Abstract
Cross-border surveillance for emerging diseases such as Ebola and other infectious diseases requires effective international collaboration. We surveyed representatives from 12 multinational disease surveillance programs between January 2013 and April 2014. Our survey identified programmatic similarities despite variation in health priorities, geography, and socioeconomic context, providing a contemporary perspective on infectious disease surveillance networks.
The 2005 International Health Regulations (IHR) require member states to report to the World Health Organization (WHO) public health events of international concern, in recognition that a broad array of infectious diseases, environmental issues, and other public health concerns cross international borders and often necessitate an international response.1 The IHR also contains articles (Article 21 on ground crossings, Article 44 on collaboration and assistance, and Article 57 on other international facilitating agreements) encouraging neighboring countries to cooperate directly in disease surveillance information sharing and coordinating responses to public health problems affecting more than one country.
The current epidemic of Ebola in west Africa exemplifies need for shared cross-border epidemiologic surveillance and coordinated disease control efforts. A relatively isolated outbreak in Guinea began in December 2013 and spread across land borders into Liberia and Sierra Leone without detection until March 2014, and shortly thereafter spun out of control.2–4 Multinational disease surveillance programs (MNDSPs), also known as regional disease surveillance networks,5 can improve public health collaboration among neighboring countries by building trust and enhancing core response capacities. MNDSPs have self-assembled on a global scale.5–8 We conducted a survey of MNDSPs operating worldwide to assess program organization, goals, operations, and evaluation.
We identified MNDSPs from January 2013 to April 2014 through Internet search and querying domestic and international colleagues. We contacted program representatives via e-mail, providing an invitation and electronic survey in English, Spanish, or Portuguese. Our survey asked about program goals, participating countries, use of standardized case definitions, and whether binational or multinational cases (as defined in the United States–Mexico Guidelines for Coordination on Public Health Events of Mutual Interest9) were reported to other participating countries at the time of survey. We also asked about communications (standard languages for official communications, emergency notification lists), laboratory testing (standardized protocols for each reportable disease, sharing of specimens or reagents), data systems, funding, and quality assurance or evaluation activities.
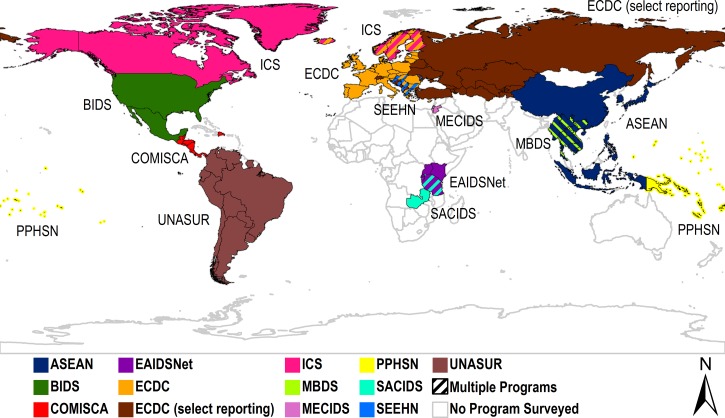
We contacted representatives from 16 MNDSPs via e-mail and obtained responses from 12 programs (response rate: 75%) spanning most continents (Figure 1 ). Among 12 programs surveyed, seven used English as a standard language for official communications (Table 1). Six programs standardized case definitions among participating countries for all reportable diseases. Common goals among responding programs included facilitating communication between members; data sharing; exchanging expertise; improving public health infrastructure and outbreak response capacity; and coordinating disease surveillance, prevention, and control.
Figure 1.
