Abstract
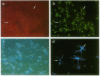
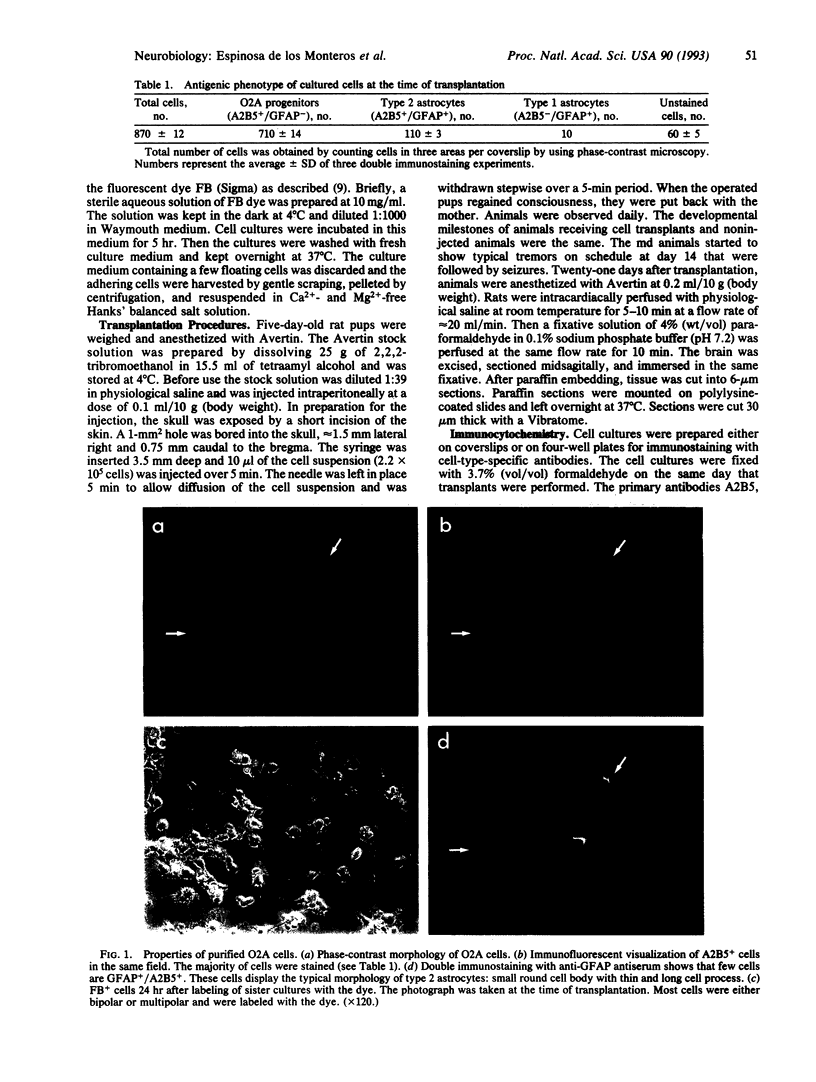
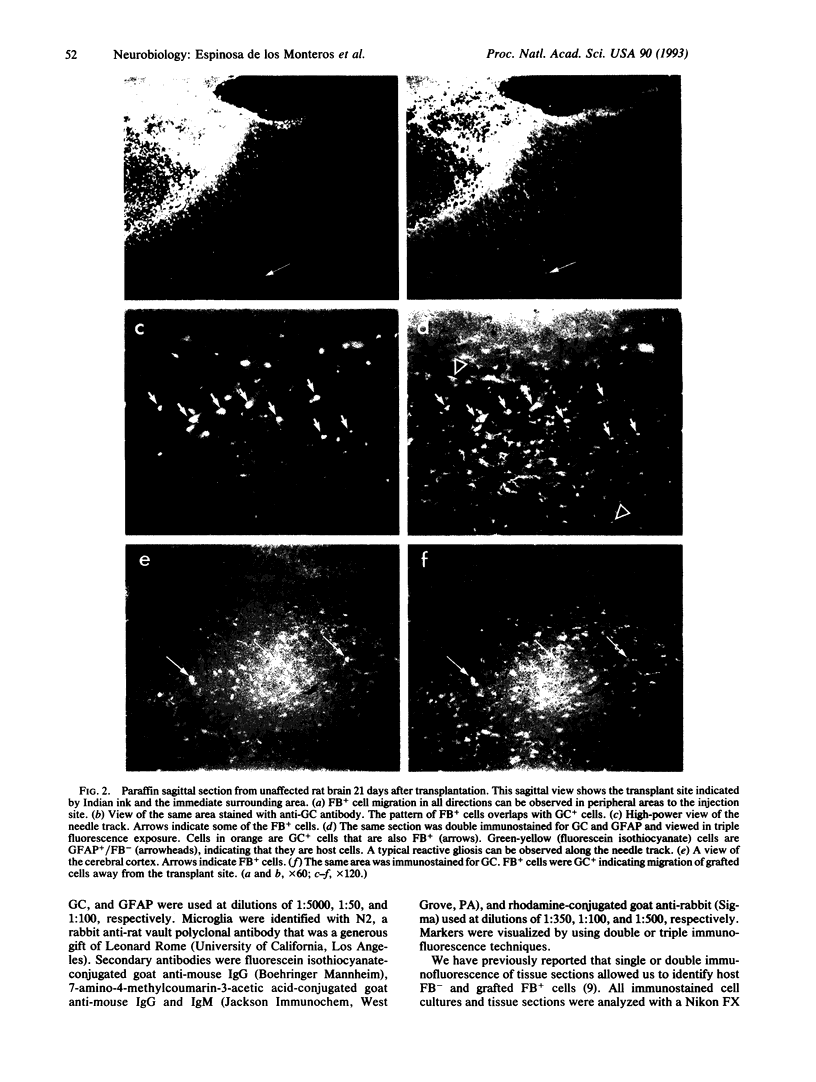
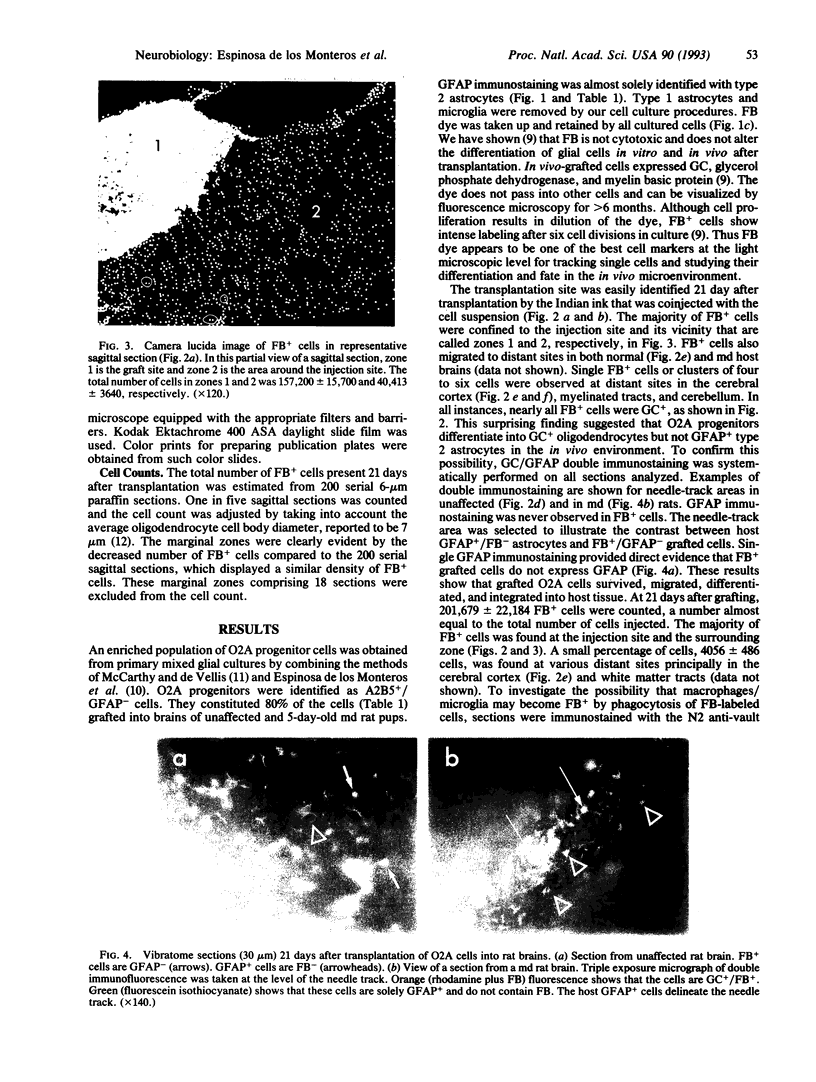
The differentiation of the bipotential O2A progenitor cell into an oligodendrocyte or a type 2 astrocyte has been well documented in cell cultures of various regions of the central nervous system. The appropriate tools to prove its existence in vivo have been lacking. We report on an in vitro-in vivo approach that combines stable labeling of an enriched population of cultured O2A progenitors by the fluorescent dye fast blue, followed by their transplantation into neonatal rat brains, which allowed us to study the influence of the brain microenvironment on their lineage decision. The grafted cells survived well and 21 days after grafting nearly all were positive for the oligodendroglial marker galactocerebroside. Surprisingly, the fast blue-positive grafted cells did not stain for the astroglial marker glial fibrillary acidic protein. These results indicate that the O2A progenitor's plasticity is restricted by the in vivo environment, resulting in the developmental exclusion of the type 2 astrocyte initially described in vitro.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aloisi F., Agresti C., D'Urso D., Levi G. Differentiation of bipotential glial precursors into oligodendrocytes is promoted by interaction with type-1 astrocytes in cerebellar cultures. Proc Natl Acad Sci U S A. 1988 Aug;85(16):6167–6171. doi: 10.1073/pnas.85.16.6167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blakemore W. F., Franklin R. J. Transplantation of glial cells into the CNS. Trends Neurosci. 1991 Aug;14(8):323–327. doi: 10.1016/0166-2236(91)90155-n. [DOI] [PubMed] [Google Scholar]
