Abstract
The effect of a parasite on the life history of its vector is important for understanding and predicting disease transmission. Chagas disease agent Trypanosoma cruzi is a generalist parasite that is diverse across scales from its genetic diversity to the 100s of mammal and vector species it infects. Its vertebrate hosts show quite variable responses to infection, however, to date there are no studies looking at how T. cruzi variability might result in variable outcomes in its invertebrate host. Therefore, we investigated the effect of different T. cruzi I strains on Rhodnius prolixus survival and development. We found significant variation between insects infected with different strains, with some strains having no effect, as compared with uninfected insects, and others with significantly lower survival and development. We also found that different variables had varying importance between strains, with the effect of time postinfection and the blood:weight ratio of the infective meal significantly affecting the survival of insects infected with some strains, but not others. Our results suggest that T. cruzi can be pathogenic not only to its vertebrate hosts but also to its invertebrate hosts.
Introduction
Chagas disease is a parasitic infection that can lead to heart failure and other related pathologies.1 Caused by the flagellate protozoan Trypanosoma cruzi, Chagas disease is found throughout the new world, mainly in areas with settings conducive to human contact with its insect vector, the triatomine bug (Hemiptera: Reduviidae). An estimated 6 million people are currently infected, and an estimated 10,000 deaths per annum are related to Chagas disease.2,3
As a generalist parasite, T. cruzi is diverse across scales, infecting a wide range of mammal host species across an expansive geographic area. In addition, T. cruzi shows such high levels of genetic diversity that it has been proposed as a complex rather than a species,4 and is currently divided into six discrete typing units (DTUs I–VI),5 each composed of genetic clusters thought to be loosely associated with different geographic regions, mammal hosts, triatomine bug genera, and clinical manifestations of Chagas disease.6–9
The diversity of T. cruzi extends to the effects it has on its hosts, with its pathogenicity in mammals being highly variable, both within and between species and individuals, ranging from asymptomatic to severe pathology.1,10–13 However, current opinion on the effect of T. cruzi on its invertebrate host assumes no change in fitness relative to uninfected individuals, except in circumstances of external stress,14,15 which is described by Schaub and others as “subpathogenic.”15–20 This idea is mainly based on studies of the triatomine species Triatoma infestans.15,21–27
Investigations into the effect of T. cruzi infection on other triatomine species have yielded variable results, with some results suggesting that T. cruzi reduces triatomine survival and or reproduction,28–32 and others finding little to no effect.33–35 Although some of the discrepancies may be due to differences in experimental conditions or design, it is also possible that the inherent variability of T. cruzi itself is a driver of the variable fitness outcomes in infected triatomines, as it is in its mammal hosts. To the best of our knowledge, this has not been tested, as all published studies on the effect of T. cruzi on triatomines have been limited to one T. cruzi strain-I triatomine species system.15,21–35
Given that a principle focus in Chagas disease prevention is the interruption of T. cruzi transmission by triatomine bugs living in and around human homes, it is important to understand the full range of outcomes that trypanosome infection can have on triatomines. Therefore, we asked, does variability exist in the effect of different T. cruzi strains on its triatomine host? To investigate this question, we measured the survival and molt outcomes of the triatomine species Rhodnius prolixus, when infected with different strains of T. cruzi DTU I. Rhodnius prolixus is considered to be the principal domestic vector of T. cruzi in Venezuela, Colombia, and parts of Central America,12,14 and thus a comprehensive understanding of the effect of this parasite on its insect host is critical to improving vector surveillance and control strategies in several areas endemic for Chagas disease.
Materials and Methods
Experiment design.
We infected 168 R. prolixus fifth instar nymphs with one of five different T. cruzi (DTU I) strains (Table 1). Thirty additional uninfected insects were used as controls. Following infection, we recorded the survival and molt outcome (successful, with deformities, or death) of each insect for up to 65–127 days.
Table 1.
Background information for each Trypanosoma cruzi strain used
| Strain name | N* | Geographic origin† | Biological origin | International ID | Ecotope |
|---|---|---|---|---|---|
| Cas15 | 29 | Villanueva, Casanare | Rhodnius prolixus | TPRX/CO/2000/CAS15 | Extradomestic |
| Cas20 | 27 | Villanueva, Casanare | R. prolixus | TPRX/CO/2000/CAS20 | Extradomestic |
| Gal61 | 29 | Galeras, Sucre | Mus spp. | XXXX/CO/91/Gal61 | Extradomestic |
| SO-8 | 27 | San Ofre, Sucre | R. pallescens | TPAC/CO/1995/ SO8 | Extradomestic |
| Sebas1 | 26 | San Sebastián, Magdalena | R. pallescens | I.RHO/CO/06/SEBAS-1.MAG | Extradomestic |
Number of insects in each group.
All geographic origins are located in Colombia, listed as “Municipality, State.”
Insects.
Rhodnius prolixus were from five different colonies in the Biología y Control de Enfermedades Infecciosas (BCEI) insectarium. Each colony was founded by R. prolixus eggs collected from Colombia. These colonies are kept under semi-controlled climate conditions (∼27°C ± 1°C and 65% ± 15% RH) in a 12-hour light/dark cycle, and insects are given the opportunity to feed twice weekly on hens, according to the animal ethics committee regulations of the Sede de Investigacion Universitaria (SIU) of the University of Antioquia.
Parasite strain selection.
All T. cruzi strains were originally isolated from Colombia, and have been characterized as sylvatic DTU I36–39 (or “Tc1”), as Tc1 is believed to be the predominant DTU in the country,9,38,40 and is associated with the genus Rhodnius.5,6,41 We used strains rather than clones to simulate the natural conditions of vector-borne T. cruzi transmission. Parasite strain background information is presented in Table 1.
Insect infection: parasite preparation.
Epimastigote forms of T. cruzi were cultured at 28°C in RPMI-1640 liquid medium (Sigma-Aldrich, St. Louis, MO) supplemented with 10% fetal bovine serum42,43 and collected in their exponential growth phase for insect infection, as in several similar studies.9,38,44–51 To calculate the parasite concentration in culture medium, we gently swished flasks containing the parasites in the medium to facilitate even parasite dispersion. We pipetted the parasites into a 0.5% formalin–phosphate-buffered saline (PBS) solution, and then counted them in a Neubauer cell counter under a compound light microscope.40,42,43,52–54 After estimating parasite concentration, we centrifuged them in 1.5 μL Eppendorf tubes for 10 minutes at 3,000 rpm to wash off the culture medium. After the centrifugation, we poured off the medium and replaced it with sterile 0.15 M NaCl, 0.01 M phosphate buffer, pH 7.2 (PBS). We gently pipetted the solution up and down to resuspend the parasites, and then repeated the process once more for a final solution of parasites resuspended in 1 mL of PBS.
Insect infection: blood preparation.
After counting and washing the parasites, we pipetted the parasite solution into a given quantity of 37.5°C defibrinated, decomplemented human blood (drawn from the same healthy donor at three different time points) supplemented with fetal bovine serum, for a final estimated concentration of 3.3–3.5 × 106 parasites per milliliter of blood.
This parasite concentration is similar to that used in several other published studies of T. cruzi infection in triatomines,9,44,46,50,54–59 and blood meals taken by triatomines at this parasite concentration lead to infective doses that fall within the range of peak parasitemias observed in mice and guinea pigs experimentally infected with T. cruzi.25,27,60–63
Insect infection: insect preparation and feeding.
