Abstract
This prospective study aimed to investigate the relationship between higher hematocrit (Hct) level and hyperuricemia (HU) incidence. A total of 27540 subjects were included. Baseline Hct was classified into four categories based on the quartile distribution of the study population. A cox proportional hazards regression was used to evaluate the risk of HU incidence across the Hct quartiles after adjusting a number of potential confounding factors. Out of the 62897 person-years of follow-up, 2745 new cases of HU were developed. In models adjusted for known risk factors of HU, higher Hct was used to predict HU incidence independently in a graded manner (p = 0.02): compared with subjects in the lowest quartile, subjects in the highest quartile of Hct (hazard ratio = 1.20; 95% confidence interval: 1.03–1.41) were n20% more likely to develop HU. Sensitivity analysis indicated that the hazard ratios increased with the extension of the minimum follow-up interval. When the minimum follow-up interval was restricted to 4 years, subjects in the highest quartile of Hct were 70% more likely to develop HU, compared with the lowest quartile. Higher Hct, a routinely measured inexpensive biomarker was independently associated with the incidence of HU even within the normal range.
Hyperuricemia (HU) has become a major public health issue worldwide because of its high and increasing prevalence in the global context1,2,3, and has long been recognized as a risk factor for the development of gout, diabetes, hypertension, stroke, artherosclerosis, cardiovascular disease, and chronic kidney disease4,5,6,7,8,9. A national survey reported that approximately 21.4% of adults in the US suffered from HU10. Meanwhile, the prevalence of HU is ranged from 13% to 25.8% in some Asian countries11,12,13,14. Therefore, the early detection, treatment and preventive methods of HU have drawn wide concern from the academic circle, but up to present, the specific pathogenesis of HU has not yet been fully elucidated.
Hematocrit (Hct), the proportion of blood volume occupied by red blood cells, is a major determinant of blood viscosity15,16. Many previous studies have proved that higher Hct or blood viscosity was positively associated with insulin resistance17,18,19,20,21,22,23,24,25, a physiological condition in which cells fail to respond to the normal actions of hormone insulin. Insulin resistance has been proved to be correlated with higher serum uric acid or HU26,27,28,29,30,31,32, although the causal effect between them remained unclear. Considering all these factors, higher Hct may be associated with HU, although it has not yet been explored by any researcher, not to mention the causal relationship. Only a few studies indicated a positive association between Hct and serum uric acid33,34,35. Therefore, the objective of this prospective study was to explore the aforementioned causal relationship based on the following hypothesis: higher blood Hct level at baseline is associated with an increased risk of HU during the follow-up period.
Methods
Study population
The study was approved by the ethics committee at Xiangya Hospital, Central South University, and was conducted in accordance with the Protocol of Helsinki. Informed consent in writing was obtained from each subject participating in this study. In the present study, subjects who underwent health examinations between 2007 and 2014 in the Department of Health Examination Center Xiangya Hospital, Central South University in Changsha, Hunan Province, China were recruited. This huge epidemiological prospective study mainly aimed to explore the risk factors (e.g. blood index) of HU and the risk factors of other disease (e.g. urinary calculus) in patients with HU. Routine health checkups are very common in China, because the Chinese government encourage people to take periodic medical examinations. Three of our screen-based cross-sectional studies have been published36,37,38. Subjects who received annual or biennial health examination twice or more (a total of 53984 subjects between 2007 and 2014) were considered to be included in this prospective study. The first health examination was considered as the baseline exposure. Then, 42505 subjects who were free of HU at baseline were identified. Subjects who were younger than 18 years old at baseline (n = 64), or with missing data of Hct level at baseline (n = 4387), or with missing data of uric acid at the subsequent health examination (n = 2594) were excluded. Subjects with missing data of important physical examination results at baseline (n = 7920), such as body mass index (BMI), blood pressure and blood glucose, were also excluded from this study. Eventually, 27540 subjects with the follow-up interval ranged from 1 to 7 years were qualified.
Baseline measurement
All blood samples were drawn after a 12-hour overnight fast and were kept at 4 °C until analysis. Hct, white blood cell count (WBC), and platelet count were detected by the Beckman Coulter LH750 automated hematology analyzer (Beckman Coulter Inc., Miami, FL, USA). Uric acid, fasting blood glucose (FBS), high-density lipoprotein cholesterol (HDL- cholesterol), low-density lipoprotein cholesterol (LDL- cholesterol), triglyceride, serum creatinine (SCr) and alanine aminotransferase (ALT) were detected by the Beckman Coulter AU 5800 (Beckman Coulter Inc., Brea, CA, USA). Systolic blood pressure (SBP) and diastolic blood pressure (DBP) were measured using an electronic sphygmomanometer. BMI was calculated as weight (kg) divided by height squared (m2).
Ascertainment of incident cases of HU
Subjects who were free of HU at baseline and received a diagnosis of HU at any subsequent health examination were defined as incident HU cases. The end point was the first recorded incidence of HU during the follow-up interval or the end of the study period (November 2014). HU was defined by the UA ≥ 416 mmol/L in male population and ≥360 mmol/L in female population.
Statistical analysis
The quantitative data were expressed as mean (standard deviation), and the qualitative data were expressed in percentage. Baseline Hct was classified into four categories based on the quartile distribution in the whole sample: <37.4%, 37.4%–40.3%, 40.4%–43.7%, and >43.7%. Differences in continuous data were evaluated by the one-way classification ANOVA (normally distributed data) or the Kruskal-Wallis H test (non-normally distributed data), while differences in qualitative data were assessed by the χ2 test. The person-years of follow-up were computed as the time interval from the first health examination (baseline) to the day of clinical diagnosis of HU at a subsequent examination, or the day of the last attended examination. The cumulative curves for the incidence of HU according to baseline Hct quartiles over the follow-up period were used; the Kaplan–Meier method and the log-rank test were used to evaluate the statistical significance of the difference among Hct quartiles. The cox proportional hazards regression was used to evaluate the risk of incident HU across the Hct quartiles. The unadjusted, age-sex-BMI adjusted, and multivariable adjusted hazard ratios (HR), as well as the related 95% confidence intervals (95%CI) were reported respectively. The following confounders from baseline were included in the multivariable model: age, sex (male or female), BMI (≥25 kg/m2 or <25 kg/m2), WBC, platelet count, LDL cholesterol, HDL cholesterol, triglyceride (log-transformed), ALT (log-transformed), estimated glomerular filtration rate (eGFR), FBS (log-transformed), SBP and DBP. eGFR was calculated by serum creatinine (Scr), sex, and patients’ age. The calculation formula was: 186 × SCr−1.154 × age−0.203 × 1.210(if black) × 0.742(if female)39. These confounding factors were chosen based on some previous studies40,41,42. Tests for linear trends were conducted based on the cox proportional hazards regression using a median variable of Hct level in each category. Sensitivity analysis was conducted by setting different minimum follow-up intervals (2, 3 and 4 years) to examine the risk of HU across the reclassified Hct quartiles. Then, subgroup analysis was conducted after stratifying data by sex. Baseline Hct was reclassified into four categories based on the quartile distribution in the male population: <41.4%, 41.4%–43.5%, 43.6%–45.5%, and >45.5%, and in the female population: <35.7%, 35.7%–37.5%, 37.6%–39.3%, and >39.3%. Analysis was performed separately for male and female subjects. All data were analyzed using SPSS 17.0; p ≤ 0.05 was considered to be statistically significant.
