Abstract
BCR-ABL1 mutations are a common, well-characterized mechanism of resistance to imatinib as first-line treatment of chronic myeloid leukemia in chronic phase (CML-CP). Less is known about mutation development during first-line treatment with dasatinib and nilotinib, despite increased use because of higher response rates compared with imatinib. Retrospective analyses were conducted to characterize mutation development in patients with newly diagnosed CML-CP treated with dasatinib (n=259) or imatinib (n=260) in DASISION (Dasatinib versus Imatinib Study in Treatment-Naive CML-CP), with 3-year minimum follow-up. Mutation screening, including patients who discontinued treatment and patients who had a clinically relevant on-treatment event (no confirmed complete cytogenetic response (cCCyR) and no major molecular response (MMR) within 12 months; fivefold increase in BCR-ABL1 with loss of MMR; loss of CCyR), yielded a small number of patients with mutations (dasatinib, n=17; imatinib, n=18). Dasatinib patients had a narrower spectrum of mutations (4 vs 12 sites for dasatinib vs imatinib), fewer phosphate-binding loop mutations (1 vs 9 mutations), fewer multiple mutations (1 vs 6 patients) and greater occurrence of T315I (11 vs 0 patients). This trial was registered at www.clinicaltrials.gov as NCT00481247.
Introduction
Chronic myeloid leukemia (CML) has become a manageable chronic disease with the advent of BCR-ABL1 inhibitors; however, mutations in the BCR-ABL1 gene may confer resistance to inhibitors, potentially limiting their effectiveness against expansion of specific leukemic clones.1, 2, 3, 4 Although the BCR-ABL1 inhibitor imatinib has demonstrated significant efficacy in newly diagnosed patients with CML in chronic phase (CML-CP), primary and secondary resistance to imatinib was observed (no initial response or loss of an established response), resulting in unfavorable long-term outcomes.5, 6 Patients treated with imatinib are susceptible to numerous mutations that reduce the binding of imatinib,7, 8, 9 and BCR-ABL1 mutations may be responsible for 9–48% of primary resistance and 10–68% of secondary or acquired resistance to imatinib.10, 11, 12, 13 Dasatinib, nilotinib, bosutinib and ponatinib have enabled many patients, including those with mutations, to overcome imatinib resistance; however, each lack efficacy against a small number of different leukemic clones, and all except ponatinib lack efficacy against T315I.3, 4, 14, 15, 16, 17
Dasatinib and nilotinib are also approved for the treatment of newly diagnosed CML-CP patients in many countries.18, 19, 20, 21 Compared with imatinib, dasatinib and nilotinib in the first-line setting are associated with faster and deeper molecular responses and reduced risk of transformation to accelerated phase/blast phase (AP/BP).22, 23 Although a narrow spectrum of mutations developing during imatinib treatment are known to confer resistance to subsequent treatment with dasatinib or nilotinib, less is known qualitatively or quantitatively regarding the spectrum of mutations emerging during first-line treatment.3, 4, 24, 25, 26
The first-line phase 3 trial DASISION (Dasatinib versus Imatinib Study in Treatment-Naive CML-CP) demonstrated that dasatinib significantly improved early cytogenetic and molecular response rates compared with imatinib in the treatment of newly diagnosed CML-CP patients.23, 24, 27 With a minimum 2-year follow-up in DASISION, mutational analyses in patients who discontinued treatment for any reason identified 10 mutations in each treatment arm affecting three amino acids in dasatinib-treated patients and nine amino acids in imatinib-treated patients.24
To identify patients potentially at higher risk for developing mutations, mutational analyses based on a minimum 3-year follow-up were conducted for patients in DASISION who had discontinued treatment for any reason and for those on treatment with clinically relevant events (defined as no confirmed complete cytogenetic response (cCCyR) and no major molecular response (MMR) within 12 months; a fivefold increase in BCR-ABL1 transcript levels with loss of MMR; loss of CCyR). Potential relationships between the development of mutations, response dynamics and long-term patient status were also explored.
Subjects and methods
DASISION (CA180-056; ClinicalTrials.gov: NCT00481247) is an ongoing, open-label, phase 3 randomized trial for which patient characteristics and eligibility criteria have been described.27 Briefly, adults with cytogenetically confirmed Philadelphia chromosome-positive (Ph+) CML-CP diagnosed within 3 months who had adequate hepatic and renal function and no serious medical conditions were eligible. With the exception of anagrelide or hydroxyurea, no prior CML therapy was permitted. The trial was approved by all institutional review boards and ethics committees, and all patients gave written informed consent before randomization in accordance with the Declaration of Helsinki.
In the study, 519 patients with newly diagnosed CML-CP were randomized 1:1 to dasatinib 100 mg once daily (n=259) or imatinib 400 mg once daily (n=260). With a two-sided α=0.05 and power of 90%, 518 subjects were needed to show a statistically significant difference in 12-month CCyR rates between the two arms when the 12-month CCyR rates in the imatinib 400 mg once daily arm and the dasatinib 100 mg once daily arm were assumed to be 69% and 81%, respectively. Study treatment was discontinued for protocol-defined disease progression (increasing white blood cell count, loss of complete hematologic response, loss of major cytogenetic response, AP/BP criteria met, death from any cause during treatment), treatment failure (no hematologic response at 3 months, no complete hematologic response or cytogenetic response at 6 months, no partial cytogenetic response at 12 months, no CCyR at 18 months),28 unacceptable toxicity, patient/investigator decision or pregnancy.