Prior to the infective feeding, we weighed and marked each R. prolixus fifth instar nymph with a small dot of nontoxic, water-based paint at the top of the pronotum64,65 for recognition after feeding, and placed it in a small jar (6 × 7 cm, 200 mL) with 5–10 other weighed and marked nymphs. Each R. prolixus fifth instar had not eaten for about 2 weeks.66,67 We placed each jar of nymphs under an artificial membrane feeder containing the parasite-blood solution at 37.5°C for about 30 minutes.68 Control insects consumed blood without parasites. We weighed each nymph after feeding to estimate the number of parasites ingested, which was calculated by subtracting each insect's pre-feeding weight from its post-feeding weight, dividing this amount by the average density of human blood (1.06 mg/mL), and multiplying the result by 3.3–3.5 × 106 flagellates per milliliter, depending on parasite treatment. Insects that did not eat were not used in the study. After infection, each insect was kept in a small glass jar (4.5 × 4.5 cm, 60 mL) with two pieces of folded carton inside and a mesh top,69 in still-air incubators (Hovabator model no. 1602N) with dimensions of 18 × 18 × 9½ inches to minimize climate variation. Climate conditions within the incubators ranged between 27.5°C ± 1.5°C and 68% ± 8% RH, in accordance with the recommended climate for rearing triatomines.69 After infection, insects were fed triweekly on hens.
Infection confirmation.
We confirmed insect infection in a subset of the insects by direct microscopic observation and polymerase chain reaction (PCR). Upon death, we macerated and centrifuged each insect, forming an insect–parasite tissue pellet as described in refs.46,70 and extracted total deoxyribonucleic acid (DNA) from each pellet with a Qiagen DNeasy blood and tissue kit. We followed the kit protocol for tissue extraction, but with an additional hour of lysis in ATL buffer at 56°C, vortexing every 15 minutes. We amplified the DNA in a PCR with the primer pair TcZ1 (5′-CGAGCTCTTGCCCACACGGGTGCT-3′) and TcZ2 (5′-CCTCCAAGCAGCGGATAGTTCAGG-3′)71 that amplifies a 188-base pair sequence of T. cruzi satellite DNA.
Molt analyses.
We evaluated four molt variables between treatment groups: 1) molt occurrence (yes or no), 2) molted but with deformities (yes or no), 3) death during molt (yes or no), and 4) molt day. “Molted but with deformities” refers to deformities resulting from the molting process that would prevent insect reproduction and/or dispersal, such as incomplete shedding of the exuviae or molting with a broken neck or crumpled wings (Supplemental Figures 1 and 2). Molt day refers to the day postinfection that molting occurred. Insect sex was determined after molting occurred, and in most cases was only possible in those individuals that molted without deformities. Therefore, differences between males and females were not tested, as the majority of insects with their sex determined had successfully molted without any problems.
Statistical analyses.
We carried out all statistical analyses using the R statistical computing environment software version 3.0372 using only nonparametric tests to avoid normality assumptions. We tested for differences between treatments in the amount of parasites or blood ingested per unit of insect mass using the Kruskal–Wallis rank sum tests, with which we also tested for an effect of the time point at which blood was drawn on the number of days lived. We tested for differences between binomial outcomes (such as death during molt) in the amount of parasites or blood ingested per unit of body weight using Wilcoxon rank sum tests. We evaluated differences between treatment groups in the variables “molt occurrence,” “molted with deformities,” and “death during molt” with Fisher's exact test for count data, and molt day between treatments with the Kruskal–Wallis rank sum test, applying the “kruskalmc” function from the “pgirmess” package73 to carry out multiple comparisons and control for family wise error when a difference was found in Kruskal–Wallis tests. This function implements comparisons between treatments, and one- and two-tailed comparisons versus control. We accepted P values < 0.05 as statistically significant.
Survival analysis.
We generated survival curves for each treatment group using the Kaplan–Meier (K–M) method in the R “survival” package.74,75 We compared survival (the probability of total time until failure) between treatment groups using the “survdiff” function in the “survival” package, a two-tailed test for censored data that implements the G-rho family of tests,76 where deaths at various times are weighted by a factor of S(t)^ρ (S = K–M estimate; t = time), where ρ is a scalar parameter that determines what type of test is used. When set at 0, all deaths are weighted equally across time and a log-rank test is used. When set at 1, deaths at the beginning of the time are more heavily weighted, and the Peto and Peto test77 is used. We set ρ at 1, to offset death events related to senescence. We carried out pairwise comparisons between K–M survival curves were with χ2 distribution tests and adjusted P values to control for the family-wise error rate using the Holm–Bonferroni correction method.78
We used Cox proportional hazards (PH) models79 to examine the main effects and two-way interactions of parasite treatment, parasite dose, and blood ingested on treatment hazard rates (the instantaneous rate of failure at any given time, given that the individual has survived up until that time). The PH assumption (i.e., hazards were proportional over time) was tested with the Coxph function in the “survival” package. Data violated the (PH) assumption, so we ran them in an extended Cox model, with data split at 28 days, which was the final molt day for the majority of the insects, and within the time when hazard curves became disproportionate (between 21 and 30 days postinfection). We created dummy variables representing two episodes, 1) up to 28 days postinfection (“early”) and 2) after 28 days postinfection (“late”), to examine hazard in each episode.
We selected model covariates using the Akaike information criterion (AIC) with the stepAIC function in the “MASS” package,80 and manual one-variable-at-a-time reduction. Final covariates included in the model were the main effects of parasite treatment and main effects of the ratio of blood ingested in the infective blood meal to the insect weight prior to feeding (referred to from here on “blood:weight”), which can also be seen as a proxy for estimated number of parasites ingested per milligram of body mass. We used the blood:weight ratio to normalize for variation in insect size. We included the interaction effects of the dummy variables representing the treatment before 28 days with blood:weight, and centered blood:weight data on the mean (6.05:1), as no insect in the study weighed nothing or consumed a volume of blood equal to 0. Blood:weight data were log2 transformed after being centered. Thus, main effects of blood:weight can be interpreted as the effects of a blood meal six times that of a given insect's weight in a given parasite treatment group. Blood:weight treatment interaction effects represent the effect of a blood meal of a specific treatment that is 3 and 12 times the insects' body weight (due to log2 transformation), depending on whether the β value given by the model is subtracted or added from the β value given for treatment main effects.
We excluded the interaction of blood:weight with treatment after 28 days, as insects took several more blood meals in this period. Parasite loads in triatomines are dramatically reduced on ingestion of an uninfected blood meal.22,26,81 Studies have shown that 1) the number of parasites in an infective blood meal decreases dramatically on ingestion due to digestive enzymes, temperature changes, and the gut microbiota community48,82; and 2) the total trypanosome population size and composition (proportion of each form present) within a triatomine fluctuates according to feeding status; significant decreases in parasite numbers can occur within 4 hours after feeding, by as much as 50% in some parts of the bug22,26,81; and 3) T. cruzi-infective dose does not correlate with the number of parasites excreted.62,83,84 These studies suggest that the number of parasites ingested is fewer than the number that the insect will face, and that, while it may lead to a temporary effect, this should change by the time the insect excretes parasites and takes its next blood meal.
We excluded sex, as it was determined after insect molt, meaning that after a majority of deaths of insects of unknown sex had already occurred. Full Cox model outputs (Supplemental Table 1) and a guide on interpreting the results are provided in Supplemental Appendix 1.
Results
Volume of blood and estimated number of parasites ingested.