Results
A total of 27540 subjects involving 62897 person-years of follow-up were included in the present study. The follow-up intervals were ranged from 1 to 7 years (mean 2.78 years). The sample was composed of 14422 males (52.4%) and 13118 females (47.6%), with an average age of 42.08 years (standard deviation 13.52) at baseline. The range of Hct in the study population was from 17.8% to 61.0% (mean 40.4%, median 40.3%). The baseline characteristics of the study population across the quartiles of Hct were shown in Tables 1 and 2. Age, sex ratio, BMI, WBC, platelet count, HDL-cholesterol, LDL-cholesterol, triglyceride, SCr, ALT, FBS, SBP and DBP were significantly different across the Hct quartiles. During the follow-up years, a total of 2745 incident cases (2068 males, 677 females) of HU were identified. The incidence of HU was 43.6 per 1000 person-years in the total sample (62.5 per 1000 person-years in males and 22.7 per 1000 person-years in females).
Table 1. Baseline characteristics of study population across the quartiles of baseline Hct.
| Quartile of baseline Hct |
P* | ||||
|---|---|---|---|---|---|
| 1 (lowest) | 2 | 3 | 4 (highest) | ||
| Median (range) | 35.5 (<37.4) | 38.8 (37.4–40.3) | 42.0 (40.4–43.7) | 45.7 (>43.7) | – |
| No. of subjects | 6908 | 6936 | 6899 | 6797 | – |
| Age (years) | 41.92 (13.76) | 42.18 (14.47) | 43.15 (13.69) | 41.07 (11.94) | 0.00 |
| Male (%) | 8.8 | 27.1 | 76.0 | 98.4 | 0.00 |
| BMI (kg/m2) | 21.83 (2.75) | 22.45 (3.03) | 23.61 (3.09) | 24.56 (3.03) | 0.00 |
| WBC (×109/L) | 5.79 (1.48) | 6.11 (1.50) | 6.41 (1.57) | 6.77 (1.67) | 0.00 |
| Platelet count (×109/L) | 193.72 (57.54) | 195.22 (51.27) | 191.37 (52.13) | 193.48 (49.60) | 0.00 |
| HDL-cholesterol (mmol/L) | 1.53 (0.34) | 1.49 (0.35) | 1.36 (0.33) | 1.27 (0.29) | 0.00 |
| LDL-cholesterol (mmol/L) | 2.47 (0.75) | 2.60 (0.77) | 2.66 (0.80) | 2.80 (0.84) | 0.00 |
| Triglyceride (mmol/L) | 0.99 (0.82) | 1.13 (0.97) | 1.51 (1.49) | 1.75 (1.52) | 0.00 |
| SCr (μmol/L) | 66.97 (21.2) | 69.78 (18.92) | 81.31 (17.16) | 85.88 (13.46) | 0.00 |
| ALT (U/L) | 17.82 (12.74) | 20.31 (14.35) | 25.99 (22.15) | 32.17 (23.87) | 0.00 |
| FBS (mmol/L) | 4.98 (0.69) | 5.07 (0.88) | 5.19 (1.09) | 5.23 (1.33) | 0.00 |
| SBP (mmHg) | 114.76 (16.41) | 117.89 (17.23) | 122.33 (16.47) | 123.80 (15.67) | 0.00 |
| DBP (mmHg) | 70.88 (10.25) | 73.65 (10.63) | 77.40 (11.11) | 79.80 (11.34) | 0.00 |
Hct, hematocrit; BMI, body mass index; WBC, white blood cell; HDL-cholesterol, high density lipoprotein cholesterol; LDL-cholesterol, low density lipoprotein cholesterol; SCr, serum creatinine; ALT, alanine aminotransferase; FBS, fasting blood glucose; SBP, systolic blood pressure; DBP, diastolic pressure.
Data are mean (Standard Deviation), unless otherwise indicated.
*P values are for test of difference across all quartiles of baseline Hct.
Table 2. Baseline characteristics of study population across the quartiles of baseline Hct in gender subgroup.
| Quartile of baseline Hct | P* | ||||
|---|---|---|---|---|---|
| 1 (lowest) | 2 | 3 | 4 (highest) | ||
| Male subgroup | |||||
| Median (range) | 39.8(<41.4) | 42.5(41.4–43.5) | 44.5(43.6–45.5) | 46.9(>45.5) | – |
| No. of subjects | 3669 | 3713 | 3461 | 3579 | – |
| age (years) | 48.35(16.25) | 43.78(13.3) | 42.09(12.42) | 40.19(11.47) | 0.00 |
| BMI (kg/m2) | 23.68(3) | 24.07(2.97) | 24.4(2.95) | 24.71(3.07) | 0.00 |
| WBC (×109/L) | 6.2(1.57) | 6.48(1.6) | 6.62(1.62) | 6.9(1.69) | 0.00 |
| Platelet count (×109/L) | 181.02(53.87) | 188.37(51.04) | 191.67(48.72) | 194.29(50.2) | 0.00 |
| HDL-cholesterol (mmol/L) | 1.31(0.32) | 1.29(0.31) | 1.27(0.29) | 1.26(0.3) | 0.00 |
| LDL-cholesterol (mmol/L) | 2.58(0.8) | 2.66(0.83) | 2.75(0.83) | 2.83(0.85) | 0.00 |
| Triglyceride (mmol/L) | 1.51(1.34) | 1.68(1.71) | 1.7(1.48) | 1.8(1.57) | 0.00 |
| SCr (μmol/L) | 90.08(26.23) | 87.33(13.52) | 86.19(13.3) | 86.34(12.93) | 0.00 |
| eGFR (ml/min/1.73 m2) | 87.62(18.71) | 91.28(18.04) | 93.34(19.15) | 93.7(17.62) | 0.00 |
| ALT (U/L) | 25.31(19.79) | 28.07(22.69) | 30.93(28.51) | 33.5(22.57) | 0.00 |
| FBS (mmol/L) | 5.25(1.12) | 5.21(1.12) | 5.21(1.19) | 5.24(1.44) | 0.00 |
| SBP (mmHg) | 125.43(17.88) | 123.38(15.76) | 123.59(15.45) | 124.08(15.76) | 0.00 |
| DBP (mmHg) | 77.17(11.34) | 78.31(11.03) | 79.17(11.17) | 80.39(11.39) | 0.00 |
| Female subgroup | |||||
| Median (range) | 34.3(<35.7) | 36.6(35.7–37.5) | 38.4(37.6–39.3) | 40.5(>39.3) | – |
| No. of subjects | 3401 | 3276 | 3276 | 3165 | – |
| age (years) | 41.07(12.64) | 40.38(12.87) | 39.65(12.76) | 40.33(13.51) | 0.00 |
| BMI (kg/m2) | 21.65(2.65) | 21.75(2.71) | 21.92(2.89) | 22.27(3.08) | 0.00 |
