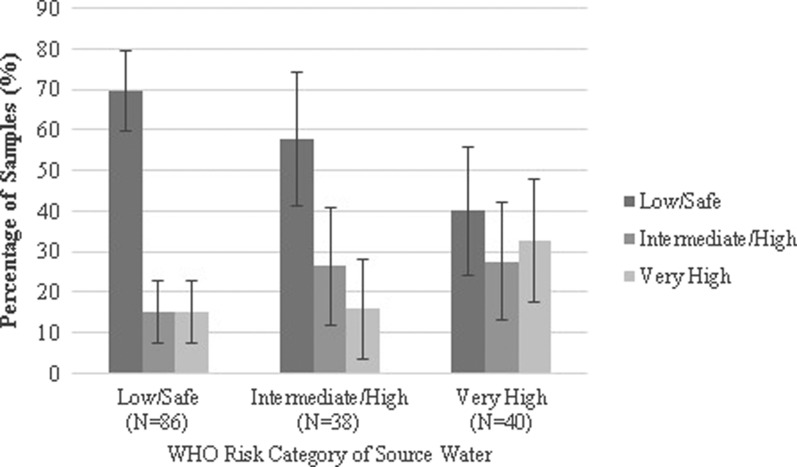
Escherichia coli was detected in 47% of source and 43% of stored water samples (P for McNemar's test = 0.14). Of 200 total stored water samples, 58% had no detectable E. coli, 22% had an intermediate/high level of contamination, and 21% were highly contaminated (> 100 MPN/100 mL) (Figure 1 ). Escherichia coli contamination was detected in a lower percentage of water samples collected from sources piped on premises (35%) than other improved water sources (57%, P = 0.007) and unimproved sources (93%, P < 0.001; Figure 2 ). All water samples from improved sources fed by irrigation channels were contaminated with E. coli; contamination of improved sources was less frequent when supplied by a well (57%, P = 0.02) or directly by the river (35%, P < 0.001). The type of water supply distributed by on-premises piped sources was similar to that of other improved sources (P = 0.26). Among participants who reported boiling their currently stored drinking water, E. coli was detected with similar frequency in paired source and stored samples (P = 0.13). The WHO health risk categories of reportedly boiled drinking water samples stratified by the health risk categories of the paired source water are shown in Figure 3 .
Figure 1.
Escherichia coli contamination of water samples by World Health Organization (WHO) health risk category. Error bars represent 95% confidence intervals.
Figure 2.
Prevalence of Escherichia coli contamination of water by source type. Error bars represent 95% confidence intervals.
Figure 3.
World Health Organization (WHO) health risk category of boiled household drinking water by WHO health risk category of source water. Error bars represent 95% confidence intervals.
Correlates of microbiologically safe stored water.
In multivariate analysis, participants who poured or used a spigot to obtain water from their storage container were 42% less likely to have contaminated stored drinking water than those who dipped an object or a hand to extract water from the container (adjusted prevalence ratio [aPR] = 0.58, 95% confidence interval [CI] = 0.42, 0.80). Container cleanliness was associated with a reduced risk of contamination (aPR = 0.67, 95% CI = 0.45, 1.00), as was correct handwashing technique (aPR = 0.62, 95% CI = 0.42, 0.90). Households that primarily used water from on-premises piped sources were less likely to have detectable E. coli in stored water than households using unimproved sources (aPR = 0.61, 95% CI = 0.37, 1.03); however, this association did not reach statistical significance. Reported water treatment was associated with a nearly 50% reduction in risk of having contaminated stored water in the unadjusted model, but the adjusted model did not converge because of small numbers (Table 2). No other factors were significantly associated with stored drinking water contamination in multivariable models. The associations between household characteristics with very highly contaminated stored water were generally similar to those associated with the presence of contamination in adjusted models, with two exceptions (Table 3). Storage containers with a cover were significantly less likely to have very highly contaminated water (aPR = 0.46, 95% CI = 0.24, 0.89), while dipping an object or a hand to extract water from the container was not associated with the risk of a very high level of contamination.