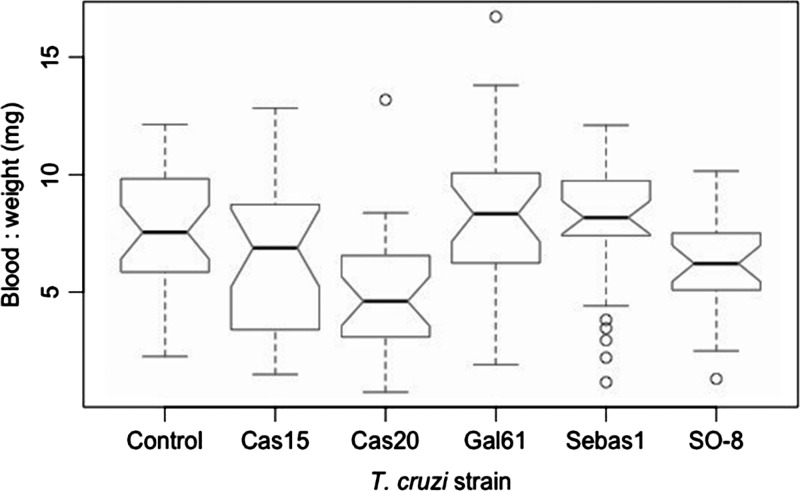
Insects ingested between 20.0 and 277.0 mg of blood (mean 172.0 mg) and an estimated 68,000–920,000 of T. cruzi parasites (mean 559,000). The ratio of the volume of blood ingested to insect pre-feeding weight ranged from 0.74 to 16.71 (mean 6.05), and the ratio of the estimated number of parasites ingested per milligram of insect biomass ranged from 2,000 to 55,000 parasites (mean 22,000). There was no significant difference in the amount of blood or the estimated number of parasites ingested between treatment groups (Kruskal–Wallis, blood: P = 0.71; parasites: P = 0.66). There was a significant difference between treatments in the ratio of the volume of blood ingested and estimated number of parasites ingested to pre-feeding weight (Kruskal–Wallis, blood: P = 1.24e−04; parasites: P = 2.85e-04, Figure 1 ), with both ratios significantly lower in the Cas20 treatment than that of the Gal61 and Sebas1 and the blood:weight ratio of Cas20 lower than the control group (Kruskalmc, P < 0.01).
Figure 1.
Blood:weight ratio distributions by treatment group. Blood:weight is the ratio of the volume of blood consumed in the infective blood meal by a given insect to its pre-feeding weight in milligram. The Cas20 group blood:weight ratio was significantly lower than that of the Gal61, Sebas1, and control groups.
Infection confirmation.
Infection was confirmed in 50% of all insects, and there was no difference in the proportion of insects with confirmed infections between treatments (Fisher's exact test, P = 0.63).
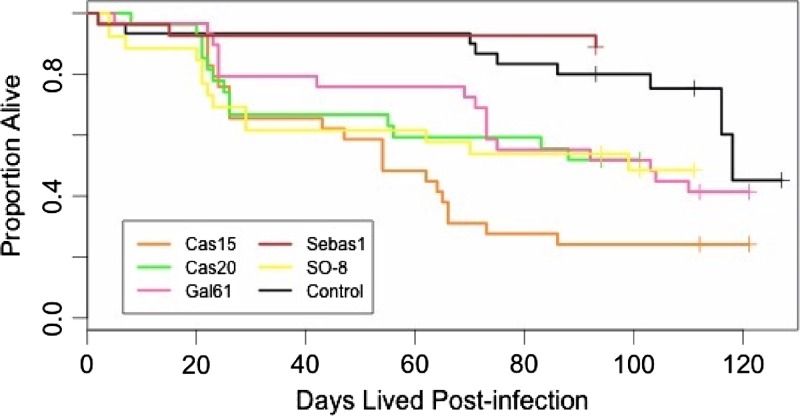
Survival.
Kaplan–Meier survival curves were not the same between treatment groups (χ2 = 25.2, 5 df, P = 1.19e-04, Figure 2 ). Pairwise comparisons revealed the difference to be between the Cas15 treatment group and the control and Sebas1 treatments (χ2 distribution comparisons, corrected P < 0.05 for comparisons of Cas15 with Sebas1 and with the control group). There was no effect of the time point of the blood drawn on insect survival (Kruskal–Wallis, P = 0.85).
Figure 2.
Kaplan–Meier (K–M) curves representing the survival of each treatment group over time. Crosses represent right-censored data.
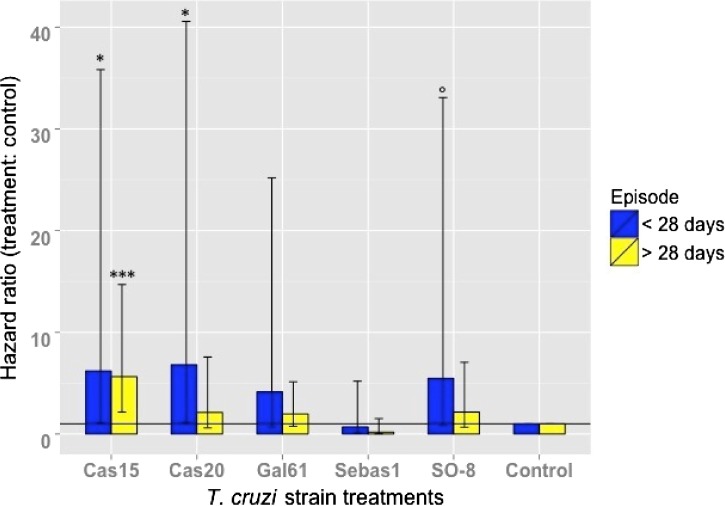
Cox model summary results suggested that treatment and blood:weight ratio significantly influenced hazard (likelihood ratio test, 62.86 on 16 df, P = 1.71e-07). Insects infected with strains Cas15 and Cas20 had statistically significant hazard ratios, with hazard being over six times higher than that of the control group in the period up to 28 days postinfection (Cas15: eβ = 6.20, P = 0.04; Cas20: eβ = 6.81, P = 0.03, Figure 3 ). After 28 days, Cas15 was the only treatment group with a statistically significant hazard ratio, with a hazard that was 5.6 times that of the control group (eβ = 5.63, P = 4.05e-04). Although not significant, there was a consistent pattern of decrease in the hazard ratio of each treatment from before 28 days to after 28 days. Main effects of blood:weight ratio significantly decreased hazard by 86.52% (eβ = 0.13, P = 1.70e-03). Cas20 significantly interacted with the blood:weight ratio, with hazard increasing with increasing volumes of blood (and/or parasites) per body mass (eβ = 7.66, P = 7.06e-03). This effect was also marginally significant for the Gal61 and Cas15 groups (Gal61: eβ = 4.70, P = 5.52e-02; Cas15: eβ = 4.02, P = 7.67e-02)
Figure 3.
Instantaneous hazard ratios (eβ) for Trypanosoma cruzi strain treatment group main effects before and after 28 days postinfection. Confidence intervals (CI) of the β value are indicated by vertical lines. The horizontal line crosses y axis at 1 to indicate where a hazard ratio of 1 lies. *P < 0.05; ***P < 0.001; °P < 0.10.
Molt.
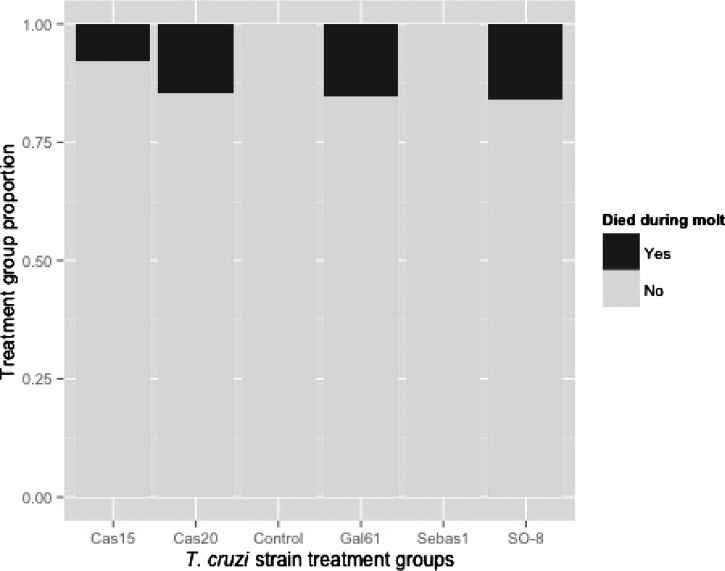
No insects in the control or Sebas1 groups died during molt or molted with deformities. The proportions of insects that died during molt and molted with deformities were globally different between treatments (Fisher's exact test, died: P = 0.03, Figure 4 ; deformities: P = 8.19e-03), however, there were no significant differences between treatment groups after pairwise comparisons were carried out between each treatment and P values were adjusted for multiple comparisons. There was no difference in the amount of blood ingested or parasites ingested per unit of body mass between insects that died during molt and those that did not, nor between insects that molted with deformities and those that did not. The proportions of insects that molted were not significantly different between treatments nor were the days that molting occurred.