| WBC (×109/L) | 5.67(1.46) | 5.88(1.42) | 6.07(1.49) | 6.25(1.5) | 0.00 |
| Platelet count (×109/L) | 195.86(59.91) | 195.86(52.24) | 200.14(50.22) | 202.8(51.93) | 0.00 |
| HDL-cholesterol (mmol/L) | 1.55(0.33) | 1.55(0.33) | 1.56(0.33) | 1.56(0.33) | 0.00 |
| LDL-cholesterol (mmol/L) | 2.42(0.73) | 2.52(0.76) | 2.6(0.75) | 2.67(0.78) | 0.00 |
| Triglyceride (mmol/L) | 0.94(0.7) | 0.97(0.82) | 0.96(0.65) | 1.05(0.81) | 0.00 |
| SCr (μmol/L) | 65.21(12.82) | 63.18(12.7) | 62.42(11.99) | 61.9(12.05) | 0.00 |
| eGFR (ml/min/1.73 m2) | 98.13(25.27) | 102.47(27.23) | 103.83(26.28) | 104.58(26.33) | 0.00 |
| ALT (U/L) | 16.94(10.38) | 17.64(10.5) | 18.14(11.07) | 19.95(12.67) | 0.00 |
| FBS (mmol/L) | 4.95(0.64) | 4.96(0.6) | 4.99(0.76) | 5.08(0.93) | 0.00 |
| SBP (mmHg) | 113.33(15.57) | 113.83(15.55) | 114.54(15.66) | 117.59(17.24) | 0.00 |
| DBP (mmHg) | 69.89(9.84) | 70.91(9.92) | 72.05(9.96) | 74.3(10.8) | 0.00 |
Hct, hematocrit; BMI, body mass index; WBC, white blood cell; HDL-cholesterol, high density lipoprotein cholesterol; LDL-cholesterol, low density lipoprotein cholesterol; SCr, serum creatinine; eGFR, estimated glomerular filtration rate; ALT, alanine aminotransferase; FBS, fasting blood glucose; SBP, systolic blood pressure; DBP, diastolic pressure.
Data are mean (Standard Deviation), unless otherwise indicated.
*P values are for test of difference across all quartiles of baseline Hct.
Figure 1 showed the cumulative incidence of HU up to 7 years of follow-up. The curves suggested that subjects in the higher quartiles of Hct exhibited higher incidence of HU, compared with those in the lower quartiles (log-rank test, p = 0.000). Cox proportional hazards regression models were used to examine the risk of HU incidence across the Hct quartiles. The outcomes were shown in Table 3. Unadjusted HRs indicated a strongly increased risk of HU in the second (HR: 1.46, 95%CI: 1.28 to 1.67), third (HR: 2.21, 95%CI: 1.96 to 2.50), and fourth quartile (HR: 3.09, 95%CI: 2.74 to 3.47), compared with the lowest quartile of Hct (p for trend = 0.00). With adjustment of age, sex and BMI, the risk of HU was still significantly increased in the second (HR: 1.16, 95%CI: 1.01 to 1.33), third (HR: 1.20, 95%CI: 1.04 to 1.39) and fourth quartile (HR: 1.44, 95%CI: 1.24 to 1.68) of Hct level, compared with the lowest quartile (p for trend = 0.00). The multivariable adjusted HR (adjusted by age, sex, BMI, WBC, platelet count, LDL cholesterol, HDL cholesterol, triglyceride, ALT, SCr, FBS, SBP and DBP) suggested a significantly increased risk of HU in the highest quartile of Hct level (HR: 1.20, 95%CI: 1.03 to 1.41), compared with the lowest quartile. The p for trend was 0.02. Relative to subjects in the lowest quartile, those in the highest quartile of Hct were 20% more likely to develop HU.
Figure 1. The cumulative curves for the incidence of HU according to Hct quartiles during 7 years of follow-up.
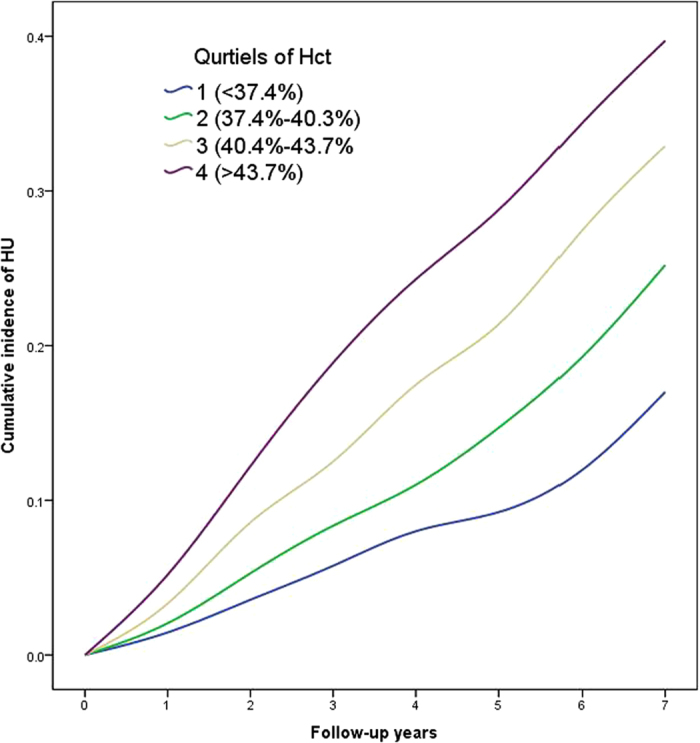
Table 3. The HRs of incident HU across the quartiles of Hct.
| Quartile of baseline Hct |
P for trend | ||||
|---|---|---|---|---|---|
| 1(lowest) | 2 | 3 | 4(highest) | ||
| Minimum follow-up = 1 year (62897 person-years) | |||||
| Median (range) | 35.5(<37.4) | 38.8(37.4–40.3) | 42.0(40.4–43.7) | 45.7(>43.7) | – |
| No. of subjects | 6908 | 6936 | 6899 | 6797 | – |
| Person-years of follow-up | 16815 | 16028 | 15856.5 | 14197.5 | – |
| Incidence of HU (per 1000 person-years) | 22.8 | 33.6 | 50.8 | 71.7 | – |
| Unadjusted HR (95%CI) | 1.00(reference) | 1.46(1.28, 1.67) | 2.21(1.96, 2.50) | 3.09(2.74, 3.47) | 0.00 |
| P value | – | 0.00 | 0.00 | 0.00 | – |
| Age, sex, BMI adjusted HR (95%CI) | 1.00(reference) | 1.16(1.01, 1.33) | 1.20(1.04, 1.39) | 1.44(1.24, 1.68) | 0.00 |
| P value | – | 0.03 | 0.02 | 0.00 | – |
| Multivariable adjusted HR (95%CI) | 1.00(reference) | 1.10(0.96, 1.26) | 1.08(0.93, 1.25) | 1.20(1.03, 1.41) | 0.02 |
| P value | – | 0.18 | 0.34 | 0.02 | – |
| Minimum follow-up = 2 years (60418 person-years) | |||||
| Multivariable adjusted HR (95%CI) | 1.00(reference) | 1.10(0.96, 1.26) | 1.09(0.94, 1.26) | 1.23(1.05, 1.44) | 0.01 |
| P value | – | 1.77 | 0.26 | 0.01 | – |
| Minimum follow-up = 3 years (48818 person-years) | |||||
| Multivariable adjusted HR (95%CI) | 1.00(reference) | 1.12(0.98, 1.28) | 1.13(0.97, 1.32) | 1.30(1.11, 1.52) | 0.00 |
| P value | – | 0.10 | 0.11 | 0.00 | |
| Minimum follow-up = 4 years (37378 person-years) | |||||
| Multivariable adjusted HR (95%CI) | 1.00(reference) | 1.27(1.11, 1.46) | 1.38(1.18, 1.61) | 1.70(1.44, 2.00) | 0.00 |
| P value | – | 0.00 | 0.00 | 0.00 | – |
Hct, hematocrit; BMI, body mass index; No., number; HR, hazard ratio.