Participating countries in multinational disease surveillance programs surveyed. ASEAN = Association of Southeast Asian Networks Plus Three Emerging Infectious Disease Surveillance; BIDS = Border Infectious Disease Surveillance; COMISCA = Council of Ministers of Health of Central America and the Dominical Republic; ICS = International Circumpolar Surveillance; EAIDSNet = East African Integrated Disease Surveillance Network; ECDC = European Center for Disease Prevention and Control; MBDS = Mekong Basin Disease Surveillance; MECIDS = Middle East Consortium on Infectious Disease Surveillance; PPHSN = Pacific Public Health Surveillance Network; SACIDS = Southern African Center for Infectious Disease Surveillance; SEEHN = Southeast European Health Network; UNASUR = Union of South American Nations. ECDC (select reporting) countries only report for select diseases, such as International Health Regulations (IHR) reportable diseases.
Table 1.
Characteristics of multinational disease surveillance programs surveyed
| Program | Global region | Standard language(s) used | Standard case definitions adopted by all countries | Multinational cases reported to other countries | Standard laboratory testing protocols for each reportable disease | Specimens or laboratory reagents shared between countries |
|---|---|---|---|---|---|---|
| ASEAN | East Asia | Not specified | IHR reportable diseases only | Yes | IHR reportable diseases only | No |
| BIDS | North America | English and Spanish | Yes | Yes | Yes | Yes* |
| COMISCA | Central America and Caribbean | Spanish | No | Yes | No | No |
| EAIDSNet | Sub-Saharan Africa | English | Yes | Yes | No | No |
| ECDC | Europe and central Asia | English | Yes | Yes | No† | Yes‡ |
| ICS | North America, western Europe | English | Yes | No | Yes | Yes |
| MBDS | East Asia | English | No | Yes | No | No |
| MECIDS | Middle East | English | Yes | No | Yes | Yes |
| PPHSN | East Asia and Pacific | No standard language§ | No | Yes | No | Yes |
| SACIDS | Sub-Saharan Africa | No standard language∥ | No | No | No | No |
| SEEHN | Western Europe | English; reports translated into national official languages | No | Yes | No | Yes |
| UNASUR | Latin America | Spanish | Yes | Yes | Yes | Yes |
ASEAN = Association of Southeast Asian Networks Plus Three Emerging Infectious Disease Surveillance; BIDS = Border Infectious Disease Surveillance; COMISCA = Council of Ministers of Health of Central America and the Dominical Republic; ICS = International Circumpolar Surveillance; IHR = International Health Regulations; EAIDSNet = East African Integrated Disease Surveillance Network; ECDC = European Center for Disease Prevention and Control; MBDS = Mekong Basin Disease Surveillance; MECIDS = Middle East Consortium on Infectious Disease Surveillance; PPHSN = Pacific Public Health Surveillance Network; SACIDS = Southern African Center for Infectious Disease Surveillance; SEEHN = Southeast European Health Network; UNASUR = Union of South American Nations.
Samples are not currently shared between the United States and Mexico on a routine basis. However, samples are exchanged in specific case scenarios (such as projects of mutual interest for which agreements are established or in cases of testing assistance).
There are a number of standardized laboratory testing protocols agreed by EU disease networks for a large number, but not all, diseases under EU surveillance.
Specimens are shared on the bases of bilateral or multilateral agreements between participating countries, and are not received by ECDC.
Reports are written in English and translated to French.
English used for Tanzania–Zambia; English, French, Kirundi, and Swahili used for Tanzania–Burundi.
Out of 12 MNDSPs, nine reported multinational cases of reportable diseases to other member countries. Among these nine programs, responses normally taken by a member country included acknowledging receipt (four programs) and sending a report of action taken via e-mail (five programs). Eleven of 12 programs, met at least annually in person or via videoconference. Common mechanisms for sharing updates and reports among members included e-mail (nine programs), websites or electronic forums (eight programs), and postal mail (seven programs). Nine programs had established emergency notification lists for member countries. Ten of 12 programs had used a central database to store epidemiologic data from participating countries. Among these 10 programs, six had manual data entry only, three had established electronic linkage with country-specific databases, and one received direct electronic submissions from data collectors.