Figure 4.
Proportion of insects in each treatment group that died during molt.
Discussion
It is well known that T. cruzi has highly variable effects on its vertebrate hosts. In humans infected with T. cruzi, 60–70% never develop symptoms of infection, whereas the other 30–40% will develop symptoms that manifest themselves in one of at least three general ways (cardiac, digestive, or both),1,13 and this is known to vary by region, with the southern cone of South America being associated with gastrointestinal problems and in the northern parts of South America associated with heart failure.85 These disease variations have been linked to the different T. cruzi genotypes circulating in these areas.5,8 T. cruzi parasitemia in mice and opossums has been found to vary with T. cruzi strain and clone,12,86–89 tissue lesions caused by T. cruzi have been found to be different between sylvatic and domestic animals,13 and differential tissue distribution was observed between different clones of T. cruzi when infecting mice.90 The length of acute parasitemia is highly variable among T. cruzi-infected vertebrate hosts, with some hosts exhibiting long-lasting high parasitemias and others moving from an acute stage to a chronic stage with low parasitemia in a matter of weeks.1,88,89 In spite of all of this attention to the varied effects of T. cruzi on vertebrate hosts, until now, there has been relatively little attention to variation in its effect on its invertebrate hosts.
Therefore, we investigated the effects of T. cruzi on the triatomine species R. prolixus when infected with different strains of T. cruzi I. Taking into account time postinfection and the volume of blood/parasites ingested per unit of body mass, we found considerable variability in the effects of T. cruzi on R. prolixus survival, which was also reflected in differences between treatments in insect molt outcomes.
Trypanosoma cruzi strain variation reflected in R. prolixus host survival outcomes.
There was a global difference between K–M survival curves of each treatment group (i.e., curves representing the probability that an individual survives longer than a given time point, adjusted for right-censored data), with marked differences between survival in the Cas15 and Sebas1 treatments, suggesting that T. cruzi strains can have highly variable effects on R. prolixus longevity. These differences in survival were confirmed in the hazards analysis, which is often considered more informative than survival function about underlying causes of death,91 as it allows for the inclusion of the main and interaction effects of covariates in the analysis, which in this case were time postinfection and the volume of blood/parasites ingested. The period between 0 and 28 days postinfection was more hazardous than the subsequent time, with insects infected with strains Cas15 and Cas20 having a hazard rate over six times that of the control group. This time is critical for both the insects and the parasite, as it includes the insect molt period, which in this study occurred between 16 and 28 days for the majority of the insects and the parasite prepatent period, which usually occurs up to 15 days postinfection.92 The increased hazard could be attributed to parasite establishment and replication along with the physiological demands of molting within this relatively short period. The imaginal molt (the molt from fifth instar to adult) is indeed very demanding, as the insect must undergo several morphological changes that do not occur in the previous four molts, including the development of wings and reproductive organs. This was reflected in the molt outcomes, with the two treatment groups with the highest survival and lowest hazards, Sebas1 and control, having no insects that died during molt or molted with deformities. After 28 days, the Cas15 treatment continued to have the highest hazard at 4.5 times higher than the control group, suggesting that negative effects of some T. cruzi strains can continue after the molt period and the initial establishment of T. cruzi infection.
In addition, the blood:weight covariate interacted with hazard differently in the control and Cas20 groups. In the control group, higher volumes of blood per milligram of insect weight significantly decreased hazard (expressed as blood:weight main effects), whereas the opposite effect occurred in the Cas20 group, with higher volumes of blood per milligram significantly increasing hazard. This suggests that, for the Cas20 strain, as the volume of blood (and in turn, number of parasites) in a T. cruzi-infected blood meal per milligram of insect weight increases, the nutritional benefits of the blood are overridden by the presence of parasites. Interestingly, Cas20 was the treatment group with significantly lower blood:weight ratios than the other groups, and the interaction between hazard and blood:weight ratio was not significant in any other group. This could be due to the existence of a threshold dose in lower parasite ranges only reached by insects in the Cas20 group, above which hazard increases, or it could be an effect of the Cas20 strain itself. Considering the variation in parasitemias demonstrated in T. cruzi-infected mammal hosts, variation in the effect of parasite dose on triatomine mortality could be an interesting area for future research and a potential predictor of triatomine mortality rates in an area where average chronic host parasitemias are known, such as in domestic and peridomestic transmission cycles consisting mainly of opossums, rodents, pets, and humans.
Impacts of T. cruzi virulence across scales.
If T. cruzi virulence were variable in field-caught triatomines, it could lead to higher average mortality rates in infected vectors than in uninfected vectors. On a population level, this might result in unstable transmission of T. cruzi, and in turn, a decreased proportion of mammal hosts infected with the parasite.93,94 On an individual level, T. cruzi virulence variation in triatomines presents the possibility that some insects may serve as filters of highly virulent strains, if the insects were to die before transmitting the strains to a new mammal host. Interestingly, in a prior study by the BCEI group, it was found that Cas15, the most virulent strain in this experiment, was highly virulent in mice as well (unpublished undergraduate thesis, Juan Fernando Rios), infecting organs uncommonly colonized by T. cruzi such as the brain and the testis. Further comparisons of the pathogenicity of a given T. cruzi strain in both of its obligate hosts, the vertebrate and invertebrate, could yield insights into the triatomine bug's potential to run “ecological interference” in the transmission of more virulent strains.95 This was tested in Anopheles stephensi mosquitoes infected with different Plasmodium chabaudi clones, but they found no correlation in parasite virulence between the mouse and in the vector.96 However, they found that the effect of P. chabaudi on mosquitoes was highly variable, depending on the P. chabaudi clone infecting the mosquito, which has also been observed in Trypanosoma brucei-infected tsetse flies.97
Future developments.
Each triatomine–T. cruzi strain pair bears a unique combination of factors belonging to both the parasite and the insect. Therefore, the survival outcome of each infected triatomine will be influenced by its own biochemical and biological variables, such as its stage, size, immune system, nutritional state, and endosymbiont population,88,92,98,99 and how these factors interact with and respond to infection by a given T. cruzi strain. In addition, other characteristics of the infection will also affect insect fitness, such as dose size, infective blood meal source and timing, inoculum frequency, and parasite form used in the infection (epimastigotes versus trypomastigotes). Therefore, some important variables to examine in future investigations on the effect of T. cruzi on triatomine life history would be insect endosymbiont populations, T. cruzi inoculum frequency and size, T. cruzi form, and T. cruzi strain origin such as domestic vs. sylvatic strains. To begin to identify the drivers within each strain of the variations in virulence we observed, it will be critical to investigate the effect of different T. cruzi I clones on triatomine survival. Finally, it is important to investigate the effect of T. cruzi on triatomines of different stages, species, and most importantly, in field-caught bugs, to confirm the patterns we observed and understand how they apply to patterns occurring in wild triatomine populations.
Summary
We found significant variation in the effect of T. cruzi strains on R. prolixus survival and development. We found that different variables had varying importance between strains, with the effect of time postinfection and the blood:weight ratio of the infective meal significantly affecting the survival of insects infected with some strains, but not others. Our results suggest that T. cruzi can be pathogenic to the triatomine species R. prolixus without apparent external stressors, suggesting that the variability in the effect of T. cruzi occurs not only in its vertebrate hosts but also in its invertebrate host.
Supplementary Material
ACKNOWLEDGMENTS
We thank Germán Rodriguez of the Office of Population Research, Princeton University, for statistical advice in the survival analyses. The Amerian Society of Tropical Medicine and Hygiene (ASTMH) assisted with publication expenses.
Footnotes
Financial support: Provided by grants from the Estrategia de sostenibilidad grupo BCEI, (CODI-Universidad de Antioquia 2014–2015), as well as graduate summer research funding from the Grand Challenges Program at Princeton University, (funded by the Woodrow Wilson School's Center for Health and Wellbeing and the Princeton Environmental Institute), and the Princeton Program in Latin American Studies.