Treatment interruptions and dose reductions were permitted for managing adverse events. Dose escalations to dasatinib 140 mg once daily or imatinib 600–800 mg/day were permitted for suboptimal response at 3–18 months.28 The primary end point was cCCyR rate by 12 months (the confirming assessment could be after 12 months). A cCCyR was defined as CCyR documented on two consecutive assessments at least 28 days apart. Key secondary end points included time in cCCyR, rates of MMR, defined as a BCR-ABL1 transcript level in peripheral blood on international scale ⩽0.1%, corresponding to ≥3-log reduction from the standardized baseline, at any time, times to cCCyR or MMR and durations of progression-free survival and overall survival. Transformation to AP/BP was defined according to the European LeukemiaNet (ELN) 2006 criteria (clonal evolution was not included).28
Mutational analysis
In DASISION, BCR-ABL1 mutational analyses were to be conducted in all patients receiving first-line dasatinib or imatinib at baseline and the end of treatment. Here, we also conducted BCR-ABL1 mutational analyses in the subset of patients who were considered more likely to have a mutation according to ELN recommendations.12 This analysis included patients on treatment who had at least one clinically relevant event (no cCCyR within 12 months; no MMR within 12 months; fivefold increase in BCR-ABL1 transcript levels with loss of MMR; loss of CCyR), and/or who discontinued treatment for any reason (Table 1). Patients may have been included in both categories having (1) a clinically relevant on-treatment event and (2) discontinued treatment. Stored specimens taken closest to the event were analyzed retrospectively for the presence of mutations (for patients who discontinued treatment, samples were analyzed within 45 days, before or after discontinuation). For those patients in whom a mutation was identified, all stored specimens from baseline to the final sample collected were retrospectively analyzed for the presence of mutations. Mutational analyses were conducted at a central independent laboratory (MolecularMD, Portland, OR, USA) using direct sequencing on peripheral blood samples after amplification of the ABL tyrosine kinase domain (amino acids 35–510) by reverse transcription PCR.29 When a mutation was initially detected in a patient sample, repeat testing from the RNA was performed to verify the presence of the identified mutation. Mutation data were blinded for samples collected at baseline and end of treatment. The remaining samples were analyzed retrospectively.
Table 1. Triggers for mutational analysis: DASISION 3-year database.
| On-treatment, clinically relevant events | Off-treatment, reason for discontinuation from study |
|---|---|
| No confirmed CCyR within 12 months | Disease progressiona |
| No MMR within 12 months | Maximum clinical benefitb |
| Fivefold increase in BCR-ABL1 transcript | Intolerance Treatment failure |
| levels with loss of MMR | |
| Loss of CCyRc | Adverse event unrelated to study drug |
| Withdrew consent | |
| Pregnancy | |
| Loss to follow-up | |
| Poor/noncompliance | |
| Subject request to discontinue |
Abbreviations: CCyR, complete cytogenetic response; DASISION, Dasatinib versus Imatinib Study in Treatment-Naive CML-CP; MMR, major molecular response.
Increasing white blood cell count, loss of complete hematologic response (CHR), loss of major cytogenetic response, transformation to accelerated phase/blast phase and death.
Any subject who received study therapy and then discontinued treatment for intolerance (recurrent ≥grade 3 hematologic toxicity or ≥grade 2 nonhematologic toxicity despite dose reduction necessitating discontinuation of therapy) or treatment failure (lack of hematologic response at 3 months, lack of CHR or CyR at 6 months, lack of partial cytogenetic response at 12 months and lack of CCyR at 18 months).
As of last patient status; patients with interim loss of CCyR who subsequently regained a CCyR were not included.
Molecular response
BCR-ABL1 transcript levels in peripheral blood were assessed using quantitative real-time PCR by MolecularMD. Patients with typical BCR-ABL1 transcripts b2a2 and b3a2 were eligible for molecular response analysis. Data are expressed on the international scale.29, 30
Patient status
For patients with mutations detected based on 3-year minimum follow-up, status was assessed based on extended follow-up (minimum 4 years), including BCR-ABL1 transcript level and current treatment dose for patients still on treatment, and included survival status (alive or dead, reason for death), current treatment and transformation to AP/BP for patients who discontinued treatment.
Results
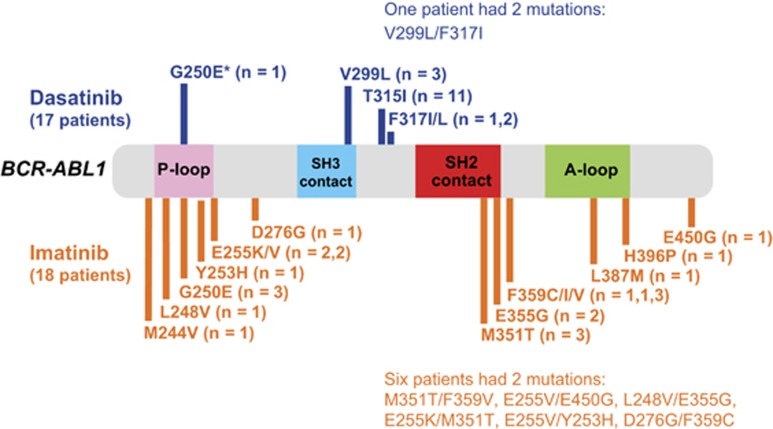
After a 3-year minimum follow-up, 169 of 259 (65%) randomized patients in the dasatinib arm and 194 of 260 (75%) in the imatinib arm of the study were identified with events that warranted mutational analysis (Table 2). BCR-ABL1 mutations were detected in a minority of patients in both arms (dasatinib, n=17/169 (10%); imatinib, n=18/194 (9%): Table 2 and Figure 1). Patients receiving dasatinib had a more narrow spectrum of mutations (affecting four sites) compared with those receiving imatinib (affecting 12 sites) and fewer phosphate-binding loop (P-loop) mutations (1 (G250E) vs 9 (G250E, n=3; L248V, n=1; E255K/V, n=4; Y253H, n=1), respectively). The G250E mutation seen with dasatinib was identified in a single patient sample collected 12 months after the start of treatment and was not detected in samples collected thereafter. The patient was screened owing to no MMR within 12 months and a fivefold increase in BCR-ABL1 transcript level with the loss of a subsequent MMR. His molecular and cytogenetic responses subsequently improved following dose escalation to 140 mg, and he remains on treatment as of last follow-up (>4 years after start of treatment). Fewer patients on dasatinib (n=1) had two BCR-ABL1 mutations detected in tandem or as separate clones compared with patients on imatinib (n=6). The T315I mutation was only identified in the dasatinib arm (n=11).
Table 2. DASISION 3-year mutational analysis.
| Dasatinib 100 mg once daily, N=259 | Imatinib 400 mg once daily, N=260 | |
|---|---|---|
| Patients identified for mutational analysis, n (%) | 169 (65) | 194 (75) |
| Patients with samples analyzed for mutations, n | 155 | 183 |
| Patients with samples not analyzed,a n | 14 | 11 |
| Patients with mutations detected (total), n | 17 | 18 |
| Mutations detectedb (n) | G250E (1), V299L (3), T315I (11), F317I/L (3) | M244V (1), L248V (1), G250E (3), Y253H (1), E255K/V (4), D276G (1), M351T (3), E355G (2), F359C/I/V (5), L387M (1), H396P (1), E450G (1) |
Abbreviation: DASISION, Dasatinib versus Imatinib Study in Treatment-Naive CML-CP.
