Abstract
Objective
To assess the value of applying MultiVane to liver T2-weighted imaging (T2WI) compared with conventional T2WIs with emphasis on detection of focal liver lesions.
Materials and Methods
Seventy-eight patients (43 men and 35 women) with 86 hepatic lesions and 20 pancreatico-biliary diseases underwent MRI including T2WIs acquired using breath-hold (BH), respiratory-triggered (RT), and MultiVane technique at 3T. Two reviewers evaluated each T2WI with respect to artefacts, organ sharpness, and conspicuity of intrahepatic vessels, hilar duct, and main lesion using five-point scales, and made pairwise comparisons between T2WI sequences for these categories. Diagnostic accuracy (Az) and sensitivity for hepatic lesion detection were evaluated using alternative free-response receiver operating characteristic analysis.
Results
MultiVane T2WI was significantly better than BH-T2WI or RT-T2WI for organ sharpness and conspicuity of intrahepatic vessels and main lesion in both separate reviews and pairwise comparisons (p < 0.001). With regard to motion artefacts, MultiVane T2WI or BH-T2WI was better than RT-T2WI (p < 0.001). Conspicuity of hilar duct was better with BH-T2WI than with MultiVane T2WI (p = 0.030) or RT-T2WI (p < 0.001). For detection of 86 hepatic lesions, sensitivity (mean, 97.7%) of MultiVane T2WI was significantly higher than that of BH-T2WI (mean, 89.5%) (p = 0.008) or RT-T2WI (mean, 84.9%) (p = 0.001).
Conclusion
Applying the MultiVane technique to T2WI of the liver is a promising approach to improving image quality that results in increased detection of focal liver lesions compared with conventional T2WI.
Keywords: Magnetic resonance imaging, 3T MRI, MultiVane, T2-weighted imaging
INTRODUCTION
Liver magnetic resonance imaging (MRI) has an advantage over other liver imaging modalities because it provides multiparametric information by combining a variety of contrast agents such as conventional gadolinium chelates and tissue-targeted agents as well as baseline or novel sequences such as diffusion-weighted imaging. Although gadolinium-enhanced T1-weighted imaging (T1WI) has been a mainstay for liver MRI, T2-weighted imaging (T2WI) is still considered to be an essential component of the liver MRI protocol because it is useful for differentiation between solid and non-solid masses, specific tumour diagnosis, and assessment of biliary disease (1,2,3,4). Technologic innovations, such as improved MRI gradient performance, multichannel surface receiver coils, parallel imaging techniques, and improved fat suppression schemes, have enabled high-quality T2WI of the liver to be obtained using breath-holding or respiratory triggering (5,6). Nevertheless, a limiting factor of liver T2WI is artefacts caused by respiratory motion or other patient motion, even during suspended respiration or respiratory triggering.
The standard T2WI is based on a turbo-spin-echo (TSE) sequence with a Cartesian k-space sampling. For T2WI free from motion artefacts, modifications to the basic protocol may include the addition of free-breathing sequences, respiratory-gating, and motion correction techniques (i.e., BLADE, PROPELLER, or radial acquisition of k-space) (7,8,9,10,11,12,13,14). Periodically rotated overlapping parallel lines with enhanced reconstruction (PROPELLER [General Electric Medical Systems, Milwaukee, WI, USA] or BLADE [Siemens Medical Systems, Erlangen, Germany]) or MultiVane (Philips Healthcare, Best, the Netherlands) is a technique that employs multiple data acquisition at the k-space center for the correction of in-plane motion by matching low-resolution images reconstructed from each set of echo train segments (10,13). MultiVane is a recently introduced motion correction technique that is similar to the PROPELLER technique (13) using rotating blades in the k-space, which makes it possible to detect in-plane motion that affects the low frequency in the center of k-space and correct in-plane motion for each blade to reduce motion effects in the images.
Several prior studies have shown that application of the PROPELLER or BLADE technique to liver imaging at 1.5T or 3T, resulted in improved image quality and lesion detection (15,16,17). However, no study with a large case series has proven sensitivity or accuracy with liver T2WI using such techniques superior to that of conventional T2WI at 3T for detection of focal liver lesions. Because applying the PROPELLER technique is time-consuming, determining its real benefits, including lesion detectability, compared to conventional techniques is clinically relevant. Therefore, we undertook this study to assess the value of MultiVane T2WI compared with conventional T2WIs in the evaluation of the liver with emphasis on detection of focal liver lesions.
MATERIALS AND METHODS
Study Sample
Our retrospective study had Institutional Review Board approval, and informed consent was waived. From July 2013 to November 2013, 78 patients (43 men and 35 women; age range, 33-80 years; mean age, 57 years) with suspected pathology in the liver and pancreatico-biliary system based on clinical and laboratory findings underwent upper abdominal MRI including T2WI acquired using MultiVane technique. Of the 78 patients, 58 had focal liver lesions as follows: 23 had 35 hepatocellular carcinoma (HCC) (size range, 0.8-9 cm), 15 had 18 hemangioma (size range, 0.6-3.0 cm), 12 had 25 metastases from colorectal cancer (size range, 0.5-3.5 cm), four had four abscesses (size range, 1.5-4.5 cm), three had three masses forming intrahepatic cholangiocarcinoma (size range, 3.5-5.5 cm), and one had focal nodular hyperplasia (size 10 cm). The remaining 20 patients had pancreatic cystic lesions including intraductal papillary mucinous neoplasm (n = 12) and serous cystic neoplasm (n = 1), common bile duct stone (n = 4), and pancreatic ducal adenocarcinoma (n = 3). All 23 patients with HCCs and one patient with cholangiocarcinoma had liver cirrhosis or chronic hepatitis associated with viral hepatitis B (n = 21) and viral hepatitis C (n = 3). The remaining patients had no underlying chronic liver disease.