Authors' addresses: Jennifer K. Peterson, Andrea L. Graham, and Andrew P. Dobson, Department of Ecology and Evolutionary Biology, Princeton University, Princeton, NJ, E-mails: jenni.peterson@gmail.com, algraham@princeton.edu, and dobson@princeton.edu. Omar Triana Chávez, Grupo BCEI, Universidad de Antioquia, Medellín, Antioquia, Colombia, E-mail: otriana@gmail.com.
References
- 1.Rassi A, Jr, Rassi A, Marin-Neto JA. Chagas disease. Lancet. 2010;375:1388–1402. doi: 10.1016/S0140-6736(10)60061-X. [DOI] [PubMed] [Google Scholar]
- 2.World Health Organization Chagas disease in Latin America: epidemiological update based on the 2010 estimates. Weekly Epidemiological Record. 2015;90:33–44. [PubMed] [Google Scholar]
- 3.WHO Expert Committee on the Control of Chagas Disease (2000: Brasilia, Brazil) Control of Chagas disease: second report of the WHO expert committee WHO Technical Report Series. 905:1–106. [Google Scholar]
- 4.Devera R, Fernandes O, Coura JR. Should Trypanosoma cruzi be called “cruzi” complex? A review of the parasite diversity and the potential of selecting population after in vitro culturing and mice infection. Mem Inst Oswaldo Cruz. 2003;98:1–12. doi: 10.1590/s0074-02762003000100001. [DOI] [PubMed] [Google Scholar]
- 5.Zingales B, Miles MA, Campbell DA, Tibayrenc M, Macedo AM, Teixeira MMG, Schijman AG, Llewellyn MS, Lages-Silva E, Machado CR, Andrade SG, Sturm NR. The revised Trypanosoma cruzi subspecific nomenclature: rationale, epidemiological relevance and research applications. Infect Genet Evol. 2012;12:240–253. doi: 10.1016/j.meegid.2011.12.009. [DOI] [PubMed] [Google Scholar]
- 6.Yeo M, Acosta N, Llewellyn M, Sánchez H, Adamson S, Miles GA, López E, González N, Patterson JS, Gaunt MW, de Arias AR, Miles MA. Origins of Chagas disease: Didelphis species are natural hosts of Trypanosoma cruzi I and armadillos hosts of Trypanosoma cruzi II, including hybrids. Int J Parasitol. 2005;35:225–233. doi: 10.1016/j.ijpara.2004.10.024. [DOI] [PubMed] [Google Scholar]
- 7.Llewellyn MS, Miles MA, Carrasco HJ, Lewis MD, Yeo M, Vargas J, Torrico F, Diosque P, Valente V, Valente SA, Gaunt MW. Genome-scale multilocus microsatellite typing of Trypanosoma cruzi discrete typing unit I reveals phylogeographic structure and specific genotypes linked to human infection. PLoS Pathog. 2009;5:e1000410. doi: 10.1371/journal.ppat.1000410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Macedo AM, Machado CR, Oliveira RP, Pena SDJ. Trypanosoma cruzi: genetic structure of populations and relevance of genetic variability to the pathogenesis of chagas disease. Mem Inst Oswaldo Cruz. 2004;99:1–12. doi: 10.1590/s0074-02762004000100001. http://www.ncbi.nlm.nih.gov/pubmed/15057339 Available at. [DOI] [PubMed] [Google Scholar]
- 9.Mejía-Jaramillo AM, Peña VH, Triana-Chávez O. Trypanosoma cruzi: biological characterization of lineages I and II supports the predominance of lineage I in Colombia. Exp Parasitol. 2009;121:83–91. doi: 10.1016/j.exppara.2008.10.002. [DOI] [PubMed] [Google Scholar]
- 10.Bahia MT, Tafuri WL, Caliari MV, Veloso VM, Carneiro CM, Coelho GLLM, de Lana M. Comparison of Trypanosoma cruzi infection in dogs inoculated with blood or metacyclic trypomastigotes of Berenice-62 and Berenice-78 strains via intraperitoneal and conjunctival routes. Rev Soc Bras Med Trop. 2002;35:339–345. doi: 10.1590/s0037-86822002000400010. http://www.ncbi.nlm.nih.gov/pubmed/12170329 Available at. [DOI] [PubMed] [Google Scholar]
- 11.Deane MP, Lenzi HL, Jansen A. Trypanosoma cruzi: vertebrate and invertebrate cycles in the same mammal host, the opossum Didelphis marsupialis. Mem Inst Oswaldo Cruz. 1984;79:513–515. doi: 10.1590/s0074-02761984000400021. [DOI] [PubMed] [Google Scholar]
- 12.Jansen A, Roque ALR. Domestic and wild mammalian reservoirs. In: Telleria J, Tibayrenc M, editors. American Trypanosomiasis Chagas Disease. Amsterdam, The Netherlands: Elsevier; 2010. pp. 249–276. [Google Scholar]
- 13.Teixeira ARL, Nascimento RJ, Sturm NR. Evolution and pathology in Chagas disease-a review. Mem Inst Oswaldo Cruz. 2006;101:463–491. doi: 10.1590/s0074-02762006000500001. http://www.ncbi.nlm.nih.gov/pubmed/17072450 Available at. [DOI] [PubMed] [Google Scholar]
- 14.Garnham P. The comparative pathogenicity of Protozoa in their vertebrate and invertebrate hosts. Symp Soc Gen Microbiol. 1955;5:191–206. [Google Scholar]
- 15.Schaub GA. Does Trypanosoma cruzi stress its vectors? Parasitol Today. 1989;5:185–188. doi: 10.1016/0169-4758(89)90142-7. [DOI] [PubMed] [Google Scholar]
- 16.Schaub GA. The effects of trypanosomatids on insects. Adv Parasitol. 1992;31:255–319. doi: 10.1016/s0065-308x(08)60023-8. http://www.ncbi.nlm.nih.gov/pubmed/1496928 Available at. [DOI] [PubMed] [Google Scholar]
- 17.Schaub GA. Pathogenicity of trypanosomatids on insects. Parasitol Today. 1994;10:463–468. doi: 10.1016/0169-4758(94)90155-4. [DOI] [PubMed] [Google Scholar]
- 18.Schaub GA, Meiser CK, Balczun C. Interactions of Trypanosoma cruzi and Triatomines. In: Mehlhorn H, editor. Progress in Parasitology. Berlin, Heidelberg: Springer Berlin Heidelberg; 2011. pp. 155–178. [Google Scholar]
- 19.Vallejo GA, Guhl F, Schaub GA. Triatominae-Trypanosoma cruzi/T. rangeli: vector-parasite interactions. Acta Trop. 2009;110:137–147. doi: 10.1016/j.actatropica.2008.10.001. [DOI] [PubMed] [Google Scholar]
- 20.Schaub GA. Interactions of trypanosomatids and triatomines. In: Simpson SJ, Casas J, editors. Advances in Insect Physiology. Volume 37. Burlington, VT: Academic Press; 2009. pp. 177–242. [Google Scholar]
- 21.Schaub GA. Development of isolated and group-reared first instars of Triatoma infestans infected with Trypanosoma cruzi. Parasitol Res. 1988;74:593–594. doi: 10.1007/BF00531641. [DOI] [PubMed] [Google Scholar]
- 22.Schaub GA, Lösch P. Trypanosoma cruzi: origin of metacyclic trypomastigotes in the urine of the vector Triatoma infestans. Exp Parasitol. 1988;65:174–186. doi: 10.1016/0014-4894(88)90121-x. http://www.ncbi.nlm.nih.gov/pubmed/3280333 Available at. [DOI] [PubMed] [Google Scholar]
- 23.Schaub G. Developmental time and mortality of larvae of Triatoma infestans infected with Trypanosoma cruzi. Trans R Soc Trop Med Hyg. 1988;82:94–96. [PubMed] [Google Scholar]
- 24.Kollien A, Schaub G. The development of Trypanosoma cruzi (Trypanosomatidae) in the Reduviid bug Triatoma infestans (Insecta): influence of starvation. J Eukaryot Microbiol. 1998;45:59–63. doi: 10.1111/j.1550-7408.1998.tb05070.x. [DOI] [PubMed] [Google Scholar]
- 25.Kollien AH, Schmidt J, Schaub GA. Modes of association of Trypanosoma cruzi with the intestinal tract of the vector Triatoma infestans. Acta Trop. 1998;70:127–141. doi: 10.1016/s0001-706x(97)00117-4. [DOI] [PubMed] [Google Scholar]