- Boison D., Stoffel W. Myelin-deficient rat: a point mutation in exon III (A----C, Thr75----Pro) of the myelin proteolipid protein causes dysmyelination and oligodendrocyte death. EMBO J. 1989 Nov;8(11):3295–3302. doi: 10.1002/j.1460-2075.1989.tb08490.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cardwell M. C., Rome L. H. RGD-containing peptides inhibit the synthesis of myelin-like membrane by cultured oligodendrocytes. J Cell Biol. 1988 Oct;107(4):1551–1559. doi: 10.1083/jcb.107.4.1551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chugani D. C., Kedersha N. L., Rome L. H. Vault immunofluorescence in the brain: new insights regarding the origin of microglia. J Neurosci. 1991 Jan;11(1):256–268. doi: 10.1523/JNEUROSCI.11-01-00256.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duncan I. D., Hammang J. P., Jackson K. F., Wood P. M., Bunge R. P., Langford L. Transplantation of oligodendrocytes and Schwann cells into the spinal cord of the myelin-deficient rat. J Neurocytol. 1988 Jun;17(3):351–360. doi: 10.1007/BF01187857. [DOI] [PubMed] [Google Scholar]
- Dutly F., Schwab M. E. Neurons and astrocytes influence the development of purified O-2A progenitor cells. Glia. 1991;4(6):559–571. doi: 10.1002/glia.440040603. [DOI] [PubMed] [Google Scholar]
- Espinosa de los Monteros A., Roussel G., Nussbaum J. L. A procedure for long-term culture of oligodendrocytes. Brain Res. 1986 Jan;389(1-2):117–125. doi: 10.1016/0165-3806(86)90179-3. [DOI] [PubMed] [Google Scholar]
- Espinosa de los Monteros A., Zhang M. S., Gordon M., Aymie M., de Vellis J. Transplantation of cultured premyelinating oligodendrocytes into normal and myelin-deficient rat brain. Dev Neurosci. 1992;14(2):98–104. doi: 10.1159/000111653. [DOI] [PubMed] [Google Scholar]
- Holmes E., Hermanson G., Cole R., de Vellis J. Developmental expression of glial-specific mRNAs in primary cultures of rat brain visualized by in situ hybridization. J Neurosci Res. 1988 Apr;19(4):389-96, 458-65. doi: 10.1002/jnr.490190402. [DOI] [PubMed] [Google Scholar]