The diagnosis of HCC was confirmed by histopathological findings of surgical specimens for seven patients (10 HCCs) and non-invasive criteria for HCC in the remaining HCCs as they showed arterial hypervascularization and early wash-out on both four-phase computed tomography and MRI (18). The confirmation of liver metastases was based on surgical resection in four patients, or a combined interpretation of all imaging modalities including follow-up multidetector CT or MRI in the remaining eight. Three cholangiocarcinomas, four pancreatic adenocarcinomas, four pancreatic cystic tumours, and one focal nodular hyperplasia were diagnosed by surgery. The diagnosis of hemangioma and pancreatic cystic lesions were based on their typical imaging finding.
MR Examination
All MRIs were acquired using a 3T whole-body MR system (Ingenia, Philips Healthcare, Best, the Netherlands) equipped with a dual-source parallel radiofrequency transmission system and quadrature body coil. The baseline MRIs included a T1-weighted turbo field-echo in-phase and opposed sequence (repetition time [TR]/first echo time [TE], second TE, 3.5 msec/1.13 msec [in-phase], 2.3 msec [opposed-phase]; flip angle, 10°; matrix size, 256 × 194; bandwidth, 1785.7 Hz/pixel). The gadoxetic acid-enhanced imaging that consisted of unenhanced, arterial-phase (20-35 seconds), portal-phase (60 seconds), 3-minute late-phase, and 20-minute hepatobiliary images were obtained using a T1-weighted three-dimensional turbo-field-echo sequence (enhanced T1 high-resolution isotropic volume examination; eTHRIVE, Philips Healthcare) (TR/TE, 3.1 msec/1.5 msec; flip angle, 10°; matrix size, 256 × 256; bandwidth, 724.1 Hz/pixel; 2-mm section thickness). The contrast agent was automatically administered intravenously at a rate of 1 mL/sec for a dose of 0.025 mmol/kg body weight using a power injector, followed by a 20-mL saline flush.
Three T2WIs were successively acquired during the long interval between the dynamic phases and the 20-minute hepatobiliary phase images (at approximately 7 minutes after administration of the contrast agent) to shorten the image acquisition time. The T2WI protocol was as follows: 1) breath-hold multi-shot T2-weighted sequence (TR/TE, 1037 msec/80 msec; flip angle, 90°; matrix size, 324 × 235; reconstructed matrix size, 384 × 384; TSE factor, 14; acceleration factor of 2; half scan factor, 0.6; maximum slices per breath hold, 10; acquisition time, 1 minute 8 seconds); 2) a respiratory-triggered multi-shot T2-weighted sequence (TR/TE, 1037 msec/80 msec; flip angle, 90°; matrix size, 376 × 270; reconstructed matrix size, 382 × 378; TSE factor, 82; acceleration factor of 4; half scan factor, 0.6; acquisition time, 3 minutes); and 3) MultiVane T2WI with respiratory triggering (TR/TE, 1554 msec/103 msec; flip angle, 90°; matrix size, 304 × 304; reconstructed matrix size, 319 × 319; TSE factor, 32; MultiVane percentage, 230%; acquisition time, 6 minutes 30 seconds), with a 5-mm section thickness, no intersection gap, and a field of view of 32-38 cm. Since motion in the liver is expected to appear through plane motion in axial imaging, gross motion correction was not applied in order to shorten examination time. The fat suppression technique was a spectral attenuated inversion recovery technique for RT-T2WI and spectral presaturation with inversion recovery for BH-T2WI and MultiVane T2WI.
Image Analysis
Assessments of all three T2WIs were performed in a blinded fashion by consensus of two readers with 4 years and 14 years of experience in abdominal MRI. Both reviewers were unaware of the sequence parameters and patient information. Adjustment of the window level and width was allowed during the qualitative assessment. At the first reading session, three T2WIs were reviewed by consensus of two reviewers with regard to respiratory motion artefact, anatomic sharpness of upper abdominal organ margin (liver, gallbladder, and pancreas), conspicuity of intrahepatic vessels (portal vein and hepatic vein) and hilar bile duct (first and second order branch), and conspicuity of main lesion on each patient using a five-point evaluation scale system in which 1 = unacceptable, 2 = poor, 3 = fair, 4 = good, and 5 = excellent. For patients with multiple lesions, the study coordinator, who was not involved in image interpretation, assigned the smallest lesion as the target lesion prior to image review. Each review was performed on three separate days separated by at least 2 weeks.