Alternate transcript; BCR-ABL1 too low to test; other.
Includes patients with two mutations: one dasatinib (V299L/F317I) and six imatinib (L248V/E355G, E255V/E450G, E255K/M351T, Y253H/E255V, D276G/F359C and M351T/F359V).
Figure 1.
Distribution of mutations detected in DASISION at 3 years by treatment arm and BCR-ABL1 location. *Identified in one sample collected 1 year after treatment start; no mutation was detected in a second sample collected the same day or in a sample collected 2 years later.
Characteristics of patients with mutations
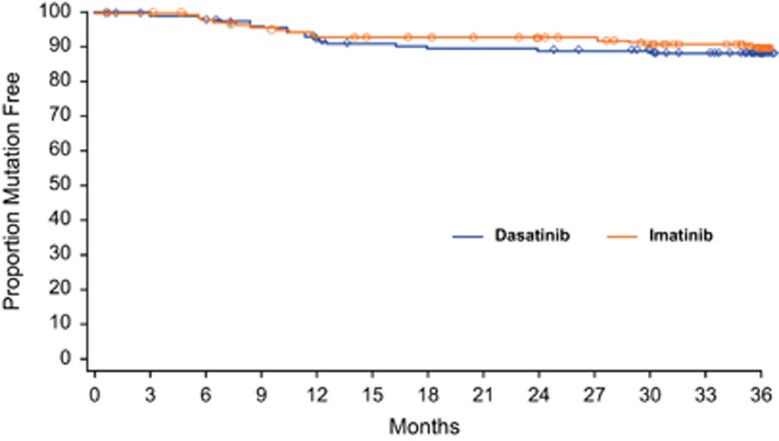
The majority of patients with mutations had their first mutation detected within 12 months of the start of treatment (Figure 2): in the dasatinib arm, 65% (11/17) had a mutation detected within 12 months and 94% (16/17) had a mutation detected within 24 months (range, 3–30 months); in the imatinib arm, 67% (12/18) had a mutation detected within 12 months and 72% (13/18) had a mutation detected within 24 months (range, 5–36 months). In addition, 76% (13/17) of dasatinib-treated patients and 61% (11/18) of imatinib-treated patients had a mutation detected shortly before the trigger for mutational analysis was observed (⩽3 months prior). The majority of patients with mutations had more than one of the defined clinically relevant on-treatment events, with most failing to achieve or maintain a molecular or cytogenetic response (Table 3).
Figure 2.
Detection of mutations over time. Kaplan–Meier curve of the detection of mutations for both dasatinib- and imatinib-treated patients tested for mutations. Dasatinib-treated patients with samples analyzed for mutations: n=155; imatinib-treated patients with samples analyzed for mutations: n=183. Patients were censored when they were no longer at risk for developing a mutation.
Table 3. Clinically relevant on-treatment events, reasons for discontinuation and current status in patients with mutations in DASISION.
| Dasatinib 100 mg once daily, n=17 | Imatinib 400 mg once daily, n=18 | |
|---|---|---|
| Clinically relevant on-treatment events, na | 14 | 16 |
| No MMR within 12 months | 12 | 16 |
| No cCCyR within 12 months | 8 | 12 |
| Loss of CCyR | 6 | 4 |
| Fivefold BCR-ABL1 increase with loss of MMR | 2 | 1 |
| Discontinued treatment, n | 14 | 14 |
| Protocol-defined disease progression | 11 | 8 |
| Lost MCyR | 4 | 4 |
| Transformed to AP/BP | 4 | 3 |
| Lost CHR | 1 | 1 |
| Increased WBC count | 1 | — |
| Death | 1 | — |
| Treatment failure | 1 | 4 |
| No PCyR, 12 months | — | 2 |
| No CCyR, 18 months | 1 | 2 |
| Other | 2b | 2c |
| Patient status (4-year minimum follow-up), n | ||
| Off treatment | 14 | 14 |
| Alive | 8 | 10 |
| Dead | 6 | 4 |
| On treatment | 3 | 4 |
| % Mutation detected at trigger plus molecular response at last analysis | 90% G250Ed; 0.02% BCR-ABL1IS | 80% F359Id; 1.03% BCR-ABL1IS |
| 40% T315Id; 0.039% BCR-ABL1IS | 100% G250Ed; 11.3% BCR-ABL1IS | |
| 80% T315Id; 0.013% BCR-ABL1IS | 90% G250Ed; 7.7% BCR-ABL1IS | |
| 20% F359Vd; 20.5% BCR- ABL1IS | ||
Abbreviations: AP/BP, accelerated phase/blast phase; cCCyR, confirmed complete cytogenetic response; CCyR, complete cytogenetic response; CHR, complete hematologic response; DASISION, Dasatinib versus Imatinib Study in Treatment-Naive CML-CP; IS, international scale; MMR, major molecular response; MCyR, major cytogenetic response; PCyR, partial cytogenetic response; WBC, white blood cell.
Patients may have had multiple events.
One no CyR, one lost CCyR.
One poor/noncompliance, one patient request.
Estimated percentage of the mutation quantified in the sample.
Among patients with mutations in the dasatinib arm, a greater percentage had high baseline Euro (Hasford) scores (41% Table 4) compared with all randomized patients (19% high, 47–48% intermediate and 33% low).27 In the dasatinib arm, Euro (Hasford) scores among patients with T315I were evenly distributed (4 high, 3 intermediate and 4 low); however, among patients with other mutations, more had high baseline scores (n=3: 1 G250E, 1 V299L and 1 V299L/F317I) than intermediate (n=2: 1 F317L and 1 V299L) or low (n=1: F317L). Euro (Hasford) scores among patients with mutations in the imatinib arm showed a similar distribution as for all randomized patients (Table 4), with no clear trends with regard to the incidence of specific mutations, double mutations or P-loop mutations. Analysis of molecular and cytogenetic response dynamics showed that most patients with mutations had little or no initial response or experienced a transient modest response often deep enough to achieve a CCyR but not an MMR (Table 4).