Four of 12 programs had established standardized laboratory protocols for all reportable diseases and three programs for select reportable diseases (such as IHR-reportable diseases). For disease detection or surveillance, the main laboratory infrastructure included national public health laboratories (all programs), state or regional laboratories (seven programs), and hospital laboratories (seven programs). Seven of 12 programs shared specimens or laboratory reagents among members. When asked about challenges for specimen or reagent sharing, responses included differing disease priorities, funding limitations for procurement and transportation, lack of formal agreements for importation and exportation, and lack of understanding about storage and transport protocols to prevent specimen deterioration.
Dedicated funding for MNDSP operation was reported by nine of 12 programs. The most common funding sources were Ministries of Health of member countries (five programs) and international donor or aid funding (four programs). Other funding mechanisms included in-kind laboratory and staff support from state and local health departments, intergovernmental body funding, and ad hoc funding requests. Three programs had not obtained any dedicated operational funding. Three of 12 MNDSPs had implemented quality assurance activities. These activities included laboratory testing assurance, data quality review, site visits, and external protocol review for new projects. Two MNDSPs reported having conducting an evaluation for program effectiveness.
Multinational disease surveillance programs can strengthen global health security by promoting timely information exchange and coordinated responses for emerging and other infectious diseases. Our survey captured MNDSPs spanning most continents, although we were unable to identify or survey programs operating in north Africa, west Africa, south Asia, or Australasia. Promisingly, we found that the majority of MNDSPs surveyed reported multinational cases to other member countries. MNDSPs might expand existing information exchange practices by using both formal and informal distribution channels (such as electronic mailing lists). In addition, only half of the programs surveyed had implemented standardized case definitions for all reportable diseases. While recognizing the need for timely collaboration on emerging public health events, inconsistent case definitions are an obstacle for comparable global infectious disease surveillance.10 Adopting case definitions recommended by the WHO11 for diseases of regional interest could facilitate standardization between programs. We also found that five of 12 programs had not shared specimens or reagents among member countries, which would particularly benefit resource-poor countries facing limitations in laboratory infrastructure and skilled personnel.12 MNDSPs might disseminate standardized laboratory protocols for reportable diseases, support laboratory specimen and reagent sharing based on international guidelines,13,14 and encourage training and transfer of technology among member countries.
Funding and sustainability are the ongoing challenges for disease surveillance networks.5 Three of 12 responding programs did not report dedicated operational funding. Program evaluations and quality assurance activities (such as data quality review, external protocol review, and laboratory testing assurance) might help demonstrate impact to secure additional domestic or international funding.
Our study has a few limitations. Although we attempted to identify and contact MNDSPs worldwide, our sample is not intended as a comprehensive list. Rather, it provides insight into the diversity and span of select global programs. Second, reliance on respondent accuracy for program characteristics, and variability in respondent roles among programs, could account for inconsistent depth and completeness of responses. Third, as our survey characterized networks rather than individual countries, we were unable to assess intra-network variability in terms of data systems, funding, and other attributes. Additional information on select networks included in our survey is available elsewhere.15–20 Fourth, our survey characterized general mechanisms for sharing program updates and reports and actions taken upon receipt of a multinational case report, but did not obtain detailed information on mechanisms for sharing case notifications. Finally, our survey instrument was offered in English, Spanish, and Portuguese only, which could account for the overrepresentation of English-speaking MNDSPs. Future assessments could expand language offerings to capture a wider array of networks.
Despite variation in health priorities, geography, and socioeconomic context, MNDSPs face common operational challenges including standardization of case definitions, sharing of laboratory capacity and training, and funding availability. MNDSPs might benefit from enhanced inter-program dialogue to actively share best practices, effective strategies, and the results of program evaluations. Connecting Organizations for Regional Disease Surveillance (CORDS), an international nongovernmental organization linked to five of the surveyed surveillance networks, is facilitating regional dialogues on cross-border surveillance including recent workshops in west Africa on Ebola.8 Funds for regional disease surveillance in west Africa are also being mobilized in response to the pressing need to rebuild the health systems in Ebola outbreak–affected countries.21 In regions with limited or no MNDSP activity, such as west Africa, health authorities might consider establishing new programs drawing on existing global experience and pursuing international funding support as needed.