After reviewing individual T2WIs, each reader independently reviewed T2WIs side-by-side for each patient for pairwise comparison followed by joint evaluation until a consensus was reached on the results. In total, 234 T2WI scans were classified into 156 pairs of MultiVane T2WI versus BH-T2WI or MultiVane T2WI versus RT-T2WI in which MultiVane T2WI was the control image. Two image sets were displayed side by side, and evaluation was performed by direct comparison. The readers assessed the image sets by considering five aspects: motion artefact, anatomic sharpness of organ margin, conspicuity of intrahepatic vessels, conspicuity of hilar bile duct, and conspicuity of main lesion. A semi-quantitative five-point scale (1 = inferior [impairing diagnosis], 2 = slightly inferior [no influence on diagnosis], 3 = equal, 4 = slightly superior [no influence on diagnosis], and 5 = superior [easing diagnosis]) (19). In addition, in patients with focal liver lesions, the observers recorded the possibility of a hepatic lesion by assigning each lesion a confidence level, based on a 4-point scale as follows: 1 = probably not a lesion, 2 = possibly not a lesion, 3 = probably a lesion, and 4 = definitely a lesion. Lesions not detected on MRI were given a rating of 0.
Statistical Analysis
Statistical analyses were performed using statistical software (SPSS version 18.0; SPSS Inc., Chicago, IL, USA) and SAS version 9.4 (SAS Institute, Cary, NC, USA). Subjective image ratings for each image were compared using the Friedman test. Dunn's multiple-comparisons test was used as the post-hoc test. The pairwise comparisons were performed using the Wilcoxon test corrected for multiple comparisons according to the Bonferroni adjustment. The diagnostic accuracy of each pulse sequence for the detection of focal hepatic lesions was determined using an alternative free-response receiver operator characteristic (ROC) curve analysis on a lesion-by-lesion basis. Considering the possible influence of lesion clustering on the diagnostic accuracy, nonparameric analysis of clustered ROC curve data was performed by using the method proposed by Obuchowski (20). The sensitivity for each set of images was evaluated according to the number of lesions assigned confidence levels of 3 or 4 from among the 86 liver lesions. We used our own method (21) for comparing sensitivity, specificity, and positive predictive value. A p value < 0.05 was considered significant. The kappa statistic for two observers was calculated to assess the inter-observer agreement regarding pairwise comparison between T2WIs.
RESULTS
Individual Analysis for Each T2WI
Table 1 summarises the results of consensus review for motion artefact; anatomic sharpness of organs margin; and conspicuity of intrahepatic vessels, hilar bile duct, and main lesion for the three different T2WI sequences. There were significant differences among the three sequences for all categories (p < 0.001). With regard to the severity of motion artefacts, MultiVane T2WI or BH-T2WI was better than RT-T2WI (p < 0.001) (Fig. 1). The mean value for the subjective assignment of anatomic sharpness of upper abdominal organ margin was highest in MultiVane T2WI (4.95), followed by BH-T2WI (4.65) and RT-T2WI (4.33), with a significant difference among the sequences (p < 0.001). The conspicuity of intrahepatic vessels and the main lesion was significantly better with MultiVane T2WI than with BH-T2WI or RT-T2WI (p < 0.001) (Fig. 2). Meanwhile, the mean value for conspicuity of hilar duct was highest in BH-T2WI (4.91), followed by MultiVane T2WI (4.77) and RT-T2WI (4.42), with a significant difference among the sequences (p < 0.001 or p = 0.030).
Table 1. Results of Subjective Image Analysis Using Five-Point Evaluation Scale System for Each MRI Sequence.
| Artefact | Organ Sharpness | Vessel Conspicuity | Hilar Duct Conspicuity | Lesion Conspicuity | |
|---|---|---|---|---|---|
| BH-T2WI | 4.92 ± 0.31 | 4.65 ± 0.66 | 4.44 ± 0.68 | 4.91 ± 0.37 | 4.54 ± 0.99 |
| RT-T2WI | 4.08 ± 0.99 | 4.33 ± 0.66 | 4.62 ± 0.67 | 4.42 ± 0.85 | 4.58 ± 0.95 |
| MultiVane T2WI | 4.95 ± 0.22 | 4.95 ± 0.22 | 4.92 ± 0.27 | 4.77 ± 0.45 | 4.92 ± 0.48 |
| P value | < 0.001*,‡, 0.570† | < 0.001*,†,‡ | 0.070*, < 0.001†,‡ | < 0.001*,‡, 0.030† | 0.550*, 0.001†,‡ |
Data represents mean values ± 1SD. *P values are BH-T2WI and RT-T2WI, †BH-T2WI and MultiVane T2WI, ‡RT-T2WI and MultiVane T2WI. BH-T2WI = breath-hold multi-shot T2-weighted image, RT-T2WI = respiratory-triggered multi-shot T2-weighted image, SD = standard deviation
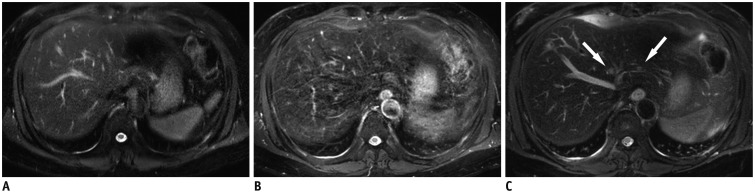
Fig. 1. 57-year-old man diagnosed with colon cancer with liver metastasis.