- 26.Kollien A, Schaub G. Trypanosoma cruzi in the rectum of the bug Triatoma infestans: effects of blood ingestion by the starved vector. Am J Trop Med Hyg. 1998;59:166–170. doi: 10.4269/ajtmh.1998.59.166. [DOI] [PubMed] [Google Scholar]
- 27.Schaub GA, Losch P. Parasite/host-interrelationships of the trypanosomatids Trypanosoma cruzi and Blastocrithidia triatomae and the reduviid bug Triatoma infestans: influence of starvation on the bug. Ann Trop Med Parasitol. 1989;83:215–223. doi: 10.1080/00034983.1989.11812335. [DOI] [PubMed] [Google Scholar]
- 28.Botto-Mahan C, Cattan PE, Medel R. Chagas disease parasite induces behavioural changes in the kissing bug Mepraia spinolai. Acta Trop. 2006;98:219–223. doi: 10.1016/j.actatropica.2006.05.005. [DOI] [PubMed] [Google Scholar]
- 29.Botto-Mahan C, Ossa CG, Medel R. Direct and indirect pathways of fitness-impact in a protozoan-infected kissing bug. Physiol Entomol. 2008;33:25–30. [Google Scholar]
- 30.Botto-Mahan C. Trypanosoma cruzi induces life-history trait changes in the wild kissing bug Mepraia spinolai: implications for parasite transmission. Vector Borne Zoonotic Dis. 2009;9:505–510. doi: 10.1089/vbz.2008.0003. [DOI] [PubMed] [Google Scholar]
- 31.Lima MM, Borges-Pereira J, Albuquerque Dos Santos JA, Teixeira Pinto Z, Vianna Braga M. Development and reproduction of Panstrongylus megistus (Hemiptera: Reduviidae) infected with Trypanosoma cruzi, under laboratory conditions. Ann Entomol Soc Am. 1992;85:458–461. [Google Scholar]
- 32.Neves D, Peres R. Aspectos da biologia do Rhodnius prolixus quando alimentado em animais sadios ou infectados com o Trypanosoma cruzi. Rev Bras Biol. 1975;35:317–320. [Google Scholar]
- 33.Dos Santos JR, Lacombe D. Estudos relativos a duracao da ecdise e oviposicao de Triatoma infestans infectado pelo Trypanosoma cruzi. An Acad Bras Cienc. 1985;57:127. [Google Scholar]
- 34.Watkins R. Ph.D. Thesis, University of California, Berkeley; 1969. Host-parasite interaction between Trypanosoma species and Rhodnius prolixus Stal (Hemiptera, Reduviidae) [Google Scholar]
- 35.Oliveira TG, Carvalho-Costa FA, Gomes TF, Sarquis O, Sposina R, Lima MM. Developmental and reproductive patterns of Triatoma brasiliensis infected with Trypanosoma cruzi under laboratory conditions. Mem Inst Oswaldo Cruz. 2010;105:1057–1060. doi: 10.1590/s0074-02762010000800018. http://www.ncbi.nlm.nih.gov/pubmed/21225206 Available at. [DOI] [PubMed] [Google Scholar]
- 36.Cura CI, Mejía-Jaramillo AM, Duffy T, Burgos JM, Rodriguero M, Cardinal MV, Kjos S, Gurgel-Gonçalves R, Blanchet D, De Pablos LM, Tomasini N, da Silva A, Russomando G, Cuba Cuba C, Aznar C, Abate T, Levin MJ, Osuna A, Gürtler RE, Diosque P, Solari A, Triana-Chávez O, Schijman AG. Trypanosoma cruzi I genotypes in different geographical regions and transmission cycles based on a microsatellite motif of the intergenic spacer of spliced-leader genes. Int J Parasitol. 2010;40:1599–1607. doi: 10.1016/j.ijpara.2010.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mejía-Jaramillo AM, Arboleda-Sánchez S, Rodríguez IB, Cura C, Salazar A, Del Mazo J, Triana-Chávez O, Schijman AG. Geographical clustering of Trypanosoma cruzi I groups from Colombia revealed by low-stringency single specific primer-PCR of the intergenic regions of spliced-leader genes. Parasitol Res. 2009;104:399–410. doi: 10.1007/s00436-008-1212-0. [DOI] [PubMed] [Google Scholar]
- 38.Jaramillo N, Moreno J, Triana O, Arcos-Burgos M, Muñoz S, Solari A. Genetic structure and phylogenetic relationships of Colombian Trypanosoma cruzi populations as determined by schizodeme and isoenzyme markers. Am J Trop Med Hyg. 1999;61:986–993. doi: 10.4269/ajtmh.1999.61.986. http://www.ncbi.nlm.nih.gov/pubmed/10674683 Available at. [DOI] [PubMed] [Google Scholar]
- 39.Olmo F, Escobedo-Orteg J, Palma P, Sánchez-Moreno M, Mejía-Jaramillo A, Triana O, Marín C. Specific primers design based on the superoxide dismutase b gene for Trypanosoma cruzi as a screening tool: validation method using strains from Colombia classified according to their discrete typing unit. Asian Pac J Trop Med. 2014;7:854–859. doi: 10.1016/S1995-7645(14)60149-8. [DOI] [PubMed] [Google Scholar]
- 40.Falla A, Herrera C, Fajardo A, Montilla M, Vallejo GA, Guhl F. Haplotype identification within Trypanosoma cruzi I in Colombian isolates from several reservoirs, vectors and humans. Acta Trop. 2009;110:15–21. doi: 10.1016/j.actatropica.2008.12.003. [DOI] [PubMed] [Google Scholar]
- 41.Gaunt M, Miles M. The ecotopes and evolution of triatomine bugs (triatominae) and their associated trypanosomes. Mem Inst Oswaldo Cruz. 2000;95:557–565. doi: 10.1590/s0074-02762000000400019. [DOI] [PubMed] [Google Scholar]
- 42.Carrasco HJ, Frame IA, Valente SA, Miles MA. Genetic exchange as a possible source of genomic diversity in sylvatic populations of Trypanosoma cruzi. Am J Trop Med Hyg. 1996;54:418–424. doi: 10.4269/ajtmh.1996.54.418. [DOI] [PubMed] [Google Scholar]
- 43.Lewis MD, Ma J, Yeo M, Carrasco HJ, Llewellyn MS, Miles MA. Genotyping of Trypanosoma cruzi: systematic selection of assays allowing rapid and accurate discrimination of all known lineages. Am J Trop Med Hyg. 2009;81:1041–1049. doi: 10.4269/ajtmh.2009.09-0305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Fellet MR, Lorenzo MG, Elliot SL, Carrasco D, Guarneri AA. Effects of Infection by Trypanosoma cruzi and Trypanosoma rangeli on the reproductive performance of the vector Rhodnius prolixus. PLoS One. 2014;9:e105255. doi: 10.1371/journal.pone.0105255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ferreira LL, Lorenzo MG, Elliot SL, Guarneri AA. A standardizable protocol for infection of Rhodnius prolixus with Trypanosoma rangeli, which mimics natural infections and reveals physiological effects of infection upon the insect. J Invertebr Pathol. 2010;105:91–97. doi: 10.1016/j.jip.2010.05.013. [DOI] [PubMed] [Google Scholar]
- 46.Castro LA, Peterson JK, Saldaña A, Perea MY, Calzada JE, Pineda V, Dobson AP, Gottdenker NL. Flight behavior and performance of Rhodnius pallescens (Hemiptera: Reduviidae) on a tethered flight mill. J Med Entomol. 2014;51:1010–1018. doi: 10.1603/me14014. [DOI] [PubMed] [Google Scholar]
- 47.Whitten MM, Mello CB, Gomes SA, Nigam Y, Azambuja P, Garcia ES, Ratcliffe NA. Role of superoxide and reactive nitrogen intermediates in Rhodnius prolixus (Reduviidae)/Trypanosoma rangeli interactions. Exp Parasitol. 2001;98:44–57. doi: 10.1006/expr.2001.4615. [DOI] [PubMed] [Google Scholar]
- 48.Azambuja P, Feder D, Garcia ES. Isolation of Serratia marcescens in the midgut of Rhodnius prolixus: impact on the establishment of the parasite Trypanosoma cruzi in the vector. Exp Parasitol. 2004;107:89–96. doi: 10.1016/j.exppara.2004.04.007. [DOI] [PubMed] [Google Scholar]