Multivariable adjusted model included: age, sex (male or female), BMI (≥25 or <25), white blood cell count, platelet count, low-density lipoprotein cholesterol, high-density lipoprotein cholesterol, triglyceride (log-transformed), alanine aminotransferase (log-transformed), Estimated glomerular filtration rate (eGFR), fasting blood glucose (log-transformed), systolic pressure and diastolic pressure.
Sensitivity analysis was conducted by restricting different minimum follow-up intervals (2, 3 and 4 years) to examine the risk of HU across the reclassified Hct quartiles (Table 3). With the minimum follow-up interval of 2 years (60418 person-years), the multivariable adjusted HR suggested a significantly increased risk of HU in the highest quartile of Hct level (HR: 1.23, 95%CI: 1.05 to 1.44), compared with the lowest quartile. P for trend was 0.01. With the minimum follow-up interval of 3 years (48818 person-years), the multivariable adjusted HR suggested a significantly increased risk of HU in the highest quartile of Hct level (HR: 1.30, 95%CI: 1.11 to 1.52), compared with the lowest quartile (p for trend = 0.00). Meanwhile, with the minimum follow-up interval of 4 years (37378 person-years), the multivariable adjusted HR also suggested a significantly increased risk of HU in the second (HR: 1.27, 95%CI: 1.11 to 1.46), third (HR: 1.38, 95%CI: 1.18 to 1.61) and the highest quartile of Hct level (HR: 1.70, 95%CI: 1.44 to 2.00), compared with the lowest quartile (p for trend = 0.00).
Subgroup analysis was conducted to assess the risk of HU across different Hct levels in the male and female population respectively (Tables 4 and 5). For male subjects, the unadjusted HRs suggested a significant higher incident HU in the third (HR: 1.13, 95%CI: 1.00 to 1.28) and fourth quartile (HR: 1.36, 95%CI: 1.21 to 1.54), compared with the reference level (p for trend = 0.00). With adjustment of age, sex and BMI, the risk of HU was still significantly increased in the fourth quartile (HR: 1.22, 95%CI: 1.08 to 1.38) of Hct level, compared with the lowest quartile (p for trend = 0.00). However, the multivariable adjusted HR just approached statistical significance (p for trend = 0.09). With the minimum follow-up interval of 3 or 4 years, the multivariable adjusted HRs suggested a significantly increased risk of HU in the highest quartile of Hct level compared with the lowest quartile (p for trend = 0.01 and 0.00, respectively). For female subjects, the unadjusted HR (HR: 1.70, 95%CI: 1.38 to 2.09), the age and BMI adjusted HR (HR: 1.51, 95%CI: 1.23 to 1.86), and the multivariable adjusted HR (HR: 1.26, 95%CI: 1.01 to 1.56) all showed a significantly increased risk in the highest quartile of Hct level, compared with the lowest quartile (p for trend = 0.00, 0.00 and 0.02, respectively).
Table 4. The HRs of incident HU across the quartiles of Hct in male population.
| Quartile of baseline Hct |
P for trend | ||||
|---|---|---|---|---|---|
| 1(lowest) | 2 | 3 | 4(highest) | ||
| Minimum follow-up = 1 year (33110 person-years) | |||||
| Median (range) | 39.8(<41.4) | 42.5(41.4–43.5) | 44.5(43.6–45.5) | 46.9(>45.5) | – |
| No. of subjects | 3669 | 3713 | 3461 | 3579 | – |
| Person-years of follow-up | 9422.5 | 8881.5 | 7727.5 | 7078.5 | – |
| Incidence of HU (per 1000 person-years) | 55.2 | 56.1 | 63.9 | 78.5 | – |
| Unadjusted HR (95%CI) | 1.00(reference) | 1.01(0.89, 1.14) | 1.13(1.00, 1.28) | 1.36(1.21, 1.54) | 0.00 |
| P value | – | 0.93 | 0.05 | 0.00 | |
| Age, BMI adjusted HR (95%CI) | 1.00(reference) | 0.95(0.84, 1.08) | 1.04(0.92, 1.18) | 1.22(1.08, 1.38) | 0.00 |
| P value | – | 0.43 | 0.57 | 0.00 | – |
| Multivariable adjusted HR (95%CI) | 1.00(reference) | 0.92(0.81, 1.04) | 0.96(0.85, 1.10) | 1.11(0.98, 1.26) | 0.09 |
| P value | – | 0.17 | 0.57 | 0.11 | – |
| Minimum follow-up = 2 years (31814 person-years) | |||||
| Multivariable adjusted HR (95%CI) | 1.00(reference) | 0.90(0.79, 1.02) | 0.98(0.86, 1.11) | 1.13(0.99, 1.28) | 0.04 |
| P value | 0.10 | 0.71 | 0.07 | ||
| Minimum follow-up = 3 years (26441 person-years) | |||||
| Multivariable adjusted HR (95%CI) | 1.00(reference) | 0.87(0.77, 0.99) | 0.96(0.88, 1.13) | 1.15(1.02, 1.31) | 0.01 |
| P value | – | 0.04 | 0.96 | 0.03 | |
| Minimum follow-up = 4 years (20756 person-years) | |||||
| Multivariable adjusted HR (95%CI) | 1.00(reference) | 0.94(0.83, 1.08) | 1.09(0.95, 1.24) | 1.40(1.23, 1.59) | 0.00 |
| P value | – | 0.40 | 0.22 | 0.00 | – |
Hct, hematocrit; BMI, body mass index; No., number; HR, hazard ratio.
Multivariable adjusted model included: age, sex (male or female), BMI (≥25 or <25), white blood cell count, platelet count, low-density lipoprotein cholesterol, high-density lipoprotein cholesterol, triglyceride (log-transformed), alanine aminotransferase (log-transformed), Estimated glomerular filtration rate (eGFR), fasting blood glucose (log-transformed), systolic pressure and diastolic pressure.