Small two liver metastases (0.3-0.4 cm in diameter) are indistinct on breath-hold T2-weighted image (A) and respiratory-triggered T2-weighted image (B), but are clearly seen on MultiVane T2-weighted image (arrows) (C). Severe motion artefact is noted in (B).
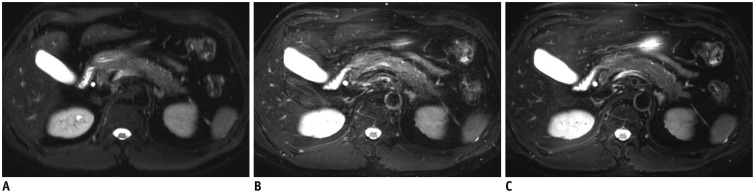
Fig. 2. Breath-hold T2-weighted image (A), respiratory-triggered T2-weighted image (B), and MultiVane T2-weighted image (C) in 36-year-old man.
Sharpness of pancreas margin was considered to be better with (C) than with (A) or (B).
Pairwise Comparison between MultiVane T2WI and BH- or RT-T2WI
In pairwise comparisons between MultiVane T2WI and BH-T2WI or RT-T2WI, the motion artefact of MultiVane T2WI was significantly lower than that of RT-T2WI (p < 0.001) (Fig. 1), but there was no difference between MultiVane T2WI and BH-T2WI (p = 0.250) (Table 2). In terms of the anatomic sharpness of upper abdominal organs and conspicuity of intrahepatic vessels, MultiVane T2WI was significantly better than BH-T2WI or RT-T2WI (p < 0.001) (Fig. 2). Similarly, for conspicuity of the main lesion, MultiVane T2WI was significantly better than BH-T2WI or RT-T2WI (p < 0.001) (Fig. 3). However, with regard to conspicuity of the hilar bile duct, BH-T2WI was significantly better than MultiVane T2WI (p = 0.005), and MultiVane T2WI was significantly better than RT-T2WI (p = 0.004). In pairwise comparison between MultiVane T2WI and BH-T2WI, two cases were judged as slightly inferior (score 2) with MultiVane T2WI compared with BH-T2WI for artefact, lesion conspicuity, and organ sharpness, and three cases had score 2 for vessel conspicuity. In the remaining cases, MultiVane T2WI was judged as being better than or equivalent to BH-T2WI (score 3-5). In pairwise comparison between MultiVane T2WI and RT-T2WI, only one case was judged as slightly inferior (score 2) with MultiVane T2WI compared with RT-T2WI for organ sharpness and vessel conspicuity. For the remaining cases, MultiVane T2WI was judged as being better than or equivalent to RT-T2WI (score 3-5).
Table 2. Result of Pairwise Comparison among MRI Sequences.
| Artefact | Organ Sharpness | Vessel Conspicuity | Hilar Duct Conspicuity | Lesion Conspicuity | ||
|---|---|---|---|---|---|---|
| MultiVane T2WI-BH-T2WI | Mean | 3.06 | 3.62 | 3.46 | 2.86 | 3.31 |
| Median | 3.0 (2.0-5.00) | 4.0 (2.0-5.0) | 3.0 (2.0-5.0) | 3.0 (2.0-4.0) | 3.0 (2.0-5.0) | |
| P value | 0.250 | < 0.001 | < 0.001 | 0.005 | < 0.001 | |
| MultiVane T2WI-RT-T2WI | Mean | 3.80 | 3.46 | 3.41 | 3.13 | 3.23 |
| Median | 3.0 (3.0-5.0) | 3.0 (2.0-5.0) | 3.0 (2.0-5.0) | 3.0 (3.0-5.0) | 3.0 (3.0-5.0) | |
| P value | < 0.001 | < 0.001 | < 0.001 | 0.004 | < 0.001 |
Numbers in parentheses represent range of scoring, respectively. BH-T2WI = breath-hold multi-shot T2-weighted image, RT-T2WI = respiratory-triggered multi-shot T2-weighted image
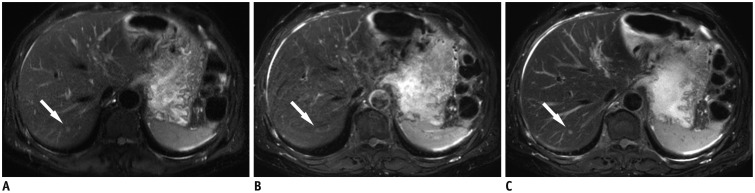
Fig. 3. 60-year-old man diagnosed with colon cancer with liver metastasis.
Small liver metastasis (0.3 cm in diameter) (arrows) is indistinct on breath-hold T2-weighted image (A) and respiratory-triggered T2-weighted image (B), however is clearly seen on MultiVane T2-weighted image (arrow) (C). Motion artefact is noted in (B). Conspicuity of intrahepatic portal vein and hepatic vein is better with (C) than with (A) or (B).