Table 2.
Prevalence ratio (PR) estimates of the association of Escherichia coli contamination of stored drinking water with water, sanitation, and hygiene practices in Pisco, Peru, 2014
| Unadjusted PR | (95% CI) | Adjusted PR* | (95% CI) | |
|---|---|---|---|---|
| Improved primary water source | 0.72 | (0.44, 1.17) | 0.79 | (0.50, 1.24) |
| Primary water source | ||||
| Unimproved source | 1.00 | – | 1.00 | – |
| Other improved source | 0.96 | (0.58, 1.59) | 0.99 | (0.62, 1.58) |
| Piped source on premises | 0.57† | (0.34, 0.96) | 0.61 | (0.37, 1.03) |
| Storage container type | ||||
| Wide-mouthed container | 1.00 | – | 1.00 | – |
| Teapot | 0.51 | (0.23, 1.13) | 0.63 | (0.28, 1.40) |
| Container covered | 0.61† | (0.39, 0.94) | 0.70 | (0.46, 1.06) |
| Container cleanliness | 0.56‡ | (0.37, 0.83) | 0.67† | (0.45, 1.00) |
| Water treatment | 0.51§ | (0.35, 0.72) | NC | – |
| Method of water extraction | ||||
| Dipped with an object or hands | 1.00 | – | 1.00 | – |
| Poured or used a spigot | 0.58§ | (0.42, 0.80) | 0.58§ | (0.42, 0.80) |
| Toilet/latrine | 0.96 | (0.64, 1.43) | 0.99 | (0.66, 1.49) |
| Correct hand washing demonstration | 0.53‡ | (0.36, 0.78) | 0.62† | (0.42, 0.90) |
CI = confidence interval; NC = model does not converge on 200 iterations.
Models adjusted for the dipping of a cup/other utensil/hands.
Significance codes: †P < 0.05; ‡P < 0.01; §P < 0.001.
Table 3.
Prevalence ratio (PR) estimates of the association of a very high risk level of fecal contamination (> 100 Escherichia coli/100 mL) of stored drinking water with water, sanitation, and hygiene practices in Pisco, Peru, 2014
| Unadjusted PR | (95% CI) | Adjusted PR* | (95% CI) | |
|---|---|---|---|---|
| Improved primary water source | 0.92 | (0.32, 2.61) | 1.04 | (0.37, 2.96) |
| Primary water source | ||||
| Unimproved source | 1.00 | – | 1.00 | – |
| Other improved source | 0.57 | (0.19, 1.75) | 0.66 | (0.22, 2.04) |
| Piped source on premises | 1.49 | (0.52, 4.30) | 1.54 | (0.54, 4.42) |
| Storage container type | ||||
| Wide-mouthed container | 1.00 | – | 1.00 | – |
| Teapot | 0.41 | (0.11, 1.56) | 0.22 | (0.03, 1.52) |
| Container covered | 0.37‡ | (0.20, 0.71) | 0.46† | (0.24, 0.89) |
| Container cleanliness | 0.42† | (0.21, 0.86) | 0.49† | (0.24, 0.98) |
| Water treatment | 0.37‡ | (0.19, 0.74) | 0.47† | (0.23, 0.97) |
| Method of water extraction | ||||
| Dipped with an object or hands | 1.00 | – | 1.00 | – |
| Poured or used a spigot | 0.69 | (0.38, 1.23) | 0.79 | (0.43, 1.46) |
| Toilet/latrine | 0.76 | (0.41, 1.41) | 0.87 | (0.47, 1.64) |
| Correct hand washing demonstration | 0.36‡ | (0.19, 0.66) | 0.50† | (0.26, 1.00) |
CI = confidence interval.
Models adjusted for the presence of a hand washing station.
Significance codes: †P < 0.05; ‡P < 0.01.