Table 5. The HRs of incident HU across the quartiles of Hct in female population.
| Quartile of baseline Hct |
P for trend | ||||
|---|---|---|---|---|---|
| 1(lowest) | 2 | 3 | 4(highest) | ||
| Minimum follow-up = 1 year (29787 person-years) | |||||
| Median (range) | 34.3(<35.7) | 36.6(35.7–37.5) | 38.4(37.6–39.3) | 40.5(>39.3) | – |
| No. of subjects | 3401 | 3276 | 3276 | 3165 | – |
| Person-years of follow-up | 8359.5 | 7700 | 7248 | 6479.5 | – |
| Incidence of HU (per 1000 person-years) | 18.9 | 18.7 | 23.2 | 31.9 | – |
| Unadjusted HR (95%CI) | 1.00(reference) | 0.99(0.79, 1.24) | 1.23(0.99, 1.53) | 1.70(1.38, 2.09) | 0.00 |
| P value | 0.92 | 0.06 | 0.00 | ||
| Age, sex, BMI adjusted HR (95%CI) | 1.00(reference) | 1.00(0.80, 1.26) | 1.20(0.96, 1.49) | 1.51(1.23, 1.86) | 0.00 |
| P value | – | 0.98 | 0.11 | 0.00 | – |
| Multivariable adjusted HR (95%CI) | 1.00(reference) | 0.94(0.75, 1.18) | 1.08(0.87, 1.35) | 1.26(1.01, 1.56) | 0.02 |
| P value | – | 0.58 | 0.48 | 0.04 | – |
| Minimum follow-up = 2 years (28603.5 person-years) | |||||
| Multivariable adjusted HR (95%CI) | 1.00(reference) | 0.92(0.73, 1.16) | 1.09(0.88, 1.36) | 1.25(1.01, 1.55) | 0.02 |
| P value | – | 0.50 | 0.42 | 0.04 | |
| Minimum follow-up = 3 years (22377 person-years) | |||||
| Multivariable adjusted HR (95%CI) | 1.00(reference) | 0.97(0.77, 1.23) | 1.10(0.87, 1.38) | 1.35(1.08, 1.68) | 0.00 |
| P value | – | 0.83 | 0.44 | 0.01 | |
| Minimum follow-up = 4 years (16622 person-years) | |||||
| Multivariable adjusted HR (95%CI) | 1.00(reference) | 1.02(0.80, 1.31) | 1.33(1.05, 1.67) | 1.61(1.29, 2.02) | 0.00 |
| P value | – | 0.88 | 0.02 | 0.00 | |
Hct, hematocrit; BMI, body mass index; No., number; HR, hazard ratio.
Multivariable adjusted model included: age, sex (male or female), BMI (≥25 or <25), white blood cell count, platelet count, low-density lipoprotein cholesterol, high-density lipoprotein cholesterol, triglyceride (log-transformed), alanine aminotransferase (log-transformed), Estimated glomerular filtration rate (eGFR), fasting blood glucose (log-transformed), systolic pressure and diastolic pressure.
Discussion
This prospective study suggested that Hct is an independent predictor of HU. This relationship was graded (p for trend < 0.05) and independent of a wide range of established HU risk factors. Compared with subjects in the lowest quartile, those in the highest quartile of Hct were nearly 30% more likely to develop HU. In addition, the probability increased with the extension of the minimum follow-up interval. For example, when the minimum follow-up interval was restricted to 4 years, subjects in the highest quartile of Hct were 76% more likely to develop HU, compared with the lowest quartile. Therefore, elevated Hct deserves attention as an emerging risk factor for HU. Hct is a marker (type 0) of natural history of HU that correlates with known clinical indices.
In addition to the studies directly suggested a positive association between Hct and serum uric acid33,34,35, some other researches indicated a positive trend between Hct and serum uric acid in a variety of settings, such as screen-based cohorts42,43, subjects with intact kidney function in community-based cohorts44, screen-based cross-sectional survey45, slightly reduced glomerular filtration rate46, and angiographically near normal coronary arteries47, even though the significance of difference remained unclear. The present study was the first one demonstrating that higher Hct level at baseline is associated with an increased risk of HU during the follow-up period.
Some studies suggested that insulin resistance was positively associated with serum uric acid in the screen-based cross-sectional survey28, and the normal glucose tolerance and normal fasting glucose subjects30. In addition, some other studies directly reported a positive relationship between insulin resistance and HU in a variety of settings, such as nondiabetic subjects with varying degrees of the metabolic syndrome26, a cross-sectional survey in young black and white adults27, and a community-based sample of Portuguese adults31. Based on these direct and indirect evidences, it was speculated that higher Hct might be a risk factor for HU, which has been proved by the present study. However, it is noteworthy that higher serum uric acid or Hu could also possibly contribute to insulin resistance. Carnethon et al.48 indicated a significantly higher risk of hyperinsulinemia in subjects with increased baseline uric acid level in a prospective study. Similarly, Krishnan et al.49 revealed that patients with HU are subjects to 1.36 times of risk for developing insulin resistance compared to normal subjects in a 15-year follow-up study. Moreover, the effect of decreased uric acid on insulin resistance was demonstrated by a mice study50. These researches implied a possible feedback effect.