Hepatic Lesion Detection
With regard to the detection of 86 hepatic lesions in 58 patients, nonparametric analysis of clustered ROC data, sensitivity, and positive predictive value are summarised in Table 3. The Az value of MultiVane T2WI (mean, 0.977) was significantly higher than that of BH-T2WI (mean, 0.570; p < 0.001) for both observers. Among 86 focal liver lesions, MultiVane T2WI allowed detection of 85 lesions (98.8%) by observer 1 and 84 lesions (97.7%) by observer 2, whereas 78 (90.7%) and 77 lesions (89.5%) were detected with BH-T2WI, and 74 (86.1%) and 73 lesions (84.9%) were detected with RT-T2WI. Thus, in terms of sensitivity, MultiVane T2WI (mean, 97.7%) yielded a significantly higher value than BH-T2WI (mean, 89.5%) (p = 0.008) or RT-T2WI (mean, 84.9%) (p = 0.001). One single HCC was not detected by all three T2WIs, even on retrospective reviewing. Seven lesions (2 HCCs and 5 metastases) were not verified by any observer on the BH-T2WI or RT-T2WI, but were clearly discerned on the MultiVane T2WI. One HCC (2.0 cm) and three metastases (0.5-1.0 cm) were not seen with BH-T2WI or RT-T2WI, even upon review (Fig. 4). Other lesions were assigned as category 1 or 2 with BH-T2WI or RT-T2WI. There was one false positive lesion on BH-T2WI caused by motion artefact.
Table 3. Diagnostic Performance of Three T2-Weighted Images for Detection of 86 Focal Liver Lesions.
| BH-T2WI | RT-T2WI | MultiVane T2WI | P | ||
|---|---|---|---|---|---|
| Az | Observer 1 | 0.605 | 0.861 | 0.988 | 0.305*, 0.128†, < 0.001‡ |
| Observer 2 | 0.570 | 0.849 | 0.977 | 0.306*, 0.127†, < 0.001‡ | |
| Mean | 0.570 | 0.849 | 0.977 | 0.306*, 0.127†, < 0.001‡ | |
| Sensitivity | Observer 1 | 90.7% (78) | 86.0% (74) | 98.8% (85) | 0.045*, 0.008†, 0.001‡ |
| Observer 2 | 89.5% (77) | 84.9% (73) | 97.7% (84) | 0.045*, 0.008†, 0.001‡ | |
| Mean | 89.5% | 84.9% | 97.7% | 0.045*, 0.008†, 0.001‡ | |
| PPV | Observer 1 | 98.7% [1] | 100.0% [0] | 100.0% [0] | 0.310*, 0.310† |
| Observer 2 | 98.7% [1] | 100.0% [0] | 100.0% [0] | 0.310*, 0.310† | |
| Mean | 98.7% | 100.0% | 100.0% | 0.310*, 0.310† |
P value was corrected by Bonferroni's method for multiple testing. Az values represent area under ROC curve. Numbers in parentheses and brackets represent number of true- and false-positive lesions, respectively. *P values are BH-T2WI and RT-T2WI, †BH-T2WI and MultiVane T2WI, ‡RT-T2WI and MultiVane T2WI. BH-T2WI = breath-hold multi-shot T2-weighted image, PPV = positive predictive value, ROC = receiver operating characteristic, RT-T2WI = respiratory-triggered multi-shot T2-weighted image
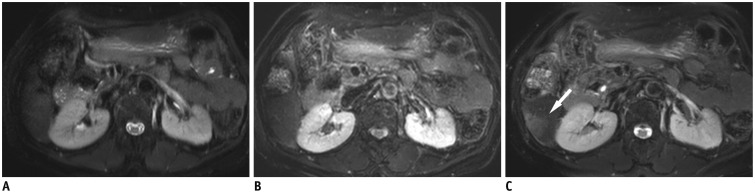
Fig. 4. 59-year-old man with 2.2 cm-sized hepatocellular carcinoma.
Hepatic mass is not depicted on breath-hold T2-weighted image (A) and respiratory-triggered T2-weighted image (B), however is clearly seen as hyperintense (arrow) on MultiVane T2-weighted image (C).
The kappa values for two observers ranged from 0.654 to 0.899 for the five MR categories, indicating good or excellent inter-observer agreement (Table 4).
Table 4. Inter-Observer Agreements Regarding Pairwise Comparison between T2WIs.
| Category | Pairwise Comparison | Kappa Value |
|---|---|---|
| Motion artefact | MultiVane T2WI vs. BH-T2WI | 0.731 |
| MultiVane T2WI vs. RT-T2WI | 0.870 | |
| Anatomic sharpness of organs | MultiVane T2WI vs. BH-T2WI | 0.874 |
| MultiVane T2WI vs. RT-T2WI | 0.654 | |
| Conspicuity of intrahepatic vessels | MultiVane T2WI vs. BH-T2WI | 0.729 |
| MultiVane T2WI vs. RT-T2WI | 0.672 | |
| Conspicuity of hilar duct | MultiVane T2WI vs. BH-T2WI | 0.842 |
| MultiVane T2WI vs. RT-T2WI | 0.899 | |
| Conspicuity of main lesion | MultiVane T2WI vs. BH-T2WI | 0.847 |
| MultiVane T2WI vs. RT-T2WI | 0.851 |
BH-T2WI = breath-hold multi-shot T2-weighted image, RT-T2WI = respiratory-triggered multi-shot T2-weighted image
DISCUSSION
For the diagnosis of focal liver lesions, the two-fold requirement for liver imaging is to improve sensitivity for malignancy detection while maintaining high specificity by accurately characterising small hemangiomas or cysts that are frequently encountered in daily practice (4). As a result of this compelling need for high-quality T2WI, there has been a steady stream of investigations on T2WI sequences that show superiority in image quality and diagnostic performance for detecting focal liver lesions among various T2WIs (6,7,11,12).