- Ingraham C. A., McCarthy K. D. Plasticity of process-bearing glial cell cultures from neonatal rat cerebral cortical tissue. J Neurosci. 1989 Jan;9(1):63–69. doi: 10.1523/JNEUROSCI.09-01-00063.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar S., Gordon M. N., Espinosa de los Monteros M. A., de Vellis J. Developmental expression of neural cell type-specific mRNA markers in the myelin-deficient mutant rat brain: inhibition of oligodendrocyte differentiation. J Neurosci Res. 1988 Oct-Dec;21(2-4):268–274. doi: 10.1002/jnr.490210219. [DOI] [PubMed] [Google Scholar]
- Kumar S., Macklin W. B., Gordon M. N., Espinosa de los Monteros A., Cole R., Scully S. A., de Vellis J. Transcriptional regulation studies of myelin-associated genes in myelin-deficient mutant rats. Dev Neurosci. 1990;12(4-5):316–325. doi: 10.1159/000111860. [DOI] [PubMed] [Google Scholar]
- Lachapelle F., Gumpel M., Baulac M., Jacque C., Duc P., Baumann N. Transplantation of CNS fragments into the brain of shiverer mutant mice: extensive myelination by implanted oligodendrocytes. I. Immunohistochemical studies. Dev Neurosci. 1983;6(6):325–334. doi: 10.1159/000112359. [DOI] [PubMed] [Google Scholar]
- Levi G., Gallo V., Ciotti M. T. Bipotential precursors of putative fibrous astrocytes and oligodendrocytes in rat cerebellar cultures express distinct surface features and "neuron-like" gamma-aminobutyric acid transport. Proc Natl Acad Sci U S A. 1986 Mar;83(5):1504–1508. doi: 10.1073/pnas.83.5.1504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lillien L. E., Sendtner M., Rohrer H., Hughes S. M., Raff M. C. Type-2 astrocyte development in rat brain cultures is initiated by a CNTF-like protein produced by type-1 astrocytes. Neuron. 1988 Aug;1(6):485–494. doi: 10.1016/0896-6273(88)90179-1. [DOI] [PubMed] [Google Scholar]