- 49.Cortez MR, Provençano A, Silva CE, Mello CB, Zimmermann LT, Schaub GA, Garcia ES, Azambuja P, Gonzalez MS. Trypanosoma cruzi: effects of azadirachtin and ecdysone on the dynamic development in Rhodnius prolixus larvae. Exp Parasitol. 2012;131:363–371. doi: 10.1016/j.exppara.2012.05.005. [DOI] [PubMed] [Google Scholar]
- 50.Mello CB, Azambuja P, Garcia ES, Ratcliffe NA. Differential in vitro and in vivo behavior of three strains of Trypanosoma cruzi in the gut and hemolymph of Rhodnius prolixus. Exp Parasitol. 1996;82:112–121. doi: 10.1006/expr.1996.0015. [DOI] [PubMed] [Google Scholar]
- 51.Mello CB, Garcia E, Ratcliffe NA, Azambuja P. Trypaonsoma cruzi and Trypanosoma rangeli: interplay with hemolymph components of Rhodius prolixus. J Invertebr Pathol. 1995;65:261–268. doi: 10.1006/jipa.1995.1040. [DOI] [PubMed] [Google Scholar]
- 52.Gaunt M, Miles M. The ecotopes and evolution of triatomine bugs (triatominae) and their associated trypanosomes. Mem Inst Oswaldo Cruz. 2000;95:557–565. doi: 10.1590/s0074-02762000000400019. http://www.ncbi.nlm.nih.gov/pubmed/10904415 Available at. [DOI] [PubMed] [Google Scholar]
- 53.Herrera C, Bargues MD, Fajardo A, Montilla M, Triana O, Vallejo GA, Guhl F. Identifying four Trypanosoma cruzi I isolate haplotypes from different geographic regions in Colombia. Infect Genet Evol. 2007;7:535–539. doi: 10.1016/j.meegid.2006.12.003. [DOI] [PubMed] [Google Scholar]
- 54.Araújo CAC, Cabello PH, Jansen AM. Growth behaviour of two Trypanosoma cruzi strains in single and mixed infections: in vitro and in the intestinal tract of the blood-sucking bug, Triatoma brasiliensis. Acta Trop. 2007;101:225–231. doi: 10.1016/j.actatropica.2007.02.004. [DOI] [PubMed] [Google Scholar]
- 55.Araújo CAC, Waniek PJ, Jansen AM. TcI/TcII co-infection can enhance Trypanosoma cruzi growth in Rhodnius prolixus. Parasit Vectors. 2014;7:94. doi: 10.1186/1756-3305-7-94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Nogueira NFS, Gonzalez MS, Gomes JE, de Souza W, Garcia E, Azambuja P, Nohara LL, Almeida IC, Zingales B, Colli W. Trypanosoma cruzi: involvement of glycoinositolphospholipids in the attachment to the luminal midgut surface of Rhodnius prolixus. Exp Parasitol. 2007;116:120–128. doi: 10.1016/j.exppara.2006.12.014. [DOI] [PubMed] [Google Scholar]
- 57.Castro DP, Moraes CS, Gonzalez MS, Ratcliffe NA, Azambuja P, Garcia ES. Trypanosoma cruzi immune response modulation decreases microbiota in Rhodnius prolixus gut and is crucial for parasite survival and development. PLoS One. 2012;7:e36591. doi: 10.1371/journal.pone.0036591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Borges EC, Machado EMM, Garcia ES, Azambuja P. Trypanosoma cruzi: effects of infection on cathepsin D activity in the midgut of Rhodnius prolixus. Exp Parasitol. 2006;112:130–133. doi: 10.1016/j.exppara.2005.09.008. [DOI] [PubMed] [Google Scholar]
- 59.Ratcliffe NA, Nigam YN, Mello CB, Garcia ES, Azambuja P. Trypanosoma cruzi and erythrocyte agglutinins : a comparative study of occurrence and properties in the gut and hemolymph of Rhodnius prolixus. Exp Parasitol. 1996;83:83–93. doi: 10.1006/expr.1996.0052. [DOI] [PubMed] [Google Scholar]
- 60.Schaub GA, Grünfelder CG, Zimmermann D, Peters W. Binding of lectin-gold conjugates by two Trypanosoma cruzi strains in ampullae and rectum of Triatoma infestans. Acta Trop. 1989;46:291–301. doi: 10.1016/0001-706x(89)90042-9. http://www.ncbi.nlm.nih.gov/pubmed/2575865 Available at. [DOI] [PubMed] [Google Scholar]
- 61.Bice DE, Zeledon R. Comparison of infectivity of strains of Trypanosoma cruzi (Chagas, 1909) J Parasitol. 1970;56:663–670. [PubMed] [Google Scholar]
- 62.Urdaneta-Morales S, Rueda IG. A comparative study of the behavior of Venezuelan and Brazilian strains of Trypanosoma (Schizotrypanum) cruzi in the Venezuelan invertebrate host (Rhodnius prolixus) Rev Inst Med Trop Sao Paulo. 1977;19:241–250. [PubMed] [Google Scholar]
- 63.Perlowagora-Szumlewicz A, Muller CA. Studies in search of a suitable experimental insect model for xenodiagnosis of hosts with Chagas disease. 1-Comparative xenodiagnosis with nine triatomine species of animals with acute infections by Trypanosoma cruzi. Mem Inst Oswaldo Cruz. 1982;77:37–53. doi: 10.1590/s0074-02761982000100005. [DOI] [PubMed] [Google Scholar]
- 64.Mac Cord JR, Jurberg P, Lima MM. Marcacao individual de tratomineos para estudos comportamentais e ecologicos. Mem Inst Oswaldo Cruz. 1983;78:473–476. [Google Scholar]
- 65.Henriques C, Castro DP, Gomes LHF, Garcia ES, De Souza W. Bioluminescent imaging of Trypanosoma cruzi infection in Rhodnius prolixus. Parasit. Vectors. 2012;5:1–15. doi: 10.1186/1756-3305-5-214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Garcia E, Subrahmanyam B, Muller T, Rembold H. Absorption, storage, organ distribution, and excretion of dietary [22, 23-3H2] dihydroazadirachtin A in the blood-feeding bug Rhodnius prolixus. J Insect Physiol. 1989;35:743–748. [Google Scholar]
- 67.Gonzalez MS, Souza MS, Garcia ES, Nogueira NFS, Mello CB, Cánepa GE, Bertotti S, Durante IM, Azambuja P, Buscaglia CA. Trypanosoma cruzi TcSMUG L-surface mucins promote development and infectivity in the triatomine vector Rhodnius prolixus. PLoS Negl Trop Dis. 2013;7:e2552. doi: 10.1371/journal.pntd.0002552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Garcia E, Azambuja P. Infection of triatomines with Trypanosoma cruzi. In: Crampton JM, Beard CB, Louis C, editors. The Molecular Biology of Insect Disease Vectors: A Methods Manual. London: Chapman & Hall; 1997. pp. 146–155. [Google Scholar]
- 69.Azambuja P, Garcia E. Care and maintenance of Triatomine colonies. In: Crampton JM, Beard CB, Louis C, editors. The Molecular Biology of Insect Disease Vectors: A Methods Manual. London: Chapman & Hall; 1997. pp. 56–64. [Google Scholar]
- 70.Calzada JE, Pineda V, Montalvo E, Alvarez D, Santamaría AM, Samudio F, Bayard V, Cáceres L, Saldaña A. Human trypanosome infection and the presence of intradomicile Rhodnius pallescens in the western border of the Panama Canal, Panama. Am J Trop Med Hyg. 2006;74:762–765. http://www.ncbi.nlm.nih.gov/pubmed/16687677 Available at. [PubMed] [Google Scholar]
- 71.Moser DR, Kirchhoff LV, Donelson JE. Detection of Trypanosoma cruzi by DNA amplification using the polymerase chain reaction. J Clin Microbiol. 1989;27:1477–1482. doi: 10.1128/jcm.27.7.1477-1482.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Core Team R . R: A Language and Environment for Statistical Computing. Vienna, Austria: R Foundation for Statistical Computing; 2014. http://www.r-project.org/ Available at. [Google Scholar]
- 73.Giraudoux P. pgirmess: Data Analysis in Ecology. 2013. http://cran.r-project.org/package=pgirmess Available at.