Discussion
In a rural population on the southern coast of Peru with 93% coverage by improved water sources, we found that 47% of source water samples were contaminated with E. coli; 22% and 25% of samples had intermediate/high and very high levels of contamination, respectively. The contamination of improved water supplies observed in this study is likely because of the use of inadequately treated water from a river and irrigation channels, both unimproved sources, in the piped water systems. These results are consistent with the findings of other studies that demonstrated improved sources are not necessarily safe.5,6,8,25–28
Although transporting water to the home from an off-premises source has been associated with contamination in other studies,15,17 in this population, microbiologic quality of stored water tended to be similar to, or better than, source water. Under conditions of relatively high-quality source water as in this study, microbial settling and time-related die-off are unlikely to yield significant improvement in water quality.29 Therefore, this finding probably resulted from the high percentage of reported water treatment in the population, which may have been a reliable indicator of treatment because of the use of gas stoves, which are more efficient and therefore boil water faster than open fires.30 A study conducted in Vietnam also found off-premises piped sources to contain more fecal contamination than on-premises piped sources, with evidence of similar stored water quality for both source types.31
Taken together, these findings highlight the need for interventions at the household level, including water treatment, safe water handling, improved storage, and hand hygiene to ensure safe drinking water, even in populations using improved supplies. In this study, reported boiling was associated with a significant reduction in contamination of stored water among households with contaminated source water, a finding that is consistent with other research.31–36 In some studies, however, reported boiling has been associated with no effect37,38 or even an increase in contamination.39 Because there is no objective measure of boiling, assessment relies on self-report, which may substantially overestimate actual practice.40,41 In addition, boiling may be ineffective if water is subsequently recontaminated via unsafe storage and handling, as was suggested in this study by the decreased risk of contamination associated with pouring or using a spigot to extract water from the storage container, rather than dipping a hand or object. This was also suggested by the association between container cleanliness and decreased contamination risk. The association between the ability of a participant to demonstrate correct handwashing procedure and having stored water with no detectable contamination highlights the importance of proper hygiene for water handling. The finding in this study that people with less than a secondary education were more likely to have contaminated stored water underscores the importance of using targeted hygiene education interventions for high-risk populations.
This study had two important limitations. First, because this assessment was limited to a rural population using primarily piped, improved water sources in an extremely arid climate, these results may not generalize to the rest of Peru or more globally. Second, the CBT serves primarily as a qualitative measure of E. coli contamination because the E. coli MPNs have wide, overlapping CIs20 and the upper limit of detection of the test of 100 E. coli/100 mL does not permit an accurate determination of the amount of contamination in highly contaminated samples. However, a qualitative measure of contamination may be a sufficient indicator of risk since it corresponds with the WHO standard for drinking water safety.3 The use of the CBT as a qualitative measure of contamination has three distinct advantages: no need for highly trained laboratory staff, a minimal need for laboratory equipment, and high sensitivity and specificity.21 An additional justification for using a qualitative measure is that the association between the level of E. coli contamination in drinking water and diarrheal disease risk is unclear. Some research has shown an association between the consumption of highly contaminated water and diarrhea,42,43 but other investigations have found no significant association between the level of fecal contamination in stored household drinking water and disease risk.44–47 A recent systematic review and meta-analysis demonstrated an association between the detection—but not the amount—of E. coli contamination and diarrhea.48
The considerable contamination of improved water sources observed in this and other studies demonstrates that the current MDG indicator for water quality should not be equated with safety. The indicator of basic water service proposed for the post-2015 monitoring period will have similar limitations. Because implementation of global microbiological testing is unlikely to be immediate, research is needed to develop more accurate indicators of safe drinking water access. Our findings underscore the importance of boiling and other adequate household water treatment methods, safe storage and handling, and information, education, and communication materials appropriate for all educational levels to ensure the safety of household drinking water. Because boiling is the only household water treatment method reported to be used at scale,49 future studies should investigate ways to improve the objective measurement and effectiveness of this method. Although there has been minimal research to investigate the determinants of boiling behavior, existing evidence suggests that technological interventions as simple as water pasteurization indicators can increase the effectiveness of heat treatment of household drinking water.50 A better understanding of the effectiveness of behavioral and technological approaches to the promotion of boiling will determine the extent to which expansion of this method can increase global access to safe drinking water.