ACKNOWLEDGMENTS
We thank representatives from each participating multinational disease surveillance program. We are grateful to Mark Smolinski for his feedback on this project. We also thank two anonymous reviewers who helped improve this manuscript. The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of CDC.
Footnotes
Financial support: This study/report was supported in part by an appointment to the Applied Epidemiology Fellowship Program administered by the Council of State and Territorial Epidemiologists (CSTE) and funded by the Centers for Disease Control and Prevention (CDC) Cooperative Agreement Number 1U380T000143-01.
Authors' addresses: Aiden K. Varan, Maureen Fonseca-Ford, and Stephen H. Waterman, Centers for Disease Control and Prevention, San Diego, CA, E-mails: vvl5@cdc.gov, mrf5@cdc.gov, and shw2@cdc.gov. Robson Bruniera-Oliveira, National School of Public Health, Rio de Janeiro, Brazil, E-mail: robson.bruniera@gmail.com. Christopher R. Peter, Consultant, El Cajon, CA, E-mail: ausgermph@gmail.com.
References
- 1.World Health Organization . International Health Regulations (2005) 2nd ed. Geneva, Switzerland: World Health Organization; 2008. http://www.who.int/ihr/publications/9789241596664/en Available at. Accessed October 23, 2014. [Google Scholar]
- 2.WHO Ebola Response Team Ebola virus disease in west Africa—the first 9 months of the epidemic and forward projections. N Engl J Med. 2014;371:1481–1495. doi: 10.1056/NEJMoa1411100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Farrar JJ, Piot P. The Ebola emergency—immediate action, ongoing strategy. N Engl J Med. 2014;371:1545–1546. doi: 10.1056/NEJMe1411471. [DOI] [PubMed] [Google Scholar]
- 4.Sack K, Fink S, Belluck P, Nossiter A. How Ebola Roared Back. New York Times. 2014. http://www.nytimes.com/2014/12/30/health/how-ebola-roared-back.html Available at. Accessed January 5, 2015.
- 5.Bond KC, Macfarlane SB, Burke C, Ungchusak K, Wibulpolprasert S. The evolution and expansion of regional disease surveillance networks and their role in mitigating the threat of infectious disease outbreaks. Emerg Health Threats J. 2013;6:19913. doi: 10.3402/ehtj.v6i0.19913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kimball AM, Moore M, French HM, Arima Y, Ungchusak K, Wibulpolprasert S, Taylor T, Touch S, Leventhal A. Regional infectious disease surveillance networks and their potential to facilitate the implementation of the international health regulations. Med Clin North Am. 2008;92:1459–1471. doi: 10.1016/j.mcna.2008.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Moore M, Bond KC, Gresham L, Rweyemamu M, Chowdhury AM, Bino S. Promising pathways for regional disease surveillance networks. Emerg Health Threats J. 2013;6:19961. doi: 10.3402/ehtj.v6i0.19961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gresham LS, Smolinski MS, Suphanchaimat R, Kimball AM, Wibulpolprasert S. Creating a global dialogue on infectious disease surveillance: connecting organizations for regional disease surveillance (CORDS) Emerg Health Threats J. 2013;6:19912. doi: 10.3402/ehtj.v6i0.19912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Centers for Disease Control and Prevention Technical Guidelines for United States–Mexico Coordination on Public Health Events of Mutual Interest. 2012. http://www.cdc.gov/usmexicohealth/pdf/us-mexico-guidelines.pdf Available at. Accessed October 23, 2014.