Table 4. Baseline Euro (Hasford) risk score and response dynamics in patients with mutations.
| Dasatinib 100 mg once daily, n=17 | Imatinib 400 mg once daily, n=18 | |
|---|---|---|
| Euro (Hasford) score, n (% of patients)a | ||
| Low (⩽780) | 5 (29) | 6 (33) |
| Patients at risk | 86 | 87 |
| Intermediate (>780 to ⩽1480) | 5 (29) | 8 (44) |
| Patients at risk | 124 | 213 |
| High (>1480) | 7 (41) | 4 (22) |
| Patients at risk | 49 | 50 |
| Best cytogenetic response, n | ||
| CCyR | 10 | 10 |
| PCyR | 1 | 4 |
| <PCyR | 6 | 4 |
| Best molecular response, n | ||
| MMR | 2 | 1 |
| BCR-ABL1 ⩽10% at 3 months | 10b | 6c |
| Treatment milestone | ||
| cCCyR by 12 months | ||
| Achieved | 6 | 4 |
| Not achieved | 11 | 14 |
| MMR at 12 months | ||
| Achieved | 0 | 0 |
| Not achieved | 17 | 18 |
Abbreviations: cCCyR, confirmed complete cytogenetic response; CCyR, complete cytogenetic response; MMR, major molecular response; PCyR, partial cytogenetic response.
Investigator-reported Euro (Hasford) risk score.
BCR-ABL1 transcript levels not available for one patient.
Three-month BCR-ABL1 transcript levels not available in two patients.
Long-term patient status
For patients with mutations identified based on 3-year minimum follow-up, an extended 4-year minimum follow-up was conducted. This extended follow-up of patients with mutations showed poor outcomes regardless of treatment (dasatinib vs imatinib) and high rates of treatment discontinuation (dasatinib, n=14/17; imatinib, n=14/18). The primary reason for discontinuation of patients with mutations was protocol-defined disease progression (dasatinib, n=11; imatinib, n=8; Table 3). Of all patients in DASISION who discontinued because of protocol-defined disease progression, patients with mutations accounted for 61% on dasatinib (n=11/18) and 42% on imatinib (n=8/19).
The patients with mutations who discontinued because of transformation to AP/BP (a subset of patients who discontinued because of disease progression) all died: four dasatinib patients (three who had T315I and one who had F317I) and three imatinib patients (two mutations each: M351T/F359V, E255K/M351T and D276G/F359C). The remaining deaths among patients with mutations (dasatinib, infection, n=2; imatinib, complication following allogenic stem cell transplant, n=1) were not related to BCR-ABL1 inhibitor therapy. The one dasatinib patient with two mutations (V299L/F317I) was the only patient with a mutation identified who transformed to AP/BP after dasatinib discontinuation; this patient had discontinued treatment because of loss of complete hematologic response, transformed after switching to nilotinib, then received allogeneic hematopoietic stem cell transplantation and was alive at last follow-up. The remaining three imatinib patients with two mutations discontinued treatment because of poor cytogenetic response (two with no partial cytogenetic response by 12 months, one with no major cytogenetic response by 18 months), switched to dasatinib (n=2) or nilotinib (n=1) and were alive at last follow-up.
Although most patients who had mutations detected were off study at the time of this analysis, there were three patients in the dasatinib arm and four patients in the imatinib arm who remained on study treatment. The three patients with mutations in the dasatinib arm who remained on treatment had a good molecular response (BCR-ABL1 transcript levels ⩽0.1% (MMR); Table 3); however, the four patients with mutations in the imatinib arm who remained on treatment had BCR-ABL1 transcript levels >1% (Table 3). The presence of a mutation and the failure to achieve BCR-ABL1 <1% represent treatment failure under the current ELN recommendations and should lead to a switch to another tyrosine-kinase inhibitor.12, 31
Discussion
This retrospective study based on 3-year minimum follow-up in the DASISION trial showed that BCR-ABL1 mutations were identified in only a small number of patients (dasatinib, n=17; imatinib, n=18), even though the criteria for mutational analysis were expanded from that used in previous reports of DASISION (clinically relevant on-treatment events, discontinuation because of any reason) to include a large number of patients in this analysis. Consistent with results of in vitro studies and previous findings in the second-line setting,3, 32, 33, 34, 35, 36, 37, 38 a narrower spectrum of mutations was detected in patients who received first-line dasatinib compared with imatinib. Notably, the number of patients who carried more than one mutation was higher with imatinib. Patients with more than one BCR-ABL1 mutation generally have a poorer outcome compared with patients harboring only one BCR-ABL1 mutation.39, 40 In this mutational analysis, all patients with two BCR-ABL1 mutations discontinued study treatment (dasatinib, n=1; imatinib, n=6) either because of protocol-defined disease progression (dasatinib, n=1; imatinib, n=3) or treatment failure (imatinib, n=3), and 3 (imatinib) subsequently died. Compared with dasatinib, more patients treated with imatinib also had P-loop mutations. Mutations in the P-loop of the BCR-ABL1 kinase domain have been shown to be associated with poor prognosis.8
Of note, the effectiveness of dasatinib against P-loop mutations varies: not all patients with P-loop mutations at the time of imatinib discontinuation have improved responses with dasatinib,11, 41, 42 and P-loop mutations have been shown to emerge during second-line treatment with dasatinib, including G250E, Q252H and L248V.3, 34, 40 Hence, the detection of a G250E mutation in the dasatinib arm is not surprising, and the absence of other P-loop mutants could be because of the small number of patients with mutations identified.
In this analysis, the T315I mutation was identified in the dasatinib arm (n=11) but not the imatinib arm (n=0). The lack of T315I mutations in the imatinib arm of DASISION is inconsistent with other reports, including the ENESTnd (Evaluating Nilotinib Efficacy and Safety in Clinical Trials–Newly Diagnosed Patients) trial.7, 8, 9, 10, 11, 25, 26, 43 With 2 and 3 years of follow-up in ENESTnd, a similar number of patients had a T315I mutation in the nilotinib and imatinib arms of the study (nilotinib 300 mg twice daily, n=3; nilotinib 400 mg twice daily, n=2; imatinib 400 mg once daily, n=3).25, 26 However, differences in methodology, patient population, small numbers and geographical distribution between DASISION and ENESTnd make interpretation of cross-study comparisons difficult.43 Mutation data from ongoing first-line trials by MD Anderson Cancer Center (CA180-040; NCT00254423)44 and the Southwest Oncology Group (SWOG S0325; NCT00070499)45 are expected to provide additional information regarding the development of mutations in patients with newly diagnosed CML-CP treated with dasatinib.