ACKNOWLEDGMENTS
We would like to express our gratitude to the study participants for their contribution to this investigation. We also thank Asociación Benéfica PRISMA for their administrative and logistical support, particularly Crisóloga Lauro Salas, Angela Huamán Gómez, Alex Fernández Díaz, Enny Herrera Mayuri, Beatriz Huamán Ccollana, and Aydee Huanaco Muñoz. We additionally thank the Peace Corps Water and Sanitation Program in Peru, particularly Jorge Izaguirre, for his assistance in facilitating this project, and Hilary Miller, for sharing her time and knowledge of the community and whose assistance in water testing and general logistical assistance were invaluable.
Disclaimer: Claudio A. Rocha, Robert E. Quick, Drake H. Tilley Jr., and Silvia M. Montano are employees of the U.S. Government. This work was prepared as part of their official duties. Title 17 U.S.C. §105 provides that “Copyright protection under this title is not available for any work of the United States Government.” Title 17 U.S.C. §101 defines a U.S. Government work as a work prepared by a military service member or employee of the U.S. Government as part of that person's official duties. The views expressed in this manuscript are those of the authors and do not necessarily reflect the official policy of position of the Department of the Navy, the Department of Defense, the Centers for Disease Control and Prevention, nor the U.S. Government.
Footnotes
Financial support: This research was supported by the Department of Defense Humanitarian Assistance Program and by NIH research training grant no. R25 TW009345 awarded to the Northern Pacific Global Health Fellows Program by the Fogarty International Center.
Authors' addresses: Kristen Heitzinger, Charles N. Mock, and Stephen E. Hawes, Department of Epidemiology, University of Washington, Seattle, WA, E-mails: heitzk@uw.edu, cmock@uw.edu, and hawes@uw.edu. Claudio A. Rocha and Silvia M. Montano, Bacteriology Department, U.S. Naval Medical Research Unit No. 6, Callao, Peru, E-mails: claudio.rocha@med.navy.mil and silvia.montano@med.navy.mil. Robert E. Quick, Waterborne Diseases Prevention Branch, U.S. Centers for Disease Control and Prevention, Atlanta, GA, E-mail: rxq1@cdc.gov. Drake H. Tilley Jr., Division of Infectious Diseases, Naval Medical Center, San Diego, CA, E-mail: drake.tilley@med.navy.mil. A. Jannet Carrasco and Ricardo M. Cabrera, Department of Environmental Health, Hospital San Juan de Dios, Pisco, Peru, E-mails: jannet09@hotmail.com and rcabrerac@hotmail.com.
References
- 1.Bain R, Cronk R, Hossain R, Bonjour S, Onda K, Wright J, Yang H, Slaymaker T, Hunter P, Pruss-Ustun A, Bartram J. Global assessment of exposure to faecal contamination through drinking water based on a systematic review. Trop Med Int Health. 2014;19:917–927. doi: 10.1111/tmi.12334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Pruss-Ustun A, Bos R, Gore F, Bartram J. Safer Water, Better Health: Costs, Benefits, and Sustainability of Interventions to Promote and Promote Health. Geneva, Switzerland: WHO; 2008. [Google Scholar]
- 3.WHO . Guidelines for Drinking Water Quality. Geneva, Switzerland: WHO; 2011. [Google Scholar]
- 4.Clasen TF. Millennium development goals water target claim exaggerates achievement. Trop Med Int Health. 2012;17:1178–1180. doi: 10.1111/j.1365-3156.2012.03052.x. [DOI] [PubMed] [Google Scholar]