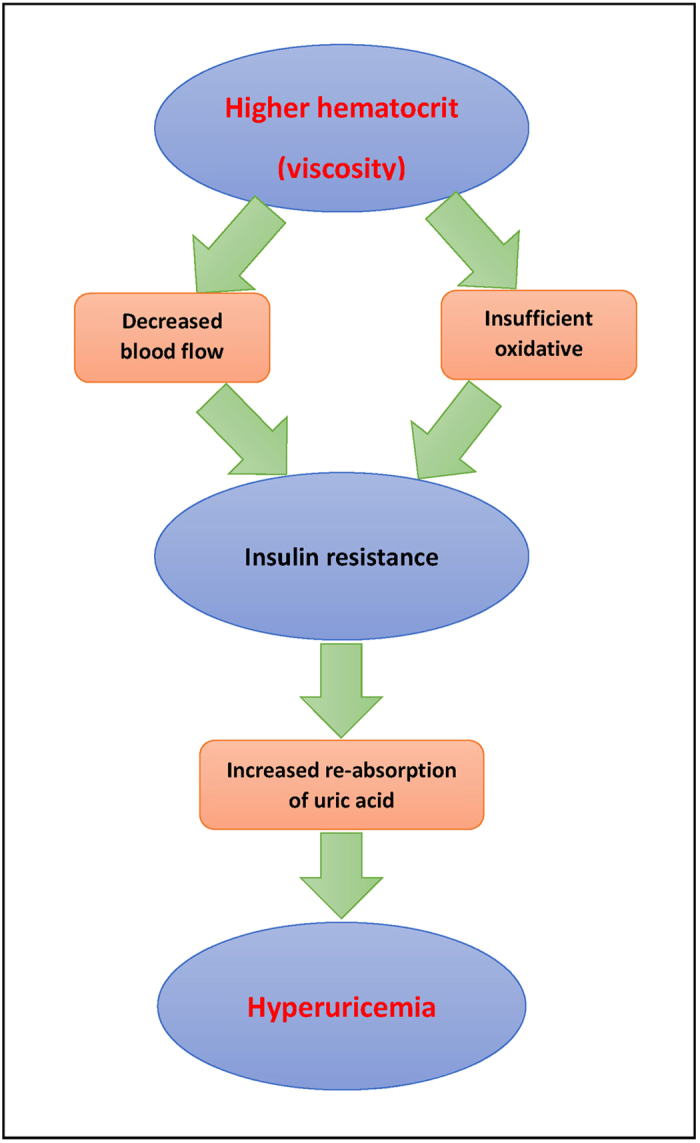
Increased blood viscosity may contribute to insulin resistance through two mechanisms: decreased blood flow and insufficient oxidative capacity51. According to Poiseuille’s law52, blood flow is inversely proportional to the whole blood viscosity. Thus, the increased blood viscosity may result in decreased blood flow, which thereby reduces the delivery of oxygen, glucose and insulin to skeletal muscle53,54. Under such a circumstance, vasodilatation and increased blood pressure which belong to the compensatory mechanisms could increase blood flow55,56,57. Once the compensatory mechanisms fail to maintain a proper blood flow, the increased blood viscosity should be sufficient to increase the glucose and insulin level51. For another side, a large number of studies suggested that insufficient oxidative capacity could be a primary cause of insulin resistance58,59,60,61,62,63,64,65,66, while oxidative capacity might be reduced by increasing blood viscosity through impaired oxygen delivery51. Once the compensatory mechanisms fail to fully restore the oxygen delivery, impaired oxidative capacity could increase insulin resistance51. Furthermore, the increased insulin level can enhance renal tubular sodium reabsorption, which in turn reduces renal excretion of uric acid67,68,69,70. Figure 2 showed the possible mechanism between Hct and HU.
Figure 2. Possible mechanisms of higher Hct serving as the risk factor for the incidence of HU.
The present study is featured with a number of strengths, such as prospective design, large sample (27540 subjects), high event rates, almost equal distribution between male and female subjects, and effect sizes adjusted for multiple confounding factors. To our best knowledge, this is the first study demonstrating that higher blood Hct is an independent predictor of HU. However, limitations should also be acknowledged. Firstly, although the incidence of HU was high, the follow-up interval (1 to 7 years) was not long enough. However, the value of HR increased with the extension of the minimum follow-up interval, suggesting that the observed association was underestimated. Secondly, the use of uric-acid-lowering drugs during the follow-up was not available, but this would also be expected to weaken the noted observed association. Meanwhile, the percentage of long-term use of uric-acid-lowering drugs was very low (3/3789), which was observed in our previous research39. Similarly, the use of antihypertensive, antilipidemic, and antidiabeteic medications was not recorded. It must be admitted that these drugs could have the potential to influence the serum uric acid level. But this would also be expected to weaken the noted observed association in most cases. Thirdly, due to the lack of information on smoking status and alcohol consumption, the influence of these factors on the association between Hct and serum uric acid level could not be determined. Fourthly, differing from the community-based survey conducted in western countries, the present study is a screen-based prospective cohort, which is similar to some other high-quality screen-based studies conducted in Asian countries, such as Japan71, Korea42 and China72. Routine health checkups have become very common in Asia. Fifthly, since estrogenic compounds can enhance the renal urate excretion and increase urate clearance, menopause should have strong impact on serum uric acid level in women, and this could affect the results of the present study, because there are both pre-menopause and post-menopause women in these participants. Sixthly, about one-third of the subjects were excluded from the present study because of many reasons, so there is a possibility that selection bias exist. Lastly, the study subjects were extracted from the Chinese population, so the findings may not be generalizable to other populations.
Conclusions
The results of this study demonstrated that higher Hct level, even within the normal range, was associated with the incidence of HU, independent of multiple risk factors. Elevated Hct, a routinely measured inexpensive biomarker, deserves attention as an emerging risk factor for HU. Further studies should be conducted to determine whether these findings are reproducible in other populations.
Additional Information
How to cite this article: Zeng, C. et al. Higher blood hematocrit predicts hyperuricemia: a prospective study of 62897 person-years of follow-up. Sci. Rep. 5, 13765; doi: 10.1038/srep13765 (2015).
Acknowledgments
This work was supported by Hunan Provincial Innovation Foundation for Postgraduate (CX2014A005), the Fundamental Research Funds for the Central Universities of Central South University, the National Natural Science Foundation of China (No. 81201420, 81272034, 81472130), the Provincial Science Foundation of Hunan (No. 14JJ3032), the Scientific Research Project of the Development and Reform Commission of Hunan Province ([2013]1199), the Scientific Research Project of Science and Technology Office of Hunan Province (2013SK2018), the Doctoral Scientific Fund Project of the Ministry of Education of China (20120162110036).
Footnotes
Author Contributions All authors had full access to the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis. G.H.L. and C.Z. conceived the study. G.H.L. and C.Z. were responsible for conception and design of the study and drafted the manuscript. H.L., T.Y. and S.G.G. contributed to data collection. Y.S.L., Y.L.X. and W.F.X. contributed to preparation and data analysis. W.L. and J.W. contributed to study retrieval. G.H.L. contributed to revision of the manuscript. All the authors contributed to the interpretation of the data and critically reviewed the manuscript for publication.