One hypothesis for the greater number of T315I mutations detected with dasatinib (n=11) compared with imatinib (n=0) derives from differences in competitive advantage between mutant clones observed in in vitro cell assays. Select mutant clones (for example, P-loop mutations Y253F, E255K) were found to have higher transformation potency and proliferation rate compared with T315I, even in the absence of BCR-ABL1 inhibitors.46 Assuming that imatinib has lower activity against these mutations compared with dasatinib,1, 24, 34, 41, 47 mutant clones with select P-loop mutations may expand more rapidly than clones with the T315I mutation when exposed to imatinib compared with dasatinib.
Consistent with this idea, some P-loop mutations have been shown to arise faster during imatinib treatment than the T315I mutation,48 and T315I is less common than all P-loop mutations in CML-CP patients with imatinib resistance.11, 42, 47, 48, 49 In addition, dasatinib suppresses P-loop mutations to a greater extent than T315I;34, 50, 51, 52, 53 therefore, T315I may be able to develop during dasatinib treatment with relatively little competition from rapidly proliferating clones.
Patients with mutations in DASISION typically had a poor outcome, with high rates of discontinuation (82% dasatinib, 78% imatinib), transformation (24 and 17%) and death (35 and 22%) by 4-year minimum follow-up. The majority of mutations were identified early, usually within the first 12 months of treatment (dasatinib: 65% range, 3–30 months; imatinib: 67% range, 5–36 months).
Despite the fact that mutations were detected in a small percentage of patients considered to be more likely to develop mutations, the data from the DASISION 3-year mutational analysis reported here suggest that patients with CML-CP who fail to achieve and maintain treatment response at key milestones should be considered for mutation screening. This is consistent with current CML treatment guidelines.12, 31, 54 Recent CML guideline updates from the National Comprehensive Cancer Network and recommendations from ELN advise mutational analysis be performed for patients with inadequate molecular or cytogenetic response.12, 31, 54 The recent CML recommendations also suggest that patients with an inadequate response or treatment failure (inclusive of mutation development) may be considered for an alternative treatment.
Mutational testing is anticipated to become more widely used to select second-line treatment in the community setting, especially with the approval of ponatinib for treatment of patients with CML or Ph+ acute lymphoblastic leukemia with resistance or intolerance to prior tyrosine-kinase inhibitor therapy. Ponatinib has demonstrated activity against T315I/A in preclinical assays,55 and patients with the T315I mutation respond well to ponatinib, unlike patients with T315I treated with other BCR-ABL1 inhibitors.15, 17 In addition, with the introduction of generic imatinib into the market in 2016, choosing the most appropriate second-line tyrosine-kinase inhibitor for patients, based on factors such as mutation status, will become increasingly important. Allowing physicians the option to choose the most suitable second-line therapy may ensure improved outcomes and decreased health-care costs.56 More sensitive detection methods (for example, next-generation sequencing) may allow mutations that were not evident through conventional sequencing to be detected following a significant rise in BCR-ABL1 levels;2 however, further data are needed to determine the clinical relevance of low-level mutations for patient outcomes.2, 40, 57, 58, 59, 60
Mutational testing provides arguably the most important measure for treatment selection in patients with CML, especially when selecting an alternative therapy because of current treatment failure. In patients with less-resistant mutations, other factors, such as patient comorbidities, the potential for cross-intolerance and adherence, should also be considered to ensure patients achieve good long-term outcomes.
Acknowledgments
We thank all participating study sites for this BMS-sponsored study and the patients and families for making this trial possible. Professional medical writing and editorial assistance was provided by Samantha L. Dwyer of StemScientific, an Ashfield Company, part of UDG Healthcare plc, funded by BMS. BMS sponsored this analysis.
Author contributions
All authors provided feedback and guidance on the analysis and interpretation of the results, contributed to the drafting of and critically reviewed the manuscript and provided final approval for submission. TPH, AH and JU designed the analysis.
TPH has received honoraria and research funding from ARIAD, Bristol-Myers Squibb (BMS) and Novartis. GS has acted as a consultant for and received honoraria from ARIAD, BMS, Celgene, Novartis and Pfizer. AQ-C has acted as a consultant for ARIAD, BMS and Novartis. MJM has received research funding from ARIAD, BMS and Novartis; and has acted as a consultant or speaker for and received honoraria from ARIAD, BMS, Novartis and Pfizer. D-WK received research funding and honoraria from ARIAD, BMS, ILYANG, Novartis and Pfizer; has received funding for travel/accommodations/meeting expenses from BMS; and has served as a consultant and board member for BMS and Novartis. JHL has received research support from ARIAD, BMS, Merck (Schering), Novartis, Pfizer (Wyeth), Roche and TEVA (ChemGenex); has served as a speaker or consultant for ARIAD, BMS, Merck, Novartis, Pfizer, Roche and TEVA; and is a stockholder of ARIAD. MBB-G is an employee of BMS and as employment benefit has received shares in BMS stock. JU was an employee of BMS at the time of analysis. AH has received research funding from ARIAD, BMS, MSD, Novartis and Pfizer. The authors did not receive financial compensation for authoring the manuscript.