- 5.Bain R, Cronk R, Wright J, Yang H, Slaymaker T, Bartram J. Fecal contamination of drinking-water in low- and middle-income countries: a systematic review and meta-analysis. PLoS Med. 2014;11:e1001644. doi: 10.1371/journal.pmed.1001644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Shaheed A, Orgill J, Montgomery MA, Jeuland MA, Brown J. Why “improved” water sources are not always safe. Bull World Health Organ. 2014;92:283–289. doi: 10.2471/BLT.13.119594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yang H, Bain R, Bartram J, Gundry S, Pedley S, Wright J. Water safety and inequality in access to drinking-water between rich and poor households. Environ Sci Technol. 2013;47:1222–1230. doi: 10.1021/es303345p. [DOI] [PubMed] [Google Scholar]
- 8.Bain RE, Gundry SW, Wright JA, Yang H, Pedley S, Bartram JK. Accounting for water quality in monitoring access to safe drinking-water as part of the Millennium development goals: lessons from five countries. Bull World Health Organ. 2012;90:228–235A. doi: 10.2471/BLT.11.094284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lim SS, Vos T, Flaxman AD, Danaei G, Shibuya K, Adair-Rohani H, Amann M, Anderson HR, Andrews KG, Aryee M, Atkinson C, Bacchus LJ, Bahalim AN, Balakrishnan K, Balmes J, Barker-Collo S, Baxter A, Bell ML, Blore JD, Blyth F, Bonner C, Borges G, Bourne R, Boussinesq M, Brauer M, Brooks P, Bruce NG, Brunekreef B, Bryan-Hancock C, Bucello C, Buchbinder R, Bull F, Burnett RT, Byers TE, Calabria B, Carapetis J, Carnahan E, Chafe Z, Charlson F, Chen H, Chen JS, Cheng AT, Child JC, Cohen A, Colson KE, Cowie BC, Darby S, Darling S, Davis A, Degenhardt L, Dentener F, Des Jarlais DC, Devries K, Dherani M, Ding EL, Dorsey ER, Driscoll T, Edmond K, Ali SE, Engell RE, Erwin PJ, Fahimi S, Falder G, Farzadfar F, Ferrari A, Finucane MM, Flaxman S, Fowkes FG, Freedman G, Freeman MK, Gakidou E, Ghosh S, Giovannucci E, Gmel G, Graham K, Grainger R, Grant B, Gunnell D, Gutierrez HR, Hall W, Hoek HW, Hogan A, Hosgood HD, 3rd, Hoy D, Hu H, Hubbell BJ, Hutchings SJ, Ibeanusi SE, Jacklyn GL, Jasrasaria R, Jonas JB, Kan H, Kanis JA, Kassebaum N, Kawakami N, Khang YH, Khatibzadeh S, Khoo JP, Kok C, Laden F, Lalloo R, Lan Q, Lathlean T, Leasher JL, Leigh J, Li Y, Lin JK, Lipshultz SE, London S, Lozano R, Lu Y, Mak J, Malekzadeh R, Mallinger L, Marcenes W, March L, Marks R, Martin R, McGale P, McGrath J, Mehta S, Mensah GA, Merriman TR, Micha R, Michaud C, Mishra V, Mohd Hanafiah K, Mokdad AA, Morawska L, Mozaffarian D, Murphy T, Naghavi M, Neal B, Nelson PK, Nolla JM, Norman R, Olives C, Omer SB, Orchard J, Osborne R, Ostro B, Page A, Pandey KD, Parry CD, Passmore E, Patra J, Pearce N, Pelizzari PM, Petzold M, Phillips MR, Pope D, Pope CA, 3rd, Powles J, Rao M, Razavi H, Rehfuess EA, Rehm JT, Ritz B, Rivara FP, Roberts T, Robinson C, Rodriguez-Portales JA, Romieu I, Room R, Rosenfeld LC, Roy A, Rushton L, Salomon JA, Sampson U, Sanchez-Riera L, Sanman E, Sapkota A, Seedat S, Shi P, Shield K, Shivakoti R, Singh GM, Sleet DA, Smith E, Smith KR, Stapelberg NJ, Steenland K, Stöckl H, Stovner LJ, Straif K, Straney L, Thurston GD, Tran JH, Van Dingenen R, van Donkelaar A, Veerman JL, Vijayakumar L, Weintraub R, Weissman MM, White RA, Whiteford H, Wiersma ST, Wilkinson JD, Williams HC, Williams W, Wilson N, Woolf AD, Yip P, Zielinski JM, Lopez AD, Murray CJ, Ezzati M, AlMazroa MA, Memish ZA. A comparative risk assessment of burden of disease and injury attributable to 67 risk factors and risk factor clusters in 21 regions, 1990–2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet. 2012;380:2224–2260. doi: 10.1016/S0140-6736(12)61766-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.WHO/UNICEF Joint Monitoring Programme (JMP) on Water Supply and Sanitation Improved and Unimproved Water and Sanitation Facilities. 2014. http://www.wssinfo.org/definitions-methods/watsan-categories/ Available at. Accessed June 3, 2014.