- Miller R. H., Ffrench-Constant C., Raff M. C. The macroglial cells of the rat optic nerve. Annu Rev Neurosci. 1989;12:517–534. doi: 10.1146/annurev.ne.12.030189.002505. [DOI] [PubMed] [Google Scholar]
- Mori S., Leblond C. P. Electron microscopic identification of three classes of oligodendrocytes and a preliminary study of their proliferative activity in the corpus callosum of young rats. J Comp Neurol. 1970 May;139(1):1–28. doi: 10.1002/cne.901390102. [DOI] [PubMed] [Google Scholar]
- Noble M. Points of controversy in the O-2A lineage: clocks and type-2 astrocytes. Glia. 1991;4(2):157–164. doi: 10.1002/glia.440040207. [DOI] [PubMed] [Google Scholar]
- Raff M. C., Abney E. R., Cohen J., Lindsay R., Noble M. Two types of astrocytes in cultures of developing rat white matter: differences in morphology, surface gangliosides, and growth characteristics. J Neurosci. 1983 Jun;3(6):1289–1300. doi: 10.1523/JNEUROSCI.03-06-01289.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raff M. C., Miller R. H., Noble M. A glial progenitor cell that develops in vitro into an astrocyte or an oligodendrocyte depending on culture medium. Nature. 1983 Jun 2;303(5916):390–396. doi: 10.1038/303390a0. [DOI] [PubMed] [Google Scholar]
- Rosenbluth J., Hasegawa M., Shirasaki N., Rosen C. L., Liu Z. Myelin formation following transplantation of normal fetal glia into myelin-deficient rat spinal cord. J Neurocytol. 1990 Oct;19(5):718–730. doi: 10.1007/BF01188040. [DOI] [PubMed] [Google Scholar]
- Skoff R. P. Gliogenesis in rat optic nerve: astrocytes are generated in a single wave before oligodendrocytes. Dev Biol. 1990 May;139(1):149–168. doi: 10.1016/0012-1606(90)90285-q. [DOI] [PubMed] [Google Scholar]
- Skoff R. P., Knapp P. E. Division of astroblasts and oligodendroblasts in postnatal rodent brain: evidence for separate astrocyte and oligodendrocyte lineages. Glia. 1991;4(2):165–174. doi: 10.1002/glia.440040208. [DOI] [PubMed] [Google Scholar]