References
- Jabbour E, Kantarjian H, Jones D, Talpaz M, Bekele N, O'Brien S, et al. Frequency and clinical significance of BCR-ABL mutations in patients with chronic myeloid leukemia treated with imatinib mesylate. Leukemia. 2006;20:1767–1773. doi: 10.1038/sj.leu.2404318. [DOI] [PubMed] [Google Scholar]
- Parker WT, Lawrence RM, Ho M, Irwin DL, Scott HS, Hughes TP, et al. Sensitive detection of BCR-ABL1 mutations in patients with chronic myeloid leukemia after imatinib resistance is predictive of outcome during subsequent therapy. J Clin Oncol. 2011;29:4250–4259. doi: 10.1200/JCO.2011.35.0934. [DOI] [PubMed] [Google Scholar]
- Müller MC, Cortes JE, Kim DW, Druker BJ, Erben P, Pasquini R, et al. Dasatinib treatment of chronic-phase chronic myeloid leukemia: analysis of responses according to preexisting BCR-ABL mutations. Blood. 2009;114:4944–4953. doi: 10.1182/blood-2009-04-214221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hughes T, Saglio G, Branford S, Soverini S, Kim DW, Müller MC, et al. Impact of baseline BCR-ABL mutations on response to nilotinib in patients with chronic myeloid leukemia in chronic phase. J Clin Oncol. 2009;27:4204–4210. doi: 10.1200/JCO.2009.21.8230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deininger M, O'Brien SG, Guilhot F, Goldman JM, Hochhaus A, Hughes TP, et al. International randomized study of interferon vs STI571 (IRIS) 8-year follow up: sustained survival and low risk for progression or events in patients with newly diagnosed chronic myeloid leukemia in chronic phase (CML-CP) treated with imatinib. Blood. 2009;114:abstract 1126. [Google Scholar]
- Hochhaus A, O'Brien SG, Guilhot F, Druker BJ, Branford S, Foroni L, et al. Six-year follow-up of patients receiving imatinib for the first-line treatment of chronic myeloid leukemia. Leukemia. 2009;23:1054–1061. doi: 10.1038/leu.2009.38. [DOI] [PubMed] [Google Scholar]
- Shah NP, Nicoll JM, Nagar B, Gorre ME, Paquette RL, Kuriyan J, et al. Multiple BCR-ABL kinase domain mutations confer polyclonal resistance to the tyrosine kinase inhibitor imatinib (STI571) in chronic phase and blast crisis chronic myeloid leukemia. Cancer Cell. 2002;2:117–125. doi: 10.1016/s1535-6108(02)00096-x. [DOI] [PubMed] [Google Scholar]
- Branford S, Rudzki Z, Walsh S, Parkinson I, Grigg A, Szer J, et al. Detection of BCR-ABL mutations in patients with CML treated with imatinib is virtually always accompanied by clinical resistance, and mutations in the ATP phosphate-binding loop (P-loop) are associated with a poor prognosis. Blood. 2003;102:276–283. doi: 10.1182/blood-2002-09-2896. [DOI] [PubMed] [Google Scholar]
- Soverini S, Martinelli G, Rosti G, Bassi S, Amabile M, Poerio A, et al. ABL mutations in late chronic phase chronic myeloid leukemia patients with up-front cytogenetic resistance to imatinib are associated with a greater likelihood of progression to blast crisis and shorter survival: a study by the GIMEMA Working Party on Chronic Myeloid Leukemia. J Clin Oncol. 2005;23:4100–4109. doi: 10.1200/JCO.2005.05.531. [DOI] [PubMed] [Google Scholar]
- de Lavallade H, Apperley JF, Khorashad JS, Milojkovic D, Reid AG, Bua M, et al. Imatinib for newly diagnosed patients with chronic myeloid leukemia: incidence of sustained responses in an intention-to-treat analysis. J Clin Oncol. 2008;26:3358–3363. doi: 10.1200/JCO.2007.15.8154. [DOI] [PubMed] [Google Scholar]
- Soverini S, Colarossi S, Gnani A, Rosti G, Castagnetti F, Poerio A, et al. Contribution of ABL kinase domain mutations to imatinib resistance in different subsets of Philadelphia-positive patients: by the GIMEMA Working Party on Chronic Myeloid Leukemia. Clin Cancer Res. 2006;12:7374–7379. doi: 10.1158/1078-0432.CCR-06-1516. [DOI] [PubMed] [Google Scholar]
- Soverini S, Hochhaus A, Nicolini FE, Gruber F, Lange T, Saglio G, et al. BCR-ABL kinase domain mutation analysis in chronic myeloid leukemia patients treated with tyrosine kinase inhibitors: recommendations from an expert panel on behalf of European LeukemiaNet. Blood. 2011;118:1208–1215. doi: 10.1182/blood-2010-12-326405. [DOI] [PubMed] [Google Scholar]
- Soverini S, Branford S, Nicolini FE, Talpaz M, Deininger MW, Martinelli G, et al. Implications of BCR-ABL1 kinase domain-mediated resistance in chronic myeloid leukemia. Leuk Res. 2014;38:10–20. doi: 10.1016/j.leukres.2013.09.011. [DOI] [PubMed] [Google Scholar]
- Cortes JE, Kantarjian HM, Brümmendorf TH, Kim DW, Turkina AG, Shen ZX, et al. Safety and efficacy of bosutinib (SKI-606) in chronic phase Philadelphia chromosome-positive chronic myeloid leukemia patients with resistance or intolerance to imatinib. Blood. 2011;118:4567–4576. doi: 10.1182/blood-2011-05-355594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cortes JE, Kim DW, Pinilla-Ibarz J, le Coutre P, Paquette R, Chuah C, et al. A phase 2 trial of ponatinib in Philadelphia chromosome-positive leukemias. N Engl J Med. 2013;369:1783–1796. doi: 10.1056/NEJMoa1306494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Redaelli S, Piazza R, Rostagno R, Magistroni V, Perini P, Marega M, et al. Activity of bosutinib, dasatinib, and nilotinib against 18 imatinib-resistant BCR/ABL mutants. J Clin Oncol. 2009;27:469–471. doi: 10.1200/JCO.2008.19.8853. [DOI] [PubMed] [Google Scholar]
- Cortes JE, Kantarjian H, Shah NP, Bixby D, Mauro MJ, Flinn I, et al. Ponatinib in refractory Philadelphia chromosome–positive leukemias. N Engl J Med. 2012;367:2075–2088. doi: 10.1056/NEJMoa1205127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bristol-Myers Squibb Company. Sprycel (dasatinib) [prescribing information]. Princeton, NJ; 2014. [Google Scholar]
- Novartis Pharmaceuticals Corporation. Tasigna (nilotinib) [prescribing information] East Hanover, NJ; 2015. [Google Scholar]
- European Medicines Agency European public assessment reports for Sprycel. . http://www.ema.europa.eu/ema/index.jsp?curl=pages/ medicines/human/medicines/000709/human_med_001062.jsp&murl= menus/medicines/medicines.jsp&mid=WC0b01ac058001d125 ( last accessed 5 February2015
- European Medicines Agency European public assessment reports for Tasigna. . http://www.ema.europa.eu/ema/index.jsp?curl=pages/ medicines/human/medicines/000798/human_med_001079.jsp&mid=WC0b01ac058001d124 ( last accessed 5 February2015
- Larson RA, Hochhaus A, Hughes TP, Clark RE, Etienne G, Kim DW, et al. Nilotinib vs imatinib in patients with newly diagnosed Philadelphia chromosome-positive chronic myeloid leukemia in chronic phase: ENESTnd 3-year follow-up. Leukemia. 2012;26:2197–2203. doi: 10.1038/leu.2012.134. [DOI] [PubMed] [Google Scholar]