Since single shot breath-hold techniques for liver T2WI has the disadvantage of relatively low spatial resolution and signal-to-noise ratio (SNR), we applied a multi-shot technique to BH-T2WI, resulting in an increased matrix size (324 × 235). Therefore, differences in matrix size were minimised among the three T2WIs for fair comparison. In the current study, MultiVane T2WI was significantly better than BH-T2WI with regard to anatomic sharpness of organ and conspicuity of intrahepatic vessels and main lesion in both separate reviews and pairwise comparison (p < 0.05), although there was no significant difference in artefacts between the sequences. In pairwise comparison between MultiVane T2WI and BH-T2WI, only two cases were judged as slightly inferior (score 2) with MultiVane T2WI compared with BH-T2WI for artefact, lesion conspicuity, and organ sharpness and three cases were slightly inferior for vessel conspicuity. In the remaining cases, MultiVane T2WI was better than or equivalent to BH-T2WI. In comparison between MultiVane T2WI and RT-T2WI, MultiVane T2WI was significantly better than RT T2WI for all five categories, both in separate reviewing and pairwise comparison (p < 0.05). Only one case was judged as slightly inferior (score 2) with MultiVane T2WI compared with RT-T2WI for organ sharpness and vessel conspicuity. In the remaining cases, MultiVane T2WI was better than or equivalent to RT-T2WI. Theoretical advantages of central k-space oversampling using BLADE, PROPELLER, or MultiVane is the reduction of artefacts associated with gross motion and a better SNR (8,9,10,13). However, motion of the upper abdomen is mainly through-plane motion from respiratory movement rather than in-plane motion that occurs in a rigid body such as the brain. Therefore, gross motion correction was not applied in our study. Nevertheless, our results showed the usefulness of applying MultiVane technique for high-quality liver T2WI, which are consistent with prior reports (8,9,10).
Liver MRI is more vulnerable to artefacts than MRI for other organs because of the proximity of the liver to lung or the gastrointestinal tract, which hinders lesion detection. In addition, clear differentiation between tiny lesions and end-on views of vessels is problematic in T2WI with poor image quality. Thus, the results for qualitative analysis regarding liver lesion detection seem to be a more relevant indicator of image contrast and quality than quantitative analysis. With regard to the detection of 86 hepatic lesions, the Az value and sensitivity of MultiVane T2WI were significantly higher than those of BH-T2WI or RT-T2WI for both observers (p < 0.05). With the popular use of gadoxetic acid for liver MRI, the role of T2WI for lesion characterisation is being emphasised because early hepatocyte uptake of gadoxetic acid might cause false-positive diagnosis of a small haemangioma as malignancy (22,23). In this regard, our observations have clinical impact. In the aforementioned cases, BH-T2WI or RT-T2WI did not suffer from severe artefacts that hindered tumour detection. In addition to the benefit of artefact-free images in MultiVane T2WI, another possible reason for our findings is higher SNR and contrast-to-noise ratio (CNR) in MultiVane T2WI as a result of acquiring multiple data at the k-space centre, which is responsible for contrast resolution (15). However, we did not acquire quantitative results because there has been no perfect noise estimation given the use of parallel imaging for BH- or RT-T2WI and T2WIs were acquired after the administration of gadoxetic acid (24).
With regard to the conspicuity of the hilar bile duct, BH-T2WI was significantly better than MultiVane T2WI (p = 0.005). Based on the above considerations, it is recommended to acquire both BH-T2WI, possibly as heavily T2WI, and MultiVane T2WI. However, the long acquisition time for MultiVane T2WI (mean, 6 minutes 30 seconds) may be a burden in a time-pressed environment. The MultiVane T2WI used in the current study was acquired with neither parallel imaging nor half scan and interleaved sampling. Further investigations with application of those techniques to MultiVane T2WI will be warranted to shorten image acquisition time while maintaining high image quality. In the gadoxetic acid-enhanced MRI protocol, introducing MultiVane T2WI during the long waiting time between early dynamic phases and the 20-minute hepatobiliary phase could be a reasonable approach to adopting a shortened protocol, because T2WIs acquired after administration of gadoxetic acid are reported to be comparable to pre-contrast T2WIs in the detection and characterization of hepatic tumours (25,26,27).
There are several limitations in our study that should be mentioned. First, owing to the retrospective study design, we acquired T2WIs during the interval between the dynamic images and the 20-minute hepatobiliary phase in order to shorten examination time. Given that signal intensity of the lesion or liver might be altered by T1- or T2 effects of administered gadoxetic acid (25,28), a bias might be introduced to the conspicuity of the hepatic lesion. However, lesion-liver contrast on postcontrast T2WI is reportedly comparable to that on precontrast T2WI (25,26,27,28). Second, there was a minimal difference in matrix size among the three T2WIs. However, despite the fact that MultiVane T2WI had the lowest reconstructed matrix size, we achieved better results with MultiVane T2WI than the other two techniques. Third, although image review was performed in a blinded manner, an experienced on-site reviewer might recognize which technique was applied. Fourth, we did not attempt to adjust parameters to optimise MultiVane T2WI for the liver by multiple testing of MultiVane percentages to balance the trade-off between image quality and acquisition time. Fifth, as previously mentioned, we did not perform quantitative measurement for SNR and CNR because of variability in image acquisition techniques among the three T2WI sequences. In addition, as a result of the retrospective study design, we did not optimise conventional BH-T2WI or RT-T2WI to maximise SNR and CNR.