- 11.WHO/UNICEF Joint Monitoring Programme (JMP) on Water Supply and Sanitation Post-2015 WASH Targets and Indicators. 2013. http://www.wssinfo.org/fileadmin/user_upload/resources/Fact_Sheets_4_eng.pdf Available at.
- 12.Miranda M, Aramburu A, Junco J, Campos M. State of the quality of drinking water in households in children under five years in Peru, 2007–2010. Rev Peru Med Exp Salud Publica. 2010;27:506–511. doi: 10.1590/s1726-46342010000400003. [DOI] [PubMed] [Google Scholar]
- 13.Demographic and Health Surveys . In: Peru: Continuous DHS, 2012. Instituto Nacional de Estadística e Informática (INEI), editor. Calverton, MD: Demographic and Health Surveys; 2012. [Google Scholar]
- 14.The World Bank . The International Benchmarking Network for Water and Sanitation Utilities (IBNET) Washington, DC: The World Bank; 2008. [Google Scholar]
- 15.Wright J, Gundry S, Conroy R. Household drinking water in developing countries: a systematic review of microbiological contamination between source and point-of-use. Trop Med Int Health. 2004;9:106–117. doi: 10.1046/j.1365-3156.2003.01160.x. [DOI] [PubMed] [Google Scholar]
- 16.Trevett AF, Carter RC, Tyrrel SF. Mechanisms leading to post-supply water quality deterioration in rural Honduran communities. Int J Hyg Environ Health. 2005;208:153–161. doi: 10.1016/j.ijheh.2005.01.024. [DOI] [PubMed] [Google Scholar]
- 17.Rufener S, Mausezahl D, Mosler HJ, Weingartner R. J Health Popul Nutr. 2010;28:34–41. doi: 10.3329/jhpn.v28i1.4521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Arya R, Antonisamy B, Kumar S. Sample size estimation in prevalence studies. Indian J Pediatr. 2012;79:1482–1488. doi: 10.1007/s12098-012-0763-3. [DOI] [PubMed] [Google Scholar]
- 19.Lemeshow S, Hosmer DW, Klar J, Lwanga SK. Adequacy of Sample Size in Health Studies. Chichester, England: Wiley; 1990. [Google Scholar]
- 20.Aquagenx Compartment Bag Test: Instructions for Use. 2013. http://www.aquagenx.com/wp-content/uploads/2013/12/Aquagenx-CBT-Instructions-v3.pdf Available at. Accessed June 3, 2014.