References
- Liu H., Zhang X. M., Wang Y. L. & Liu B. C. Prevalence of hyperuricemia among Chinese adults: a national cross-sectional survey using multistage, stratified sampling. J Nephrol Apr 1 (2014). [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- Trifirò, et al. Epidemiology of gout and hyperuricaemia in Italy during the years 2005-2009: a nationwide population-based study. Ann Rheum Dis 75, 694–700 (2013). [DOI] [PubMed] [Google Scholar]
- Wallace K. L., Riedel A. A., Joseph-ridge N. & Wortmann R. Increasing prevalence of gout and hyperuricemia over 10 years among older adults in a managed care population. J Rheumatol 31, 1582–1587 (2004). [PubMed] [Google Scholar]
- Kim S. Y. et al. Hyperuricemia and risk of stroke: a systematic review and meta-analysis. Arthritis Rheum 61, 885–892 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sluijs I. et al. Plasma uric acid is associated with increased risk of type 2 diabetes independent of diet and metabolic risk factors. J Nutr 143, 80–85 (2013). [DOI] [PubMed] [Google Scholar]
- Filiopoulos V., Hadjiyannakos D. & Vlassopoulos D. New insights into uric Acid effects on the progression and prognosis of chronic kidney disease. Ren Fail 34, 510–520 (2012). [DOI] [PubMed] [Google Scholar]
- de Oliveira E. P. & Burini R. C. High plasma uric acid concentration: causes and consequences. Diabetol Metab Syndr 4, 12 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puddu P., Puddu G. M., Cravero E., Vizioli L. & Muscari A. The relationships among hyperuricemia, endothelial dysfunction, and cardiovascular diseases: molecular mechanisms and clinical implications. J Cardiol 59, 235–242 (2012). [DOI] [PubMed] [Google Scholar]
- Kawano Y. Uric acid and blood pressure. Circ J 75, 2755–2756 (2011). [DOI] [PubMed] [Google Scholar]
- Zhu Y., Pandya B. J. & Choi H. K. Prevalence of gout and hyperuricemia in the US general population: the National Health and Nutrition Examination Survey 2007-2008. Arthritis Rheum 63, 3136–3141 (2011). [DOI] [PubMed] [Google Scholar]
- Uaratanawong S., Suraamornkul S., Angkeaw S. & Uaratanawong R. Prevalence of hyperuricemia in Bangkok population. Clin Rheumatol 30, 887–893 (2011). [DOI] [PubMed] [Google Scholar]
- Roddy E. & Doherty M. Epidemiology of gout. Arthritis Res Ther 12, 223 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miao Z. et al. Dietary and lifestyle changes associated with high prevalence of hyperuricemia and gout in the Shandong coastal cities of Eastern China. J Rheumatol 35, 1859–1864 (2008). [PubMed] [Google Scholar]
- Naqahama K. et al. Hyperuricemia and cardiovascular risk factor clustering in a screened cohort in Okinawa, Japan. Hypertens Res 27, 227–233 (2004). [DOI] [PubMed] [Google Scholar]
- Braekkan S. K., Mathiesen E. B., Njølstad I., Wilsqaard T. & Hansen J. B. Hematocrit and risk of venous thromboembolism in a general population. The Tromso study. Haematologica 95, 270–275 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paul L. et al. Hematocrit predicts long-term mortality in a nonlinear and sex-specific manner in hypertensive adults. Hypertension 60, 631–638 (2012). [DOI] [PubMed] [Google Scholar]
- Moan A., Nordby G., Os I., Birkeland K. I. & Kidldsen S. E. Relationshio between hemorrheologic factors and insulin sensitivity in healthy young men. Metabolism 43, 423–427 (1994). [DOI] [PubMed] [Google Scholar]
- Nordby G., Moan A., Kjeldsen S. E. & Os I. Relationship between hemorheological factors and insulin sensitivity in normotensive and hypertensive premenopausal women. Am J Hypertens 8, 439–444 (1995). [DOI] [PubMed] [Google Scholar]
- Wannamethee S. G., Perry I. J. & Shaper A. G. Hematocrit and risk of NIDDM. Diabetes 45, 576–579 (1996). [DOI] [PubMed] [Google Scholar]
- Fossum E. et al. Relationship between insulin sensitivity and maximal forearm blood flow in young men. Hypertension 32, 838–843 (1998). [DOI] [PubMed] [Google Scholar]
- Høieggen A., Fossum E., Moan A., Enger E. & Kjeldsen S. E. Whole-blood viscosity and the insulin-resistance syndrome. J Hypertens 16, 203–210 (1998). [DOI] [PubMed] [Google Scholar]
- Facchini F. S., Carantoni M., Jappesen J. & Reaven G. M. Hematocrit and hemoglobin are independently related to insulin resistance and compensatory hyperinsulinemia in healthy, non-obese men and women. Metabolism 47, 831–835 (1998). [DOI] [PubMed] [Google Scholar]
- Høieggen A., Fossum E., Nesbitt S. D., Palmieri V. & Kjelden S. E. Blood viscosity, plasma adrenaline and fasting insulin in hypertensive patients with left ventricular hypertrophy. ICARUS, a LIFE Substudy. Insulin CARotids US Scandinavica. Blood Press 9, 83–90 (2000). [DOI] [PubMed] [Google Scholar]
- Solá E. et al. Fibrinogen, plasma viscosity and blood viscosity in obesity. Relationship with insulin resistance. Clin Hemorheol Microcirc 37, 309–318 (2007). [PubMed] [Google Scholar]
- Vervita V. et al. Obesity and insulin resistance increase plasma viscosity in young women with polycystic ovary syndrome. Gynecol Endocrinol 25, 640–646 (2009). [DOI] [PubMed] [Google Scholar]
- Vuorinen-Markkola H. & Yki-Järvinen H. Hyperuricemia and insulin resistance. J Clin Endocrinol Metab 78, 25–29 (1994). [DOI] [PubMed] [Google Scholar]
- Rathmann W., Funkhouser E., Dyer A. R. & Roseman J. M. Relations of hyperuricemia with the various components of the insulin resistance syndrome in young black and white adults: the CARDIA study. Coronary Artery Risk Development in Young Adults. Ann Epidemiol 8, 250–261 (1998). [DOI] [PubMed] [Google Scholar]
- Yoo T. W. et al. Relationship between serum uric acid concentration and insulin resistance and metabolic syndrome. Circ J 69, 928–933 (2005). [DOI] [PubMed] [Google Scholar]
- Tsouli S. G., Liberopoulos E. N., Mikhailidis D. P., Athyros V. G. & Elisaf M. S. Elevated serum uric acid levels in metabolic syndrome: an active component or an innocent bystander? Metabolism 55, 1293–1301 (2006). [DOI] [PubMed] [Google Scholar]
- Meshkani R., Zargari M. & Larijani B. The relationship between uric acid and metabolic syndrome in normal glucose tolerance and normal fasting glucose subjects. Acta Diabetol 48, 79–88 (2011). [DOI] [PubMed] [Google Scholar]
- Abreu E., Fonseca M. J. & Santos A. C. Association between hyperuricemia and insulin resistance. Acta Med Port 24, 565–574 (2011). [PubMed] [Google Scholar]
- Li C., Hsieh M. C. & Chang S. J. Metabolic syndrome, diabetes, and hyperuricemia. Curr Opin Rheumatol 25, 210–216 (2013). [DOI] [PubMed] [Google Scholar]
- Jefferson J. A. et al. Hyperuricemia, hypertension, and proteinuria associated with high-altitude polycythemia. Am J Kidney Dis 39, 1135–1142 (2002). [DOI] [PubMed] [Google Scholar]
- Chamorro A. et al. Prognostic significance of uric acid serum concentration in patients with acute ischemic stroke. Stroke 33, 1048–1052 (2002). [DOI] [PubMed] [Google Scholar]
- Höieggen A., Fossum E., Reims H. & Kjeldsen S. E. Serum uric acid and hemorheology in borderline hypertensives and in subjects with established hypertension and left ventricular hypertrophy. Blood Press 12, 104–110 (2003). [PubMed] [Google Scholar]