- 74.Therneau T. A Package for Survival Analysis in S. 2014. http://cran.r-project.org/package=survival Available at.
- 75.Therneau T, Grambsch P. Modeling Survival Data: Extending the Cox Model. New York, NY: Springer; 2000. [Google Scholar]
- 76.Harrington DP, Fleming TR. A class of rank test procedures for censored survival data. Biometrika. 1982;69:553–566. [Google Scholar]
- 77.Peto R, Peto J. Asymptotically efficient rank invariant test procedures. J R Stat Soc. 1972;135:185–207. [Google Scholar]
- 78.Holm S. A simple sequentially rejective multiple test procedure. Scand J Stat. 1979;6:65–70. [Google Scholar]
- 79.Cox DR. Regression models and life-tables. J R Stat Soc. 1972;34:187–220. [Google Scholar]
- 80.Venables WN, Ripley BD. Modern Applied Statistics with S. 4th edition. New York, NY: Springer; 2002. [Google Scholar]
- 81.Schaub GA. Trypanosoma cruzi : quantitative studies of development of two strains in small intestine and rectum of the vector Triatoma infestans. Exp Parasitol. 1989;68:260–273. doi: 10.1016/0014-4894(89)90108-2. [DOI] [PubMed] [Google Scholar]
- 82.Azambuja P, Garcia ES, Ratcliffe NA. Gut microbiota and parasite transmission by insect vectors. Trends Parasitol. 2005;21:568–572. doi: 10.1016/j.pt.2005.09.011. [DOI] [PubMed] [Google Scholar]
- 83.Chowdury M, Fistein B. Excretion of Trypanosoma cruzi by various stages of Rhodnius prolixus. Int J Parasitol. 1986;16:353–359. doi: 10.1016/0020-7519(86)90114-1. [DOI] [PubMed] [Google Scholar]
- 84.Wood SF. Environmental temperature as a factor in development of Trypanosoma cruzi in Triatoma protracta. Exp Parasitol. 1954;3:227–233. doi: 10.1016/0014-4894(54)90021-1. [DOI] [PubMed] [Google Scholar]
- 85.Zafra G, Mantilla JC, Valadares HM, Macedo AM, González CI. Evidence of Trypanosoma cruzi II infection in Colombian chagasic patients. Parasitol Res. 2008;103:731–734. doi: 10.1007/s00436-008-1034-0. [DOI] [PubMed] [Google Scholar]
- 86.Andrade SG, Campos RF, Castro Sobral KS, Magalhães JB, Pereira Guedes RS, Guerreiro ML. Reinfections with strains of Trypanosoma cruzi, of different biodemes as a factor of aggravation of myocarditis and myositis in mice. Rev Soc Bras Med Trop. 2006;39:1–8. doi: 10.1590/s0037-86822006000100001. [DOI] [PubMed] [Google Scholar]
- 87.Lima V, Mangia R, Carreira J, Marchevsky R, Jansen A. Trypanosoma cruzi: correlations of biological aspects of the life cycle in mice and triatomines. Mem Inst Oswaldo Cruz. 1999;94:397–402. doi: 10.1590/s0074-02761999000300021. http://www.ncbi.nlm.nih.gov/pubmed/10348990 Available at. [DOI] [PubMed] [Google Scholar]
- 88.Noireau F, Diosque P, Jansen AM. Trypanosoma cruzi: adaptation to its vectors and its hosts. Vet Res. 2009;40:26. doi: 10.1051/vetres/2009009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Rodrigues CM, Valadares HMS, Francisco AF, Arantes JM, Campos CF, Teixeira-Carvalho A, Martins-Filho OA, Araujo MSS, Arantes RME, Chiari E, Franco GR, Machado CR, Pena SDJ, Faria AMC, Macedo AM. Coinfection with different Trypanosoma cruzi strains interferes with the host immune response to infection. PLoS Negl Trop Dis. 2010;4:e846. doi: 10.1371/journal.pntd.0000846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Andrade LO, Machado CR, Chiari E, Pena SD, Macedo AM. Differential tissue distribution of diverse clones of Trypanosoma cruzi in infected mice. Mol Biochem Parasitol. 1999;100:163–172. doi: 10.1016/s0166-6851(99)90035-x. http://www.ncbi.nlm.nih.gov/pubmed/10391378 Available at. [DOI] [PubMed] [Google Scholar]
- 91.Tableman M, Sung Kim J. Survival Analysis Using S. Boca Raton, FL: Chapman and Hall/CRC; 2005. [Google Scholar]
- 92.Brener Z. Biology of Trypanosoma cruzi. Annu Rev Microbiol. 1973;27:347–382. doi: 10.1146/annurev.mi.27.100173.002023. [DOI] [PubMed] [Google Scholar]
- 93.Aron J, May RM. The population dynamics of malaria. In: Anderson RA, editor. The Population Dynamics of Infectious Diseases: Theory and Applications. London: Chapman and Hall; 1982. pp. 139–179. [Google Scholar]
- 94.Dobson A. Population dynamics of pathogens with multiple host species. Am Nat. 2004;164(Suppl 5):S64–S78. doi: 10.1086/424681. [DOI] [PubMed] [Google Scholar]
- 95.Rohani P, Green CJ, Mantilla-Beniers NB, Grenfell BT. Ecological interference between fatal diseases. Nature. 2003;422:885–888. doi: 10.1038/nature01542. [DOI] [PubMed] [Google Scholar]
- 96.Ferguson HM, Mackinnon MJ, Chan BH, Read AF. Mosquito mortality and the evolution of malaria virulence. Evolution. 2003;57:2792–2804. doi: 10.1111/j.0014-3820.2003.tb01521.x. http://www.ncbi.nlm.nih.gov/pubmed/14761058 Available at. [DOI] [PubMed] [Google Scholar]
- 97.Peacock L, Ferris V, Bailey M, Gibson W. Dynamics of infection and competition between two strains of Trypanosoma brucei brucei in the tsetse fly observed using fluorescent markers. Kinetoplastid Biol Dis. 2007;6:4. doi: 10.1186/1475-9292-6-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Schaub GA, Eichler S. The effects of aposymbiosis and of an infection with Blastocrithidia triatomae (Trypanosomatidae) on the tracheal system of the reduviid bugs Rhodnius prolixus and Triatoma infestans. J Insect Physiol. 1998;44:131–140. doi: 10.1016/s0022-1910(97)00095-4. http://www.ncbi.nlm.nih.gov/pubmed/12769885 Available at. [DOI] [PubMed] [Google Scholar]
- 99.Eichler S, Schaub GA. Development of symbionts in triatomine bugs and the effects of infections with trypanosomatids. Exp Parasitol. 2002;100:17–27. doi: 10.1006/expr.2001.4653. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.