- 21.Stauber C, Miller C, Cantrell B, Kroell K. Evaluation of the compartment bag test for the detection of Escherichia coli in water. J Microbiol Methods. 2014;99:66–70. doi: 10.1016/j.mimet.2014.02.008. [DOI] [PubMed] [Google Scholar]
- 22.Vyas S, Kumaranayake L. Constructing socio-economic status indices: how to use principal components analysis. Health Policy Plan. 2006;21:459–468. doi: 10.1093/heapol/czl029. [DOI] [PubMed] [Google Scholar]
- 23.Houweling TA, Kunst AE, Mackenbach JP. Measuring health inequality among children in developing countries: does the choice of the indicator of economic status matter? Int J Equity Health. 2003;2:8. doi: 10.1186/1475-9276-2-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.WHO/UNICEF . Core Questions on Drinking-Water and Sanitation for Household Surveys. Geneva, Switzerland: World Health Organization; 2006. [Google Scholar]
- 25.Patrick M, Berendes D, Murphy J, Bertrand F, Husain F, Handzel T. Access to safe water in rural Artibonite, Haiti 16 months after the onset of the cholera epidemic. Am J Trop Med Hyg. 2013;89:647–653. doi: 10.4269/ajtmh.13-0308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Godfrey S, Labhasetwar P, Wate S, Pimpalkar S. How safe are the global water coverage figures? Case study from Madhya Pradesh, India. Environ Monit Assess. 2011;176:561–574. doi: 10.1007/s10661-010-1604-3. [DOI] [PubMed] [Google Scholar]
- 27.Shaheed A, Orgill J, Ratana C, Montgomery MA, Jeuland MA, Brown J. Water quality risks of ‘improved’ water sources: evidence from Cambodia. Trop Med Int Health. 2014;19:186–194. doi: 10.1111/tmi.12229. [DOI] [PubMed] [Google Scholar]
- 28.Pickering AJ, Davis J, Walters SP, Horak HM, Keymer DP, Mushi D, Strickfaden R, Chynoweth JS, Liu J, Blum A, Rogers K, Boehm AB. Hands, water, and health: fecal contamination in Tanzanian communities with improved, non-networked water supplies. Environ Sci Technol. 2010;44:3267–3272. doi: 10.1021/es903524m. [DOI] [PubMed] [Google Scholar]
- 29.Levy K, Nelson KL, Hubbard A, Eisenberg JN. Following the water: a controlled study of drinking water storage in northern coastal Ecuador. Environ Health Perspect. 2008;116:1533–1540. doi: 10.1289/ehp.11296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Smith KR, Uma R, Kishore VVN, Lata K, Joshi V, Zhang J, Rasmussen RA, Khalil MAK. Greenhouse Gases from Small-scale Combustion Devices in Developing Countries, Phase IIa: Household Stoves in India. Washington, DC: U.S. Environmental Protection Agency, Office of Research and Development; 2000. [Google Scholar]
- 31.Brown J, Hien VT, McMahan L, Jenkins MW, Thie L, Liang K, Printy E, Sobsey MD. Relative benefits of on-plot water supply over other ‘improved’ sources in rural Vietnam. Trop Med Int Health. 2013;18:65–74. doi: 10.1111/tmi.12010. [DOI] [PubMed] [Google Scholar]
- 32.Clasen TF, Thao do H, Boisson S, Shipin O. Microbiological effectiveness and cost of boiling to disinfect drinking water in rural Vietnam. Environ Sci Technol. 2008;42:4255–4260. doi: 10.1021/es7024802. [DOI] [PubMed] [Google Scholar]
- 33.Clasen T, McLaughlin C, Nayaar N, Boisson S, Gupta R, Desai D, Shah N. Microbiological effectiveness and cost of disinfecting water by boiling in semi-urban India. Am J Trop Med Hyg. 2008;79:407–413. [PubMed] [Google Scholar]
- 34.Rosa G, Miller L, Clasen T. Microbiological effectiveness of disinfecting water by boiling in rural Guatemala. Am J Trop Med Hyg. 2010;82:473–477. doi: 10.4269/ajtmh.2010.09-0320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sodha SV, Menon M, Trivedi K, Ati A, Figueroa ME, Ainslie R, Wannemuehler K, Quick R. Microbiologic effectiveness of boiling and safe water storage in South Sulawesi, Indonesia. J Water Health. 2011;9:577–585. doi: 10.2166/wh.2011.255. [DOI] [PubMed] [Google Scholar]