- Zeng C. et al. Relationship between serum magnesium concentration and radiographic knee osteoarthritis. J Rheumatol Jun 1 (2015). [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- Zeng C. et al. Association between dietary magnesium intake and radiographic knee osteoarthritis. PLoS One 10, e0127666 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y. et al. Relationship between cigarette smoking and radiographic knee osteoarthritis in Chinese population: a cross-sectional study. Rheumatol Int 35, 1211–1217 (2015). [DOI] [PubMed] [Google Scholar]
- National Kidney Foundation. K/DOQI clinical practice guidelines for chronic kidney disease: evaluation, classification, and stratification. Am J Kidney Dis 39, S1–266 (2002). [PubMed] [Google Scholar]
- Luo M. et al. Relationship between red cell distribution width and serum uric acid in patients with untreated essential hypertension. Sci Rep 4, 7291 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryu S. et al. A cohort study of hyperuricemia in middle-aged South Korean men. Am J Epidemiol 175, 133–143 (2012). [DOI] [PubMed] [Google Scholar]
- Jae S. Y. et al. Higher blood hematocrit predicts hypertension in men. J Hypertens 32, 245–250 (2014). [DOI] [PubMed] [Google Scholar]
- Iseki K. et al. Significance of hyperuricemia as a risk factor for developing ESRD in a screened cohort. Am J Kidney Dis 44, 642–650 (2004). [PubMed] [Google Scholar]
- Weiner D. E. et al. Uric acid and incident kidney disease in the community. J Am Soc Nephrol 19, 1204–1211 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu X. et al. Association of hematocrit and pre-hypertension among Chinese adults: the CRC study. Cell Biochem Biophys Dec 5 (2014). [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- Corrêa Leite M. L. Fibrinogen, hematocrit, platelets in mild kidney dysfunction and the role of uric acid: an Italian male population study. Clin Appl Thromb Hemost 17, 58–65 (2011). [DOI] [PubMed] [Google Scholar]
- Wang S. et al. Correlation between the hematocrit and slow coronary flow. Clin Hemorheol Microcirc 58, 297–305 (2014). [DOI] [PubMed] [Google Scholar]
- Carnethon M. R. et al. Risk factors for progression to incident hyperinsulinemia: the atherosclerosis risk in communities study, 1987-1998. Am J Epidemiol 158, 1058–1067 (2003). [DOI] [PubMed] [Google Scholar]
- Krishnan E., Pandya B. J., Chung L., Hriri A. & Dabbous O. Hyperuricemia in young adults and risk of insulin resistance, prediabetes, and diabetes: a 15-year follow-up study. Am J Epidemiol 176, 108–116 (2012). [DOI] [PubMed] [Google Scholar]
- Baldwin W. et al. Hyperuricemia as a mediator of the proinflammatory endocrine imbalance in the adipose tissue in a murine model of the metabolic syndrome. Diabetes 60, 1258–1269 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamariz L. J. et al. Blood viscosity and hematocrit as risk factors for type 2 diabetes mellitus: the atherosclerosis risk in communities (ARIC) study. Am J Epidemiol 168, 1153–1160 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guyton A. & Hall J. Texbook of Medical Physiology. 10th ed. Philadelphia, PA: WB Saunders Company; 2000. [Google Scholar]
- Yang Y. J., Hope I. D., Ader M. & Bergman R. N. Insulin transport across capillaries is rate limiting for insulin action in dogs. J Clin Invest 84, 1620–1628 (1989). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schultz T. A., Lewis S. B., Westbie D. K., Wallin J. D. & Gerich J. E. Glucose delivery: a modulator of glucose uptake in contracting skeletal muscle. Am J Physiol 233, E514–518 (1977). [DOI] [PubMed] [Google Scholar]
- Cinar Y., Senyol A. M. & Duman K. Blood viscosity and blood pressure: role of temperature and hyperglycemia. Am J Hypertens 14, 433–438 (2001). [DOI] [PubMed] [Google Scholar]
- Cinar Y., Demir G., Pac M. & Cinar A. B. Effect of hematocrit on blood pressure via hyperviscosity. Am J Hypertens 12, 739–743 (1999). [DOI] [PubMed] [Google Scholar]
- Clark M. G. et al. Blood flow and muscle metabolism: a focus on insulin action. Am J Physiol Endocrinol Metab 284, E241–258 (2003). [DOI] [PubMed] [Google Scholar]
- Trounce I., Byrne E. & Marzuki S. Decline in skeletal muscle mitochondrial respiratory chain function: possible factor in ageing. Lancet 1, 637–639 (1989). [DOI] [PubMed] [Google Scholar]
- Petersen K. F. et al. Mitochondrial dysfunction in the elderly: possible role in insulin resistance. Science 300, 1140–1142 (2003). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iossa S. et al. A possible link between skeletal muscle mitochondrial efficiency and age-induced insulin resistance. Diabetes 53, 2861–2866 (2004). [DOI] [PubMed] [Google Scholar]
- Morino K. et al. Reduced mitochondrial density and increased IRS-1 serine phosphorylation in muscle of insulin-resistant offspring of type 2 diabetic parents. J Clin Invest 115, 3587–3593 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mootha V. K. et al. PGC-1alpha-responsive genes involved in oxidative phosphorylation are coordinately downregulated in human diabetes. Nat Genet 34, 267–273 (2003). [DOI] [PubMed] [Google Scholar]
- Petersen K. F., Dufour S., Befroy D., Garcia R. & Shulman G. I. Impaired mitochondrial activity in the insulin-resistant offspring of patients with type 2 diabetes. N Engl J Med 350, 664–671 (2004). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lowell B. B. & Shulman G. I. Mitochondrial dysfunction and type 2 diabetes. Science 307, 384–387 (2005). [DOI] [PubMed] [Google Scholar]
- Short K. R., Nair K. S. & Stump C. S. Impaired mitochondrial activity and insulin-resistance offspring of patients with type 2 diabetes. N Engl J Med 350, 2419–2421 (2004). [DOI] [PubMed] [Google Scholar]
- Wisløff U. et al. Cardiovascular risk factors emerge after artificial selection for low aerobic capacity. Science 307, 418–420 (2005). [DOI] [PubMed] [Google Scholar]
- Cappuccio F. P., Strazzullo P., Farinaro E. & Trevisan M. Uric acid metabolism and tubular sodium handling. Result from a population-based study. JAMA 270, 354–359 (1993). [PubMed] [Google Scholar]
- Peters T. et al. Mouse model of foreign body reaction that alters the submesothelium and transperitoneal transport. Am J Physiol Renal Physiol 300, F283–289 (2011). [DOI] [PubMed] [Google Scholar]
- Muscelli E. et al. Effect of insulin on renal sodium and uric acid handling in essential hypertension. Am J Hypertens 9, 746–752 (1996). [DOI] [PubMed] [Google Scholar]
- Ter Maaten J. C. et al. Renal handling of urate and sodium during acute physiological hyperinsulinaemia in healthy subjects. Clin Sci (Lond) 92, 51–58 (1997). [DOI] [PubMed] [Google Scholar]
- Heianza Y. et al. HbA1c 5.7–6.4% and impaired fasting plasma glucose for diagnosis of prediabetes and risk of progression to diabetes in Japan (TOPICS 3): a longitudinal cohort study. Lancet 378, 147–155 (2011). [DOI] [PubMed] [Google Scholar]
- Zhou J. Y. et al. Neck circumference as an independent predictive contributor to cardio-metabolic syndrome. Cardiovasc Diabetol 12, 76 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]