- 10.World Health Organization WHO Report on Global Surveillance of Epidemic-Prone Infectious Diseases. 2000. http://apps.who.int/iris/handle/10665/66485 Available at. Accessed October 23, 2014.
- 11.World Health Organization WHO Recommended Surveillance Standards. 1999. http://apps.who.int/iris/handle/10665/65517 Available at. Accessed October 28, 2014.
- 12.Nkengasong JN, Nsubuga P, Nwanyanwu O, Gershy-Damet G-M, Roscigno G, Bulterys M, Schoub B, DeCock KM, Birx D. Laboratory systems and services are critical in global health time to end the neglect? Am J Clin Pathol. 2010;134:368–373. doi: 10.1309/AJCPMPSINQ9BRMU6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.World Health Organization Guidance on Regulations for the Transport of Infectious Substances 2013–2014. 2012. http://www.who.int/ihr/publications/who_hse_ihr_2012.12/en/ Available at. Accessed December 31, 2014.
- 14.Pearson JE. Regulatory constraints for the transport of samples and compliance with the World Organisation for Animal Health (OIE) standards for biosecurity and biocontainment. Dev Biol (Basel) 2007;128:59–68. [PubMed] [Google Scholar]
- 15.Bino S, Cavaljuga S, Kunchev A, Lausevic D, Kaic B, Pistol A, Kon P, Karadjovski Z, Georghita S, Cicevalieva S. Southeastern European Health Network (SEEHN) Communicable Diseases Surveillance: a decade of bridging trust and collaboration. Emerg Health Threats J. 2013;6 doi: 10.3402/ehtj.v6i0.19950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Phommasack B, Jiraphongsa C, Ko Oo M, Bond KC, Phaholyothin N, Suphanchaimat R, Ungchusak K, Macfarlane SB. Mekong Basin Disease Surveillance (MBDS): a trust-based network. Emerg Health Threats J. 2013;6 doi: 10.3402/ehtj.v6i0.19944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rweyemamu MM, Mmbuji P, Karimuribo E, Paweska J, Kambarage D, Neves L, Kayembe JM, Mweene A, Matee M. The Southern African Centre for Infectious Disease Surveillance: a one health consortium. Emerg Health Threats J. 2013;6 doi: 10.3402/ehtj.v6i0.19958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ope M, Sonoiya S, Kariuki J, Mboera LE, Gandham RN, Schneidman M, Kimura M. Regional initiatives in support of surveillance in east Africa: the East Africa Integrated Disease Surveillance Network (EAIDSNet) experience. Emerg Health Threats J. 2013;6 doi: 10.3402/ehtj.v6i0.19948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Weinberg M, Waterman S, Lucas CA, Falcon VC, Morales PK, Lopez LA, Peter C, Gutierrez AE, Gonzalez ER, Flisser A, Bryan R, Valle EN, Rodriguez A, Hernandez GA, Rosales C, Ortiz JA, Landen M, Vilchis H, Rawlings J, Leal FL, Ortega L, Flagg E, Conyer RT, Cetron M. The U.S.–Mexico Border Infectious Disease Surveillance project: establishing bi-national border surveillance. Emerg Infect Dis. 2003;9:97–102. doi: 10.3201/eid0901.020047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Leventhal A, Ramlawi A, Belbiesi A, Sheikh S, Haddadin A, Husseini S, Abdeen Z, Cohen D. Enhanced surveillance for detection and management of infectious diseases: regional collaboration in the Middle East. Emerg Health Threats J. 2013;6 doi: 10.3402/ehtj.v6i0.19955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.World Bank Ebola: World Bank Group Provides New Financing to Help Guinea, Liberia and Sierra Leone Recover from Ebola Emergency. 2015. http://www.worldbank.org/en/news/press-release/2015/04/17/ebola-world-bank-group-provides-new-financing-to-help-guinea-liberia-sierra-leone-recover-from-ebola-emergency Available at. Accessed April 21, 2015.