- Jabbour E, Kantarjian HM, Saglio G, Steegmann JL, Shah NP, Boqué C, et al. Early response with dasatinib or imatinib in chronic myeloid leukemia: 3-year follow-up from a randomized phase 3 trial (DASISION) Blood. 2014;123:494–500. doi: 10.1182/blood-2013-06-511592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kantarjian HM, Shah NP, Cortes JE, Baccarani M, Agarwal MB, Undurraga MS, et al. Dasatinib or imatinib in newly diagnosed chronic-phase chronic myeloid leukemia: 2-year follow-up from a randomized phase 3 trial (DASISION) Blood. 2012;119:1123–1129. doi: 10.1182/blood-2011-08-376087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kantarjian HM, Hochhaus A, Saglio G, De Souza C, Flinn IW, Stenke L, et al. Nilotinib versus imatinib for the treatment of patients with newly diagnosed chronic phase, Philadelphia chromosome-positive, chronic myeloid leukaemia: 24-month minimum follow-up of the phase 3 randomised ENESTnd trial Lancet Oncol 201112841–851.[published erratum appears inLancet Oncol 201112989]. [DOI] [PubMed] [Google Scholar]
- Hochhaus A, Saglio G, Larson RA, Kim DW, Etienne G, Rosti G, et al. Nilotinib is associated with a reduced incidence of BCR-ABL mutations vs imatinib in patients with newly diagnosed chronic myeloid leukemia in chronic phase. Blood. 2013;121:3703–3708. doi: 10.1182/blood-2012-04-423418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kantarjian H, Shah NP, Hochhaus A, Cortes J, Shah S, Ayala M, et al. Dasatinib versus imatinib in newly diagnosed chronic-phase chronic myeloid leukemia. N Engl J Med. 2010;362:2260–2270. doi: 10.1056/NEJMoa1002315. [DOI] [PubMed] [Google Scholar]
- Baccarani M, Saglio G, Goldman J, Hochhaus A, Simonsson B, Appelbaum F, et al. Evolving concepts in the management of chronic myeloid leukemia: recommendations from an expert panel on behalf of the European LeukemiaNet. Blood. 2006;108:1809–1820. doi: 10.1182/blood-2006-02-005686. [DOI] [PubMed] [Google Scholar]
- Hughes T, Deininger M, Hochhaus A, Branford S, Radich J, Kaeda J, et al. Monitoring CML patients responding to treatment with tyrosine kinase inhibitors: review and recommendations for harmonizing current methodology for detecting BCR-ABL transcripts and kinase domain mutations and for expressing results. Blood. 2006;108:28–37. doi: 10.1182/blood-2006-01-0092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Müller MC, Cross NC, Erben P, Schenk T, Hanfstein B, Ernst T, et al. Harmonization of molecular monitoring of CML therapy in Europe. Leukemia. 2009;23:1957–1963. doi: 10.1038/leu.2009.168. [DOI] [PubMed] [Google Scholar]
- Baccarani M, Deininger MW, Rosti G, Hochhaus A, Soverini S, Apperley JF, et al. European LeukemiaNet recommendations for the management of chronic myeloid leukemia: 2013. Blood. 2013;122:872–884. doi: 10.1182/blood-2013-05-501569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Hare T, Walters DK, Stoffregen EP, Jia T, Manley PW, Mestan J, et al. In vitro activity of Bcr-Abl inhibitors AMN107 and BMS-354825 against clinically relevant imatinib-resistant Abl kinase domain mutants. Cancer Res. 2005;65:4500–4505. doi: 10.1158/0008-5472.CAN-05-0259. [DOI] [PubMed] [Google Scholar]
- Bradeen HA, Eide CA, O'Hare T, Johnson KJ, Willis SG, Lee FY, et al. Comparison of imatinib mesylate, dasatinib (BMS-354825), and nilotinib (AMN107) in an N-ethyl-N-nitrosourea (ENU)-based mutagenesis screen: high efficacy of drug combinations. Blood. 2006;108:2332–2338. doi: 10.1182/blood-2006-02-004580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cortes J, Jabbour E, Kantarjian H, Yin CC, Shan J, O'Brien S, et al. Dynamics of BCR-ABL kinase domain mutations in chronic myeloid leukemia after sequential treatment with multiple tyrosine kinase inhibitors. Blood. 2007;110:4005–4011. doi: 10.1182/blood-2007-03-080838. [DOI] [PubMed] [Google Scholar]
- Shah NP, Skaggs BJ, Branford S, Hughes TP, Nicoll JM, Paquette RL, et al. Sequential ABL kinase inhibitor therapy selects for compound drug-resistant BCR-ABL mutations with altered oncogenic potency. J Clin Invest. 2007;117:2562–2569. doi: 10.1172/JCI30890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soverini S, Gnani A, Colarossi S, Castagnetti F, Abruzzese E, Paolini S, et al. Philadelphia-positive patients who already harbor imatinib-resistant Bcr-Abl kinase domain mutations have a higher likelihood of developing additional mutations associated with resistance to second- or third-line tyrosine kinase inhibitors. Blood. 2009;114:2168–2171. doi: 10.1182/blood-2009-01-197186. [DOI] [PubMed] [Google Scholar]
- Jabbour E, Kantarjian HM, Jones D, Reddy N, O'Brien S, Garcia-Manero G, et al. Characteristics and outcome of chronic myeloid leukemia patients with F317L BCR-ABL kinase domain mutation after therapy with tyrosine kinase inhibitors. Blood. 2008;112:4839–4842. doi: 10.1182/blood-2008-04-149948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khorashad JS, Milojkovic D, Mehta P, Anand M, Ghorashian S, Reid AG, et al. In vivo kinetics of kinase domain mutations in CML patients treated with dasatinib after failing imatinib. Blood. 2008;111:2378–2381. doi: 10.1182/blood-2007-06-096396. [DOI] [PubMed] [Google Scholar]
- Quintás-Cardama A, Kantarjian H, Shah NP, Schiffer CA, le Coutre P, Saglio G, et al. Patients with chronic myeloid leukemia in chronic phase carrying more than one BCR-ABL kinase domain mutation exhibit poorer response rates and outcomes to second-line dasatinib compared to those with no or only one BCR-ABL mutation Blood 2010116abstract 2297. [Google Scholar]
- Parker WT, Ho M, Scott HS, Hughes TP, Branford S. Poor response to second-line kinase inhibitors in chronic myeloid leukemia patients with multiple low-level mutations, irrespective of their resistance profile. Blood. 2012;119:2234–2238. doi: 10.1182/blood-2011-08-375535. [DOI] [PubMed] [Google Scholar]
- Talpaz M, Shah NP, Kantarjian H, Donato N, Nicoll J, Paquette R, et al. Dasatinib in imatinib-resistant Philadelphia chromosome-positive leukemias. N Engl J Med. 2006;354:2531–2541. doi: 10.1056/NEJMoa055229. [DOI] [PubMed] [Google Scholar]
- Apperley JF. Part I: mechanisms of resistance to imatinib in chronic myeloid leukaemia. Lancet Oncol. 2007;8:1018–1029. doi: 10.1016/S1470-2045(07)70342-X. [DOI] [PubMed] [Google Scholar]
- Nicolini FE, Corm S, Lê QH, Roche-Listienne C, Preudhomme C. The prognosis impact of BCR-ABL P-loop mutations: worse or not worse. Leukemia. 2007;21:193–194. doi: 10.1038/sj.leu.2404490. [DOI] [PubMed] [Google Scholar]
- ClinicalTrials.gov: A service of the U.S. National Institutes of Health Study of dasatinib in patients with chronic myelogenous leukemia http://clinicaltrials.gov/show/NCT00254423last accessed 1 October 2014.