In conclusion, application of the MultiVane technique to T2WI of the liver is a promising approach to improving image quality that leads to increased detection of focal liver lesions compared with conventional T2WIs.
References
- 1.Hussain HK, Syed I, Nghiem HV, Johnson TD, Carlos RC, Weadock WJ, et al. T2-weighted MR imaging in the assessment of cirrhotic liver. Radiology. 2004;230:637–644. doi: 10.1148/radiol.2303020921. [DOI] [PubMed] [Google Scholar]
- 2.Kim YK, Lee YH, Kim CS, Han YM. Added diagnostic value of T2-weighted MR imaging to gadolinium-enhanced three-dimensional dynamic MR imaging for the detection of small hepatocellular carcinomas. Eur J Radiol. 2008;67:304–310. doi: 10.1016/j.ejrad.2007.07.001. [DOI] [PubMed] [Google Scholar]
- 3.Maetani Y, Itoh K, Watanabe C, Shibata T, Ametani F, Yamabe H, et al. MR imaging of intrahepatic cholangiocarcinoma with pathologic correlation. AJR Am J Roentgenol. 2001;176:1499–1507. doi: 10.2214/ajr.176.6.1761499. [DOI] [PubMed] [Google Scholar]
- 4.McFarland EG, Mayo-Smith WW, Saini S, Hahn PF, Goldberg MA, Lee MJ. Hepatic hemangiomas and malignant tumors: improved differentiation with heavily T2-weighted conventional spin-echo MR imaging. Radiology. 1994;193:43–47. doi: 10.1148/radiology.193.1.8090920. [DOI] [PubMed] [Google Scholar]
- 5.Reinig JW. Breath-hold fast spin-echo MR imaging of the liver: a technique for high-quality T2-weighted images. Radiology. 1995;194:303–304. doi: 10.1148/radiology.194.2.7824701. [DOI] [PubMed] [Google Scholar]
- 6.Tang Y, Yamashita Y, Namimoto T, Abe Y, Takahashi M. Liver T2-weighted MR imaging: comparison of fast and conventional half-Fourier single-shot turbo spin-echo, breath-hold turbo spin-echo, and respiratory-triggered turbo spin-echo sequences. Radiology. 1997;203:766–772. doi: 10.1148/radiology.203.3.9169702. [DOI] [PubMed] [Google Scholar]
- 7.Augui J, Vignaux O, Argaud C, Coste J, Gouya H, Legmann P. Liver: T2-weighted MR imaging with breath-hold fast-recovery optimized fast spin-echo compared with breath-hold half-Fourier and non-breath-hold respiratory-triggered fast spin-echo pulse sequences. Radiology. 2002;223:853–859. doi: 10.1148/radiol.2233011011. [DOI] [PubMed] [Google Scholar]
- 8.Hirokawa Y, Isoda H, Maetani YS, Arizono S, Shimada K, Togashi K. Evaluation of motion correction effect and image quality with the periodically rotated overlapping parallel lines with enhanced reconstruction (PROPELLER) (BLADE) and parallel imaging acquisition technique in the upper abdomen. J Magn Reson Imaging. 2008;28:957–962. doi: 10.1002/jmri.21538. [DOI] [PubMed] [Google Scholar]
- 9.Hirokawa Y, Isoda H, Maetani YS, Arizono S, Shimada K, Togashi K. MRI artifact reduction and quality improvement in the upper abdomen with PROPELLER and prospective acquisition correction (PACE) technique. AJR Am J Roentgenol. 2008;191:1154–1158. doi: 10.2214/AJR.07.3657. [DOI] [PubMed] [Google Scholar]
- 10.Kiryu S, Watanabe M, Kabasawa H, Akahane M, Aoki S, Ohtomo K. Evaluation of super paramagnetic iron oxide-enhanced diffusion-weighted PROPELLER T2-fast spin echo magnetic resonance imaging: preliminary experience. J Comput Assist Tomogr. 2006;30:197–200. doi: 10.1097/00004728-200603000-00005. [DOI] [PubMed] [Google Scholar]
- 11.Klessen C, Asbach P, Kroencke TJ, Fischer T, Warmuth C, Stemmer A, et al. Magnetic resonance imaging of the upper abdomen using a free-breathing T2-weighted turbo spin echo sequence with navigator triggered prospective acquisition correction. J Magn Reson Imaging. 2005;21:576–582. doi: 10.1002/jmri.20293. [DOI] [PubMed] [Google Scholar]
- 12.Lee SS, Byun JH, Hong HS, Park SH, Won HJ, Shin YM, et al. Image quality and focal lesion detection on T2-weighted MR imaging of the liver: comparison of two high-resolution free-breathing imaging techniques with two breath-hold imaging techniques. J Magn Reson Imaging. 2007;26:323–330. doi: 10.1002/jmri.21002. [DOI] [PubMed] [Google Scholar]
- 13.Pipe JG. Motion correction with PROPELLER MRI: application to head motion and free-breathing cardiac imaging. Magn Reson Med. 1999;42:963–969. doi: 10.1002/(sici)1522-2594(199911)42:5<963::aid-mrm17>3.0.co;2-l. [DOI] [PubMed] [Google Scholar]