- 36.Brown J, Sobsey MD. Boiling as household water treatment in Cambodia: a longitudinal study of boiling practice and microbiological effectiveness. Am J Trop Med Hyg. 2012;87:394–398. doi: 10.4269/ajtmh.2012.11-0715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Gupta SK, Suantio A, Gray A, Widyastuti E, Jain N, Rolos R, Hoekstra RM, Quick R. Factors associated with E. coli contamination of household drinking water among tsunami and earthquake survivors, Indonesia. Am J Trop Med Hyg. 2007;76:1158–1162. [PubMed] [Google Scholar]
- 38.Oswald WE, Lescano AG, Bern C, Calderon MM, Cabrera L, Gilman RH. Fecal contamination of drinking water within peri-urban households, Lima, Peru. Am J Trop Med Hyg. 2007;77:699–704. [PubMed] [Google Scholar]
- 39.Psutka R, Peletz R, Michelo S, Kelly P, Clasen T. Assessing the microbiological performance and potential cost of boiling drinking water in urban Zambia. Environ Sci Technol. 2011;45:6095–6101. doi: 10.1021/es2004045. [DOI] [PubMed] [Google Scholar]
- 40.Arnold B, Arana B, Mausezahl D, Hubbard A, Colford JM., Jr Evaluation of a pre-existing, 3-year household water treatment and handwashing intervention in rural Guatemala. Int J Epidemiol. 2009;38:1651–1661. doi: 10.1093/ije/dyp241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Rosa G, Huaylinos ML, Gil A, Lanata C, Clasen T. Assessing the consistency and microbiological effectiveness of household water treatment practices by urban and rural populations claiming to treat their water at home: a case study in Peru. PLoS One. 2014;9:e114997. doi: 10.1371/journal.pone.0114997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Moe CL, Sobsey MD, Samsa GP, Mesolo V. Bacterial indicators of risk of diarrhoeal disease from drinking-water in the Philippines. Bull World Health Organ. 1991;69:305–317. [PMC free article] [PubMed] [Google Scholar]
- 43.Brown JM, Proum S, Sobsey MD. Escherichia coli in household drinking water and diarrheal disease risk: evidence from Cambodia. Water Sci Technol. 2008;58:757–763. doi: 10.2166/wst.2008.439. [DOI] [PubMed] [Google Scholar]
- 44.Gundry S, Wright J, Conroy R. A systematic review of the health outcomes related to household water quality in developing countries. J Water Health. 2004;2:1–13. [PubMed] [Google Scholar]
- 45.Jensen PK, Jayasinghe G, van der Hoek W, Cairncross S, Dalsgaard A. Is there an association between bacteriological drinking water quality and childhood diarrhoea in developing countries? Trop Med Int Health. 2004;9:1210–1215. doi: 10.1111/j.1365-3156.2004.01329.x. [DOI] [PubMed] [Google Scholar]
- 46.Khush RS, Arnold BF, Srikanth P, Sudharsanam S, Ramaswamy P, Durairaj N, London AG, Ramaprabha P, Rajkumar P, Balakrishnan K, Colford JM., Jr H2S as an indicator of water supply vulnerability and health risk in low-resource settings: a prospective cohort study. Am J Trop Med Hyg. 2013;89:251–259. doi: 10.4269/ajtmh.13-0067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Levy K, Nelson KL, Hubbard A, Eisenberg JN. Rethinking indicators of microbial drinking water quality for health studies in tropical developing countries: case study in northern coastal Ecuador. Am J Trop Med Hyg. 2012;86:499–507. doi: 10.4269/ajtmh.2012.11-0263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Gruber JS, Ercumen A, Colford JM., Jr Coliform bacteria as indicators of diarrheal risk in household drinking water: systematic review and meta-analysis. PLoS One. 2014;9:e107429. doi: 10.1371/journal.pone.0107429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Rosa G, Clasen T. Estimating the scope of household water treatment in low- and medium-income countries. Am J Trop Med Hyg. 2010;82:289–300. doi: 10.4269/ajtmh.2010.09-0382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Iijima Y, Karama M, Oundo JO, Honda T. Prevention of bacterial diarrhea by pasteurization of drinking water in Kenya. Microbiol Immunol. 2001;45:413–416. doi: 10.1111/j.1348-0421.2001.tb02639.x. [DOI] [PubMed] [Google Scholar]