- ClinicalTrials.gov: A service of the U.S. National Institutes of Health Imatinib mesylate or dasatinib in treating patients with chronic phase chronic myelogenous leukemia http://clinicaltrials.gov/show/NCT00070499last accessed 1 October 2014.
- Griswold IJ, MacPartlin M, Bumm T, Goss VL, O'Hare T, Lee KA, et al. Kinase domain mutants of Bcr-Abl exhibit altered transformation potency, kinase activity, and substrate utilization, irrespective of sensitivity to imatinib. Mol Cell Biol. 2006;26:6082–6093. doi: 10.1128/MCB.02202-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Branford S, Melo JV, Hughes TP. Selecting optimal second-line tyrosine kinase inhibitor therapy for chronic myeloid leukemia patients after imatinib failure: does the BCR-ABL mutation status really matter. Blood. 2009;114:5426–5435. doi: 10.1182/blood-2009-08-215939. [DOI] [PubMed] [Google Scholar]
- Ernst T, Erben P, Müller MC, Paschka P, Schenk T, Hoffmann J, et al. Dynamics of BCR-ABL mutated clones prior to hematologic or cytogenetic resistance to imatinib. Haematologica. 2008;93:186–192. doi: 10.3324/haematol.11993. [DOI] [PubMed] [Google Scholar]
- Jabbour E, Jones D, Kantarjian HM, O'Brien S, Tam C, Koller C, et al. Long-term outcome of patients with chronic myeloid leukemia treated with second-generation tyrosine kinase inhibitors after imatinib failure is predicted by the in vitro sensitivity of BCR-ABL kinase domain mutations. Blood. 2009;114:2037–2043. doi: 10.1182/blood-2009-01-197715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim WS, Kim D, Kim DW, Kweon IY, Kim SH, Goh HG, et al. Dynamic change of T315I BCR-ABL kinase domain mutation in Korean chronic myeloid leukaemia patients during treatment with Abl tyrosine kinase inhibitors. Hematol Oncol. 2010;28:82–88. doi: 10.1002/hon.918. [DOI] [PubMed] [Google Scholar]
- Ahn JS, Kim YK, Lee SR, Yu L, Yang DH, Cho SH, et al. Coexisting with clonal evolution and BCR-ABL mutant in CML patients treated with second-generation tyrosine kinase inhibitors predict the discrepancy of in vitro drug sensitivity. Cancer Res Treat. 2010;42:37–41. doi: 10.4143/crt.2010.42.1.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Branford S, Hochhaus A, Mueller M, Bahceci E, Ploughman L, Mukhopadhyay J, et al. Analysis of molecular data and the emergence of mutations for chronic-phase chronic myelogenous leukemia (CML-CP) patients treated with dasatinib after imatinib failure. Blood. 2009;114:abstract 3282. [Google Scholar]
- Gruber FX, Ernst T, Porkka K, Engh RA, Mikkola I, Maier J, et al. Dynamics of the emergence of dasatinib and nilotinib resistance in imatinib-resistant CML patients. Leukemia. 2012;26:172–177. doi: 10.1038/leu.2011.187. [DOI] [PubMed] [Google Scholar]
- National Comprehensive Cancer Network NCCN Clinical Practice Guidelines in Oncology—Chronic Myelogenous Leukemia v1.2015 . http://www.nccn.org/professionals/physician_gls/f_guidelines.asplast accessed 1 October 2014. [DOI] [PubMed]
- O'Hare T, Shakespeare WC, Zhu X, Eide CA, Rivera VM, Wang F, et al. AP24534, a pan-BCR-ABL inhibitor for chronic myeloid leukemia, potently inhibits the T315I mutant and overcomes mutation-based resistance. Cancer Cell. 2009;16:401–412. doi: 10.1016/j.ccr.2009.09.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jabbour EJ, Makenbaeva D, Lingohr-Smith M, Lin J.Potential economic consequences of genetic mutations and restricted acccess to tyrosine kinase inhibitor treatments among patients with chronic myelogenous leukemia in the USA Blood 2014124abstract 2645. [Google Scholar]
- Iqbal Z, Aleem A, Iqbal M, Naqvi MI, Gill A, Taj AS, et al. Sensitive detection of pre-existing BCR-ABL kinase domain mutations in CD34+ cells of newly diagnosed chronic-phase chronic myeloid leukemia patients is associated with imatinib resistance: implications in the post-imatinib era. PLoS One. 2013;8:e55717. doi: 10.1371/journal.pone.0055717. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Lange T, Ernst T, Gruber FX, Maier J, Cross M, Müller MC, et al. The quantitative level of T315I mutated BCR-ABL predicts for major molecular response to second-line nilotinib or dasatinib treatment in patients with chronic myeloid leukemia. Haematologica. 2013;98:714–717. doi: 10.3324/haematol.2012.068890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willis SG, Lange T, Demehri S, Otto S, Crossman L, Niederwieser D, et al. High-sensitivity detection of BCR-ABL kinase domain mutations in imatinib-naive patients: correlation with clonal cytogenetic evolution but not response to therapy. Blood. 2005;106:2128–2137. doi: 10.1182/blood-2005-03-1036. [DOI] [PubMed] [Google Scholar]
- Jiang X, Forrest D, Nicolini F, Turhan A, Guilhot J, Yip C, et al. Properties of CD34+ CML stem/progenitor cells that correlate with different clinical responses to imatinib mesylate. Blood. 2010;116:2112–2121. doi: 10.1182/blood-2009-05-222471. [DOI] [PMC free article] [PubMed] [Google Scholar]