- 14.Zech CJ, Herrmann KA, Huber A, Dietrich O, Stemmer A, Herzog P, et al. High-resolution MR-imaging of the liver with T2-weighted sequences using integrated parallel imaging: comparison of prospective motion correction and respiratory triggering. J Magn Reson Imaging. 2004;20:443–450. doi: 10.1002/jmri.20127. [DOI] [PubMed] [Google Scholar]
- 15.Hirokawa Y, Isoda H, Maetani YS, Arizono S, Shimada K, Okada T, et al. Hepatic lesions: improved image quality and detection with the periodically rotated overlapping parallel lines with enhanced reconstruction technique--evaluation of SPIO-enhanced T2-weighted MR images. Radiology. 2009;251:388–397. doi: 10.1148/radiol.2512081360. [DOI] [PubMed] [Google Scholar]
- 16.Haneder S, Dinter D, Gutfleisch A, Schoenberg SO, Michaely HJ. Image quality of T2w-TSE of the abdomen and pelvis with Cartesian or BLADE-type k-space sampling: a retrospective interindividual comparison study. Eur J Radiol. 2011;79:177–182. doi: 10.1016/j.ejrad.2009.12.028. [DOI] [PubMed] [Google Scholar]
- 17.Rosenkrantz AB, Mannelli L, Mossa D, Babb JS. Breath-hold T2-weighted MRI of the liver at 3T using the BLADE technique: impact upon image quality and lesion detection. Clin Radiol. 2011;66:426–433. doi: 10.1016/j.crad.2010.10.018. [DOI] [PubMed] [Google Scholar]
- 18.Bruix J, Sherman M; Management of hepatocellular carcinoma: an update. Hepatology. 2011;53:1020–1022. doi: 10.1002/hep.24199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Deák Z, Grimm JM, Treitl M, Geyer LL, Linsenmaier U, Körner M, et al. Filtered back projection, adaptive statistical iterative reconstruction, and a model-based iterative reconstruction in abdominal CT: an experimental clinical study. Radiology. 2013;266:197–206. doi: 10.1148/radiol.12112707. [DOI] [PubMed] [Google Scholar]
- 20.Obuchowski NA. Nonparametric analysis of clustered ROC curve data. Biometrics. 1997;53:567–578. [PubMed] [Google Scholar]
- 21.Kwak M, Um SW, Jung SH. Comparison of operational characteristics for binary tests with clustered data. Stat Med. 2015 May 20; doi: 10.1002/sim.6485. [Epub] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tamada T, Ito K, Ueki A, Kanki A, Higaki A, Higashi H, et al. Peripheral low intensity sign in hepatic hemangioma: diagnostic pitfall in hepatobiliary phase of Gd-EOB-DTPA-enhanced MRI of the liver. J Magn Reson Imaging. 2012;35:852–858. doi: 10.1002/jmri.23514. [DOI] [PubMed] [Google Scholar]
- 23.Tamada T, Ito K, Yamamoto A, Sone T, Kanki A, Tanaka F, et al. Hepatic hemangiomas: evaluation of enhancement patterns at dynamic MRI with gadoxetate disodium. AJR Am J Roentgenol. 2011;196:824–830. doi: 10.2214/AJR.10.5113. [DOI] [PubMed] [Google Scholar]
- 24.Heverhagen JT. Noise measurement and estimation in MR imaging experiments. Radiology. 2007;245:638–639. doi: 10.1148/radiol.2453062151. [DOI] [PubMed] [Google Scholar]
- 25.Kim YK, Kwak HS, Kim CS, Han YM. Detection and characterization of focal hepatic tumors: a comparison of T2-weighted MR images before and after the administration of gadoxectic acid. J Magn Reson Imaging. 2009;30:437–443. doi: 10.1002/jmri.21839. [DOI] [PubMed] [Google Scholar]
- 26.Song KD, Kim YK, Lee WJ, Lee MW, Park MJ, Hwang J, et al. Detection and characterization of small focal hepatic lesions (≤ 2.5 cm in diameter): a comparison of diffusion-weighted images before and after administration of gadoxetic acid disodium at 3.0T. Acta Radiol. 2012;53:485–493. doi: 10.1258/ar.2012.110437. [DOI] [PubMed] [Google Scholar]
- 27.Muhi A, Ichikawa T, Motosugi U, Sou H, Sano K, Araki T. Diffusion- and T2-weighted MR imaging of the liver: effect of intravenous administration of gadoxetic acid disodium. Magn Reson Med Sci. 2012;11:185–191. doi: 10.2463/mrms.11.185. [DOI] [PubMed] [Google Scholar]
- 28.Jeong YY, Mitchell DG, Holland GA. Liver lesion conspicuity: T2-weighted breath-hold fast spin-echo MR imaging before and after gadolinium enhancement--initial experience. Radiology. 2001;219:455–460. doi: 10.1148/radiology.219.2.r01ma09455. [DOI] [PubMed] [Google Scholar]